In Vitro Inhibition of Endoplasmic Reticulum Stress: A Promising Therapeutic Strategy for Patients with Crohn’s Disease
Abstract
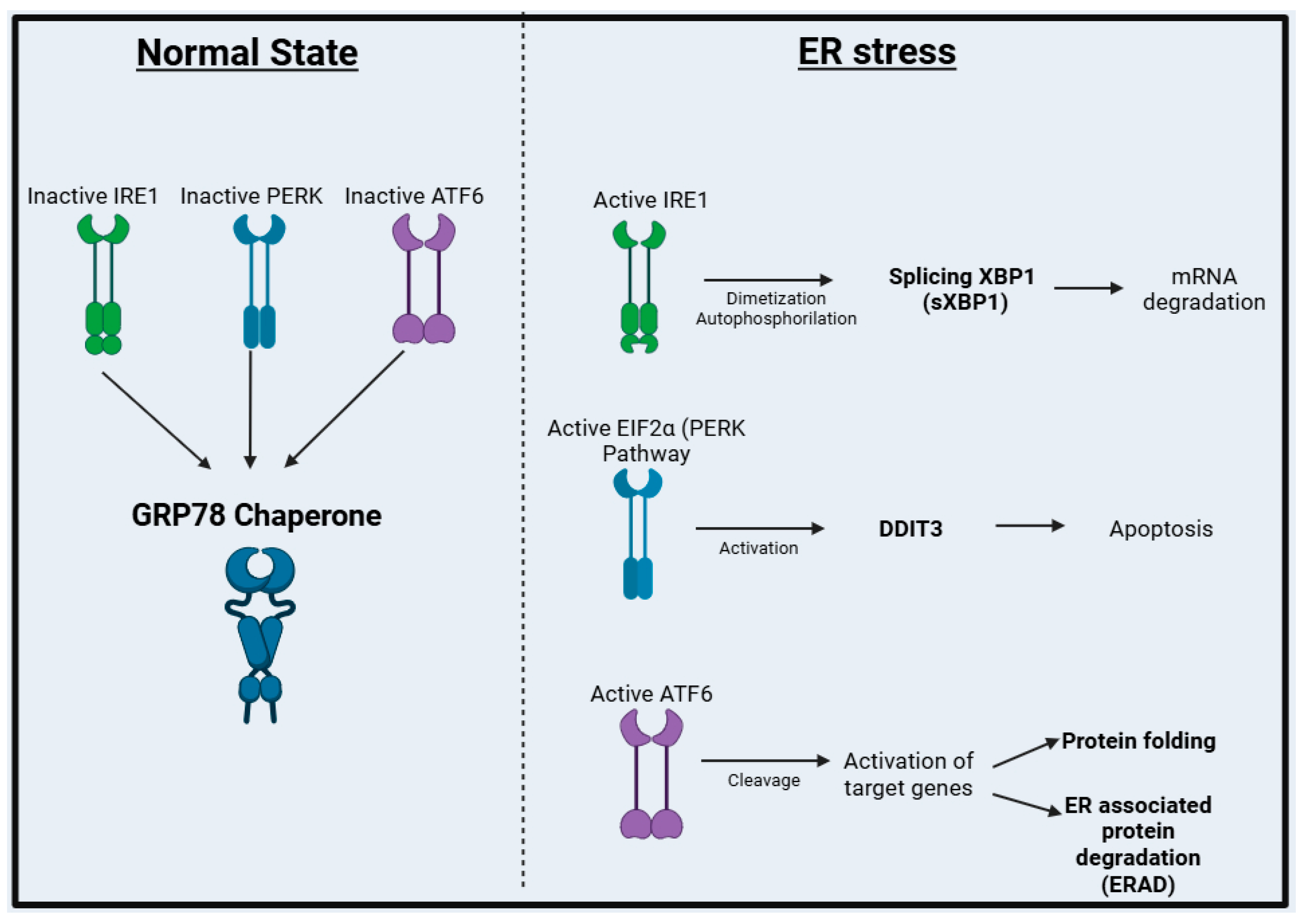
1. Introduction
2. Materials and Methods
2.1. Patients and Ethics Statement
2.2. Immunochemistry
2.3. Explant Culture
2.4. RNA Extraction and cDNA Synthesis
2.5. Quantitative Real-Time PCR (qPCR)
2.6. Cytokines Quantification
2.7. Statistics
3. Results
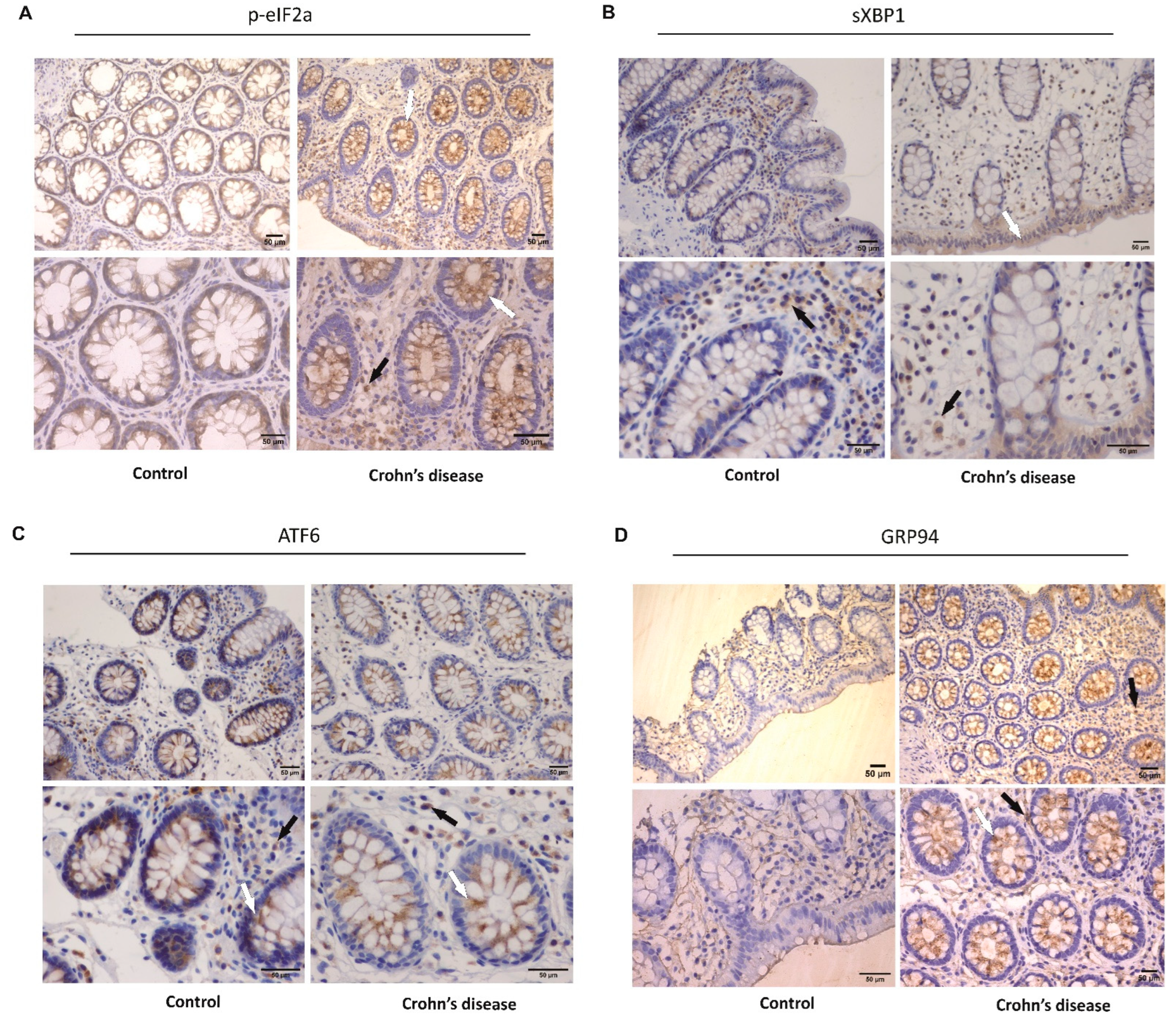
3.1. Activation of ER Stress in the Intestinal Mucosa of Crohn’s Disease Patients
3.2. Blockade of Intestinal Mucosa ER Stress of Crohn’s Disease by a Chemical Chaperone
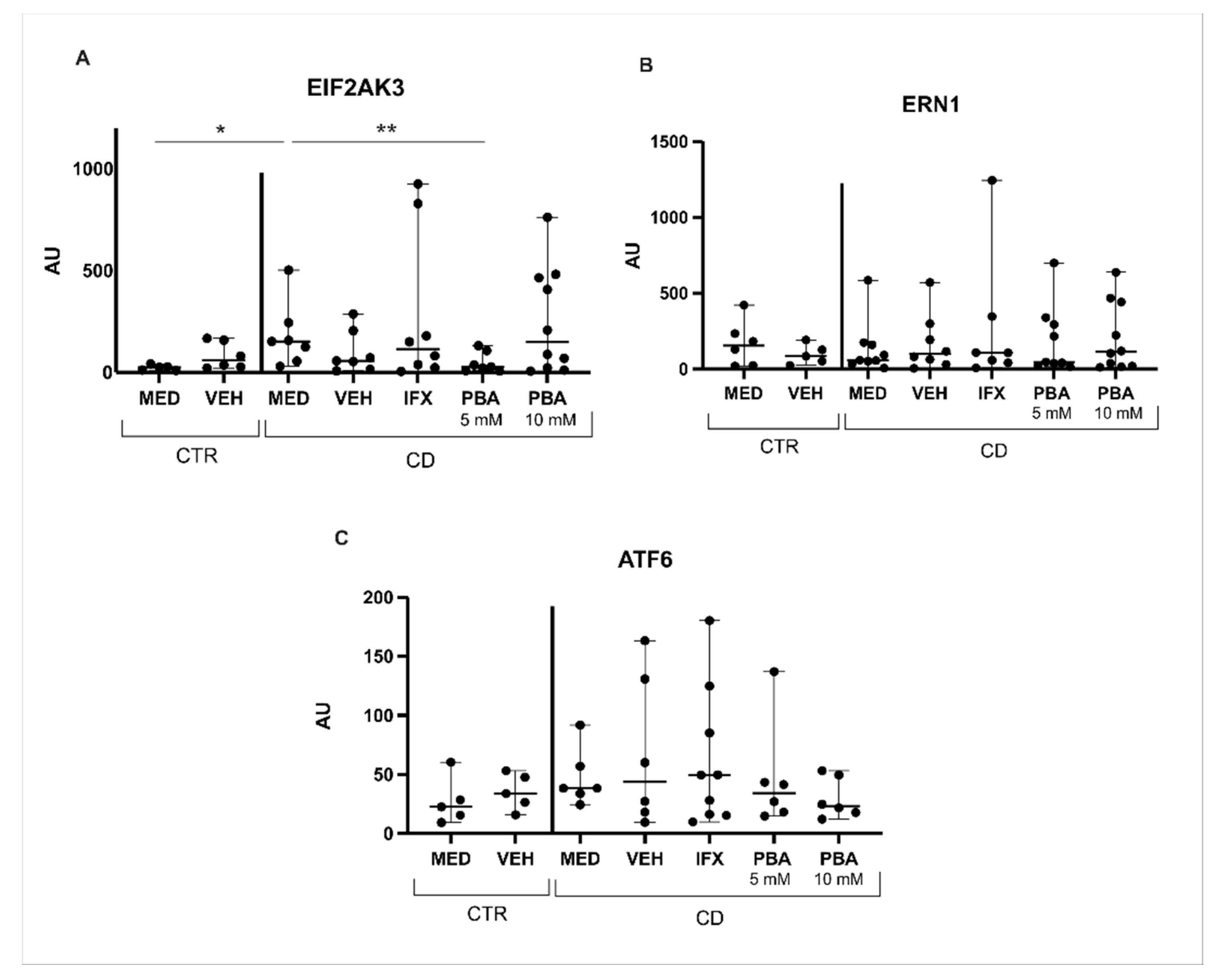
3.3. Chaperones and Genes Responsive to UPR Were Blocked with PBA in the Intestinal Mucosa of Crohn’s Disease Patients
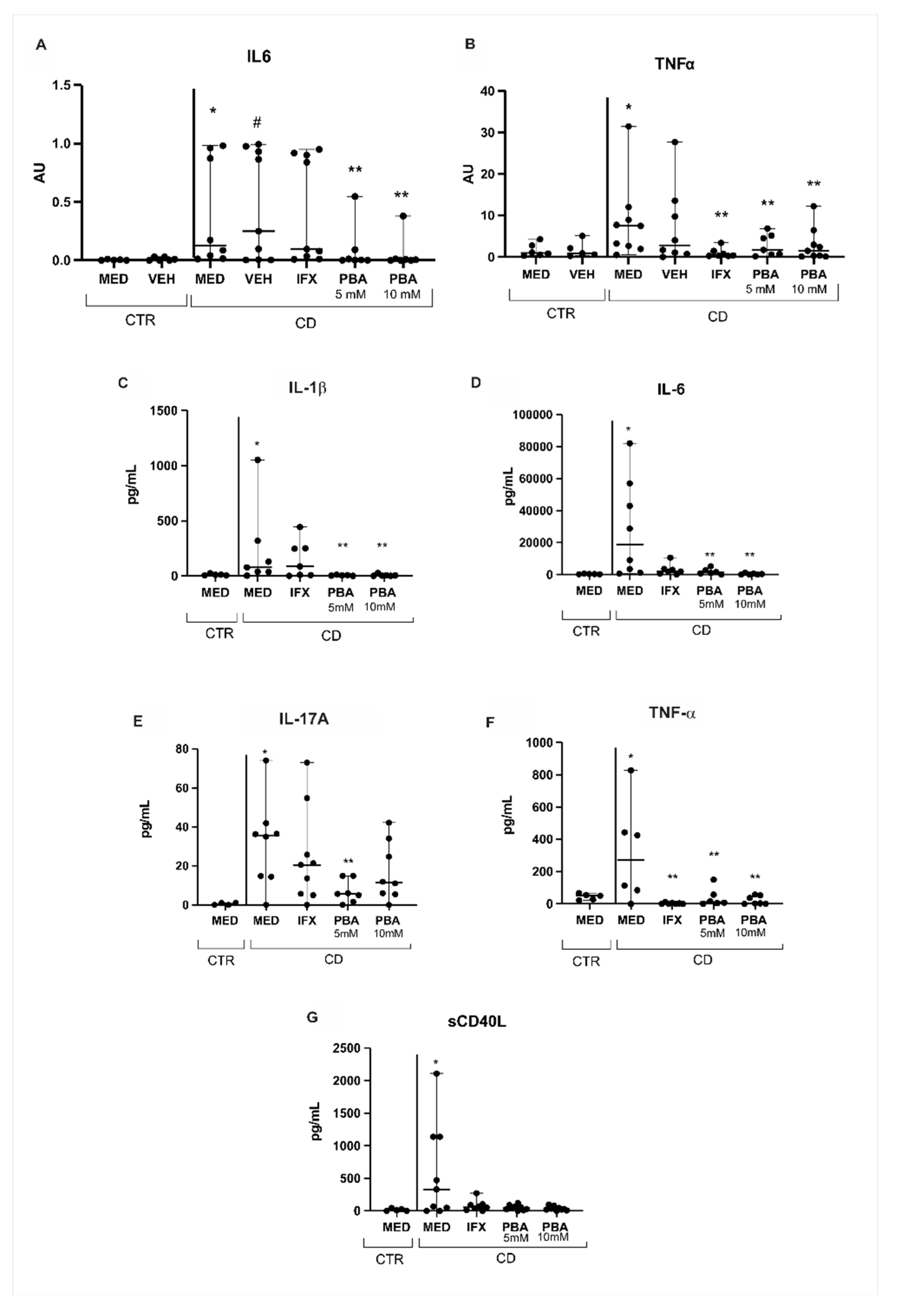
3.4. PBA as an Immune Modulator in Crohn’s Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, J.T. Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Shivashankar, R.; Tremaine, W.J.; Harmsen, W.S.; Loftus, E.V., Jr. Incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin. Gastroenterol. Hepatol. 2017, 15, 857–863. [Google Scholar] [CrossRef]
- Hooper, K.M.; Barlow, P.G.; Henderson, P.; Stevens, C. Interactions between autophagy and the unfolded protein response: Implications for inflammatory bowel disease. Inflamm. Bowel. Dis. 2019, 25, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Li, C. Unfolded Protein Response and Crohn’s Diseases: A Molecular Mechanism of Wound Healing in the Gut. Gastrointest. Disord. 2021, 3, 31–43. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J. The Effect of Protein Nutritional Support on Inflammatory Bowel Disease and Its Potential Mechanisms. Nutrients 2024, 16, 2302. [Google Scholar] [CrossRef]
- Senft, D.; Ronai, Z.A. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 2015, 40, 141–148. [Google Scholar] [CrossRef]
- Cao, S.S. Endoplasmic reticulum stress and unfolded protein response in inflammatory bowel disease. Inflamm. Bowel. Dis. 2015, 21, 636–644. [Google Scholar] [CrossRef] [PubMed]
- So, J.-S. Roles of endoplasmic reticulum stress in immune responses. Mol. Cells 2018, 41, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Tirasophon, W.; Shen, X.; Michalak, M.; Prywes, R.; Okada, T.; Yoshida, H.; Mori, K.; Kaufman, R.J. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002, 16, 452–466. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta. 2013, 1833, 3460–3470. [Google Scholar] [CrossRef] [PubMed]
- Urra, H.; Dufey, E.; Lisbona, F.; Rojas-Rivera, D.; Hetz, C. When ER stress reaches a dead end. Biochim. Biophys. Acta. 2013, 1833, 3507–3517. [Google Scholar] [CrossRef] [PubMed]
- Namba, T.; Ishihara, T.; Tanaka, K.; Hoshino, T.; Mizushima, T. Transcriptional activation of ATF6 by endoplasmic reticulum stressors. Biochem. Biophys. Res. Commun. 2007, 355, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef]
- Ma, X.; Dai, Z.; Sun, K.; Zhang, Y.; Chen, J.; Yang, Y.; Tso, P.; Wu, G.; Wu, Z. Intestinal Epithelial Cell Endoplasmic Reticulum Stress and Inflammatory Bowel Disease Pathogenesis: An Update Review. Front. Immunol. 2017, 8, 1271. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Zimmermann, E.M.; Chuang, B.M.; Song, B.; Nwokoye, A.; Wilkinson, J.E.; Eaton, K.A.; Kaufman, R.J. The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice. Gastroenterology. 2013, 144, 989–1000.e6. [Google Scholar] [CrossRef]
- Zhang, H.S.; Chen, Y.; Fan, L.; Xi, Q.L.; Wu, G.H.; Li, X.X.; Yuan, T.L.; He, S.Q.; Yu, Y.; Shao, M.L.; et al. The Endoplasmic Reticulum Stress Sensor IRE1α in Intestinal Epithelial Cells Is Essential for Protecting against Colitis. J. Biol. Chem. 2015, 290, 15327–15336. [Google Scholar] [CrossRef]
- Coope, A.; Pascoal, L.B.; Botezelli, J.D.; da Silva, F.A.R.; Ayrizono, M.L.S.; Rodrigues, B.L.; Milanski, M.; Carvalho, R.B.; Fagundes, J.J.; Velloso, L.A.; et al. ER stress activation in the intestinal mucosa but not in mesenteric adipose tissue is associated with inflammation in Crohn’s disease patients. PLoS ONE 2019, 14, e0223105. [Google Scholar] [CrossRef]
- Rodrigues, B.L.; Dotti, I.; Pascoal, L.B.; Morari, J.; Esteller, M.; Coope, A.; Ayrizono, M.L.S.; Salas, A.; Leal, R.F. Endoplasmic Reticulum Stress in Colonic Mucosa of Ulcerative Colitis Patients Is Mediated by PERK and IRE1 Pathway Activation. Mediat. Inflamm. 2022, 2022, 6049500. [Google Scholar] [CrossRef]
- Basseri, S.; Lhoták, S.; Sharma, A.M.; Austin, R.C. The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response. J. Lipid Res. 2009, 50, 2486–2501. [Google Scholar] [CrossRef]
- Carlisle, R.E.; Brimble, E.; Werner, K.E.; Cruz, G.L.; Ask, K.; Ingram, A.J.; Dickhout, J.G. 4-Phenylbutyrate inhibits tunicamycin-induced acute kidney injury via CHOP/GADD153 repression. PLoS ONE 2014, 9, e84663. [Google Scholar] [CrossRef] [PubMed]
- Vilatoba, M.; Eckstein, C.; Bilbao, G.; Smyth, C.A.; Jenkins, S.; Thompson, J.A.; Eckhoff, D.E.; Contreras, J.L. Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum-stress mediated apoptosis. Surgery 2005, 138, 342–351. [Google Scholar] [CrossRef]
- Ayala, P.; Montenegro, J.; Vivar, R.; Letelier, A.; Urroz, P.A.; Copaja, M.; Pivet, D.; Humeres, C.; Troncoso, R.; Vicencio, J.M.; et al. Attenuation of endoplasmic reticulum stress using the chemical chaperone 4-phenylbutyric acid prevents cardiac fibrosis induced by isoproterenol. Exp. Mol. Pathol. 2012, 121, 104669. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Giacca, A.; Lewis, G.F. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans. Diabetes 2011, 60, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Nimura, S.; Nishinakagawa, T.; Hideshima, Y.; Enjyoji, M.; Nabeshima, K.; Nakashima, M. Sodium 4-phenylbutyrate suppresses the development of dextran sulfate sodium-induced colitis in mice. Exp. Ther. Med. 2014, 7, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Nimura, S.; Hideshima, Y.; Nabeshima, K.; Nakashima, M. Orally administered sodium 4-phenylbutyrate suppresses the development of dextran sulfate sodium-induced colitis in mice. Exp. Ther. Med. 2017, 14, 5485–5490. [Google Scholar] [CrossRef]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J.F. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Fucilini, L.M.P.; Genaro, L.M.; Sousa, D.C.E.; Coy, C.S.R.; Leal, R.F.; Ayrizono, M.L.S. Epidemiological Profile and Clinical Characteristics of Inflammatory Bowel Diseases In a Brazilian Referral Center. Arq. Gastroenterol. 2021, 58, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Solberg, I.C.; Vatn, M.H.; Høie, O.; Stray, N.; Sauar, J.; Jahnsen, J.; Moum, B.; Lygren, I.; IBSEN Study Group. Clinical course in Crohn’s disease: Results of a Norwegian population-based ten-year follow-up study. Clin. Gastroenterol. Hepatol. 2007, 55, 430–1438. [Google Scholar] [CrossRef]
- Jess, T.; Riis, L.; Vind, I.; Winther, K.V.; Borg, S.; Binder, V.; Langholz, E.; Thomsen, O.Ø.; Munkholm, P. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: A population-based study from Copenhagen, Denmark. Inflamm. Bowel. Dis. 2007, 13, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, L.; Kiss, L.S.; David, G.; Pandur, T.; Erdelyi, Z.; Mester, G.; Balogh, M.; Szipocs, I.; Molnar, C.; Komaromi, E.; et al. Incidence, disease phenotype at diagnosis, and early disease course in inflammatory bowel diseases in Western Hungary, 2002–2006. Inflamm. Bowel. Dis. 2011, 17, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; He, C.; Hua, R.; Guo, Y.; Wang, B.; Liang, C.; Gao, L.; Shang, H.; Xu, J.D. Endoplasmic Reticulum Stress of Gut Enterocyte and Intestinal Diseases. Front. Mol. Biosci. 2022, 24, 817392. [Google Scholar] [CrossRef] [PubMed]
- Jian, L.; Lu, Y.; Lu, S.; Lu, C. Chemical chaperone 4-phenylbutyric acid protects H9c2 cardiomyocytes from ischemia/reperfusion injury by attenuating endoplasmic reticulum stress-induced apoptosis. Mol. Med. Rep. 2016, 13, 4386–4392. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Sang, W.; Chen, S.; Chen, R.; Zhang, H.; Xue, F.; Li, Z.; Liu, Y.; Gong, Y.; Zhang, H.; et al. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol. Lett. 2017, 5, 26–37. [Google Scholar] [CrossRef]
- Tang, W.; Cai, D.; Fu, Y.; Zheng, Z.; Huang, X.; Khouzam, R.N.; Song, Y.; Lian, J. 4-phenylbutyric acid re-trafficking hERG/G572R channel protein by modulating the endoplasmic reticulum stress-associated chaperones and endoplasmic reticulum-associated degradation gene. J. Thorac. Dis. 2023, 15, 4472–4485. [Google Scholar] [CrossRef] [PubMed]
- Schlussel, A.T.; Steele, S.R.; Alavi, K. Current challenges in the surgical management of Crohn’s disease: A systematic review. Am. J. Surg. 2016, 212, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Dorrington, A.M.; Selinger, C.P.; Parkes, G.C.; Smith, M.; Pollok, R.C.; Raine, T. The Historical Role and Contemporary Use of Corticosteroids in Inflammatory Bowel Disease. J. Crohns Colitis 2020, 14, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Panaccione, R.; Loftus EVJr Binion, D.; McHugh, K.; Alam, S.; Chen, N.; Guerette, B.; Mulani, P.; Chao, J. Efficacy and safety of adalimumab in Canadian patients with moderate to severe Crohn’s disease: Results of the Adalimumab in Canadian SubjeCts with ModErate to Severe Crohn’s DiseaSe (ACCESS) trial. Can. J. Gastroenterol. 2011, 25, 419–425. [Google Scholar] [CrossRef]
- Samoilă, I.; Dinescu, S.; Costache, M. Interplay between Cellular and Molecular Mechanisms Underlying Inflammatory Bowel Diseases Development-A Focus on Ulcerative Colitis. Cells 2020, 9, 1674. [Google Scholar] [CrossRef] [PubMed]
- Deka, D.; D’Incà, R.; Sturniolo, G.C.; Das, A.; Pathak, S.; Banerjee, A. Role of ER Stress Mediated Unfolded Protein Responses and ER Stress Inhibitors in the Pathogenesis of Inflammatory Bowel Disease. Dig. Dis. Sci. 2022, 67, 5392–5406. [Google Scholar] [CrossRef]
- Eugene, S.P.; Reddy, V.S.; Trinath, J. Endoplasmic Reticulum Stress and Intestinal Inflammation: A Perilous Union. Front. Immunol. 2020, 11, 543022. [Google Scholar] [CrossRef]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic reticulum stress signalling—From basic mechanisms to clinical applications. Febs. J. 2019, 286, 241–278. [Google Scholar] [CrossRef]
- Todd, D.J.; Lee, A.H.; Glimcher, L.H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol 2008, 8, 663–674. [Google Scholar] [CrossRef]
- Hosomi, S.; Kaser, A.; Blumberg, R.S. Role of endoplasmic reticulum stress and autophagy as interlinking pathways in the pathogenesis of inflammatory bowel disease. Curr. Opin. Gastroenterol. 2015, 31, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Younes, O.A.; Elsherbiny, D.M.; Hanna, D.M.F.; Gad, A.M.; Azab, S.S. Tocilizumab unfolds colo-protective and immunomodulatory effect in experimentally induced ulcerative colitis via mitigating autophagy and ER stress signaling. Inflammopharmacology 2024, 32, 3881–3898. [Google Scholar] [CrossRef]
- Delong, T.; Baker, R.L.; Reisdorph, N.; Reisdorph, R.; Powell, R.L.; Armstrong, M.; Barbour, G.; Bradley, B.; Haskins, K. Islet amyloid polypeptide is a target antigen for diabetogenic CD4+ T cells. Diabetes 2011, 60, 2325–2330. [Google Scholar] [CrossRef]
- Fan, Z.; Xu, L.; Gao, Y.; Cao, Y.; Tian, Y.; Pan, Z.; Wei, L.; Chen, S.; Zhang, X.; Liu, M.; et al. The cytoplasmic-nuclear transport of DDX3X promotes immune-mediated liver injury in mice regulated by endoplasmic reticulum stress. Cell Death Dis. 2024, 15, 702. [Google Scholar] [CrossRef]
- Ikebe, E.; Matsuoka, S.; Tezuka, K.; Kuramitsu, M.; Okuma, K.; Nakashima, M.; Kobayashi, S.; Makiyama, J.; Yamagishi, M.; Oyadomari, S.; et al. Activation of PERK-ATF4-CHOP pathway as a novel therapeutic approach for efficient elimination of HTLV-1-infected cells. Blood Adv. 2020, 4, 1845–1858. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Li, H.; Zhang, Y.; Ron, D.; Walter, P. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS ONE 2009, 4, e4170. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T.; Williams, M. Defining and characterizing drug/compound function. Biochem. Pharmacol. 2014, 87, 40–63. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsson, J.; Andersson, R.; Jirstrand, M.; Hjorth, S. Dose-Response-Time Data Analysis: An Underexploited Trinity. Pharmacol. Rev. 2019, 71, 89–122. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, E.G.; Jeong, J.H.; Yoo, W.H. 4-Phenylbutyric acid, a potent endoplasmic reticulum stress inhibitor, attenuates the severity of collagen-induced arthritis in mice via inhibition of proliferation and inflammatory responses of synovial fibroblasts. Kaohsiung J. Med. Sci. 2021, 37, 604–615. [Google Scholar] [CrossRef]
- Tsuru, A.; Fujimoto, N.; Takahashi, S.; Saito, M.; Nakamura, D.; Iwano, M.; Iwawaki, T.; Kadokura, H.; Ron, D.; Kohno, K. Negative feedback by IRE1β optimizes mucin production in goblet cells. Proc. Natl. Acad. Sci. USA 2013, 110, 2864–2869. [Google Scholar] [CrossRef] [PubMed]
- Kokame, K.; Kato, H.; Miyata, T. Identification of ERSE-II, a new cis-acting element responsible for the ATF6-dependent mammalian unfolded protein response. J. Biol. Chem 2001, 276, 9199–9205. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, R.L.; Mesgarzadeh, J.S.; Hendershot, L.M. Reshaping endoplasmic reticulum quality control through the unfolded protein response. Mol. Cell 2022, 82, 1477–1491. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Li, H.; Yasumura, D.; Cohen, H.R.; Zhang, C.; Panning, B.; Shokat, K.M.; Lavail, M.M.; Walter, P. IRE1 signaling affects cell fate during the unfolded protein response. Science 2007, 318, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Heazlewood, C.K.; Cook, M.C.; Eri, R.; Price, G.R.; Tauro, S.B.; Taupin, D.; Thornton, D.J.; Png, C.W.; Crockford, T.L.; Cornall, R.J.; et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008, 5, e54. [Google Scholar] [CrossRef]
- Heijmans, J.; van Lidth de Jeude, J.F.; Koo, B.K.; Rosekrans, S.L.; Wielenga, M.C.; van de Wetering, M.; Ferrante, M.; Lee, A.S.; Onderwater, J.J.; Paton, J.C.; et al. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Rep. 2013, 3, 1128–1139. [Google Scholar] [CrossRef]
- Chotikatum, S.; Naim, H.Y.; El-Najjar, N. Inflammation induced ER stress affects absorptive intestinal epithelial cells function and integrity. Int. Immunopharmacol. 2018, 55, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Rees, W.D.; Stahl, M.; Jacobson, K.; Bressler, B.; Sly, L.M.; Vallance, B.A.; Steiner, T.S. Enteroids derived from inflammatory bowel disease patients display dysregulated endoplasmic reticulum stress pathways, leading to differential inflammatory responses and dendritic cell maturation. J. Crohns Colitis 2020, 14, 948–961. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Grider, J.R.; Murthy, K.S.; Bohl, J.; Rivet, E.; Wieghard, N.; Kuemmerle, J.F. Endoplasmic reticulum stress in subepithelial myofibroblasts increases the TGF-β1 activity that regulates fibrosis in Crohn’s disease. Inflamm. Bowel. Dis. 2020, 26, 809–819. [Google Scholar] [CrossRef]
- Verjan Garcia, N.; Hong, K.U.; Matoba, N. The unfolded protein response and its implications for novel therapeutic strategies in inflammatory bowel disease. Biomedicines 2023, 11, 2006. [Google Scholar] [CrossRef] [PubMed]
- Berns, M.; Hommes, D.W. Anti-TNF-α therapies for the treatment of Crohn’s disease: The past, present and future. Expert Opin. Investig. Drugs 2016, 25, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Alhendi, A.; Naser, S.A. The dual role of interleukin-6 in Crohn’s disease pathophysiology. Front. Immunol. 2023, 14, 1295230. [Google Scholar] [CrossRef]
- Vebr, M.; Pomahačová, R.; Sýkora, J.; Schwarz, J. A Narrative Review of Cytokine Networks: Pathophysiological and Therapeutic Implications for Inflammatory Bowel Disease Pathogenesis. Biomedicines 2023, 11, 3229. [Google Scholar] [CrossRef] [PubMed]

| CTR | CD | |
|---|---|---|
| Number of patients | 7 | 8 |
| Gender (male/female) | 3/4 | 2/6 |
| Age (median–min/max) | 38.5 years old (28–51) | 51 years old (27–57) |
| Montreal classification | ||
| Age of diagnosis (A1/A2/A3) | ----- | 2/6/0 |
| Location (L1/L2/L3/L4) | ----- | 2/3/3/0 |
| Behavior (B1/B2/B3) | ----- | 4/2/2 |
| Perianal disease (yes/no) | ----- | 3/5 |
| Disease duration (median–min/max) | ----- | 13.5 years (1–21) |
| CDEIS (median–min/max) | ----- | 6.62 (0–22.1) |
| Medications (number of patients) | ----- | Infliximab (1) Adalimumab (3) Azatioprine (5) |
| CTRL | CD | |
|---|---|---|
| Number of patients | 6 | 10 |
| Gender (male/female) | 2/4 | 5/5 |
| Age (median–min/max) | 64 years old (51–69) | 36 years old (23–72) |
| Montreal classification | ||
| Age of diagnosis (A1/A2/A3) | ----- | 2/6/2 |
| Location (L1/L2/L3/L4) | ----- | 2/1/7/0 |
| Behavior (B1/B2/B3) | ----- | 5/4/1 |
| Perianal disease (yes/no) | ----- | 2/8 |
| Disease duration (median–min/max) | ----- | 9.5 years (5–25) |
| CDEIS (median–min/max) | ----- | 6.18 (1.24–29.5) |
| Medications (number of patients) | ----- | Infliximab (4) Vedolizumb (1) Adalimumab (4) Mesalazine (2) Azatioprine (8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, B.L.; Pascoal, L.B.; Genaro, L.M.; Warrak, L.S.C.A.; Rodrigues, B.A.G.; Coope, A.; Camargo, M.G.; Oliveira, P.d.S.P.; Ayrizono, M.d.L.S.; Velloso, L.A.; et al. In Vitro Inhibition of Endoplasmic Reticulum Stress: A Promising Therapeutic Strategy for Patients with Crohn’s Disease. Cells 2025, 14, 270. https://doi.org/10.3390/cells14040270
Rodrigues BL, Pascoal LB, Genaro LM, Warrak LSCA, Rodrigues BAG, Coope A, Camargo MG, Oliveira PdSP, Ayrizono MdLS, Velloso LA, et al. In Vitro Inhibition of Endoplasmic Reticulum Stress: A Promising Therapeutic Strategy for Patients with Crohn’s Disease. Cells. 2025; 14(4):270. https://doi.org/10.3390/cells14040270
Chicago/Turabian StyleRodrigues, Bruno Lima, Lívia Bitencourt Pascoal, Lívia Moreira Genaro, Leonardo Saint Clair Assad Warrak, Beatriz Alves Guerra Rodrigues, Andressa Coope, Michel Gardere Camargo, Priscilla de Sene Portel Oliveira, Maria de Lourdes Setsuko Ayrizono, Lício Augusto Velloso, and et al. 2025. "In Vitro Inhibition of Endoplasmic Reticulum Stress: A Promising Therapeutic Strategy for Patients with Crohn’s Disease" Cells 14, no. 4: 270. https://doi.org/10.3390/cells14040270
APA StyleRodrigues, B. L., Pascoal, L. B., Genaro, L. M., Warrak, L. S. C. A., Rodrigues, B. A. G., Coope, A., Camargo, M. G., Oliveira, P. d. S. P., Ayrizono, M. d. L. S., Velloso, L. A., & Leal, R. F. (2025). In Vitro Inhibition of Endoplasmic Reticulum Stress: A Promising Therapeutic Strategy for Patients with Crohn’s Disease. Cells, 14(4), 270. https://doi.org/10.3390/cells14040270







