Differences in Formation of Prepuce and Urethral Groove During Penile Development Between Guinea Pigs and Mice Are Controlled by Differential Expression of Shh, Fgf10 and Fgfr2
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Organ Culture and In Vitro Cyclopamine, BGJ-398, Shh, and Fgf-10 Administration
2.3. Histology and Immunohistochemistry
2.4. In Situ Hybridization
2.5. Quantitative Gene Expression Analysis Using RT-QPCR
2.6. Statistical Analysis
3. Results
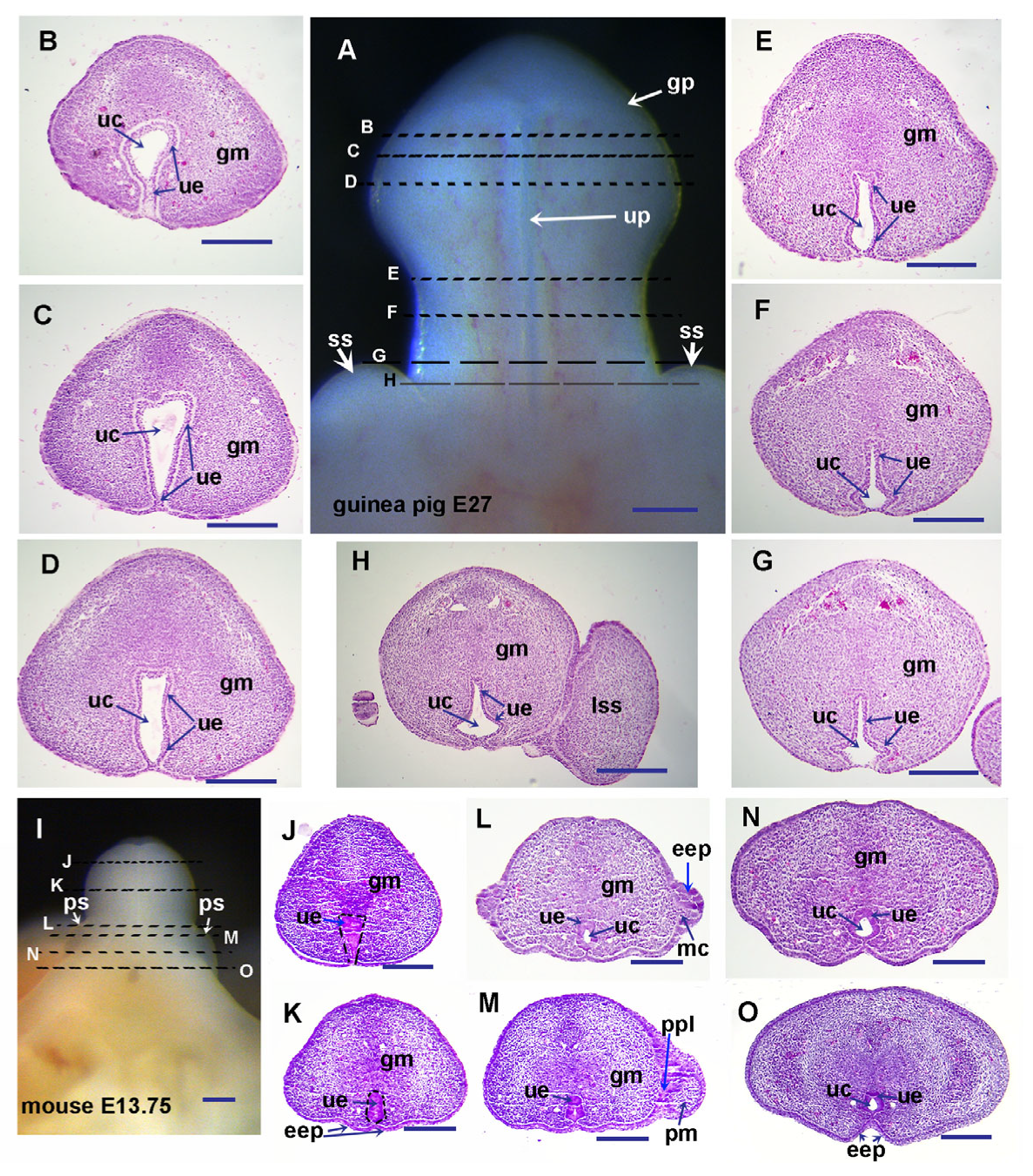
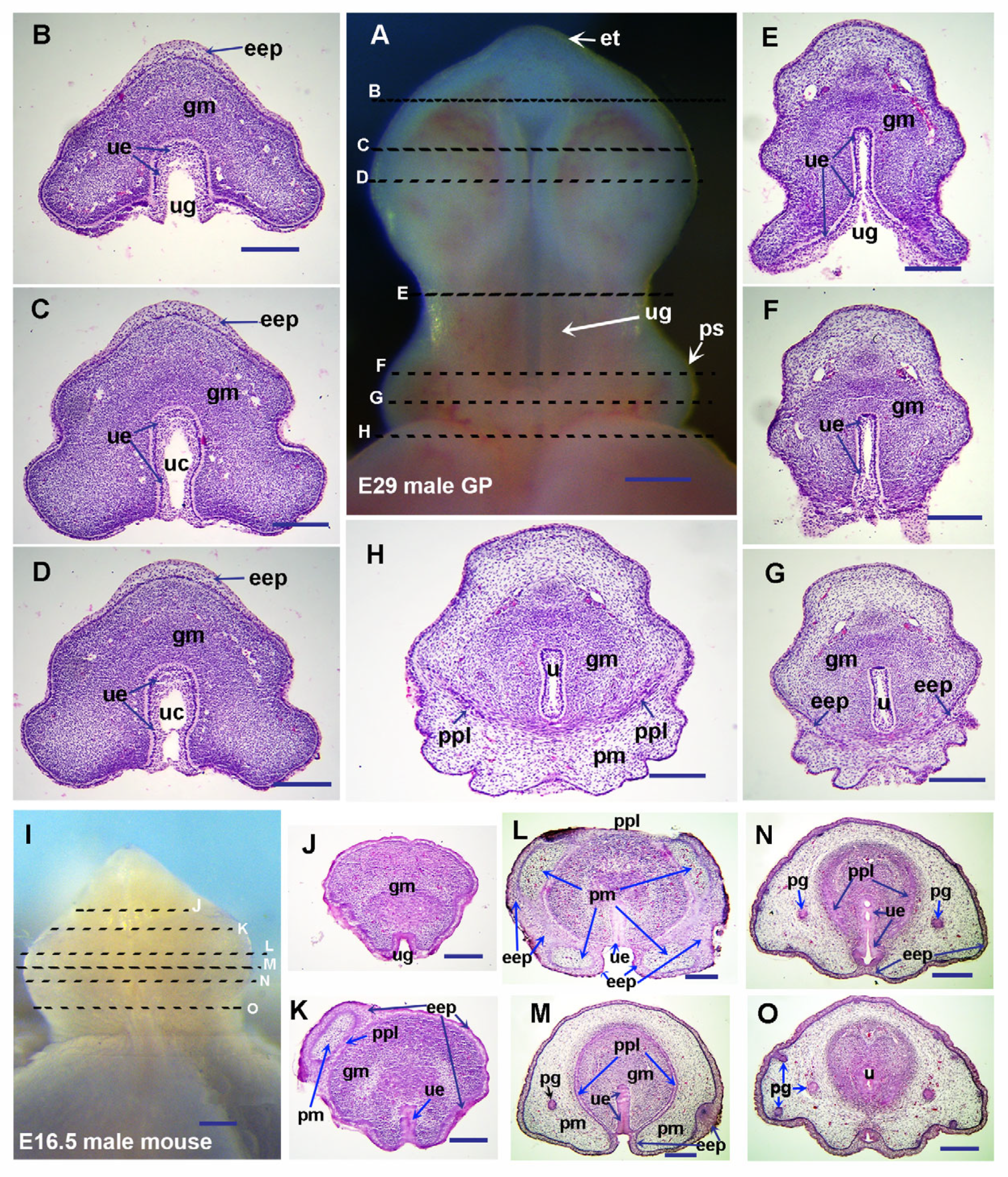
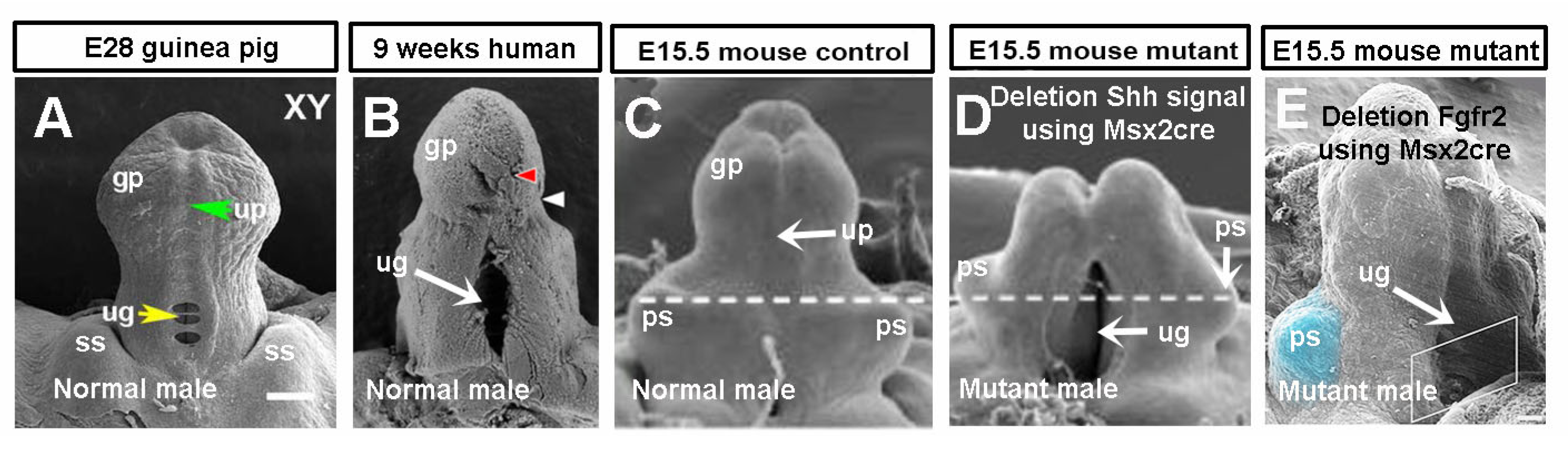
3.1. Guinea Pigs Show Delayed Preputial Formation During the Glans and Preputial Development Compared with Mice
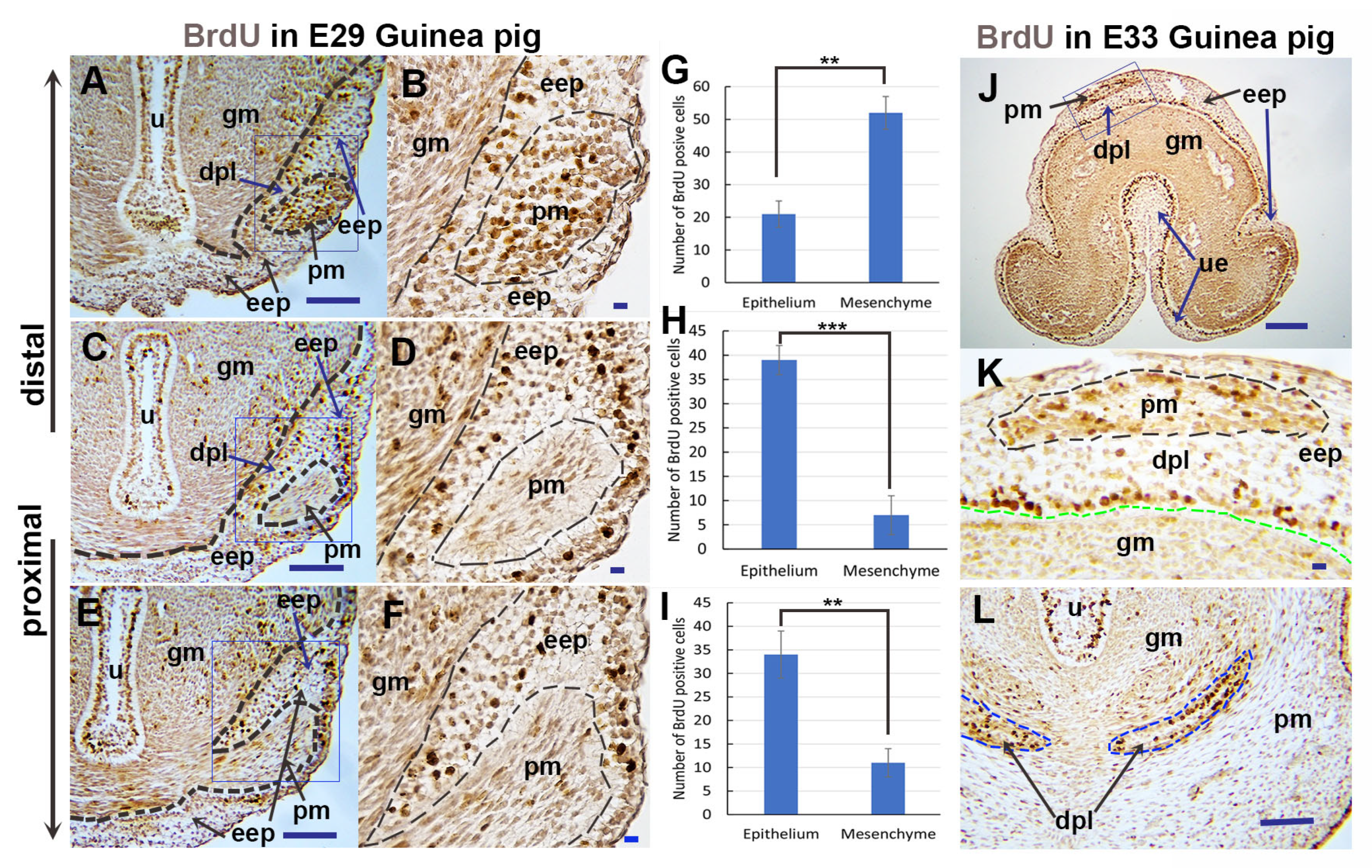
3.2. Differential Cell Proliferation in Epithelium and Mesenchyme Contributes to Preputial Development
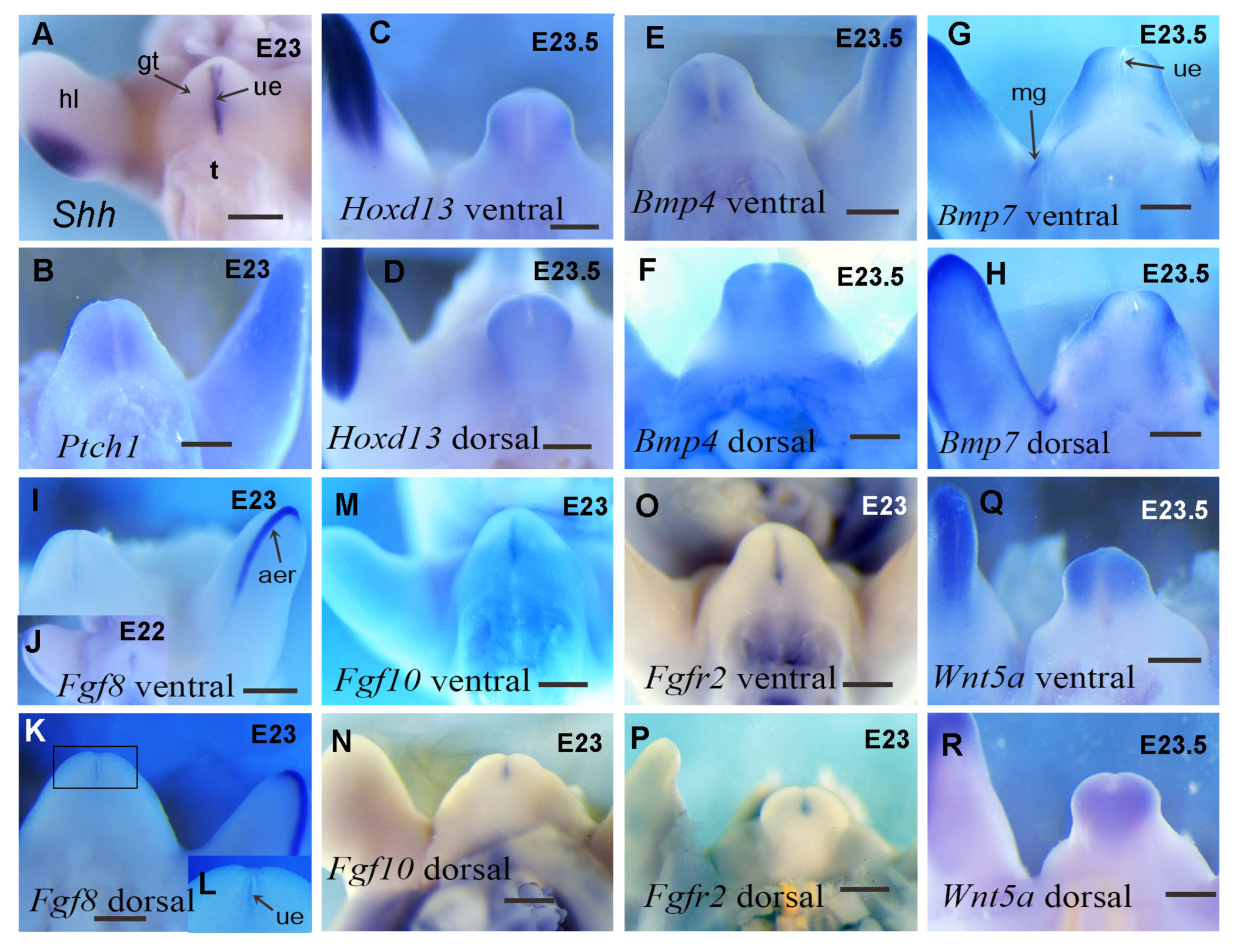
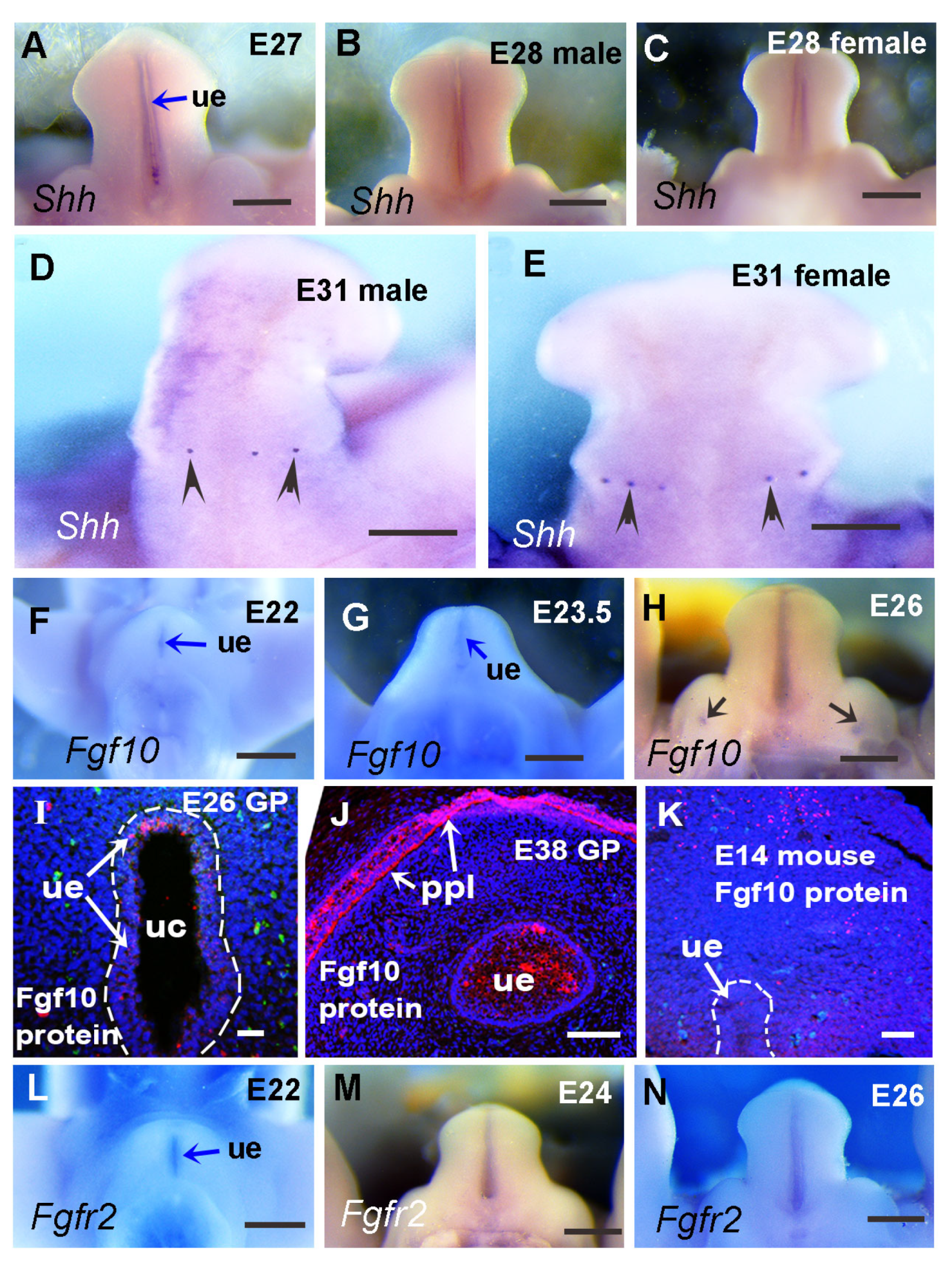
3.3. The Expression Patterns of Key Developmental Genes in Guinea Pig Genital Tubercle
3.4. Relative Expression Levels of Shh, Fgf10, and Fgfr2 in Developing Guinea Pig GT Are Reduced Compared with the Comparable Stage of Mouse GT
3.5. Shh and Fgf10/Fgfr2 Play Key Roles in Preputial and Urethral Groove Formation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Sinclair, A.; Cao, M.; Shen, J.; Choudhry, S.; Botta, S.; Cunha, G.; Baskin, L. Canalization of the urethral plate precedes fusion of the urethral folds during male penile urethral development: The double zipper hypothesis. J. Urol. 2015, 193, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Armfield, B.A.; Cohn, M.J. Timing of androgen receptor disruption and estrogen exposure underlies a spectrum of congenital penile anomalies. Proc. Natl. Acad. Sci. USA 2015, 112, E7194–E7203. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, M.; Zhu, D.; Mathews, R.; Zheng, Z. External Genital Development, Urethra Formation, and Hypospadias Induction in Guinea Pig: A Double Zipper Model for Human Urethral Development. Urology 2018, 113, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.A.; Clark, R.L. External genitalia of the rat: Normal development and the histogenesis of 5 alpha-reductase inhibitor-induced abnormalities. Teratology 1990, 42, 483–496. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Z. Differential cell proliferation and cell death during the urethral groove formation in guinea pig model. Pediatr. Res. 2019, 86, 452–459. [Google Scholar] [CrossRef]
- Perriton, C.L.; Powles, N.; Chiang, C.; Maconochie, M.K.; Cohn, M.J. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev. Biol. 2002, 247, 26–46. [Google Scholar] [CrossRef]
- Hunter, R.H. Notes on the Development of the Prepuce. J. Anat. 1935, 70, 68–75. [Google Scholar]
- Glenister, T.W. A consideration of the processes involved in the development of the prepuce in man. Br. J. Urol. 1956, 28, 243–249. [Google Scholar] [CrossRef]
- Cunha, G.R.; Sinclair, A.; Cao, M.; Baskin, L.S. Development of the human prepuce and its innervation. Differentiation 2020, 111, 22–40. [Google Scholar] [CrossRef]
- Liu, X.; Liu, G.; Shen, J.; Yue, A.; Isaacson, D.; Sinclair, A.; Cao, M.; Liaw, A.; Cunha, G.R.; Baskin, L. Human glans and preputial development. Differentiation 2018, 103, 86–99. [Google Scholar] [CrossRef]
- Cohn, M.J. Development of the external genitalia: Conserved and divergent mechanisms of appendage patterning. Dev. Dyn. 2011, 240, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, S.; Zheng, Z. Etiology of Hypospadias: A Comparative Review of Genetic Factors and Developmental Processes Between Human and Animal Models. Res. Rep. Urol. 2020, 12, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Haller, M.; Ma, L. Temporal, spatial, and genetic regulation of external genitalia development. Differentiation 2019, 110, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Haid, B.; Pechriggl, E.; Nagele, F.; Dudas, J.; Webersinke, G.; Rammer, M.; Fritsch, H.; Oswald, J. FGF8, FGF10 and FGF receptor 2 in foreskin of children with hypospadias: An analysis of immunohistochemical expression patterns and gene transcription. J. Pediatr. Urol. 2020, 16, 41.e1–41.e10. [Google Scholar] [CrossRef]
- Haraguchi, R.; Suzuki, K.; Murakami, R.; Sakai, M.; Kamikawa, M.; Kengaku, M.; Sekine, K.; Kawano, H.; Kato, S.; Ueno, N.; et al. Molecular analysis of external genitalia formation: The role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development 2000, 127, 2471–2479. [Google Scholar] [CrossRef]
- Gredler, M.L.; Seifert, A.W.; Cohn, M.J. Tissue-specific roles of Fgfr2 in development of the external genitalia. Development 2015, 142, 2203–2212. [Google Scholar] [CrossRef]
- Kajioka, D.; Suzuki, K.; Nakada, S.; Matsushita, S.; Miyagawa, S.; Takeo, T.; Nakagata, N.; Yamada, G. Bmp4 is an essential growth factor for the initiation of genital tubercle (GT) outgrowth. Congenit. Anom. 2020, 60, 15–21. [Google Scholar] [CrossRef]
- Ching, S.T.; Infante, C.R.; Du, W.; Sharir, A.; Park, S.; Menke, D.B.; Klein, O.D. Isl1 mediates mesenchymal expansion in the developing external genitalia via regulation of Bmp4, Fgf10 and Wnt5a. Hum. Mol. Genet. 2018, 27, 107–119. [Google Scholar] [CrossRef]
- Lin, C.; Yin, Y.; Long, F.; Ma, L. Tissue-specific requirements of beta-catenin in external genitalia development. Development 2008, 135, 2815–2825. [Google Scholar] [CrossRef]
- Miyagawa, S.; Satoh, Y.; Haraguchi, R.; Suzuki, K.; Iguchi, T.; Taketo, M.M.; Nakagata, N.; Matsumoto, T.; Takeyama, K.; Kato, S.; et al. Genetic interactions of the androgen and Wnt/beta-catenin pathways for the masculinization of external genitalia. Mol. Endocrinol. 2009, 23, 871–880. [Google Scholar] [CrossRef]
- Alcantara, M.C.; Suzuki, K.; Acebedo, A.R.; Sakamoto, Y.; Nishita, M.; Minami, Y.; Kikuchi, A.; Yamada, G. Stage-dependent function of Wnt5a during male external genitalia development. Congenit. Anom. 2021, 61, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Person, A.D.; Beiraghi, S.; Sieben, C.M.; Hermanson, S.; Neumann, A.N.; Robu, M.E.; Schleiffarth, J.R.; Billington, C.J., Jr.; van Bokhoven, H.; Hoogeboom, J.M.; et al. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev. Dyn. 2010, 239, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Warot, X.; Fromental-Ramain, C.; Fraulob, V.; Chambon, P.; Dolle, P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development 1997, 124, 4781–4791. [Google Scholar] [CrossRef] [PubMed]
- Goodman, F.R.; Bacchelli, C.; Brady, A.F.; Brueton, L.A.; Fryns, J.P.; Mortlock, D.P.; Innis, J.W.; Holmes, L.B.; Donnenfeld, A.E.; Feingold, M.; et al. Novel HOXA13 mutations and the phenotypic spectrum of hand-foot-genital syndrome. Am. J. Hum. Genet. 2000, 67, 197–202. [Google Scholar] [CrossRef]
- Tuzel, E.; Samli, H.; Kuru, I.; Turkmen, S.; Demir, Y.; Maralcan, G.; Guler, C. Association of hypospadias with hypoplastic synpolydactyly and role of HOXD13 gene mutations. Urology 2007, 70, 161–164. [Google Scholar] [CrossRef]
- Seifert, A.W.; Zheng, Z.; Ormerod, B.K.; Cohn, M.J. Sonic hedgehog controls growth of external genitalia by regulating cell cycle kinetics. Nat. Commun. 2010, 1, 23. [Google Scholar] [CrossRef]
- Sarac, M.; Canpolat, S.; Onalan Etem, E.; Tektemur, A.; Tartar, T.; Bakal, U.; Kazez, A. The role of sonic hedgehog homologue signal pathway in hypospadias aetiology. J. Pediatr. Urol. 2021, 17, 630.e631–630.e637. [Google Scholar] [CrossRef]
- Depreux, F.F.; Czech, L.; Whitlon, D.S. Sex Genotyping of Archival Fixed and Immunolabeled Guinea Pig Cochleas. Sci. Rep. 2018, 8, 5156. [Google Scholar] [CrossRef]
- Wang, S.; Lawless, J.; Zheng, Z. Prenatal low-dose methyltestosterone, but not dihydrotestosterone, treatment induces penile formation in female mice and guinea pigsdagger. Biol. Reprod. 2020, 102, 1248–1260. [Google Scholar] [CrossRef]
- Nieto, M.A.; Patel, K.; Wilkinson, D.G. In situ hybridization analysis of chick embryos in whole mount and tissue sections. Methods Cell Biol. 1996, 51, 219–235. [Google Scholar] [CrossRef]
- Tan, H.; Wu, G.; Wang, S.; Lawless, J.; Sinn, A.; Chen, D.; Zheng, Z. Prenatal exposure to atrazine induces cryptorchidism and hypospadias in F1 male mouse offspring. Birth Defects Res. 2021, 113, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.W.; Bouldin, C.M.; Choi, K.S.; Harfe, B.D.; Cohn, M.J. Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development 2009, 136, 3949–3957. [Google Scholar] [CrossRef] [PubMed]
- Scott, V.; Morgan, E.A.; Stadler, H.S. Genitourinary functions of Hoxa13 and Hoxd13. J. Biochem. 2005, 137, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.W.; Yamaguchi, T.; Cohn, M.J. Functional and phylogenetic analysis shows that Fgf8 is a marker of genital induction in mammals but is not required for external genital development. Development 2009, 136, 2643–2651. [Google Scholar] [CrossRef][Green Version]
- Yamaguchi, T.P.; Bradley, A.; McMahon, A.P.; Jones, S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 1999, 126, 1211–1223. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef]
- Wanek, N.; Muneoka, K.; Holler-Dinsmore, G.; Burton, R.; Bryant, S.V. A staging system for mouse limb development. J. Exp. Zool. 1989, 249, 41–49. [Google Scholar] [CrossRef]
- Harfe, B.D.; Scherz, P.J.; Nissim, S.; Tian, H.; McMahon, A.P.; Tabin, C.J. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 2004, 118, 517–528. [Google Scholar] [CrossRef]
- Brady, M.V.; Vaccarino, F.M. Role of SHH in Patterning Human Pluripotent Cells towards Ventral Forebrain Fates. Cells 2021, 10, 914. [Google Scholar] [CrossRef]
- Lin, C.; Yin, Y.; Veith, G.M.; Fisher, A.V.; Long, F.; Ma, L. Temporal and spatial dissection of Shh signaling in genital tubercle development. Development 2009, 136, 3959–3967. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Haraguchi, R.; Wright, T.J.; Mansour, S.L.; Partanen, J.; Hajihosseini, M.K.; Eswarakumar, V.P.; Lonai, P.; Yamada, G. Regulation of external genitalia development by concerted actions of FGF ligands and FGF receptors. Anat. Embryol. 2004, 208, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, X.; Shen, J.; Sinclair, A.; Baskin, L.; Cunha, G.R. Contrasting mechanisms of penile urethral formation in mouse and human. Differentiation 2018, 101, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Overland, M.; Sinclair, A.; Cao, M.; Yue, X.; Cunha, G.; Baskin, L. Complex epithelial remodeling underlie the fusion event in early fetal development of the human penile urethra. Differentiation 2016, 92, 169–182. [Google Scholar] [CrossRef][Green Version]
- Beleza-Meireles, A.; Lundberg, F.; Lagerstedt, K.; Zhou, X.; Omrani, D.; Frisen, L.; Nordenskjold, A. FGFR2, FGF8, FGF10 and BMP7 as candidate genes for hypospadias. Eur. J. Hum. Genet. 2007, 15, 405–410. [Google Scholar] [CrossRef]
- Carmichael, S.L.; Ma, C.; Choudhry, S.; Lammer, E.J.; Witte, J.S.; Shaw, G.M. Hypospadias and genes related to genital tubercle and early urethral development. J. Urol. 2013, 190, 1884–1892. [Google Scholar] [CrossRef]
- Tarulli, G.A.; Cripps, S.M.; Pask, A.J.; Renfree, M.B. Spatiotemporal map of key signaling factors during early penis development. Dev. Dyn. 2022, 251, 609–624. [Google Scholar] [CrossRef]
- Petiot, A.; Perriton, C.L.; Dickson, C.; Cohn, M.J. Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development 2005, 132, 2441–2450. [Google Scholar] [CrossRef]
- Bangs, F.; Welten, M.; Davey, M.G.; Fisher, M.; Yin, Y.; Downie, H.; Paton, B.; Baldock, R.; Burt, D.W.; Tickle, C. Identification of genes downstream of the Shh signalling in the developing chick wing and syn-expressed with Hoxd13 using microarray and 3D computational analysis. Mech. Dev. 2010, 127, 428–441. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, A.R.; Yakushiji-Kaminatsui, N.; Atsuta, Y.; Andrey, G.; Schorderet, P.; Duboule, D.; Tabin, C.J. Integration of Shh and Fgf signaling in controlling Hox gene expression in cultured limb cells. Proc. Natl. Acad. Sci. USA 2017, 114, 3139–3144. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zheng, Z. Differences in Formation of Prepuce and Urethral Groove During Penile Development Between Guinea Pigs and Mice Are Controlled by Differential Expression of Shh, Fgf10 and Fgfr2. Cells 2025, 14, 348. https://doi.org/10.3390/cells14050348
Wang S, Zheng Z. Differences in Formation of Prepuce and Urethral Groove During Penile Development Between Guinea Pigs and Mice Are Controlled by Differential Expression of Shh, Fgf10 and Fgfr2. Cells. 2025; 14(5):348. https://doi.org/10.3390/cells14050348
Chicago/Turabian StyleWang, Shanshan, and Zhengui Zheng. 2025. "Differences in Formation of Prepuce and Urethral Groove During Penile Development Between Guinea Pigs and Mice Are Controlled by Differential Expression of Shh, Fgf10 and Fgfr2" Cells 14, no. 5: 348. https://doi.org/10.3390/cells14050348
APA StyleWang, S., & Zheng, Z. (2025). Differences in Formation of Prepuce and Urethral Groove During Penile Development Between Guinea Pigs and Mice Are Controlled by Differential Expression of Shh, Fgf10 and Fgfr2. Cells, 14(5), 348. https://doi.org/10.3390/cells14050348







