Development of Antibody–Drug Conjugates for Malignancies of the Uterine Corpus: A Review
Abstract
1. Introduction
2. General Overview of ADC
2.1. Structure and Mechanism of Action
2.2. Antibody and Target Antigen
2.3. Payload
2.4. Linker
3. ADC for Endometrial Carcinoma
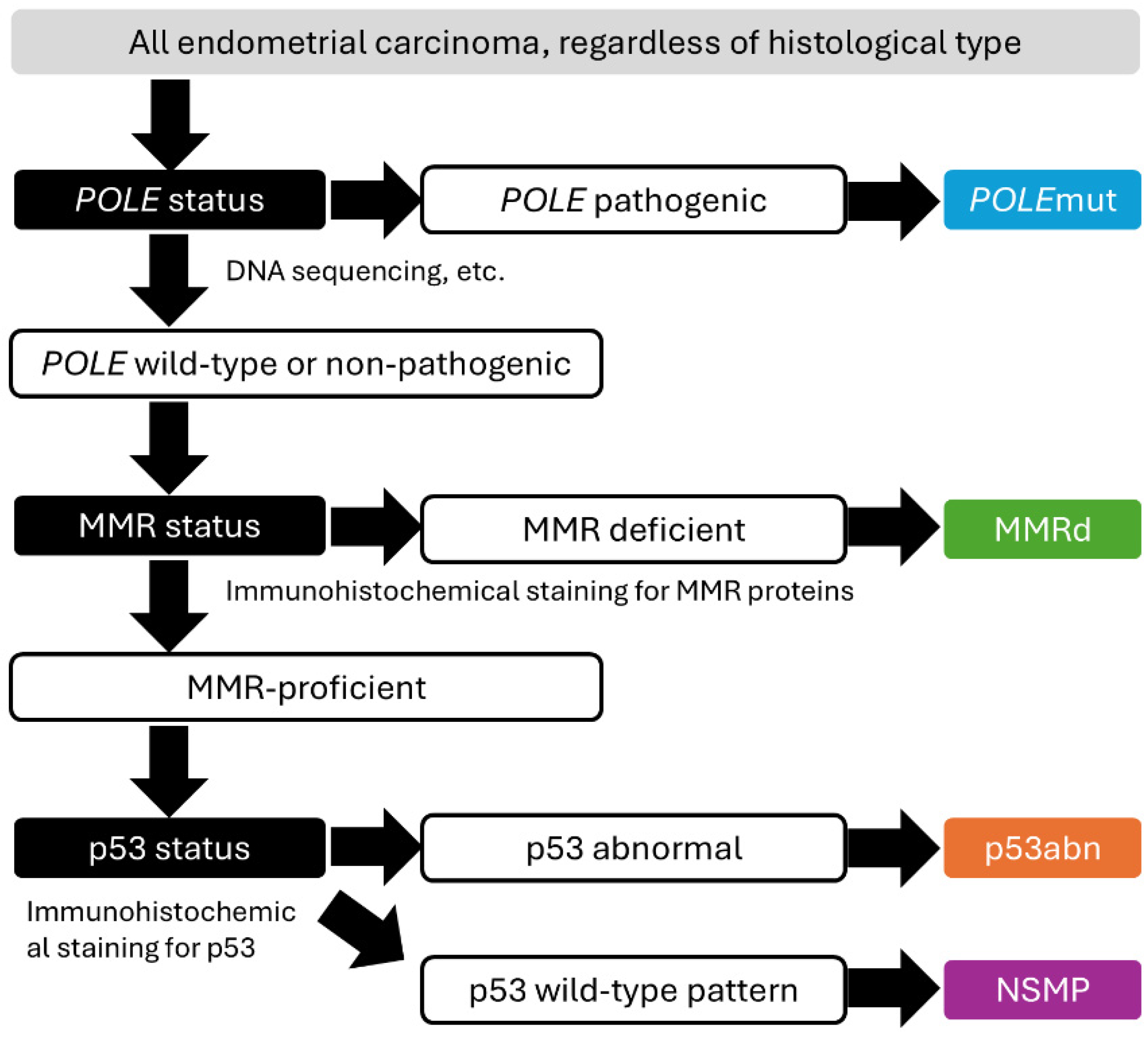
3.1. Overview of the Histological and Molecular Features of Endometrial Carcinoma (Figure 1)
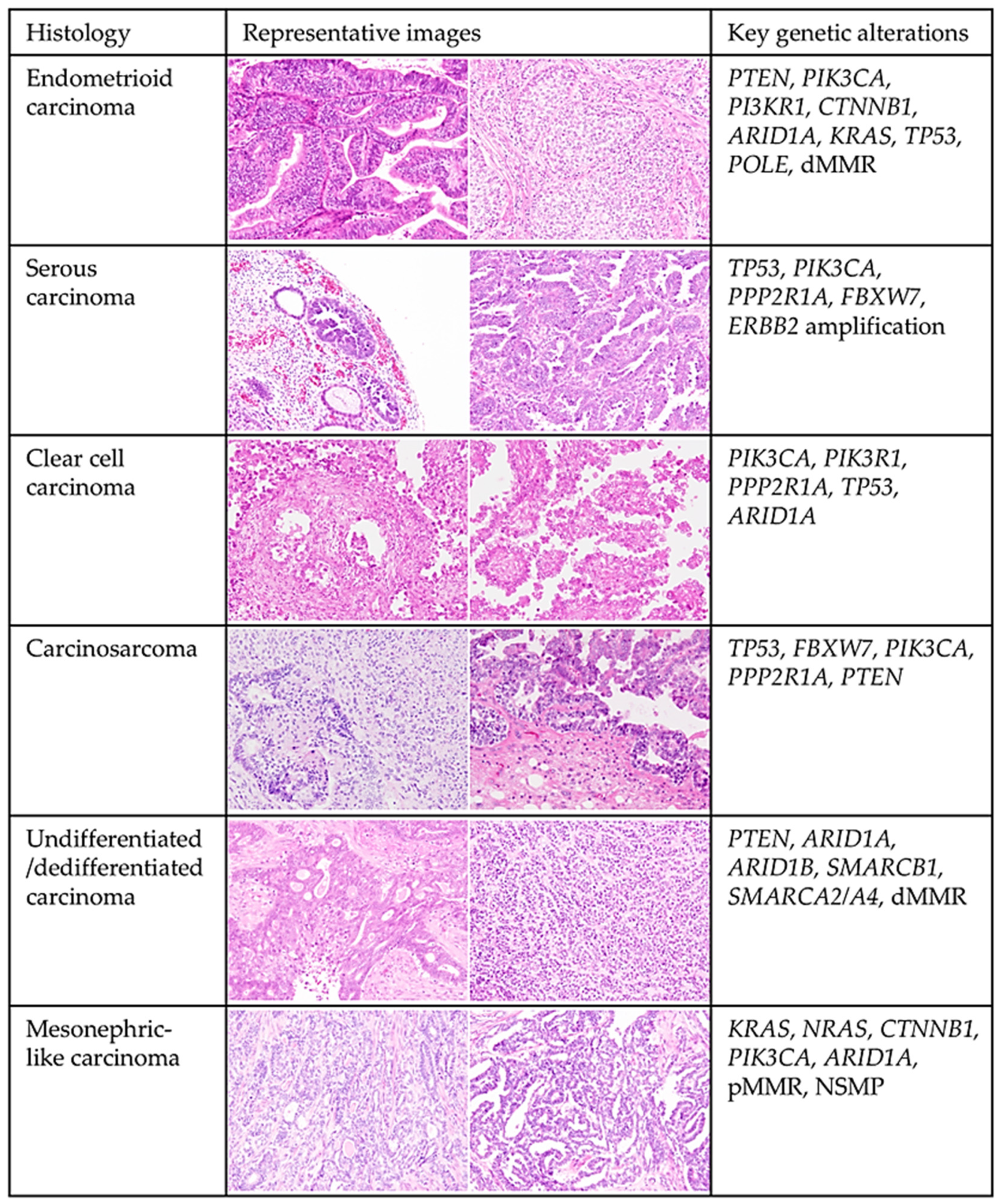
3.2. Molecular Characterization of Endometrial Cancer
3.3. The Current State of Standard Systemic Therapies for Endometrial Carcinoma
3.4. Promising Target for Endometrial Carcinoma
3.4.1. HER2
- Trastuzumab Deruxtecan
- 2.
- Trastuzumab Duocarmazine
- 3.
- BNT323/DB-1303
3.4.2. TROP2
- Sacituzumab Govitecan
- 2.
- Datopotamab Deruxtecan
- 3.
- Sacituzumab Tirumotecan
3.4.3. FRα
- Mirvetuximab Soravtansine (MIRV)
- 2.
- Farletuzumab Ecteribulin (MORAb-202)
- 3.
- Luveltamab Tazevibulin
- 4.
- Rinatabart Sesutecan
3.4.4. B7-H4
- SGN-B7H4V
- 2.
- AZD8205 (Puxitatug Samrotecan)
- 3.
- XMT-1660 (Emiltatug Ledadotin)
- 4.
- HS-20089
3.4.5. Other Targets
4. ADC for Uterine Sarcoma
4.1. Overview of the Histological and Molecular Features of Uterine Sarcoma (Figure 3)

4.2. The Current State of Standard Systemic Therapies for Uterine Sarcoma
4.3. Promising Targets for Uterine Sarcoma
4.3.1. AXL
- Mecbotamab Vedotin (BA3011)
- 2.
- Enapotamab Vedotin
4.3.2. B7-H3
- Ifinatamab Deruxtecan (DS7300a)
4.3.3. GD2
- M3554
4.3.4. Leucine-Rich Repeat Containing 15
- ABBV-085
5. Future Directions
5.1. Mechanisms of Resistance to ADCs
5.2. Sequencing Strategies
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J Clin 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Biagioli, E.; Harano, K.; Galli, F.; Hudson, E.; Antill, Y.; Choi, C.H.; Rabaglio, M.; Marme, F.; Marth, C.; et al. Atezolizumab and chemotherapy for advanced or recurrent endometrial cancer (AtTEnd): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2024, 25, 1135–1146. [Google Scholar] [CrossRef]
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B.; Mannel, R.; Shahin, M.S.; Cantuaria, G.H.; Girda, E.; et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Colombo, N.; Casado Herraez, A.; Santin, A.D.; Colomba, E.; Miller, D.S.; Fujiwara, K.; Pignata, S.; Baron-Hay, S.; Ray-Coquard, I.; et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N. Engl. J. Med. 2022, 386, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; dePont Christensen, R.; Novak, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef] [PubMed]
- Westin, S.N.; Moore, K.; Chon, H.S.; Lee, J.Y.; Thomes Pepin, J.; Sundborg, M.; Shai, A.; de la Garza, J.; Nishio, S.; Gold, M.A.; et al. Durvalumab Plus Carboplatin/Paclitaxel Followed by Maintenance Durvalumab With or Without Olaparib as First-Line Treatment for Advanced Endometrial Cancer: The Phase III DUO-E Trial. J. Clin. Oncol. 2024, 42, 283–299. [Google Scholar] [CrossRef]
- Brooks, S.E.; Zhan, M.; Cote, T.; Baquet, C.R. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989–1999. Gynecol. Oncol. 2004, 93, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Reichert, J.M.; Senter, P.D.; Lambert, J.M.; Beck, A. Antibody-drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 2023, 22, 641–661. [Google Scholar] [CrossRef]
- Colombo, R.; Tarantino, P.; Rich, J.R.; LoRusso, P.M.; de Vries, E.G.E. The Journey of Antibody-Drug Conjugates: Lessons Learned from 40 Years of Development. Cancer Discov. 2024, 14, 2089–2108. [Google Scholar] [CrossRef] [PubMed]
- Udofa, E.; Sankholkar, D.; Mitragotri, S.; Zhao, Z. Antibody-drug conjugates in the clinic. Bioeng. Transl. Med. 2024, 9, e10677. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Grants Accelerated Approval to Fam-Trastuzumab Deruxtecan-Nxki for Unresectable or Metastatic HER2-Positive Solid Tumors. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2 (accessed on 5 September 2024).
- Staudacher, A.H.; Brown, M.P. Antibody drug conjugates and bystander killing: Is antigen-dependent internalisation required? Br. J. Cancer 2017, 117, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, F.; Corti, C.; Tarantino, P.; Michelini, F.; Curigliano, G. Bystander effect of antibody-drug conjugates: Fact or fiction? Curr. Oncol. Rep. 2022, 24, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaia, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Flynn, P.; Suryaprakash, S.; Grossman, D.; Panier, V.; Wu, J. The antibody-drug conjugate landscape. Nat. Rev. Drug Discov. 2024, 23, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.T.; Moody, A.; Schorzman, A.N.; Zamboni, W.C. Importance and Considerations of Antibody Engineering in Antibody-Drug Conjugates Development from a Clinical Pharmacologist’s Perspective. Antibodies 2021, 10, 30. [Google Scholar] [CrossRef]
- Sheyi, R.; de la Torre, B.G.; Albericio, F. Linkers: An Assurance for Controlled Delivery of Antibody-Drug Conjugate. Pharmaceutics 2022, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Russo, M.; Broach, J.; Sheldon, K.; Houser, K.R.; Liu, D.J.; Kesterson, J.; Phaeton, R.; Hossler, C.; Hempel, N.; Baker, M.; et al. Clonal evolution in paired endometrial intraepithelial neoplasia/atypical hyperplasia and endometrioid adenocarcinoma. Hum. Pathol. 2017, 67, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Baak, J.P.; Mutter, G.L.; Robboy, S.; van Diest, P.J.; Uyterlinde, A.M.; Orbo, A.; Palazzo, J.; Fiane, B.; Lovslett, K.; Burger, C.; et al. The molecular genetics and morphometry-based endometrial intraepithelial neoplasia classification system predicts disease progression in endometrial hyperplasia more accurately than the 1994 World Health Organization classification system. Cancer 2005, 103, 2304–2312. [Google Scholar] [CrossRef]
- Jarboe, E.A.; Mutter, G.L. Endometrial intraepithelial neoplasia. Semin. Diagn. Pathol. 2010, 27, 215–225. [Google Scholar] [CrossRef]
- Zaino, R.J.; Kurman, R.J.; Diana, K.L.; Morrow, C.P. The utility of the revised International Federation of Gynecology and Obstetrics histologic grading of endometrial adenocarcinoma using a defined nuclear grading system. A Gynecologic Oncology Group study. Cancer 1995, 75, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee, F.W.s.C.C. FIGO staging of endometrial cancer: 2023. Int. J. Gynecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Xiang, L.; Fadare, O.; Kong, B. A proposed model for endometrial serous carcinogenesis. Am. J. Surg. Pathol. 2011, 35, e1–e14. [Google Scholar] [CrossRef]
- Huang, C.Y.; Tang, Y.H.; Chiang, Y.C.; Wang, K.L.; Fu, H.C.; Ke, Y.M.; Lau, H.Y.; Hsu, K.F.; Wu, C.H.; Cheng, W.F. Impact of management on the prognosis of pure uterine papillary serous cancer—A Taiwanese Gynecologic Oncology Group (TGOG) study. Gynecol. Oncol. 2014, 133, 221–228. [Google Scholar] [CrossRef]
- Tate, K.; Yoshida, H.; Ishikawa, M.; Uehara, T.; Ikeda, S.I.; Hiraoka, N.; Kato, T. Prognostic factors for patients with early-stage uterine serous carcinoma without adjuvant therapy. J. Gynecol. Oncol. 2018, 29, e34. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Watanabe, J.; Kawaguchi, M.; Kamata, Y.; Nishimura, Y.; Jobo, T.; Kuramoto, H. Clear cell adenocarcinoma of the endometrium is a biologically distinct entity from endometrioid adenocarcinoma. Int. J. Gynecol. Cancer 2006, 16, 391–395. [Google Scholar] [CrossRef] [PubMed]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Klar, M.; Matsuzaki, S.; Roman, L.D.; Sood, A.K.; Matsuo, K. Uterine carcinosarcoma: Contemporary clinical summary, molecular updates, and future research opportunity. Gynecol. Oncol. 2021, 160, 586–601. [Google Scholar] [CrossRef] [PubMed]
- Gopinatha Pillai, M.S.; Shaw, P.; Dey Bhowmik, A.; Bhattacharya, R.; Rao, G.; Dhar Dwivedi, S.K. Uterine carcinosarcoma: Unraveling the role of epithelial-to-mesenchymal transition in progression and therapeutic potential. FASEB J. 2024, 38, e70132. [Google Scholar] [CrossRef]
- Lee, E.K.; Liu, J.F. Uterine serous carcinoma and uterine carcinosarcoma: Molecular features, clinical advances, and emerging therapies. Clin. Adv. Hematol. Oncol. 2024, 22, 301–310. [Google Scholar] [PubMed]
- Stewart, C.J.; Crook, M.L. SWI/SNF complex deficiency and mismatch repair protein expression in undifferentiated and dedifferentiated endometrial carcinoma. Pathology 2015, 47, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Coatham, M.; Li, X.; Karnezis, A.N.; Hoang, L.N.; Tessier-Cloutier, B.; Meng, B.; Soslow, R.A.; Blake Gilks, C.; Huntsman, D.G.; Stewart, C.J.; et al. Concurrent ARID1A and ARID1B inactivation in endometrial and ovarian dedifferentiated carcinomas. Mod. Pathol. 2016, 29, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, P.; Croce, S.; McCluggage, W.G. Loss of expression of SMARCA4 (BRG1), SMARCA2 (BRM) and SMARCB1 (INI1) in undifferentiated carcinoma of the endometrium is not uncommon and is not always associated with rhabdoid morphology. Histopathology 2017, 70, 359–366. [Google Scholar] [CrossRef]
- Wang, Y.; Hoang, L.; Ji, J.X.; Huntsman, D.G. SWI/SNF Complex Mutations in Gynecologic Cancers: Molecular Mechanisms and Models. Annu. Rev. Pathol. 2020, 15, 467–492. [Google Scholar] [CrossRef] [PubMed]
- McFarland, M.; Quick, C.M.; McCluggage, W.G. Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: Report of a series of mesonephric-like adenocarcinomas. Histopathology 2016, 68, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.M.; Fix, D.J.; Sebastiao, A.P.M.; Selenica, P.; Ferrando, L.; Kim, S.H.; Stylianou, A.; Da Cruz Paula, A.; Pareja, F.; Smith, E.S.; et al. Mesonephric and mesonephric-like carcinomas of the female genital tract: Molecular characterization including cases with mixed histology and matched metastases. Mod. Pathol. 2021, 34, 1570–1587. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G. Mesonephric-like Adenocarcinoma of the Female Genital Tract: From Morphologic Observations to a Well-characterized Carcinoma With Aggressive Clinical Behavior. Adv. Anat. Pathol. 2022, 29, 208–216. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N.; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Jumaah, A.S.; Salim, M.M.; Al-Haddad, H.S.; McAllister, K.A.; Yasseen, A.A. The frequency of POLE-mutation in endometrial carcinoma and prognostic implications: A systemic review and meta-analysis. J. Pathol. Transl. Med. 2020, 54, 471–479. [Google Scholar] [CrossRef] [PubMed]
- McConechy, M.K.; Talhouk, A.; Leung, S.; Chiu, D.; Yang, W.; Senz, J.; Reha-Krantz, L.J.; Lee, C.H.; Huntsman, D.G.; Gilks, C.B.; et al. Endometrial Carcinomas with POLE Exonuclease Domain Mutations Have a Favorable Prognosis. Clin. Cancer Res. 2016, 22, 2865–2873. [Google Scholar] [CrossRef]
- Post, C.C.B.; Stelloo, E.; Smit, V.; Ruano, D.; Tops, C.M.; Vermij, L.; Rutten, T.A.; Jurgenliemk-Schulz, I.M.; Lutgens, L.; Jobsen, J.J.; et al. Prevalence and Prognosis of Lynch Syndrome and Sporadic Mismatch Repair Deficiency in Endometrial Cancer. J. Natl. Cancer Inst. 2021, 113, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Kurnit, K.C.; Kim, G.N.; Fellman, B.M.; Urbauer, D.L.; Mills, G.B.; Zhang, W.; Broaddus, R.R. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod. Pathol. 2017, 30, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Kommoss, S.; McConechy, M.K.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Kommoss, F.; Grevenkamp, F.; Karnezis, A.; et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.; Sugihara, K.; Jikoh, T.; Abe, Y.; Agatsuma, T. The Latest Research and Development into the Antibody-Drug Conjugate, [fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem. Pharm Bull 2019, 67, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.Y.; Banerjee, S.; Gonzalez-Martin, A.; Jung, K.H.; Lugowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef]
- Nishikawa, T.; Hasegawa, K.; Matsumoto, K.; Mori, M.; Hirashima, Y.; Takehara, K.; Ariyoshi, K.; Kato, T.; Yagishita, S.; Hamada, A.; et al. Trastuzumab Deruxtecan for Human Epidermal Growth Factor Receptor 2-Expressing Advanced or Recurrent Uterine Carcinosarcoma (NCCH1615): The STATICE Trial. J. Clin. Oncol. 2023, 41, 2789–2799. [Google Scholar] [CrossRef]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Sabanathan, D.; Du, Y.; Duan, H.; Li, X.; Wang, F.; Marathe, O.; Yang, H.; Makker, V.; Growdon, W.; et al. Safety and efficacy of DB-1303 in patients with advanced/metastatic solid tumors: A multicenter, open-label, first-in-human, phase 1/2a study. J. Clin. Oncol. 2023, 41, 3023. [Google Scholar] [CrossRef]
- Moore, K.; Makker, V.; Yeku, O.; Pothuri, B. #430 DB-1303, a HER2-targeting ADC, for patients with advanced/metastatic endometrial cancer: Preliminary clinical results from an ongoing phase 1/2a trial (NCT05150691). Int. J. Gynecol. Cancer 2023, 33 (Suppl. S3), A9–A10. [Google Scholar] [CrossRef]
- Bignotti, E.; Zanotti, L.; Calza, S.; Falchetti, M.; Lonardi, S.; Ravaggi, A.; Romani, C.; Todeschini, P.; Bandiera, E.; Tassi, R.A.; et al. Trop-2 protein overexpression is an independent marker for predicting disease recurrence in endometrioid endometrial carcinoma. BMC Clin. Pathol. 2012, 12, 22. [Google Scholar] [CrossRef]
- Cardillo, T.M.; Govindan, S.V.; Sharkey, R.M.; Trisal, P.; Arrojo, R.; Liu, D.; Rossi, E.A.; Chang, C.H.; Goldenberg, D.M. Sacituzumab Govitecan (IMMU-132), an Anti-Trop-2/SN-38 Antibody-Drug Conjugate: Characterization and Efficacy in Pancreatic, Gastric, and Other Cancers. Bioconjug Chem. 2015, 26, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.M.; Sharkey, R.M. Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy. Expert Opin. Biol. Ther. 2020, 20, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Bardia, A.; Marme, F.; Cortes, J.; Schmid, P.; Loirat, D.; Tredan, O.; Ciruelos, E.; Dalenc, F.; Pardo, P.G.; et al. Sacituzumab Govitecan in Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. J. Clin. Oncol. 2022, 40, 3365–3376. [Google Scholar] [CrossRef]
- Santin, A.D.; Corr, B.R.; Spira, A.; Willmott, L.; Butrynski, J.; Tse, K.Y.; Patel, J.; Mekan, S.; Wu, T.; Lin, K.W.; et al. Efficacy and Safety of Sacituzumab Govitecan in Patients With Advanced Solid Tumors (TROPiCS-03): Analysis in Patients With Advanced Endometrial Cancer. J. Clin. Oncol. 2024, 42, 3421–3429. [Google Scholar] [CrossRef]
- Okajima, D.; Yasuda, S.; Maejima, T.; Karibe, T.; Sakurai, K.; Aida, T.; Toki, T.; Yamaguchi, J.; Kitamura, M.; Kamei, R.; et al. Datopotamab Deruxtecan, a Novel TROP2-directed Antibody-drug Conjugate, Demonstrates Potent Antitumor Activity by Efficient Drug Delivery to Tumor Cells. Mol. Cancer Ther. 2021, 20, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Jhaveri, K.; Im, S.A.; Pernas, S.; De Laurentiis, M.; Wang, S.; Martinez Janez, N.; Borges, G.; Cescon, D.W.; Hattori, M.; et al. Datopotamab Deruxtecan Versus Chemotherapy in Previously Treated Inoperable/Metastatic Hormone Receptor-Positive Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer: Primary Results From TROPION-Breast01. J. Clin. Oncol. 2025, 43, 285–296. [Google Scholar] [CrossRef]
- FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-datopotamab-deruxtecan-dlnk-unresectable-or-metastatic-hr-positive-her2-negative-breast (accessed on 19 January 2025).
- Cheng, Y.; Yuan, X.; Tian, Q.; Huang, X.; Chen, Y.; Pu, Y.; Long, H.; Xu, M.; Ji, Y.; Xie, J.; et al. Preclinical profiles of SKB264, a novel anti-TROP2 antibody conjugated to topoisomerase inhibitor, demonstrated promising antitumor efficacy compared to IMMU-132. Front. Oncol. 2022, 12, 951589. [Google Scholar] [CrossRef]
- Xu, B.; Yin, Y.; Fan, Y.; Ouyang, Q.; Song, L.; Wang, X.; Li, W.; Li, M.; Yan, X.; Wang, S.; et al. Sacituzumab tirumotecan (SKB264/MK-2870) in patients (pts) with previously treated locally recurrent or metastatic triple-negative breast cancer (TNBC): Results from the phase III OptiTROP-Breast01 study. J. Clin. Oncol. 2024, 42, 104. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Leamon, C.P.; Reddy, J.A. The folate receptor is a rational therapeutic target for personalized cancer treatment. Drug Resist. Updat. 2014, 17, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.A.; Canevari, S.; Thigpen, T. Targeting the folate receptor: Diagnostic and therapeutic approaches to personalize cancer treatments. Ann. Oncol. 2015, 26, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.L.; Xiong, N.; Tayob, N.; Polak, M.; Sawyer, H.; Hayes, M.; Gardner, J.; Campos, S.; Horowitz, N.; Krasner, C.; et al. Abstract CT008: A phase 2, two-stage study of mirvetuximab soravtansine (IMGN853) in combination with pembrolizumab in patients with microsatellite stable (MSS) recurrent or persistent endometrial cancer. Cancer Res. 2024, 84, CT008. [Google Scholar] [CrossRef]
- Saito, A.; Nishikawa, T.; Yoshida, H.; Mizoguchi, C.; Kitadai, R.; Yamamoto, K.; Yazaki, S.; Kojima, Y.; Ishikawa, M.; Kato, T.; et al. Folate receptor alpha is widely expressed and a potential therapeutic target in uterine and ovarian carcinosarcoma. Gynecol. Oncol. 2023, 176, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Angelergues, A.; Konecny, G.E.; Garcia, Y.; Banerjee, S.; Lorusso, D.; Lee, J.Y.; Moroney, J.W.; Colombo, N.; Roszak, A.; et al. Mirvetuximab Soravtansine in FRalpha-Positive, Platinum-Resistant Ovarian Cancer. N. Engl. J. Med. 2023, 389, 2162–2174. [Google Scholar] [CrossRef] [PubMed]
- FDA. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761310Origs005lbl.pdf (accessed on 19 January 2025).
- Moore, K.N.; Borghaei, H.; O’Malley, D.M.; Jeong, W.; Seward, S.M.; Bauer, T.M.; Perez, R.P.; Matulonis, U.A.; Running, K.L.; Zhang, X.; et al. Phase 1 dose-escalation study of mirvetuximab soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, in patients with solid tumors. Cancer 2017, 123, 3080–3087. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Fujiwara, Y.; Yonemori, K.; Koyama, T.; Sato, J.; Tamura, K.; Shimomura, A.; Ikezawa, H.; Nomoto, M.; Furuuchi, K.; et al. First-in-Human Phase 1 Study of MORAb-202, an Antibody-Drug Conjugate Comprising Farletuzumab Linked to Eribulin Mesylate, in Patients with Folate Receptor-alpha-Positive Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3905–3915. [Google Scholar] [CrossRef] [PubMed]
- Pothuri, B.; Naumann, R.W.; Martin, L.P.; O’Malley, D.; Uyar, D.; Moroney, J.W.; Diaz, J.; Garcia-Donas, J.; Redondo Sanchez, A.; Gonzalez Martin, A.; et al. 741MO Luveltamab tazevibulin (STRO-002), an anti-folate receptor alpha (FolRα) antibody drug conjugate (ADC), demonstrates clinical activity in recurrent/progressive epithelial endometrial cancer (EEC): STRO-002-GM1 phase I dose expansion. Ann. Oncol. 2023, 34, S508. [Google Scholar] [CrossRef]
- Lee, E.K.; Yeku, O.; Winer, I.; Hamilton, E.P.; Richardson, D.L.; Zhang, J.; Konecny, G.E.; Anderson, I.C.; Wu, X.; Orr, D.; et al. 719MO A phase I/II study of rinatabart sesutecan (Rina-S) in patients with advanced ovarian or endometrial cancer. Ann. Oncol. 2024, 35, S550. [Google Scholar] [CrossRef]
- Gitto, S.B.; Whicker, M.; Davies, G.; Kumar, S.; Kinneer, K.; Xu, H.; Lewis, A.; Mamidi, S.; Medvedev, S.; Kim, H.; et al. A B7-H4-Targeting Antibody-Drug Conjugate Shows Antitumor Activity in PARPi and Platinum-Resistant Cancers with B7-H4 Expression. Clin. Cancer Res. 2024, 30, 1567–1581. [Google Scholar] [CrossRef]
- Kinneer, K.; Wortmann, P.; Cooper, Z.A.; Dickinson, N.J.; Masterson, L.; Cailleau, T.; Hutchinson, I.; Vijayakrishnan, B.; McFarlane, M.; Ball, K.; et al. Design and Preclinical Evaluation of a Novel B7-H4-Directed Antibody-Drug Conjugate, AZD8205, Alone and in Combination with the PARP1-Selective Inhibitor AZD5305. Clin. Cancer Res. 2023, 29, 1086–1101. [Google Scholar] [CrossRef] [PubMed]
- Podojil, J.R.; Miller, S.D. Potential targeting of B7-H4 for the treatment of cancer. Immunol. Rev. 2017, 276, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.; Ulrich, M.; Epp, A.; Younan, P.; Sahetya, D.; Hensley, K.; Allred, S.; Huang, L.Y.; Hahn, J.; Gahnberg, K.; et al. SGN-B7H4V, an investigational vedotin ADC directed to the immune checkpoint ligand B7-H4, shows promising activity in preclinical models. J. Immunother. Cancer 2023, 11, e007572. [Google Scholar] [CrossRef]
- Perez, C.A.; Henry, J.T.; Lakhani, N.; Call, J.A.; Hamilton, E.P.; Colon-Otero, G.; Diamond, J.R.; O’Neil, B.; Kalyan, A.; Sonpavde, G.P.; et al. 660MO First-in-human study of SGN-B7H4V, a B7-H4-directed vedotin ADC, in patients with advanced solid tumors: Preliminary results of a phase I study (SGNB7H4V-001). Ann. Oncol. 2023, 34, S464–S465. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Naito, Y.; Gaillard, S.; Shimoi, T.; Chung, V.; Davis, A.A.; Proia, T.; Ayyoub, A.; Kulkarni, M.; Upadhyay, S.; et al. 606O Initial results from a first-in-human study of the B7-H4-directed antibody-drug conjugate (ADC) AZD8205 (puxitatug samrotecan) in patients with advanced/metastatic solid tumors. Ann. Oncol. 2024, 35, S485–S486. [Google Scholar] [CrossRef]
- Toader, D.; Fessler, S.P.; Collins, S.D.; Conlon, P.R.; Bollu, R.; Catcott, K.C.; Chin, C.N.; Dirksen, A.; Du, B.; Duvall, J.R.; et al. Discovery and Preclinical Characterization of XMT-1660, an Optimized B7-H4-Targeted Antibody-Drug Conjugate for the Treatment of Cancer. Mol. Cancer Ther. 2023, 22, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, J.; Li, H.; Wang, X.; Zhang, Q.Y.; Shi, Y.; yan, M.; Pan, Y.; Shen, A.; Chen, Q.; et al. 381O First-in-human/phase I trial of HS-20089, a B7-H4 ADC, in patients with advanced solid tumors. Ann. Oncol. 2023, 34, S336. [Google Scholar] [CrossRef]
- Mbatani, N.; Olawaiye, A.B.; Prat, J. Uterine sarcomas. Int. J. Gynecol. Obstet. 2018, 143 (Suppl. S2), 51–58. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Prat, J. Uterine sarcomas: A review. Gynecol. Oncol. 2010, 116, 131–139. [Google Scholar] [CrossRef]
- Board, W.C.o.T.E. Female Genital Tumours, WHO Classification of Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020; Volume 4.
- Niu, S.; Zheng, W. Endometrial stromal tumors: Diagnostic updates and challenges. Semin. Diagn. Pathol. 2022, 39, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Howitt, B.E.; Sholl, L.M.; Dal Cin, P.; Jia, Y.; Yuan, L.; MacConaill, L.; Lindeman, N.; Kuo, F.; Garcia, E.; Nucci, M.R.; et al. Targeted genomic analysis of Mullerian adenosarcoma. J. Pathol. 2015, 235, 37–49. [Google Scholar] [CrossRef]
- Yoshida, H.; Kikuchi, A.; Tsuda, H.; Sakamoto, A.; Fukunaga, M.; Kaku, T.; Yoshida, M.; Shikama, A.; Kogata, Y.; Terao, Y.; et al. Discrepancies in pathological diagnosis of endometrial stromal sarcoma: A multi-institutional retrospective study from the Japanese Clinical Oncology Group. Hum. Pathol. 2022, 124, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Tate, K.; Watanabe, R.; Yoshida, H.; Shimizu, H.; Uehara, T.; Ishikawa, M.; Ikeda, S.I.; Hiraoka, N.; Kato, T. Uterine adenosarcoma in Japan: Clinicopathologic features, diagnosis and management. Asia Pac. J. Clin. Oncol. 2018, 14, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.; Cotzia, P.; Hyman, D.M.; Drilon, A.; Tap, W.D.; Zhang, L.; Hechtman, J.F.; Frosina, D.; Jungbluth, A.A.; Murali, R.; et al. NTRK Fusions Define a Novel Uterine Sarcoma Subtype With Features of Fibrosarcoma. Am. J. Surg. Pathol. 2018, 42, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Szalai, L.; Vereczkey, I.; Szemes, M.; Rokusz, A.; Csernak, E.; Toth, E.; Melegh, Z. NTRK-rearranged spindle cell sarcoma of the uterine cervix with a novel NUMA1::NTRK1 fusion. Virchows Arch. 2024, 484, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Kolin, D.L.; Dong, F.; Baltay, M.; Lindeman, N.; MacConaill, L.; Nucci, M.R.; Crum, C.P.; Howitt, B.E. SMARCA4-deficient undifferentiated uterine sarcoma (malignant rhabdoid tumor of the uterus): A clinicopathologic entity distinct from undifferentiated carcinoma. Mod. Pathol. 2018, 31, 1442–1456. [Google Scholar] [CrossRef]
- Seddon, B.; Strauss, S.J.; Whelan, J.; Leahy, M.; Woll, P.J.; Cowie, F.; Rothermundt, C.; Wood, Z.; Benson, C.; Ali, N.; et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): A randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1397–1410. [Google Scholar] [CrossRef]
- Pautier, P.; Italiano, A.; Piperno-Neumann, S.; Chevreau, C.; Penel, N.; Firmin, N.; Boudou-Rouquette, P.; Bertucci, F.; Lebrun-Ly, V.; Ray-Coquard, I.; et al. Doxorubicin-Trabectedin with Trabectedin Maintenance in Leiomyosarcoma. N. Engl. J. Med. 2024, 391, 789–799. [Google Scholar] [CrossRef]
- Ingham, M.; Allred, J.B.; Chen, L.; Das, B.; Kochupurakkal, B.; Gano, K.; George, S.; Attia, S.; Burgess, M.A.; Seetharam, M.; et al. Phase II Study of Olaparib and Temozolomide for Advanced Uterine Leiomyosarcoma (NCI Protocol 10250). J. Clin. Oncol. 2023, 41, 4154–4163. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.W.; Schulz, A.S.; Steenvoorden, A.C.; Schmidberger, M.; Strehl, S.; Ambros, P.F.; Bartram, C.R. A novel putative tyrosine kinase receptor with oncogenic potential. Oncogene 1991, 6, 2113–2120. [Google Scholar] [PubMed]
- Pollack, S.; Conley, A.P.; Tap, W.D.; Yen, C.C.; Charlson, J.; Davis, L.; Chalmers, A.; Druta, M.; Loggers, E.; Grewal, J.S.; et al. 53O Results from a phase II part I trial of mecbotamab vedotin (BA3011), a CAB-AXL-ADC, in patients with advanced refractory sarcoma. ESMO Open 2024, 9, 102443. [Google Scholar] [CrossRef]
- De Wispelaere, W.; Annibali, D.; Tuyaerts, S.; Lambrechts, D.; Amant, F. Resistance to Immune Checkpoint Blockade in Uterine Leiomyosarcoma: What Can We Learn from Other Cancer Types? Cancers 2021, 13, 40. [Google Scholar] [CrossRef]
- Van Renterghem, B.; Wozniak, A.; Castro, P.G.; Franken, P.; Pencheva, N.; Sciot, R.; Schoffski, P. Enapotamab Vedotin, an AXL-Specific Antibody-Drug Conjugate, Demonstrates Antitumor Efficacy in Patient-Derived Xenograft Models of Soft Tissue Sarcoma. Int. J. Mol. Sci. 2022, 23, 7493. [Google Scholar] [CrossRef] [PubMed]
- Ameratunga, M.; Harvey, R.D.; Mau-Sørensen, M.; Thistlethwaite, F.; Forssmann, U.; Gupta, M.; Johannsdottir, H.; Ramirez-Andersen, T.; Bohlbro, M.L.; Losic, N.; et al. First-in-human, dose-escalation, phase (ph) I trial to evaluate safety of anti-Axl antibody-drug conjugate (ADC) enapotamab vedotin (EnaV) in solid tumors. J. Clin. Oncol. 2019, 37, 2525. [Google Scholar] [CrossRef]
- Kontos, F.; Michelakos, T.; Kurokawa, T.; Sadagopan, A.; Schwab, J.H.; Ferrone, C.R.; Ferrone, S. B7-H3: An Attractive Target for Antibody-based Immunotherapy. Clin. Cancer Res. 2021, 27, 1227–1235. [Google Scholar] [CrossRef]
- Lynch, M.M.; Al-Marayaty, R.; Obeidin, F.; Alexiev, B.A.; Chen, E.Y.; Viveiros, P.; Schroeder, B.A.; Hudkins, K.; Fan, T.M.; Redman, M.W.; et al. B7-H3 is widely expressed in soft tissue sarcomas. BMC Cancer 2024, 24, 1336. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Byers, L.A.; Cheng, Y.; Hayashi, H.; Paz-Ares, L.G.; Perol, M.; Turner, J.; Qian, M.; Garcia, C.R.; Godard, J.; et al. IDeate-Lung02: A phase 3, randomized, open-label study of ifinatamab deruxtecan (I-DXd) vs treatment of physician’s choice (TPC) in relapsed small cell lung cancer (SCLC). J. Clin. Oncol. 2024, 42, TPS8126. [Google Scholar] [CrossRef]
- Ziebarth, A.J.; Felder, M.A.; Harter, J.; Connor, J.P. Uterine leiomyosarcoma diffusely express disialoganglioside GD2 and bind the therapeutic immunocytokine 14.18-IL2: Implications for immunotherapy. Cancer Immunol. Immunother. 2012, 61, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Amendt, C.; Knuehl, C.; Crandall, T.; Sloot, W.; Toister-Achituv, M.; Sweeney-Lasch, S.; Pereira, J.N.; Dotterweich, J.; Boehm, H.H.; Ma, J.; et al. Abstract ND08: M3554, a novel anti-GD2 antibody drug conjugate. Cancer Res. 2024, 84, ND08. [Google Scholar] [CrossRef]
- Demetri, G.D.; Luke, J.J.; Hollebecque, A.; Powderly, J.D., 2nd; Spira, A.I.; Subbiah, V.; Naumovski, L.; Chen, C.; Fang, H.; Lai, D.W.; et al. First-in-Human Phase I Study of ABBV-085, an Antibody-Drug Conjugate Targeting LRRC15, in Sarcomas and Other Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3556–3566. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Rugo, H.S.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Kalinsky, K.; Cortes, J.; Shaughnessy, J.O.; et al. Final Results From the Randomized Phase III ASCENT Clinical Trial in Metastatic Triple-Negative Breast Cancer and Association of Outcomes by Human Epidermal Growth Factor Receptor 2 and Trophoblast Cell Surface Antigen 2 Expression. J. Clin. Oncol. 2024, 42, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Klumper, N.; Tran, N.K.; Zschabitz, S.; Hahn, O.; Buttner, T.; Roghmann, F.; Bolenz, C.; Zengerling, F.; Schwab, C.; Nagy, D.; et al. NECTIN4 Amplification Is Frequent in Solid Tumors and Predicts Enfortumab Vedotin Response in Metastatic Urothelial Cancer. J. Clin. Oncol. 2024, 42, 2446–2455. [Google Scholar] [CrossRef] [PubMed]
- Poumeaud, F.; Morisseau, M.; Cabel, L.; Goncalves, A.; Rivier, C.; Tredan, O.; Volant, E.; Frenel, J.S.; Ladoire, S.; Jacot, W.; et al. Efficacy of administration sequence: Sacituzumab Govitecan and Trastuzumab Deruxtecan in HER2-low metastatic breast cancer. Br. J. Cancer 2024, 131, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, R.; Chen, R.; Pan, F.; Shen, X.; Li, H.; Rong, Q.; An, X.; Xue, C.; Shi, Y. Optimal Sequential Strategies for Antibody-Drug Conjugate in Metastatic Breast Cancer: Evaluating Efficacy and Cross-Resistance. Oncologist 2024, 29, e957–e966. [Google Scholar] [CrossRef]
| Antibody Target | Agent | Payload | Type of Payload | DAR | Linker | Phase | Clinical Trials |
|---|---|---|---|---|---|---|---|
| HER2 | Trastuzumab deruxtecan | Deruxtecan | Topoisomerase I inhibitor | 8 | Cleavable | II | NCT04482309 |
| HER2 | Trastuzumab duocarmazine | Duocarmazine | DNA targeting agent | 2.8 | Cleavable | II | NCT04205630 |
| HER2 | BNT323/DB-1303 | P1003 | Topoisomerase I inhibitor | 8 | Cleavable | I/IIA | NCT05150691 |
| III | ENGOT en25/GOG 3105/BNT323 01 | ||||||
| HER2 | Disitamab vedotin | MMAE | Anti microtubule agent | 4 | Cleavable | I | NCT02881190 |
| HER2 | IBI354 | NT3 | Topoisomerase I inhibitor | 8 | Cleavable | I | NCT05636215 |
| TROP2 | Sacituzumab govitecan | SN-38 | Topoisomerase I inhibitor | 7 | Cleavable | III | ASCENT-GYN-01/GOG-3104/ENGOT-en26 |
| TROP2 | Sacituzumab tirumotican | Tirumotican | Topoisomerase I inhibitor | 7.4 | Cleavable | III | MK-2870–005/ENGOT-en23/GOG-3095 |
| TROP2 | Datopotamab deruxtecan | Deruxtecan | Topoisomerase I inhibitor | 4 | Cleavable | II | NCT05489211 |
| FRα | Mirvetuximab soravtansine | DM4 | Anti-tubulin maytansinoid agent | 3.5 | Cleavable | II | NCT03832361 |
| FRα | Farletuzumab ecteribulin (MORAb202) | Eribulin mesylate | Anti microtubule agent | 4 | Cleavable | I/II | NCT04300556 |
| FRα | Luveltamab tazevibulin (STRO-002) | Hemiasterlin | Anti microtubule agent | 4 | Cleavable | I/II | NCT03748186, NCT06238687 |
| FRα | Rinatabart sesutecan (Rina S, PRO1184) | Exatecan | Topoisomerase I inhibitor | 8 | Cleavable | I/II | NCT05579366 |
| FRα | IMGN151 | DM21 | Anti-tubulin maytansinoid agent | 3.5 | Cleavable | I | NCT05527184 |
| FRα | LY4170156 | Exatecan | Topoisomerase I inhibitor | 8 | Cleavable | I | NCT06400472 |
| B7-H4 | AZD8205 | AZ14170133 | Topoisomerase I inhibitor | 8 | Cleavable | I/IIA | NCT05123482 |
| B7-H4 | HS-20089 | - | Topoisomerase I inhibitor | 6 | Cleavable | I | NCT05263479 |
| B7-H4 | SGN-B7H4V | MMAE | Anti microtubule agent | 4 | Cleavable | I | NCT05194072 |
| B7-H4 | XMT-1660 | Auristatin F | Anti microtubule agent | 6 | Cleavable | I | NCT05377996 |
| TF | Tisotumab vedotin | MMAE | Anti microtubule agent | 4 | Cleavable | II | |
| Claudin6 | TORL-1-23 | MMAE | Anti microtubule agent | ~4 | Cleavable | I | NCT05103683 |
| Nectin4 | LY4101174 | Exatecan | Topoisomerase I inhibitor | 8 | Cleavable | I | NCT06400472 |
| Antibody Target | Agent | Payload | Type of Payload | DAR | Linker | Phase | Clinical Trials |
|---|---|---|---|---|---|---|---|
| AXL | Mecbotamab vedotin (BA3011) | MMAE | Anti microtubule agent | 1.8 | Cleavable | I/II | NCT03425279 |
| AXL | Enapotamab vedotin | MMAE | Anti microtubule agent | 4 | Cleavable | I/II | NCT02988817 |
| B7-H3 | Ifinatamab deruxtecan (DS7300a) | Deruxtecan | Topoisomerase I inhibitor | 4 | Cleavable | IB/II | NCT06330064 |
| GD2 | M3554 | Exatecan | Topoisomerase I inhibitor | 2 | Cleavable | I | NCT06641908 |
| LRRC15 | ABBV-085 | MMAE | Anti microtubule agent | 2 | Cleavable | I | NCT02565758 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamanaka, T.; Nishikawa, T.; Yoshida, H. Development of Antibody–Drug Conjugates for Malignancies of the Uterine Corpus: A Review. Cells 2025, 14, 333. https://doi.org/10.3390/cells14050333
Yamanaka T, Nishikawa T, Yoshida H. Development of Antibody–Drug Conjugates for Malignancies of the Uterine Corpus: A Review. Cells. 2025; 14(5):333. https://doi.org/10.3390/cells14050333
Chicago/Turabian StyleYamanaka, Taro, Tadaaki Nishikawa, and Hiroshi Yoshida. 2025. "Development of Antibody–Drug Conjugates for Malignancies of the Uterine Corpus: A Review" Cells 14, no. 5: 333. https://doi.org/10.3390/cells14050333
APA StyleYamanaka, T., Nishikawa, T., & Yoshida, H. (2025). Development of Antibody–Drug Conjugates for Malignancies of the Uterine Corpus: A Review. Cells, 14(5), 333. https://doi.org/10.3390/cells14050333





