Preclinical Evaluation of the Safety, Toxicity and Efficacy of Genetically Modified Wharton’s Jelly Mesenchymal Stem/Stromal Cells Expressing the Antimicrobial Peptide SE-33
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmid Vector Construction
2.2. Mesenchymal Stem/Stromal Cell Culture and Transfection
2.3. Antimicrobial Activity Assessment
2.4. High-Performance Liquid Chromatography
2.5. Animal Studies
2.6. Administration of Genetically Modified WJ-MSCs to Animals
2.7. Mortality and Clinical Signs
2.8. Body Weight and Temperature
2.9. Necropsy and Histopathological Studies
- (1)
- Infiltration or aggregation of inflammatory cells in the airspace or vessel wall: 0—none; 1—wall only; 2—a few cells (1–5) in the airspace; 3—intermediate; 4—severe (airspace is congested).
- (2)
- Interstitial congestion and hyaline membrane formation: 1—normal lung; 2—moderate (<25% of the lung area); 3—intermediate (25–50% of the lung area); 4—severe (>50% of the lung area).
- (3)
- Hemorrhage: 0—absent; 1—present.
2.10. Functional Assessment of the Central Nervous System
2.11. Hematological and Biochemical Blood Analysis
2.12. Renal Function Assessment
2.13. Assessment of Local Tolerance
2.14. Assessment of Systemic Anaphylactic Response
2.15. Immunotoxicity and Immunogenicity Studies
2.15.1. Humoral Immune Response
2.15.2. Cellular Immune Response
2.15.3. Phagocytic Activity Assessment
2.15.4. Lymphocyte Blast Transformation Assessment
2.15.5. Immunogenicity Studies of WJ-MSC-SE33
2.16. Induction of Infectious Lung Disease and Sample Collection
2.17. CFU Quantification in BALF
2.18. Protein Quantification in BALF (Lowry Method)
2.19. Leukocyte Quantification in BALF
2.20. Statistical Analysis
3. Results
3.1. Toxicological Evaluation of Genetically Modified MSCs Expressing the SE-33 Peptide in Animals Following Single and Repeated Administrations
3.1.1. Evaluation of the SE-33 Peptide Level in WJ-MSC-SE33
3.1.2. Clinical Signs and Mortality Following WJ-MSC-SE33 Administration
3.1.3. Effects of WJ-MSC-SE33 on Body Weight and Rectal Temperature
3.1.4. Effects of WJ-MSC-SE33 on Central Nervous System Functional State
3.1.5. Effects of WJ-MSC-SE33 on Hematological Parameters
3.1.6. Effects of WJ-MSC-SE33 on Blood Biochemical Parameters
3.1.7. Effects of WJ-MSC-SE33 on Renal Function Parameters
3.1.8. Effects of WJ-MSC-SE33 on Organ Mass Coefficients
3.1.9. Macroscopic and Histological Analysis of Internal Organs
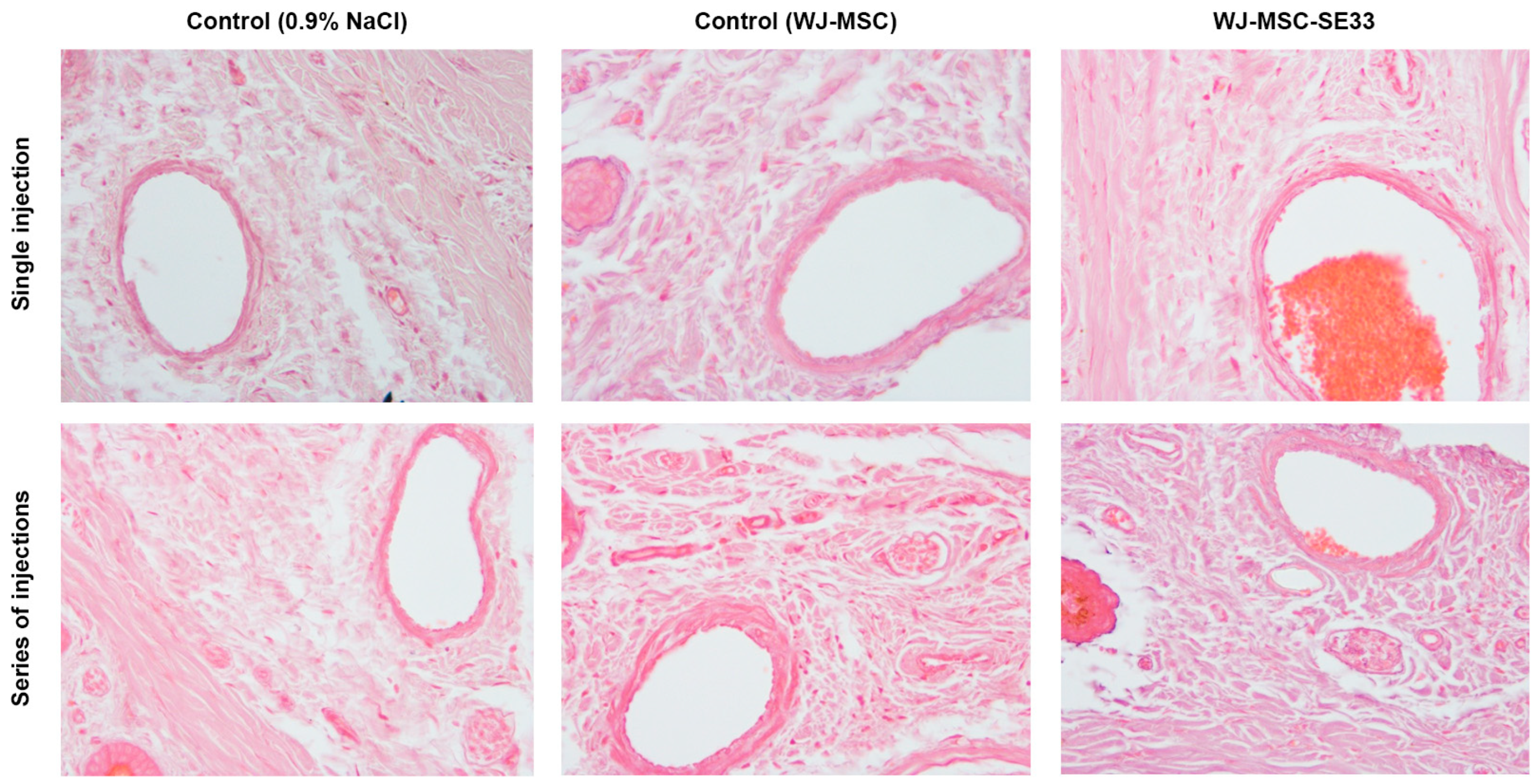
3.1.10. Local Tolerance at the Intravenous Injection Site of WJ-MSC-SE33 in Animals
3.2. Sensitization Study of WJ-MSC-SE33 in Animals
3.3. Immunotoxicity Assessment of WJ-MSC-SE33 in Animals
3.3.1. Humoral Immune Response in Mice: Hemagglutination Reaction
3.3.2. Cellular Immune Response in Mice: DTH Reaction to Hapten
3.3.3. Phagocytic Activity of Peritoneal Macrophages
3.3.4. Lymphocyte Blast Transformation
3.4. Immunogenicity of WJ-MSC-SE33 in Animals
3.5. Study of the Antimicrobial Effect of WJ-MSC-SE33 Against S. aureus-Induced Bacterial Pneumonia in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of action of living, apoptotic, and dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Meirelles, L.; Caplan, A.I.; Nardi, N.B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 2008, 26, 2287–2299. [Google Scholar] [CrossRef]
- Schu, S.; Nosov, M.; O’Flynn, L.; Shaw, G.; Treacy, O.; Barry, F.; Murphy, M.; O’Brien, T.; Ritter, T. Immunogenicity of allogeneic mesenchymal stem cells. J. Cell. Mol. Med. 2012, 16, 2094–2103. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef]
- Bharti, D.; Shivakumar, S.B.; Park, J.K.; Ullah, I.; Subbarao, R.B.; Park, J.S.; Lee, S.L.; Park, B.W.; Rho, G.J. Comparative analysis of human Wharton’s jelly mesenchymal stem cells derived from different parts of the same umbilical cord. Cell Tissue Res. 2018, 372, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Guillot, P.V.; Gotherstrom, C.; Chan, J.; Kurata, H.; Fisk, N.M. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells 2007, 25, 646–654. [Google Scholar] [CrossRef]
- Zhuang, W.Z.; Lin, Y.H.; Su, L.J.; Wu, M.S.; Jeng, H.Y.; Chang, H.C.; Huang, Y.H.; Ling, T.Y. Mesenchymal stem/stromal cell-based therapy: Mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 2021, 28, 38. [Google Scholar] [CrossRef]
- Liau, L.L.; Ruszymah, B.H.I.; Ng, M.H.; Law, J.X. Characteristics and clinical applications of Wharton’s jelly-derived mesenchymal stromal cells. Curr. Res. Transl. Med. 2020, 68, 5–16. [Google Scholar] [CrossRef]
- Mebarki, M.; Iglicki, N.; Marigny, C.; Abadie, C.; Nicolet, C.; Churlaud, G.; Maheux, C.; Boucher, H.; Monsel, A.; Menasché, P.; et al. Development of a human umbilical cord-derived mesenchymal stromal cell-based advanced therapy medicinal product to treat immune and/or inflammatory diseases. Stem Cell Res. Ther. 2021, 12, 571. [Google Scholar] [CrossRef]
- Dong, H.; Li, G.; Shang, C.; Yin, H.; Luo, Y.; Meng, H.; Li, X.; Wang, Y.; Lin, L.; Zhao, M. Umbilical cord mesenchymal stem cell (UC-MSC) transplantations for cerebral palsy. Am. J. Transl. Res. 2018, 10, 901–906. [Google Scholar] [PubMed]
- Chang, D.; Fan, T.; Gao, S.; Jin, Y.; Zhang, M.; Ono, M. Application of mesenchymal stem cell sheet to treatment of ischemic heart disease. Stem Cell Res. Ther. 2021, 12, 384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Fan, L.; Zhang, F.; Li, L. The clinical application of mesenchymal stem cells in liver disease: The current situation and potential future. Ann. Transl. Med. 2020, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Rota, C.; Morigi, M.; Imberti, B. Stem Cell Therapies in Kidney Diseases: Progress and Challenges. Int. J. Mol. Sci. 2019, 20, 2790. [Google Scholar] [CrossRef]
- Shaw, T.D.; Krasnodembskaya, A.D.; Schroeder, G.N.; Zumla, A.; Maeurer, M.; O’Kane, C.M. Mesenchymal Stromal Cells: An Antimicrobial and Host-Directed Therapy for Complex Infectious Diseases. Clin. Microbiol. Rev. 2021, 34, e00064-21. [Google Scholar] [CrossRef]
- Marrazzo, P.; Crupi, A.N.; Alviano, F.; Teodori, L.; Bonsi, L. Exploring the roles of MSCs in infections: Focus on bacterial diseases. J. Mol. Med. 2019, 97, 437–450. [Google Scholar] [CrossRef]
- Shaw, T.D.; Krasnodembskaya, A.D.; Schroeder, G.N.; Doherty, D.F.; Silva, J.D.; Tandel, S.M.; Su, Y.; Butler, D.; Ingram, R.J.; O’Kane, C.M. Human mesenchymal stromal cells inhibit Mycobacterium avium replication in clinically relevant models of lung infection. Thorax 2024, 79, 778–787. [Google Scholar] [CrossRef]
- Curley, G.F.; Jerkic, M.; Dixon, S.; Hogan, G.; Masterson, C.; O’toole, D.; Devaney, J.; Laffey, J.G. Cryopreserved, Xeno-Free Human Umbilical Cord Mesenchymal Stromal Cells Reduce Lung Injury Severity and Bacterial Burden in Rodent Escherichia coli-Induced Acute Respiratory Distress Syndrome. Crit. Care Med. 2017, 45, e202–e212. [Google Scholar] [CrossRef]
- Yang, H.; Xu, F.; Zheng, X.; Yang, S.; Ren, Z.; Yang, J. Human Umbilical Cord Mesenchymal Stem Cells Prevent Bacterial Biofilm Formation. BioMed Res. Int. 2022, 2022, 1530525. [Google Scholar] [CrossRef]
- Ren, Z.; Zheng, X.; Yang, H.; Zhang, Q.; Liu, X.; Zhang, X.; Yang, S.; Xu, F.; Yang, J. Human umbilical-cord mesenchymal stem cells inhibit bacterial growth and alleviate antibiotic resistance in neonatal imipenem-resistant Pseudomonas aeruginosa infection. Innate Immun. 2020, 26, 215–221. [Google Scholar] [CrossRef]
- Liang, B.; Chen, J.; Li, T.; Wu, H.; Yang, W.; Li, Y.; Li, J.; Yu, C.; Nie, F.; Ma, Z.; et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine 2020, 99, E21429. [Google Scholar] [CrossRef]
- Josse, J.; Velard, F.; Mechiche Alami, S.; Brun, V.; Guillaume, C.; Kerdjoudj, H.; Lamkhioued, B.; Gangloff, S.C. Increased internalization of Staphylococcus aureus and cytokine expression in human Wharton’s jelly mesenchymal stem cells. Biomed. Mater. Eng. 2014, 24, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Merakou, C.; Schaefers, M.M.; Flett, K.B.; Martini, S.; Lu, R.; Blumenthal, J.A.; Webster, S.S.; Cross, A.R.; Al Ahmar, R.; et al. Rapid expansion and extinction of antibiotic resistance mutations during treatment of acute bacterial respiratory infections. Nat. Commun. 2022, 13, 1231. [Google Scholar] [CrossRef] [PubMed]
- Sfeir, M.M. Diagnosis of Multidrug-Resistant Pathogens of Pneumonia. Diagnostics 2021, 11, 2287. [Google Scholar] [CrossRef] [PubMed]
- Kato, H. Antibiotic therapy for bacterial pneumonia. J. Pharm. Heal. Care Sci. 2024, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, T.R.; Wells, T.J.; Souza-Fonseca-Guimaraes, F. Towards efficient immunotherapy for bacterial infection. Trends Microbiol. 2021, 30, 158–169. [Google Scholar] [CrossRef]
- Laroye, C.; Gibot, S.; Reppel, L.; Bensoussan, D. Concise Review: Mesenchymal Stromal/Stem Cells: A New Treatment for Sepsis and Septic Shock? Stem Cells 2017, 35, 2331–2339. [Google Scholar] [CrossRef]
- Moeinabadi-Bidgoli, K.; Rezaee, M.; Rismanchi, H.; Mohammadi, M.M.; Babajani, A. Mesenchymal Stem Cell-Derived Antimicrobial Peptides as Potential Anti-Neoplastic Agents: New Insight into Anticancer Mechanisms of Stem Cells and Exosomes. Front. Cell Dev. Biol. 2022, 10, 900418. [Google Scholar] [CrossRef]
- Krasnodembskaya, A.; Song, Y.; Fang, X.; Gupta, N.; Serikov, V.; Lee, J.W.; Matthay, M.A. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 2010, 28, 2229–2238. [Google Scholar] [CrossRef]
- Manna, C.; Das, K.; Mandal, D.; Banerjee, D.; Mukherjee, J.; Ganguly, I.; Naskar, S.; Bag, S. Canine umbilical cord tissue derived mesenchymal stem cells naturally express mRNAs of some antimicrobial peptides. Vet. Res. Commun. 2023, 47, 2229–2233. [Google Scholar] [CrossRef]
- McCarthy, S.D.; Horgan, E.; Ali, A.; Masterson, C.; Laffey, J.G.; MacLoughlin, R.; O’Toole, D. Nebulized Mesenchymal Stem Cell Derived Conditioned Medium Retains Antibacterial Properties Against Clinical Pathogen Isolates. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Mezey, É. Human Mesenchymal Stem/Stromal Cells in Immune Regulation and Therapy. Stem Cells Transl. Med. 2022, 11, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Y.; Wang, B.; Li, J.; Peng, Z. The safety and efficacy of mesenchymal stromal cells in ARDS: A meta-analysis of randomized controlled trials. Crit. Care 2023, 27, 31. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, D.; Masterson, C.; Brady, J.; Horie, S.; McCarthy, S.D.; Gonzalez, H.; O’Toole, D.; Laffey, J. Delayed MSC therapy enhances resolution of organized pneumonia induced by antibiotic resistant Klebsiella pneumoniae infection. Front. Med. 2023, 10, 1132749. [Google Scholar] [CrossRef] [PubMed]
- Hum, C.; Tahir, U.; Mei, S.H.J.; Champagne, J.; Fergusson, D.A.; Lalu, M.; Stewart, D.J.; Walley, K.; Marshall, J.; Santos, C.C.D.; et al. Efficacy and Safety of Umbilical Cord-Derived Mesenchymal Stromal Cell Therapy in Preclinical Models of Sepsis: A Systematic Review and Meta-analysis. Stem Cells Transl. Med. 2024, 13, 346–361. [Google Scholar] [CrossRef]
- Zhu, X.; Ma, D.; Yang, B.; An, Q.; Zhao, J.; Gao, X.; Zhang, L. Research progress of engineered mesenchymal stem cells and their derived exosomes and their application in autoimmune/inflammatory diseases. Stem Cell Res. Ther. 2023, 14, 71. [Google Scholar] [CrossRef]
- Shahbaz, S.K.; Mansourabadi, A.H.; Jafari, D. Genetically engineered mesenchymal stromal cells as a new trend for treatment of severe acute graft-versus-host disease. Clin. Exp. Immunol. 2022, 208, 12–24. [Google Scholar] [CrossRef]
- Chen, H.; Tang, S.; Liao, J.; Liu, M.; Lin, Y. VEGF165 gene-modified human umbilical cord blood mesenchymal stem cells protect against acute liver failure in rats. J. Gene Med. 2021, 23, 3369. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Monteiro, A.P.T.; Tsoporis, J.N.; Mei, S.H.J.; Stewart, D.J.; dos Santos, C.C. Genetically Modified Mesenchymal Stromal/Stem Cells: Application in Critical Illness. Stem Cell Rev. Rep. 2020, 16, 812–827. [Google Scholar] [CrossRef]
- Li, J.; Li, N.; Wei, J.; Feng, C.; Chen, Y.; Chen, T.; Ai, Z.; Zhu, X.; Ji, W.; Li, T. Genetically engineered mesenchymal stem cells with dopamine synthesis for Parkinson’s disease in animal models. NPJ Park. Dis. 2022, 8, 175. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, Y.; Yang, L.; Sun, Y.; Han, Y.; Zhao, Z.; Wang, Y.; Liu, Y.; Ma, Y.; Zhang, T.; et al. Genetically engineered distal airway stem cell transplantation protects mice from pulmonary infection. EMBO Mol. Med. 2020, 12, e10233. [Google Scholar] [CrossRef] [PubMed]
- Shahror, R.A.; Wu, C.C.; Chiang, Y.H.; Chen, K.Y. Genetically Modified Mesenchymal Stem Cells: The Next Generation of Stem Cell-Based Therapy for TBI. Int. J. Mol. Sci. 2020, 21, 4051. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, Y.; Yuan, P.; Guo, W.; Hu, X.; Xing, W.; Ao, L.; Tan, Y.; Wu, X.; Ao, X.; et al. Antibacterial Fusion Protein BPI21/LL-37 Modification Enhances the Therapeutic Efficacy of hUC-MSCs in Sepsis. Mol. Ther. 2020, 28, 1806–1817. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef]
- Lysenko, E.S.; Gould, J.; Bals, R.; Wilson, J.M.; Weiser, J.N. Bacterial Phosphorylcholine Decreases Susceptibility to the Antimicrobial Peptide LL-37/hCAP18 Expressed in the Upper Respiratory Tract. Infect. Immun. 2000, 68, 1664–1671. [Google Scholar] [CrossRef]
- Koprivnjak, T.; Peschel, A. Bacterial resistance mechanisms against host defense peptides. Cell. Mol. Life Sci. 2011, 68, 2243–2254. [Google Scholar] [CrossRef]
- Ridyard, K.E.; Overhage, J. The potential of human peptide ll-37 as an antimicrobial and anti-biofilm agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef]
- Takahashi, T.; Kulkarni, N.N.; Lee, E.Y.; Zhang, L.J.; Wong, G.C.L.; Gallo, R.L. Cathelicidin promotes inflammation by enabling binding of self-RNA to cell surface scavenger receptors. Sci. Rep. 2018, 8, 4032. [Google Scholar] [CrossRef]
- Zhan, N.; Zhang, L.; Yang, H.; Zheng, Y.; Wei, X.; Wang, J.; Shan, A. Design and heterologous expression of a novel dimeric LL37 variant in Pichia pastoris. Microb. Cell Fact. 2021, 20, 143. [Google Scholar] [CrossRef]
- Kim, E.Y.; Rajasekaran, G.; Shin, S.Y. LL-37-derived short antimicrobial peptide KR-12-a5 and its d-amino acid substituted analogs with cell selectivity, anti-biofilm activity, synergistic effect with conventional antibiotics, and anti-inflammatory activity. Eur. J. Med. Chem. 2017, 136, 428–441. [Google Scholar] [CrossRef]
- Gasanov, V.; Vorotelyak, E.; Vasiliev, A. Expression of the Antimicrobial Peptide SE-33-A2P, a Modified Analog of Cathelicidin, and an Analysis of Its Properties. Antibiotics 2024, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Gasanov, V.A.; Vorotelyak, E.A.; Vasiliev, A.V. Production of Antimicrobial Peptides (Cathelicidin Analogues) and Evaluation of Their Biological Properties. Biol. Bull. 2022, 49, S148–S151. [Google Scholar] [CrossRef]
- Trenin, A.S.; Arzumanian, V.G.; Zhmak, M.N.; Shelukhina, I.V.; Makarova, Y.V.; Ivanov, I.A.; Bychkova, O.P.; Budikhina, A.S.; Balyasova, L.S.; Tsetlin, V.I. Synthesis and Antimicrobial Activity of a New Drug Based on Retro-Analog Cathelicidin-Polypeptide SE-33. Russ. J. Bioorganic Chem. 2019, 45, 252–264. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, S.; Zhou, L.; Cai, J.; Tan, J.; Gao, X.; Zeng, Z.; Li, D. Thromboembolism Induced by Umbilical Cord Mesenchymal Stem Cell Infusion: A Report of Two Cases and Literature Review. Transplant. Proc. 2017, 49, 1656–1658. [Google Scholar] [CrossRef]
- Yang, B.; Long, Y.; Zhang, A.; Wang, H.; Chen, Z.; Li, Q. Procoagulant Properties of Mesenchymal Stem Cells and Extracellular Vesicles: A Novel Aspect of Thrombosis Pathogenesis. Stem Cells 2024, 42, 98–106. [Google Scholar] [CrossRef]
- National Research Council (US) Committee. Guide for the Care and Use of Laboratory Animals; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Shao, J.; Xia, L.; Ye, Z.; Yang, Q.; Zhang, C.; Shi, Y.; Zhang, L.; Gu, L.; Xu, C.; Chen, Y.; et al. A repeat-dose toxicity study of human umbilical cord mesenchymal stem cells in NOG mice by intravenous injection. Expert Opin. Drug Metab. Toxicol. 2023, 19, 857–866. [Google Scholar] [CrossRef]
- Weigle, W.O.; Cochrane, C.G.; Dixon, F.J. Anaphylactogenic Properties of Soluble Antigen-Antibody Complexes in the Guinea Pig and Rabbit. J. Immunol. 1960, 85, 469–477. [Google Scholar] [CrossRef]
- Asahi, H.; Kawabata, M.; Sendo, F.; Naiki, M.; Hosaka, Y.; Kobayakawa, T. The presence of anti-sheep red blood cell heterophile antibodies and their characteristics in murine schistosomiasis japonica. Microbiol. Immunol. 1984, 28, 1241–1256. [Google Scholar] [CrossRef]
- Liu, Z.X.; Liu, G.Q.; Lin, Z.X.; Chen, Y.Q.; Chen, P.; Hu, Y.J.; Yu, B.; Jiang, N. Effects of Staphylococcus aureus on stem cells and potential targeted treatment of inflammatory disorders. Stem Cell Res. Ther. 2024, 15, 187. [Google Scholar] [CrossRef]
- Sutton, M.T.; Fletcher, D.; Ghosh, S.K.; Weinberg, A.; Van Heeckeren, R.; Kaur, S.; Sadeghi, Z.; Hijaz, A.; Reese, J.; Lazarus, H.M.; et al. Antimicrobial Properties of Mesenchymal Stem Cells: Therapeutic Potential for Cystic Fibrosis Infection, and Treatment. Stem Cells Int. 2016, 2016, 5303048. [Google Scholar] [CrossRef]
- Qian, J.; Hu, Y.; Zhao, L.; Xia, J.; Li, C.; Shi, L.; Xu, F. Protective Role of Adipose-Derived Stem Cells in Staphylococcus aureus-Induced Lung Injury is Mediated by RegIIIγ Secretion. Stem Cells 2016, 34, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, X.; Deng, Y.; Yuan, X.; Shi, J.; Huang, W.; Huang, J.; Chen, X.; Zheng, S.; Chen, J.; et al. Mesenchymal stromal cells alleviate acute respiratory distress syndrome through the cholinergic anti-inflammatory pathway. Signal Transduct. Target. Ther. 2022, 7, 307. [Google Scholar] [CrossRef] [PubMed]
- Subramani, P.; Kannaiyan, J.; Khare, S.; Balaji, P.; Atif, A.A.; Saad, S.A.; Alsulami, M.O.; Al-Amer, O.M.; Alzahrani, O.R.; Altayar, M.A.; et al. Toxicity, Safety, and Efficacy Studies on Mesenchymal Stem Cells Derived from Decidua basalis in Wistar Albino Rats by Intravenous and Subcutaneous Routes. Curr. Issues Mol. Biol. 2022, 44, 4045–4058. [Google Scholar] [CrossRef] [PubMed]
- Pichardo, A.H.; Amadeo, F.; Wilm, B.; Lévy, R.; Ressel, L.; Murray, P.; Sée, V. Optical Tissue Clearing to Study the Intra-Pulmonary Biodistribution of Intravenously Delivered Mesenchymal Stromal Cells and Their Interactions with Host Lung Cells. Int. J. Mol. Sci. 2022, 23, 14171. [Google Scholar] [CrossRef]
- Chin, S.P.; Marzuki, M.; Tai, L.; Mohamed Shahrehan, N.A.; Ricky, C.; Fanty, A.; Salleh, A.; Low, C.T.; Then, K.Y.; Hoe, S.L.L.; et al. Dynamic tracking of human umbilical cord mesenchymal stem cells (hUC-MSCs) following intravenous administration in mice model. Regen. Ther. 2024, 25, 273–283. [Google Scholar] [CrossRef]
- Sadeghi, B.; Moretti, G.; Arnberg, F.; Samén, E.; Kohein, B.; Catar, R.; Kamhieh-Milz, J.; Geissler, S.; Moll, G.; Holmin, S.; et al. Preclinical Toxicity Evaluation of Clinical Grade Placenta-Derived Decidua Stromal Cells. Front. Immunol. 2019, 10, 2685. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Z.B.; Ma, J.; Zuo, C.; Geng, J.; Gong, W.; Sun, Y.; Li, H.; Wang, B.; Zhang, L.; et al. A toxicity study of multiple-administration human umbilical cord mesenchymal stem cells in cynomolgus monkeys. Stem Cells Dev. 2012, 21, 1401–1408. [Google Scholar] [CrossRef]
- Pan, W.; Gu, L.; Yang, H.; Xu, C.; Yang, Z.; Lu, Q.; Shi, Y.; Zhang, L.; Shao, J.; Chen, Y.; et al. Repeat-dose toxicity study of human umbilical cord mesenchymal stem cells in cynomolgus monkeys by intravenous and subcutaneous injection. Front. Cell Dev. Biol. 2023, 11, 1273723. [Google Scholar] [CrossRef]
- He, J.; Liu, B.; Du, X.; Wei, Y.; Kong, D.; Feng, B.; Guo, R.; Asiamah, E.A.; Griffin, M.D.; Hynes, S.O.; et al. Amelioration of diabetic nephropathy in mice by a single intravenous injection of human mesenchymal stromal cells at early and later disease stages is associated with restoration of autophagy. Stem Cell Res. Ther. 2024, 15, 66. [Google Scholar] [CrossRef]
- Yuan, M.; Yao, L.; Chen, P.; Wang, Z.; Liu, P.; Xiong, Z.; Hu, X.; Li, L.; Jiang, Y. Human umbilical cord mesenchymal stem cells inhibit liver fibrosis via the microRNA-148a-5p/SLIT3 axis. Int. Immunopharmacol. 2023, 125, 111134. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, K.; Qi, Q.; Zhou, W.; Yu, F.; Zhang, Y. Human umbilical cord-derived mesenchymal stem cells attenuate hepatic stellate cells activation and liver fibrosis. Mol. Biol. Rep. 2024, 51, 734. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Cui, R.; Peng, L.; Ma, J.; Chen, X.; Xie, R.J.; Li, B. Mesenchymal stem cells, not conditioned medium, contribute to kidney repair after ischemia-reperfusion injury. Stem Cell Res. Ther. 2014, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yang, I.Y.; Kim, J.; Lee, K.Y.; Jang, Y.S. Antimicrobial peptide LL-37 promotes antigen-specific immune responses in mice by enhancing Th17-skewed mucosal and systemic immunities. Eur. J. Immunol. 2015, 45, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Marquina, M.; Collado, J.A.; Pérez-Cruz, M.; Fernández-Pernas, P.; Fafián-Labora, J.; Blanco, F.J.; Máñez, R.; Arufe, M.C.; Costa, C. Biodistribution and immunogenicity of allogeneic mesenchymal stem cells in a rat model of intraarticular chondrocyte xenotransplantation. Front. Immunol. 2017, 8, 1465. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, X.; Zhang, R.; Koci, M.; Si, D.; Ahmad, B.; Cheng, J.; Wang, J. Development of a Highly Efficient Hybrid Peptide That Increases Immunomodulatory Activity Via the TLR4-Mediated Nuclear Factor-κB Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 6161. [Google Scholar] [CrossRef]
- Franquesa, M.; Hoogduijn, M.J.; Bestard, O.; Grinyó, J.M. Immunomodulatory effect of mesenchymal stem cells on B cells. Front. Immunol. 2012, 3, 26840. [Google Scholar] [CrossRef]
- Jerkic, M.; Gagnon, S.; Rabani, R.; Ward-Able, T.; Masterson, C.; Otulakowski, G.; Curley, G.F.; Marshall, J.; Kavanagh, B.P.; Laffey, J.G. Human Umbilical Cord Mesenchymal Stromal Cells Attenuate Systemic Sepsis in Part by Enhancing Peritoneal Macrophage Bacterial Killing Via Heme Oxygenase-1 Induction in Rats. Anesthesiology 2020, 132, 140–154. [Google Scholar] [CrossRef]
- Wan, M.; van der Does, A.M.; Tang, X.; Lindbom, L.; Agerberth, B.; Haeggström, J.Z. Antimicrobial peptide LL-37 promotes bacterial phagocytosis by human macrophages. J. Leukoc. Biol. 2014, 95, 971–981. [Google Scholar] [CrossRef]
- Lu, D.; Xu, Y.; Liu, Q.; Zhang, Q. Mesenchymal Stem Cell-Macrophage Crosstalk and Maintenance of Inflammatory Microenvironment Homeostasis. Front. Cell Dev. Biol. 2021, 9, 681171. [Google Scholar] [CrossRef]
- Adutler-Lieber, S.; Ben-Mordechai, T.; Naftali-Shani, N.; Asher, E.; Loberman, D.; Raanani, E.; Leor, J. Human macrophage regulation via interaction with cardiac adipose tissue-derived mesenchymal stromal cells. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 78–86. [Google Scholar] [CrossRef]
- Huang, B.; Cheng, X.; Wang, H.; Huang, W.; Hu, Z.l.G.; Wang, D.; Zhang, K.; Zhang, H.; Xue, Z.; Da, Y.; et al. Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J. Transl. Med. 2016, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Hu, C.; Chen, J.; Cen, P.; Wang, J.; Li, L. Interaction between Mesenchymal Stem Cells and B-Cells. Int. J. Mol. Sci. 2016, 17, 650. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.L.; Chagastelles, P.C.; Sesterheim, P.; Pranke, P. In Vivo Immunogenic Response to Allogeneic Mesenchymal Stem Cells and the Role of Preactivated Mesenchymal Stem Cells Cotransplanted with Allogeneic Islets. Stem Cells Int. 2017, 2017, 9824698. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef]
- Oloyo, A.K.; Ambele, M.A.; Pepper, M.S. Contrasting Views on the Role of Mesenchymal Stromal/Stem Cells in Tumour Growth: A Systematic Review of Experimental Design. Adv. Exp. Med. Biol. 2018, 1083, 103–124. [Google Scholar] [CrossRef]
- Mebarki, M.; Abadie, C.; Larghero, J.; Cras, A. Human umbilical cord-derived mesenchymal stem/stromal cells: A promising candidate for the development of advanced therapy medicinal products. Stem Cell Res. Ther. 2021, 12, 152. [Google Scholar] [CrossRef]
- Drobiova, H.; Sindhu, S.; Ahmad, R.; Haddad, D.; Al-Mulla, F.; Al Madhoun, A. Wharton’s jelly mesenchymal stem cells: A concise review of their secretome and prospective clinical applications. Front. Cell Dev. Biol. 2023, 11, 1211217. [Google Scholar] [CrossRef]
- Lee, M.; Jeong, S.Y.; Ha, J.; Kim, M.; Jin, H.J.; Kwon, S.J.; Chang, J.W.; Choi, S.J.; Oh, W.; Yang, Y.S.; et al. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2014, 446, 983–989. [Google Scholar] [CrossRef]
- Moroncini, G.; Paolini, C.; Orlando, F.; Capelli, C.; Grieco, A.; Tonnini, C.; Agarbati, S.; Mondini, E.; Saccomanno, S.; Goteri, G.; et al. Mesenchymal stromal cells from human umbilical cord prevent the development of lung fibrosis in immunocompetent mice. PLoS ONE 2018, 13, e0196048. [Google Scholar] [CrossRef]
- Hasanpour, A.H.; Sepidarkish, M.; Mollalo, A.; Ardekani, A.; Almukhtar, M.; Mechaal, A.; Hosseini, S.R.; Bayani, M.; Javanian, M.; Rostami, A. The global prevalence of methicillin-resistant Staphylococcus aureus colonization in residents of elderly care centers: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2023, 12, 4. [Google Scholar] [CrossRef]
- Kraitchman, D.L.; Tatsumi, M.; Gilson, W.D.; Ishimori, T.; Kedziorek, D.; Walczak, P.; Segars, W.P.; Chen, H.H.; Fritzges, D.; Izbudak, I.; et al. Dynamic Imaging of Allogeneic Mesenchymal Stem Cells Trafficking to Myocardial Infarction. Circulation 2005, 112, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Ma, C.; Peng, H.; Wu, Z.; Xu, H.; Wu, J.; Zhang, N.; Jiang, Q.; Ma, C.; Huang, R.; et al. Define Mesenchymal Stem Cell from Its Fate: Biodisposition of Human Mesenchymal Stem Cells in Normal and Concanavalin A-Induced Liver Injury Mice. J. Pharmacol. Exp. Ther. 2021, 379, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Bao, Y.; El-Hashash, A. Mesenchymal stromal cell-based therapy in lung diseases; from research to clinic. Am. J. Stem Cells 2024, 13, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Krasnodembskaya, A.; Kapetanaki, M.; Mouded, M.; Tan, X.; Serikov, V.; Matthay, M.A. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 2012, 67, 533–539. [Google Scholar] [CrossRef]
- Mei, S.H.J.; Haitsma, J.J.; Dos Santos, C.C.; Deng, Y.; Lai, P.F.H.; Slutsky, A.S.; Liles, W.C.; Stewart, D.J. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am. J. Respir. Crit. Care Med. 2010, 182, 1047–1057. [Google Scholar] [CrossRef]
- Matthay, M.A. Therapeutic potential of mesenchymal stromal cells for acute respiratory distress syndrome. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. S1), S54–S57. [Google Scholar] [CrossRef]
- Lee, J.W.; Fang, X.; Krasnodembskaya, A.; Howard, J.P.; Matthay, M.A. Concise review: Mesenchymal stem cells for acute lung injury: Role of paracrine soluble factors. Stem Cells 2011, 29, 913–919. [Google Scholar] [CrossRef]
- Harrell, C.R.; Sadikot, R.; Pascual, J.; Fellabaum, C.; Jankovic, M.G.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Based Therapy of Inflammatory Lung Diseases: Current Understanding and Future Perspectives. Stem Cells Int. 2019, 2019, 4236973. [Google Scholar] [CrossRef]
- Nagaoka, I.; Tamura, H.; Reich, J. Therapeutic Potential of Cathelicidin Peptide LL-37, an Antimicrobial Agent, in a Murine Sepsis Model. Int. J. Mol. Sci. 2020, 21, 5973. [Google Scholar] [CrossRef]
- Kumagai, Y.; Murakami, T.; Kuwahara-Arai, T.; Iba, T.; Reich, J.; Nagaoka, I. Antimicrobial peptide LL-37 ameliorates a murine sepsis model via the induction of microvesicle release from neutrophils. Innate Immun. 2020, 26, 565–579. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, W.; Zhou, L.; Liu, F.; Ren, X.; Yu, L.; Chen, H.; Jiang, Z. LL-37 improves sepsis-induced acute lung injury by suppressing pyroptosis in alveolar epithelial cells. Int. Immunopharmacol. 2024, 129, 111580. [Google Scholar] [CrossRef]








| Observation Period | Sex | Control (0.9% NaCl) (n = 12) | Control (WJ-MSC 2.5 × 107 Cells/kg) (n = 12) | WJ-MSC-SE33 | ||
|---|---|---|---|---|---|---|
| 0.5 × 107 Cells/kg (n = 12) | 1.25 × 107 Cells/kg (n = 12) | 2.5 × 107 Cells/kg (n = 12) | ||||
| 0 days | Male | 18.92 ± 0.21 | 19.00 ± 0.21 | 18.92 ± 0.23 | 18.92 ± 0.28 | 18.88 ± 0.18 |
| Female | 18.88 ± 0.25 | 18.54 ± 0.22 | 18.83 ± 0.26 | 18.54 ± 0.18 | 18.79 ± 0.23 | |
| 7 days | Male | 19.17 ± 0.23 | 19.21 ± 0.23 | 19.25 ± 0.23 | 19.17 ± 0.25 | 19.21 ± 0.14 |
| Female | 19.04 ± 0.28 | 18.75 ± 0.21 | 19.13 ± 0.24 | 18.75 ± 0.16 | 19.13 ± 0.23 | |
| 14 days | Male | 19.38 ± 0.22 | 19.42 ± 0.23 | 19.50 ± 0.17 | 19.50 ± 0.20 | 19.50 ± 0.14 |
| Female | 19.33 ± 0.30 | 19.13 ± 0.16 | 19.17 ± 0.20 | 19.04 ± 0.18 | 19.54 ± 0.20 | |
| Observation Period | Sex | Control (0.9% NaCl) (n = 12) | Control (WJ-MSC 2.5 × 107 Cells/kg) (n = 12) | WJ-MSC-SE33 | ||
|---|---|---|---|---|---|---|
| 0.5 × 107 Cells/kg (n = 12) | 1.25 × 107 Cells/kg (n = 12) | 2.5 × 107 Cells/kg (n = 12) | ||||
| 0 days | Male | 37.58 ± 0.07 | 37.55 ± 0.05 | 37.56 ± 0.06 | 37.64 ± 0.05 | 37.63 ± 0.06 |
| Female | 37.66 ± 0.07 | 37.64 ± 0.05 | 37.57 ± 0.06 | 37.61 ± 0.06 | 37.63 ± 0.05 | |
| 7 days | Male | 37.64 ± 0.07 | 37.64 ± 0.07 | 37.58 ± 0.07 | 37.62 ± 0.07 | 37.62 ± 0.06 |
| Female | 37.53 ± 0.05 | 37.59 ± 0.08 | 37.58 ± 0.05 | 37.69 ± 0.07 | 37.59 ± 0.07 | |
| 14 days | Male | 37.54 ± 0.07 | 37.58 ± 0.06 | 37.63 ± 0.05 | 37.54 ± 0.07 | 37.58 ± 0.06 |
| Female | 37.58 ± 0.06 | 37.53 ± 0.05 | 37.60 ± 0.07 | 37.61 ± 0.05 | 37.56 ± 0.06 | |
| Observation Period | Sex | Control (0.9% NaCl) (n = 12) | Control (WJ-MSC 2.5 × 107 Cells/kg) (n = 12) | WJ-MSC-SE33 | ||
|---|---|---|---|---|---|---|
| 0.5 × 107 Cells/kg (n = 12) | 1.25 × 107 Cells/kg (n = 12) | 2.5 × 107 Cells/kg (n = 12) | ||||
| Horizontal activity (duration, sec) | ||||||
| 0 days | Male | 208.68 ± 5.21 | 208.65 ± 5.50 | 202.1 ± 3.62 | 210.49 ± 5.96 | 206.08 ± 5.02 |
| Female | 198.22 ± 6.09 | 203.31 ± 4.15 | 203.26 ± 5.96 | 203.46 ± 5.82 | 201.24 ± 6.41 | |
| 14 days | Male | 205.13 ± 5.24 | 209.73 ± 4.58 | 208.99 ± 4.34 | 202.79 ± 6.00 | 210.49 ± 5.23 |
| Female | 205.16 ± 5.84 | 202.33 ± 5.88 | 198.05 ± 5.68 | 205.06 ± 5.71 | 196.53 ± 6.07 | |
| Vertical activity (number of vertical stands) | ||||||
| 0 days | Male | 29.00 ± 0.55 | 28.33 ± 0.57 | 28.42 ± 0.47 | 28.83 ± 0.42 | 28.00 ± 0.59 |
| Female | 28.67 ± 0.59 | 28.08 ± 0.47 | 27.83 ± 0.47 | 28.50 ± 0.50 | 28.42 ± 0.48 | |
| 14 days | Male | 28.25 ± 0.48 | 29.17 ± 0.53 | 28.67 ± 0.50 | 28.17 ± 0.46 | 28.58 ± 0.43 |
| Female | 28.17 ± 0.49 | 28.33 ± 0.47 | 28.92 ± 0.53 | 28.58 ± 0.53 | 28.50 ± 0.48 | |
| Grooming duration, sec | ||||||
| 0 days | Male | 3.31 ± 0.06 | 3.29 ± 0.06 | 3.29 ± 0.06 | 3.23 ± 0.05 | 3.23 ± 0.04 |
| Female | 3.25 ± 0.06 | 3.21 ± 0.06 | 3.30 ± 0.05 | 3.26 ± 0.05 | 3.27 ± 0.05 | |
| 14 days | Male | 3.29 ± 0.05 | 3.32 ± 0.08 | 3.24 ± 0.04 | 3.22 ± 0.07 | 3.30 ± 0.06 |
| Female | 3.31 ± 0.06 | 3.27 ± 0.05 | 3.30 ± 0.06 | 3.32 ± 0.05 | 3.23 ± 0.04 | |
| Duration of freezing reaction, sec | ||||||
| 0 days | Male | 0.67 ± 0.03 | 0.62 ± 0.03 | 0.65 ± 0.04 | 0.68 ± 0.04 | 0.62 ± 0.04 |
| Female | 0.67 ± 0.05 | 0.72 ± 0.04 | 0.69 ± 0.03 | 0.69 ± 0.04 | 0.63 ± 0.05 | |
| 14 days | Male | 0.70 ± 0.03 | 0.65 ± 0.04 | 0.69 ± 0.04 | 0.63 ± 0.03 | 0.64 ± 0.04 |
| Female | 0.68 ± 0.04 | 0.67 ± 0.04 | 0.63 ± 0.04 | 0.67 ± 0.04 | 0.69 ± 0.04 | |
| Duration of sniffing reaction, sec | ||||||
| 0 days | Male | 44.69 ± 3.09 | 45.33 ± 2.11 | 43.46 ± 2.11 | 44.33 ± 3.04 | 48.13 ± 2.27 |
| Female | 45.04 ± 2.75 | 43.96 ± 2.62 | 48.93 ± 2.98 | 45.34 ± 1.74 | 44.85 ± 2.31 | |
| 14 days | Male | 46.3 ± 2.76 | 46.87 ± 1.84 | 50.01 ± 2.49 | 47.09 ± 3.02 | 49.38 ± 2.58 |
| Female | 43.46 ± 2.32 | 47.71 ± 2.88 | 44.59 ± 2.9 | 48.33 ± 2.19 | 44.26 ± 2.82 | |
| Observation Period | Sex | Control (0.9% NaCl) (n = 6) | Control (WJ-MSC 2.5 × 107 Cells/kg) (n = 6) | WJ-MSC-SE33 | ||
|---|---|---|---|---|---|---|
| 0.5 × 107 Cells/kg (n = 6) | 1.25 × 107 Cells/kg (n = 6) | 2.5 × 107 Cells/kg (n = 6) | ||||
| Hemoglobin, g/L | ||||||
| 15 days | Male | 143.83 ± 3.93 | 131.17 ± 6.93 | 142.50 ± 1.73 | 144.67 ± 1.45 | 145.33 ± 3.82 |
| Female | 142.33 ± 3.14 | 140.17 ± 3.38 | 143.83 ± 1.49 | 146.83 ± 2.12 | 144.00 ± 4.66 | |
| Erythrocytes, ×1012/L | ||||||
| 15 days | Male | 8.43 ± 0.26 | 7.82 ± 0.42 | 8.50 ± 0.12 | 8.78 ± 0.11 | 8.68 ± 0.25 |
| Female | 8.32 ± 0.23 | 8.33 ± 0.23 | 8.54 ± 0.15 | 8.71 ± 0.19 | 8.59 ± 0.27 | |
| Leukocytes, ×109/L | ||||||
| 15 days | Male | 7.25 ± 0.70 | 6.50 ± 0.96 | 6.68 ± 0.92 | 5.95 ± 0.92 | 6.08 ± 0.89 |
| Female | 6.72 ± 0.95 | 5.10 ± 0.87 | 5.13 ± 0.63 | 5.37 ± 0.99 | 5.02 ± 0.76 | |
| Platelets, ×109/L | ||||||
| 15 days | Male | 655.67 ± 37.83 | 650.00 ± 23.56 | 597.33 ± 48.78 | 619.33 ± 43.46 | 653.00 ± 32.06 |
| Female | 664.67 ± 50.31 | 772.17 ± 35.20 | 621.00 ± 70.47 | 702.00 ± 37.60 | 661.83 ± 40.47 | |
| ESR, mm/h | ||||||
| 15 days | Male | 1.17 ± 0.17 | 1.17 ± 0.17 | 1.33 ± 0.33 | 1.17 ± 0.17 | 1.17 ± 0.17 |
| Female | 1.50 ± 0.34 | 1.17 ± 0.17 | 1.17 ± 0.17 | 1.17 ± 0.17 | 1.33 ± 0.21 | |
| Observation Period | Sex | Control (0.9% NaCl) (n = 6) | Control (WJ-MSC 2.5 × 107 Cells/kg) (n = 6) | WJ-MSC-SE33 | ||
|---|---|---|---|---|---|---|
| 0.5 × 107 Cells/kg (n = 6) | 1.25 × 107 Cells/kg (n = 6) | 2.5 × 107 Cells/kg (n = 6) | ||||
| Band neutrophils, % | ||||||
| 15 days | Male | 0 | 0 | 0.17±0.17 | 0.17±0.17 | 0 |
| Female | 0.17 ± 0.17 | 0 | 0 | 0.17 ± 0.17 | 0 | |
| Segmented neutrophils, % | ||||||
| 15 days | Male | 19.17 ± 1.14 | 20.00 ± 3.89 | 20.83 ± 2.70 | 22.67 ± 2.39 | 21.00 ± 2.13 |
| Female | 18.67 ± 1.82 | 21.00 ± 3.59 | 21.33 ± 1.82 | 21.00 ± 2.29 | 19.00 ± 1.69 | |
| Eosinophils, % | ||||||
| 15 days | Male | 2.67 ± 0.49 | 1.67 ± 0.71 | 2.50 ± 0.56 | 2.00 ± 0.58 | 2.83 ± 0.48 |
| Female | 2.33 ± 0.67 | 2.17 ± 0.60 | 2.50 ± 0.85 | 2.00 ± 0.86 | 2.67 ± 0.49 | |
| Basophils, % | ||||||
| 15 days | Male | 0 | 0 | 0 | 0 | 0 |
| Female | 0 | 0 | 0 | 0 | 0 | |
| Monocytes, % | ||||||
| 15 days | Male | 3.50 ± 0.62 | 3.33 ± 0.76 | 3.17 ± 0.95 | 2.83 ± 0.83 | 3.50 ± 0.67 |
| Female | 3.17 ± 0.87 | 3.50 ± 0.72 | 2.50 ± 0.56 | 3.00 ± 0.97 | 2.83 ± 0.48 | |
| Lymphocytes, % | ||||||
| 15 days | Male | 74.67 ± 1.45 | 75.00 ± 3.40 | 73.33 ± 2.74 | 72.33 ± 2.93 | 72.67 ± 2.39 |
| Female | 75.67 ± 1.61 | 73.33 ± 4.38 | 73.67 ± 1.23 | 73.83 ± 2.15 | 75.50 ± 1.95 | |
| Observation Period | Sex | Control (0.9% NaCl) (n = 6) | Control (WJ-MSC 2.5 × 107 Cells/kg) (n = 6) | WJ-MSC-SE33 | ||
|---|---|---|---|---|---|---|
| 0.5 × 107 Cells/kg (n = 6) | 1.25 × 107 Cells/kg (n = 6) | 2.5 × 107 Cells/kg (n = 6) | ||||
| Total protein, g/L | ||||||
| 15 days | Male | 56.55 ± 1.20 | 57.37 ± 1.42 | 56.63 ± 1.20 | 57.02 ± 1.24 | 56.37 ± 1.63 |
| Female | 56.34 ± 1.52 | 58.68 ± 1.32 | 57.38 ± 1.49 | 58.54 ± 1.28 | 57.07 ± 1.26 | |
| Albumin, g/L | ||||||
| 15 days | Male | 34.09 ± 0.98 | 33.98 ± 0.89 | 32.12 ± 0.97 | 33.40 ± 0.87 | 33.28 ± 1.09 |
| Female | 34.44 ± 1.13 | 34.23 ± 0.17 | 33.65 ± 0.86 | 35.19 ± 1.69 | 33.80 ± 1.28 | |
| Urea, mmol/L | ||||||
| 15 days | Male | 6.62 ± 0.10 | 6.57 ± 0.09 | 6.63 ± 0.08 | 6.63 ± 0.12 | 6.69 ± 0.10 |
| Female | 6.69 ± 0.12 | 6.64 ± 0.11 | 6.56 ± 0.07 | 6.57 ± 0.10 | 6.68 ± 0.12 | |
| Creatinine, µmol/L | ||||||
| 15 days | Male | 74.80 ± 0.94 | 74.36 ± 1.15 | 73.66 ± 1.01 | 72.91 ± 0.75 | 75.09 ± 0.77 |
| Female | 74.89 ± 1.05 | 74.53 ± 0.88 | 75.30 ± 0.66 | 74.31 ± 0.76 | 73.08 ± 0.92 | |
| Total cholesterol, mmol/L | ||||||
| 15 days | Male | 2.00 ± 0.12 | 2.17 ± 0.06 | 2.02 ± 0.08 | 2.06 ± 0.06 | 1.98 ± 0.13 |
| Female | 1.95 ± 0.11 | 1.85 ± 0.10 | 2.00 ± 0.05 | 2.01 ± 0.12 | 2.07 ± 0.16 | |
| Triglycerides, mmol/L | ||||||
| 15 days | Male | 1.02 ± 0.12 | 1.08 ± 0.14 | 1.05 ± 0.11 | 1.08 ± 0.12 | 1.06 ± 0.13 |
| Female | 1.07 ± 0.16 | 1.06 ± 0.10 | 1.10 ± 0.11 | 1.05 ± 0.09 | 1.12 ± 0.012 | |
| Aspartate aminotransferase, U/L | ||||||
| 15 days | Male | 225.30 ± 22.29 | 242.07 ± 26.58 | 261.48 ± 16.69 | 257.86 ± 25.55 | 248.88 ± 19.64 |
| Female | 247.72 ± 28.14 | 266.51 ± 33.87 | 257.74 ± 42.20 | 277.79 ± 32.04 | 256.82 ± 26.73 | |
| Alanine aminotransferase, U/L | ||||||
| 15 days | Male | 54.24 ± 6.33 | 57.36 ± 9.81 | 63.86 ± 5.81 | 66.87 ± 7.38 | 67.66 ± 8.04 |
| Female | 62.85 ± 8.44 | 70.89 ± 10.76 | 62.67 ± 3.47 | 72.85 ± 8.71 | 69.62 ± 8.29 | |
| Alkaline phosphatase, U/L | ||||||
| 15 days | Male | 105.93 ± 16.46 | 103.74 ± 5.92 | 106.45 ± 11.34 | 101.21 ± 6.22 | 101.93 ± 10.01 |
| Female | 106.95 ± 9.39 | 106.03 ± 14.07 | 111.81 ± 6.46 | 113.64 ± 6.22 | 110.76 ± 7.49 | |
| Glucose, mmol/L | ||||||
| 15 days | Male | 4.59 ± 0.10 | 4.57 ± 0.10 | 4.52 ± 0.09 | 4.62 ± 0.10 | 4.53 ± 0.06 |
| Female | 4.61 ± 0.13 | 4.60 ± 0.06 | 4.54 ± 0.09 | 4.58 ± 0.06 | 4.56 ± 0.09 | |
| Total bilirubin, µmol/L | ||||||
| 15 days | Male | 1.46 ± 0.36 | 1.98 ± 0.58 | 2.19 ± 0.58 | 1.69 ± 0.56 | 1.61 ± 0.40 |
| Female | 1.66 ± 0.47 | 1.70 ± 0.59 | 2.01 ± 0.60 | 1.74 ± 0.54 | 1.95 ± 0.39 | |
| Potassium, mmol/L | ||||||
| 15 days | Male | 6.51 ± 0.08 | 6.53 ± 0.12 | 6.49 ± 0.06 | 6.57 ± 0.10 | 6.52 ± 0.09 |
| Female | 6.48 ± 0.09 | 6.50 ± 0.11 | 6.45 ± 0.11 | 6.51 ± 0.11 | 6.47 ± 0.10 | |
| Calcium, mmol/L | ||||||
| 15 days | Male | 2.29 ± 0.07 | 2.27 ± 0.08 | 2.31 ± 0.07 | 2.32 ± 0.07 | 2.33 ± 0.06 |
| Female | 2.31 ± 0.05 | 2.33 ± 0.10 | 2.33 ± 0.07 | 2.28 ± 0.08 | 2.30 ± 0.07 | |
| Sodium, mmol/L | ||||||
| 15 days | Male | 139.34 ± 0.45 | 139.57 ± 0.59 | 139.10 ± 0.49 | 138.86 ± 0.61 | 139.45 ± 0.62 |
| Female | 138.91 ± 0.59 | 139.16 ± 0.61 | 139.02 ± 0.61 | 139.27 ± 0.40 | 137.74 ± 0.53 | |
| Observation Period | Sex | Control (0.9% NaCl) (n = 12) | Control (WJ-MSC 2.5 × 107 Cells/kg) (n = 12) | WJ-MSC-SE33 | ||
|---|---|---|---|---|---|---|
| 0.5 × 107 Cells/kg (n = 12) | 1.25 × 107 Cells/kg (n = 12) | 2.5 × 107 Cells/kg (n = 12) | ||||
| рН | ||||||
| 15 days | Male | 6.33 ± 0.17 | 6.42 ± 0.15 | 6.33 ± 0.17 | 6.42 ± 0.15 | 6.42 ± 0.15 |
| Female | 6.58 ± 0.15 | 6.50 ± 0.18 | 6.58 ± 0.15 | 6.42 ± 0.15 | 6.33 ± 0.11 | |
| Specific gravity, g/ml | ||||||
| 15 days | Male | 1.003 ± 0.002 | 1.003 ± 0.002 | 1.003 ± 0.002 | 1.003 ± 0.002 | 1.004 ± 0.002 |
| Female | 1.003 ± 0.002 | 1.004 ± 0.002 | 1.004 ± 0.002 | 1.002 ± 0.002 | 1.004 ± 0.002 | |
| Leukocytes, number per field | ||||||
| 15 days | Male | 0-0 | 0-1 | 0-1 | 0-1 | 0-1 |
| Female | 0-0 | 1-1 | 1-0 | 0-1 | 0-0 | |
| Erythrocytes, number per field | ||||||
| 15 days | Male | 0-0 | 1-1 | 0-1 | 1-1 | 1-0 |
| Female | 0-0 | 0-0 | 0-0 | 0-0 | 0-0 | |
| Glucose, mmol/L | ||||||
| 15 days | Male | negative | negative | negative | negative | negative |
| Female | negative | negative | negative | negative | negative | |
| Ketone bodies, mmol/L | ||||||
| 15 days | Male | negative | negative | negative | negative | negative |
| Female | negative | negative | negative | negative | negative | |
| Bilirubin, µmol/L | ||||||
| 15 days | Male | negative | negative | negative | negative | negative |
| Female | negative | negative | negative | negative | negative | |
| Total protein, g/L | ||||||
| 15 days | Male | 0.00 ± 0.00 | 0.05 ± 0.05 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Female | 0.00 ± 0.00 | 0.05 ± 0.05 | 0.05 ± 0.05 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Observation Period | Sex | Control (0.9% NaCl) (n = 12) | Control (WJ-MSC 2.5 × 107 Cells/kg) (n = 12) | WJ-MSC-SE33 | ||
|---|---|---|---|---|---|---|
| 0.5 × 107 Cells/kg (n = 12) | 1.25 × 107 Cells/kg (n = 12) | 2.5 × 107 Cells/kg (n = 12) | ||||
| Heart | ||||||
| 15 days | Male | 4.94 ± 0.18 | 5.04 ± 0.18 | 4.93 ± 0.17 | 4.99 ± 0.11 | 5.06 ± 0.14 |
| Female | 4.97 ± 0.19 | 4.86 ± 0.16 | 5.06 ± 0.19 | 4.95 ± 0.15 | 4.79 ± 0.14 | |
| Lungs (both) | ||||||
| 15 days | Male | 9.80 ± 0.17 | 9.54 ± 0.35 | 9.42 ± 0.30 | 9.51 ± 0.37 | 9.73 ± 0.32 |
| Female | 9.62 ± 0.31 | 9.48 ± 0.26 | 9.38 ± 0.26 | 9.60 ± 0.18 | 9.78 ± 0.30 | |
| Thymus | ||||||
| 15 days | Male | 3.62 ± 0.08 | 3.58 ± 0.06 | 3.66 ± 0.08 | 3.60 ± 0.09 | 3.62 ± 0.08 |
| Female | 3.73 ± 0.11 | 3.69 ± 0.09 | 3.70 ± 0.07 | 3.70 ± 0.09 | 3.60 ± 0.09 | |
| Spleen | ||||||
| 15 days | Male | 6.29 ± 0.10 | 6.30 ± 0.17 | 6.06 ± 0.16 | 6.24 ± 0.11 | 6.24 ± 0.10 |
| Female | 6.33 ± 0.14 | 6.26 ± 0.13 | 6.30 ± 0.12 | 6.47 ± 0.11 | 6.21 ± 0.11 | |
| Liver | ||||||
| 15 days | Male | 58.50 ± 0.73 | 57.58 ± 1.05 | 59.07 ± 0.72 | 58.22 ± 1.02 | 59.16 ± 0.59 |
| Female | 59.25 ± 1.53 | 59.64 ± 0.98 | 59.26 ± 1.00 | 59.10 ± 1.09 | 59.63 ± 1.02 | |
| Kidneys (both) | ||||||
| 15 days | Male | 12.35 ± 0.32 | 12.57 ± 0.26 | 12.65 ± 0.26 | 12.30 ± 0.27 | 12.17 ± 0.25 |
| Female | 12.40 ± 0.28 | 12.37 ± 0.27 | 12.48 ± 0.27 | 12.64 ± 0.39 | 12.29 ± 0.25 | |
| Brain | ||||||
| 15 days | Male | 20.57 ± 0.34 | 20.75 ± 0.35 | 20.78 ± 0.29 | 20.40 ± 0.24 | 20.35 ± 0.14 |
| Female | 20.67 ± 0.30 | 21.00 ± 0.26 | 20.81 ± 0.27 | 20.94 ± 0.32 | 20.61 ± 0.23 | |
| Negative Control (0.9% NaCl) | Positive Control (0.6% HEW) | WJ-MSC-SE33 | |||||
|---|---|---|---|---|---|---|---|
| 0.5 × 107 Cells/kg (n = 6) | 2.5 × 107 Cells/kg (n = 6) | ||||||
| Male (n = 6) | Female (n = 6) | Male (n = 6) | Female (n = 6) | Male (n = 6) | Female (n = 6) | Male (n = 6) | Female (n = 6) |
| Endolaryngeal administration | |||||||
| 0 | 0 | 3.33 ± 0.33 * | 3.50 ± 0.34 * | 0 | 0 | 0 | 0 |
| Intravenous administration | |||||||
| 0 | 0 | 3.00 ± 0.37 * | 3.17 ± 0.31 * | 0 | 0 | 0 | 0 |
| Observation Period | Control (0.9% NaCl) | Control (Native WJ-MSCs at 0.5 × 107 Cells/kg) | WJ-MSC-SE33 | ||
|---|---|---|---|---|---|
| 0.5 × 107 Cells/kg | 1 × 107 Cells/kg | 1.5 × 107 Cells/kg | |||
| Infiltration or aggregation of inflammatory cells in the airspace or vessel walls | |||||
| 5 days | 2.20 ± 0.13 | 0.90 ± 0.18 * | 0.90 ± 0.18 * | 0.80 ± 0.20 * | 0.80 ± 0.25 * |
| 8 days | 2.50 ± 0.22 | 1.00 ± 0.19 * | 0.88 ± 0.23 * | 0.89 ± 0.20 * | 0.89 ± 0.26 * |
| Interstitial congestion and hyaline membrane formation | |||||
| 5 days | 3.30 ± 0.15 | 1.50 ± 0.17 * | 1.40 ± 0.16 * | 1.40 ± 0.22 * | 1.30 ± 0.21 * |
| 8 days | 3.50 ± 0.22 | 1.50 ± 0.19 * | 1.38 ± 0.18 * | 1.44 ± 0.18 * | 1.33 ± 0.24 * |
| Hemorrhage | |||||
| 5 days | 1.00 ± 0.00 | 0.40 ± 0.16 * | 0.30 ± 0.15 * | 0.20 ± 0.13 * | 0.30 ± 0.15 * |
| 8 days | 1.00 ± 0.00 | 0.38 ± 0.18 * | 0.38 ± 0.18 * | 0.33 ± 0.17 * | 0.22 ± 0.15 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasanov, V.A.o.; Kashirskikh, D.A.; Khotina, V.A.; Kuzmina, D.M.; Nikitochkina, S.Y.; Mukhina, I.V.; Vorotelyak, E.A.; Vasiliev, A.V. Preclinical Evaluation of the Safety, Toxicity and Efficacy of Genetically Modified Wharton’s Jelly Mesenchymal Stem/Stromal Cells Expressing the Antimicrobial Peptide SE-33. Cells 2025, 14, 341. https://doi.org/10.3390/cells14050341
Gasanov VAo, Kashirskikh DA, Khotina VA, Kuzmina DM, Nikitochkina SY, Mukhina IV, Vorotelyak EA, Vasiliev AV. Preclinical Evaluation of the Safety, Toxicity and Efficacy of Genetically Modified Wharton’s Jelly Mesenchymal Stem/Stromal Cells Expressing the Antimicrobial Peptide SE-33. Cells. 2025; 14(5):341. https://doi.org/10.3390/cells14050341
Chicago/Turabian StyleGasanov, Vagif Ali oglu, Dmitry Alexandrovich Kashirskikh, Victoria Alexandrovna Khotina, Daria Mikhailovna Kuzmina, Sofya Yurievna Nikitochkina, Irina Vasilievna Mukhina, Ekaterina Andreevna Vorotelyak, and Andrey Valentinovich Vasiliev. 2025. "Preclinical Evaluation of the Safety, Toxicity and Efficacy of Genetically Modified Wharton’s Jelly Mesenchymal Stem/Stromal Cells Expressing the Antimicrobial Peptide SE-33" Cells 14, no. 5: 341. https://doi.org/10.3390/cells14050341
APA StyleGasanov, V. A. o., Kashirskikh, D. A., Khotina, V. A., Kuzmina, D. M., Nikitochkina, S. Y., Mukhina, I. V., Vorotelyak, E. A., & Vasiliev, A. V. (2025). Preclinical Evaluation of the Safety, Toxicity and Efficacy of Genetically Modified Wharton’s Jelly Mesenchymal Stem/Stromal Cells Expressing the Antimicrobial Peptide SE-33. Cells, 14(5), 341. https://doi.org/10.3390/cells14050341








