Unveiling the Molecular Mechanisms Underlying the Success of Simple Limbal Epithelial Transplantation (SLET)
Abstract
1. Introduction
2. Method of Literature Search
- Inclusion criteria: randomized control clinical trials, review articles, prospective and retrospective case series, cohort studies, case control studies, animal and laboratory studies.
- Exclusion criteria: letters, conference abstracts and editorials.
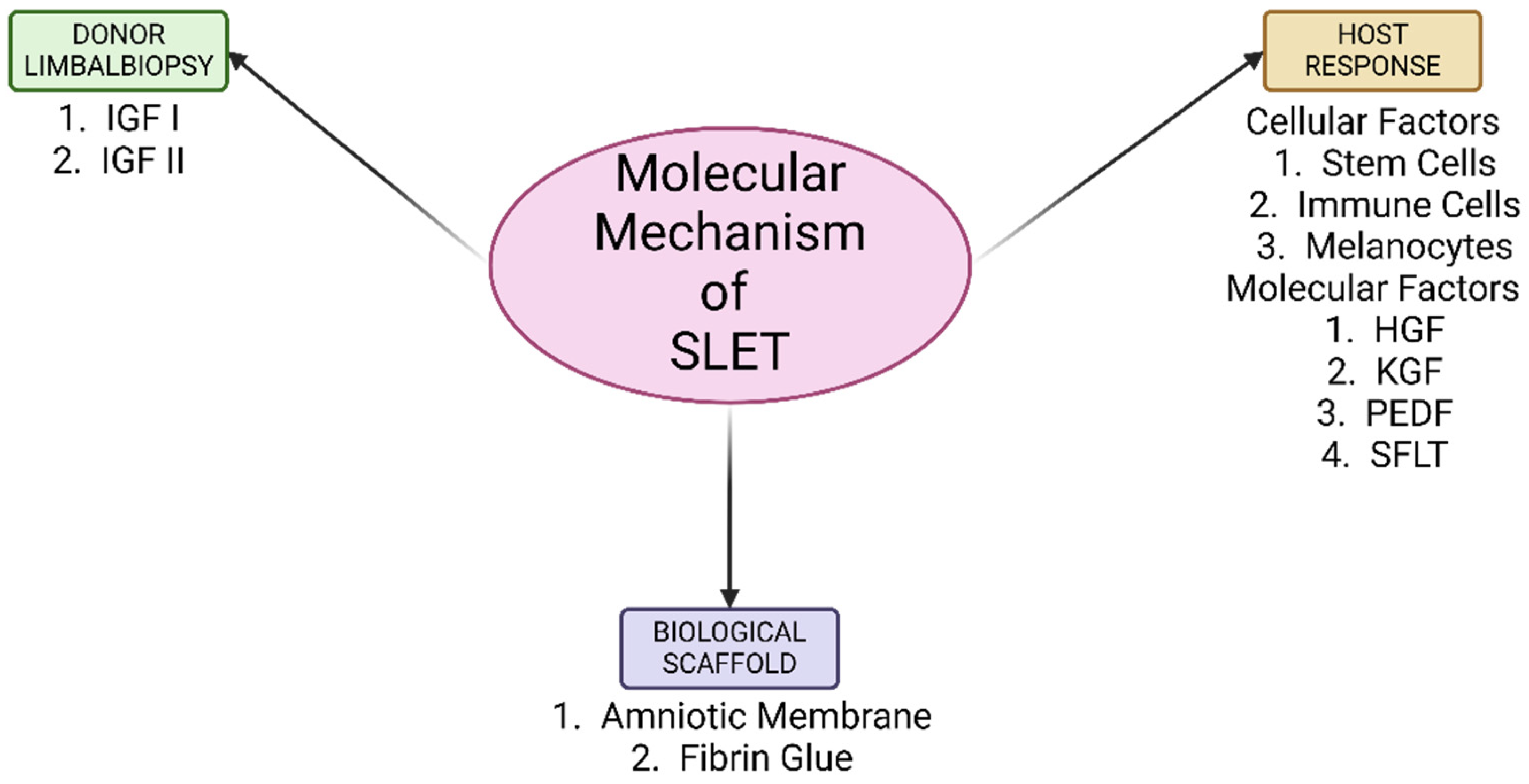
3. Proposed Molecular Mechanisms of the Donor Limbal Biopsies
3.1. Limbal Niche
3.2. Stem Cells
3.2.1. Hepatocyte Growth Factor (HGF)
3.2.2. Soluble Fms-like Tyrosine Kinase-1 (sFLT-1)
3.2.3. Pigment Epithelium-Derived Factor (PEDF)
3.3. Keratinocyte Growth Factor (KGF)
3.4. Limbal Fibroblasts
3.5. Melanocytes
3.6. Immune Cells
3.7. Adhesion Molecules
4. Proposed Molecular Mechanisms by the Host
4.1. Insulin-like Growth Factor (IGF)
4.2. Risk Factors Contributing to SLET Failure
5. Biological Scaffold for SLET
5.1. Amniotic Membrane
5.2. Fibrin Glue
6. Recent Advances, Modifications and Future Implications of SLET
6.1. SLET Without Scaffold
6.2. Mini-Simple Limbal Epithelial Transplantation (Mini-SLET) Technique
6.3. Glueless-Simple Limbal Epithelial Transplantation (G-SLET)
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghareeb, A.E.; Lako, M.; Figueiredo, F.C. Recent Advances in Stem Cell Therapy for Limbal Stem Cell Deficiency: A Narrative Review. Ophthalmol. Ther. 2020, 9, 809–831. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Inoue, T.; Takamatsu, F.; Kobayashi, T.; Shiraishi, A.; Maeda, N.; Ohashi, Y.; Nishida, K. Differences Between Niche Cells and Limbal Stromal Cells in Maintenance of Corneal Limbal Stem Cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hayashida, Y.; Chen, Y.T.; Tseng, S.C.G. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007, 17, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.Y.; Xie, H.T.; Zhao, X.Y.; Zhang, M.C. Limbal niche cells: A novel feeder cell for autologous cultivated oral mucosal epithelial transplantation. Regen. Med. 2019, 14, 49–62. [Google Scholar] [CrossRef]
- Le, Q.; Xu, J.; Deng, S.X. The diagnosis of limbal stem cell deficiency. Ocul. Surf. 2018, 16, 58–69. [Google Scholar] [CrossRef]
- Chen, J.J.; Tseng, S.C. Corneal epithelial wound healing in partial limbal deficiency. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1301–1314. [Google Scholar]
- Sangwan, V.S.; Sharp, J.A.H. Simple limbal epithelial transplantation. Curr. Opin. Ophthalmol. 2017, 28, 382–386. [Google Scholar] [CrossRef]
- Sangwan, V.S.; Basu, S.; MacNeil, S.; Balasubramanian, D. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br. J. Ophthalmol. 2012, 96, 931–934. [Google Scholar] [CrossRef]
- Bhalekar, S.; Basu, S.; Sangwan, V.S. Successful management of immunological rejection following allogeneic simple limbal epithelial transplantation (SLET) for bilateral ocular burns. BMJ Case Rep. 2013, 2013, bcr2013009051. [Google Scholar] [CrossRef]
- Basu, S.; Sureka, S.P.; Shanbhag, S.S.; Kethiri, A.R.; Singh, V.; Sangwan, V.S. Simple Limbal Epithelial Transplantation: Long-Term Clinical Outcomes in 125 Cases of Unilateral Chronic Ocular Surface Burns. Ophthalmology 2016, 123, 1000–1010. [Google Scholar] [CrossRef]
- Vazirani, J.; Ali, M.H.; Sharma, N.; Gupta, N.; Mittal, V.; Atallah, M.; Amescua, G.; Chowdhury, T.; Abdala-Figuerola, A.; Ramirez-Miranda, A.; et al. Autologous simple limbal epithelial transplantation for unilateral limbal stem cell deficiency: Multicentre results. Br. J. Ophthalmol. 2016, 100, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Joshi, J.; Farooqui, J.H.; Mathur, U. Results of simple limbal epithelial transplantation in unilateral ocular surface burn. Indian. J. Ophthalmol. 2018, 66, 45–52. [Google Scholar] [CrossRef]
- Prabhasawat, P.; Chirapapaisan, C.; Ngowyutagon, P.; Ekpo, P.; Tangpagasit, W.; Lekhanont, K.; Sikarinkul, R.; Matamnan, S.; Boonwong, C.; Pinitpuwadol, W.; et al. Efficacy and outcome of simple limbal epithelial transplantation for limbal stem cell deficiency verified by epithelial phenotypes integrated with clinical evaluation. Ocul. Surf. 2021, 22, 27–37. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Yang, K.; Zhang, Y.; Deng, S.; Wang, Z.; Li, S.; Tian, L.; Jie, Y. Clinical outcomes of modified simple limbal epithelial transplantation for limbal stem cell deficiency in Chinese population: A retrospective case series. Stem Cell Res. Ther. 2021, 12, 259. [Google Scholar] [CrossRef]
- Iyer, G.; Srinivasan, B.; Agarwal, S.; Tarigopula, A. Outcome of allo simple limbal epithelial transplantation (alloSLET) in the early stage of ocular chemical injury. Br. J. Ophthalmol. 2017, 101, 828–833. [Google Scholar] [CrossRef]
- Shanbhag, S.S.; Patel, C.N.; Goyal, R.; Donthineni, P.R.; Singh, V.; Basu, S. Simple limbal epithelial transplantation (SLET): Review of indications, surgical technique, mechanism, outcomes, limitations, and impact. Indian J. Ophthalmol. 2019, 67, 1265–1277. [Google Scholar]
- Thokala, P.; Singh, A.; Singh, V.K.; Rathi, V.M.; Basu, S.; Singh, V.; Basu, S. Economic, clinical and social impact of simple limbal epithelial transplantation for limbal stem cell deficiency. Br. J. Ophthalmol. 2022, 106, 923–928. [Google Scholar] [CrossRef]
- Xie, H.T.; Chen, S.Y.; Li, G.G.; Tseng, S.C.G. Limbal epithelial stem/progenitor cells attract stromal niche cells by SDF-1/CXCR4 signaling to prevent differentiation. Stem Cells 2011, 29, 1874–1885. [Google Scholar] [CrossRef]
- Guo, P.; Sun, H.; Zhang, Y.; Tighe, S.; Chen, S.; Su, C.; Liu, Y.; Zhao, H.; Hu, M.; Zhu, Y. Limbal niche cells are a potent resource of adult mesenchymal progenitors. J. Cell. Mol. Med. 2018, 22, 3315–3322. [Google Scholar] [CrossRef]
- Tuori, A.; Uusitalo, H.; Burgeson, R.E.; Terttunen, J.; Virtanen, I. The immunohistochemical composition of the human corneal basement membrane. Cornea 1996, 15, 286–294. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Burgeson, R.E.; Butkowski, R.J.; Michael, A.F.; Sun, T.T.; Kenney, M.C. Human corneal basement membrane heterogeneity: Topographical differences in the expression of type IV collagen and laminin isoforms. Lab. Investig. J. Tech. Methods Pathol. 1995, 72, 461–473. [Google Scholar]
- Tseng, S.C.G.; He, H.; Zhang, S.; Chen, S.Y. Niche Regulation of Limbal Epithelial Stem Cells: Relationship between Inflammation and Regeneration. Ocul. Surf. 2016, 14, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Kethiri, A.R.; Basu, S.; Shukla, S.; Sangwan, V.S.; Singh, V. Optimizing the role of limbal explant size and source in determining the outcomes of limbal transplantation: An in vitro study. PLoS ONE 2017, 12, e0185623. [Google Scholar] [CrossRef]
- Polisetty, N.; Fatima, A.; Madhira, S.L.; Sangwan, V.S.; Vemuganti, G.K. Mesenchymal cells from limbal stroma of human eye. Mol. Vis. 2008, 14, 431–442. [Google Scholar]
- Eslani, M.; Putra, I.; Shen, X.; Hamouie, J.; Afsharkhamseh, N.; Besharat, S.; Rosenblatt, M.I.; Dana, R.; Hematti, P.; Djalilian, A.R. Corneal Mesenchymal Stromal Cells Are Directly Antiangiogenic via PEDF and sFLT-1. Invest. Ophthalmol. Vis. Sci. 2017, 58, 5507–5517. [Google Scholar] [CrossRef]
- Amin, S.; Jalilian, E.; Katz, E.; Frank, C.; Yazdanpanah, G.; Guaiquil, V.H.; Rosenblatt, M.I.; Djalilian, A.R. The Limbal Niche and Regenerative Strategies. Vision 2021, 5, 43. [Google Scholar] [CrossRef]
- Basu, S.; Hertsenberg, A.J.; Funderburgh, M.L.; Burrow, M.K.; Mann, M.M.; Du, Y.; Lathrop, K.L.; Syed-Picard, F.N.; Adams, S.M.; Birk, D.E.; et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci. Transl. Med. 2014, 6, 266ra172. [Google Scholar] [CrossRef]
- Sahu, A.; Foulsham, W.; Amouzegar, A.; Mittal, S.K.; Chauhan, S.K. The therapeutic application of mesenchymal stem cells at the ocular surface. Ocul. Surf. 2019, 17, 198–207. [Google Scholar] [CrossRef]
- Nakamura, T.; Mizuno, S. The discovery of Hepatocyte Growth Factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 588–610. [Google Scholar] [CrossRef]
- Omoto, M.; Suri, K.; Amouzegar, A.; Li, M.; Katikireddy, K.R.; Mittal, S.K.; Chauhan, S.K. Hepatocyte Growth Factor Suppresses Inflammation and Promotes Epithelium Repair in Corneal Injury. Mol. Ther. 2017, 25, 1881–1888. [Google Scholar] [CrossRef]
- Mittal, S.K.; Omoto, M.; Amouzegar, A.; Sahu, A.; Rezazadeh, A.; Katikireddy, K.R.; Shah, D.I.; Sahu, S.K.; Chauhan, S.K. Restoration of Corneal Transparency by Mesenchymal Stem Cells. Stem Cell Rep. 2016, 7, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Farooqui, J.H.; Dziasko, M.A.; Daniels, J.T.; Umang, M.; Sangwan, V.S. Reappearance of limbal pigmentation post-simple limbal epithelial transplant. Indian. J. Ophthalmol. 2020, 68, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Hajrasouliha, A.R.; Sadrai, Z.; Ueno, H.; Chauhan, S.K.; Dana, R. Nerves and Neovessels Inhibit Each Other in the Cornea. Investig. Ophthalmol. Vis. Sci. 2013, 54, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Nishino, Y.; Maeda, S.; Yamagishi, S.-i. PEDF-derived peptide inhibits corneal angiogenesis by suppressing VEGF expression. Microvasc. Res. 2012, 84, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.W.; Volpert, O.V.; Gillis, P.; Crawford, S.E.; Xu, H.; Benedict, W.; Bouck, N.P. Pigment epithelium-derived factor: A potent inhibitor of angiogenesis. Science 1999, 285, 245–248. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Ma, J.; Tian, H.; Jiao, Y.; Zhang, R.; Huang, Z.; Xiao, J.; Zhao, B.; Qian, H.; et al. Effects of keratinocyte growth factor-2 on corneal epithelial wound healing in a rabbit model of carbon dioxide laser injury. Biol. Pharm. Bull. 2010, 33, 971–976. [Google Scholar] [CrossRef]
- Chen, T.C.; Chang, S.W. Effect of mitomycin C on IL-1R expression, IL-1-related hepatocyte growth factor secretion and corneal epithelial cell migration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1389–1396. [Google Scholar] [CrossRef]
- Amirjamshidi, H.; Milani, B.Y.; Sagha, H.M.; Movahedan, A.; Shafiq, M.A.; Lavker, R.M.; Yue, B.Y.T.; Djalilian, A.R. Limbal fibroblast conditioned media: A non-invasive treatment for limbal stem cell deficiency. Mol. Vis. 2011, 17, 658–666. [Google Scholar]
- Katikireddy, K.R.; Dana, R.; Jurkunas, U.V. Differentiation potential of limbal fibroblasts and bone marrow mesenchymal stem cells to corneal epithelial cells. Stem Cells 2014, 32, 717–729. [Google Scholar] [CrossRef]
- Blazejewska, E.A.; Schlötzer-Schrehardt, U.; Zenkel, M.; Bachmann, B.; Chankiewitz, E.; Jacobi, C.; Kruse, F.E. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells 2009, 27, 642–652. [Google Scholar] [CrossRef]
- Higa, K.; Shimmura, S.; Miyashita, H.; Shimazaki, J.; Tsubota, K. Melanocytes in the corneal limbus interact with K19-positive basal epithelial cells. Exp. Eye Res. 2005, 81, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Polisetti, N.; Gießl, A.; Zenkel, M.; Heger, L.; Dudziak, D.; Naschberger, E.; Stich, L.; Steinkasserer, A.; Kruse, F.E.; Schlötzer-Schrehardt, U. Melanocytes as emerging key players in niche regulation of limbal epithelial stem cells. Ocul. Surf. 2021, 22, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Polisetti, N.; Zenkel, M.; Menzel-Severing, J.; Kruse, F.E.; Schlötzer-Schrehardt, U. Cell Adhesion Molecules and Stem Cell-Niche-Interactions in the Limbal Stem Cell Niche. Stem Cells 2016, 34, 203–219. [Google Scholar] [CrossRef]
- Dziasko, M.A.; Tuft, S.J.; Daniels, J.T. Limbal melanocytes support limbal epithelial stem cells in 2D and 3D microenvironments. Exp. Eye Res. 2015, 138, 70–79. [Google Scholar] [CrossRef]
- Micera, A.; Jirsova, K.; Esposito, G.; Balzamino, B.O.; Di Zazzo, A.; Bonini, S. Mast Cells Populate the Corneoscleral Limbus: New Insights for Our Understanding of Limbal Microenvironment. Investig. Ophthalmol. Vis. Sci. 2020, 61, 43. [Google Scholar] [CrossRef]
- Nazari, M.; Ni, N.C.; Lüdke, A.; Li, S.H.; Guo, J.; Weisel, R.D.; Li, R.-K. Mast cells promote proliferation migration inhibit differentiation of mesenchymal stem cells through, PDGF. J. Mol. Cell. Cardiol. 2016, 94, 32–42. [Google Scholar] [CrossRef]
- Raymond, K.; Deugnier, M.A.; Faraldo, M.M.; Glukhova, M.A. Adhesion within the stem cell niches. Curr. Opin. Cell Biol. 2009, 21, 623–629. [Google Scholar] [CrossRef]
- Dziasko, M.A.; Armer, H.E.; Levis, H.J.; Shortt, A.J.; Tuft, S.; Daniels, J.T. Localisation of epithelial cells capable of holoclone formation in vitro and direct interaction with stromal cells in the native human limbal crypt. PLoS ONE 2014, 9, e94283. [Google Scholar] [CrossRef]
- González, S.; Deng, S.X. Presence of native limbal stromal cells increases the expansion efficiency of limbal stem/progenitor cells in culture. Exp. Eye Res. 2013, 116, 169–176. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Dietrich, T.; Saito, K.; Sorokin, L.; Sasaki, T.; Paulsson, M.; Kruse, F.E. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp. Eye Res. 2007, 85, 845–860. [Google Scholar] [CrossRef]
- Mei, H.; Gonzalez, S.; Deng, S.X. Extracellular Matrix is an Important Component of Limbal Stem Cell Niche. J. Funct. Biomater. 2012, 3, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Stepp, M.A. Corneal integrins and their functions. Exp. Eye Res. 2006, 83, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Yamato, M.; Sugiyama, H.; Sumide, T.; Yang, J.; Okano, T.; Tano, Y.; Nishida, K. N-Cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem Cells 2007, 25, 289–296. [Google Scholar] [CrossRef]
- Trosan, P.; Svobodova, E.; Chudickova, M.; Krulova, M.; Zajicova, A.; Holan, V. The key role of insulin-like growth factor I in limbal stem cell differentiation and the corneal wound-healing process. Stem Cells Dev. 2012, 21, 3341–3350. [Google Scholar] [CrossRef]
- Ko, J.A.; Yanai, R.; Nishida, T. IGF-1 released by corneal epithelial cells induces up-regulation of N-cadherin in corneal fibroblasts. J. Cell. Physiol. 2009, 221, 254–261. [Google Scholar] [CrossRef]
- Jester, J.V.; Ho-Chang, J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp. Eye Res. 2003, 77, 581–592. [Google Scholar] [CrossRef]
- Yamada, N.; Yanai, R.; Nakamura, M.; Inui, M.; Nishida, T. Role of the C domain of IGFs in synergistic promotion, with a substance P-derived peptide, of rabbit corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1125–1131. [Google Scholar] [CrossRef][Green Version]
- Grueterich, M.; Espana, E.M.; Tseng, S.C.G. Ex vivo expansion of limbal epithelial stem cells: Amniotic membrane serving as a stem cell niche. Surv. Ophthalmol. 2003, 48, 631–646. [Google Scholar] [CrossRef]
- Grueterich, M.; Espana, E.; Tseng, S.C.G. Connexin 43 expression and proliferation of human limbal epithelium on intact and denuded amniotic membrane. Investig. Ophthalmol. Vis. Sci. 2002, 43, 63–71. [Google Scholar]
- Koizumi, N.J.; Inatomi, T.J.; Sotozono, C.J.; Fullwood, N.J.; Quantock, A.J.; Kinoshita, S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr. Eye Res. 2000, 20, 173–177. [Google Scholar] [CrossRef]
- Touhami, A.; Grueterich, M.; Tseng, S.C.G. The role of NGF signaling in human limbal epithelium expanded by amniotic membrane culture. Investig. Ophthalmol. Vis. Sci. 2002, 43, 987–994. [Google Scholar]
- Lord, S.T. Molecular mechanisms affecting fibrin structure and stability. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Kumar, S.; Kumar, A.; Bansal, R.; Bhartiya, S. Fibrin glue in ophthalmology. Indian J. Ophthalmol. 2009, 57, 371–379. [Google Scholar] [CrossRef]
- Jain, N.; Mittal, V.; Sanandiya, D. Outcomes of Simple Limbal Epithelial Transplantation Without Amniotic Membrane Grafting in Unilateral Limbal Stem Cell Deficiency: A Case Series of 6 Patients. Cornea 2025, 44, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Pannu, A.; Sati, A.; Mishra, S.K.; Kumar, S.; Dhar, S. Innovative technique of mini-simple limbal epithelial transplantation in pediatric patients. Indian J. Ophthalmol. 2021, 69, 2222–2224. [Google Scholar] [CrossRef] [PubMed]
- Malyugin, B.E.; Gerasimov, M.Y.; Borzenok, S.A. Glueless Simple Limbal Epithelial Transplantation: The Report of the First 2 Cases. Cornea 2020, 39, 1588–1591. [Google Scholar] [CrossRef]
- Malyugin, B.E.; Kalinnikova, S.Y.; Knyazer, B.; Gerasimov, M.Y. Midterm Outcomes of Autologous Glueless Simple Limbal Epithelial Transplantation for Unilateral Limbal Stem Cell Deficiency. Cornea 2024, 43, 45–51. [Google Scholar] [CrossRef]
- Malyugin, B.; Svetlana, K.; Fabian, M.; Werner, B.; Boris, K.; Maksim, G. Femtosecond Laser-Assisted Autologous Glueless Simple Limbal Epithelial Transplantation in Unilateral Limbal Stem Cell Deficiency: 12-Month Outcome of the First Clinical Cases. Cornea 2025, 44, 262–270. [Google Scholar] [CrossRef]
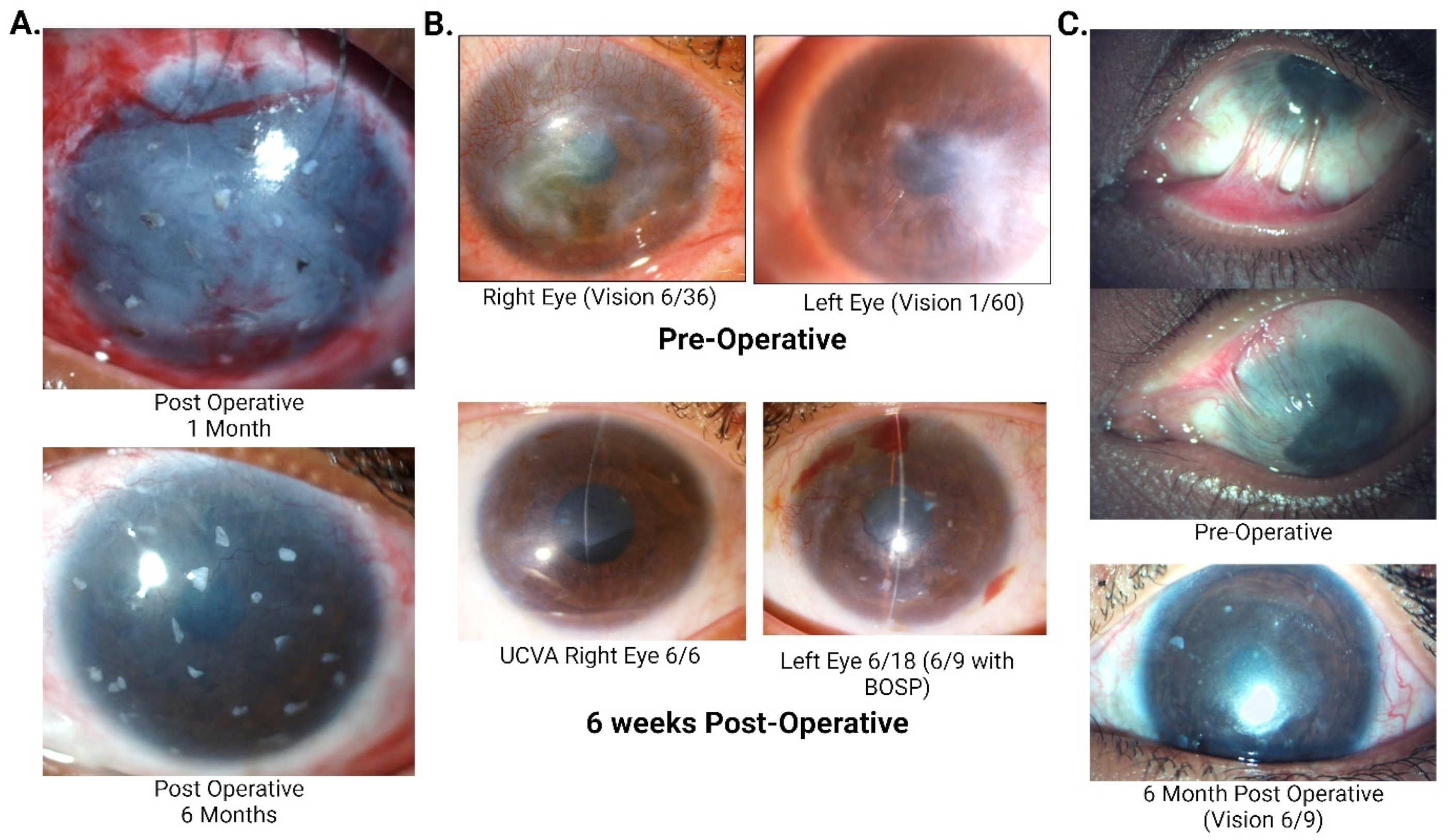
| Authors | Study Title | Sample Size | Outcomes of SLET |
|---|---|---|---|
| Basu et al. [10] | Long-Term Clinical Outcomes in 125 Cases of Unilateral Chronic Ocular Surface Burns | One hundred twenty-five eyes | Two-line improvement in visual acuity was seen in 75.2% of eyes over a mean follow-up of 35.5 months. A proportion of 67% of the successful cases attained a visual acuity of ≥20/60 (p < 0.0001). Progressive conjunctivalization occurred in 18.4% of eyes. |
| Vazirani et al. [11] | Autologous Simple Limbal Epithelial Transplantation for Unilateral Limbal Stem Cell Deficiency: Multicentre Results | Sixty-eight eyes underwent autologous SLET across eight centres in three countries. | Success rate was 83.8% over a mean follow up of 12 months. A proportion of 64.7% eyes achieved a visual acuity of ≥20/200. A proportion of 64.7% gained a visual acuity of ≥2 Snellen lines. Focal recurrences of pannus were noted in 36.8% cases with clinical success. |
| Gupta et al. [12] | Results of Simple Limbal Epithelial Transplantation in Unilateral Ocular Surface Burn | Thirty eyes (eighteen adults and twelve children) | Visual acuity gain (≥20/200) was reported in 71.4% of successful cases over a mean follow up of 1.1 years. |
| Prabhasawat et al. [13] | Efficacy and Outcome of Simple Limbal Epithelial Transplantation for Limbal Stem Cell Deficiency Verified by Epithelial Phenotypes Integrated with Clinical Evaluation | Twenty-eight eyes of twenty-six patients (eleven autologous SLET and seventeen living-related allogenic LSET) | The survival rate was 89.3% at 2 years and 75.6% at 3 years, with statistically significant improvement in visual acuity. No statistically significant difference was noted between autoSLET and Lr-alloSLET. |
| Wang et al. [14] | Clinical Outcomes of Modified Simple Limbal Epithelial Transplantation for Limbal Stem Cell Deficiency in Chinese Population: A Retrospective Case Series | Thirteen eyes with LSCD. Ten eyes underwent autologous SLET. Three eyes underwent allogeneic modified SLET. | At an approximate 6–7 months follow-up, 77% of the eyes maintained a successful outcome. Two-line improvement was noted in 60% of the cases. |
| Iyer et al. [15] | Outcome of Allo Simple Limbal Epithelial Transplantation (alloSLET) in the Early Stage of Ocular Chemical Injury | Eighteen eyes of seventeen patients with ocular chemical injury. | Corneal phenotype with complete epithelialisation was achieved in the immediate postoperative period in 17 of the 18 eyes (94.11%) i.e., 22.5 ± 9.14 days. |
| Shanbhag SS et al. [16] | Simple Limbal Epithelial Transplantation (SLET): Review of Indications, Surgical Technique, Mechanism, Outcomes, Limitations, and Impact | Thirty eyes with LSCD. Sixteen eyes underwent living-related allogeneic SLET. Fourteen eyes underwent cadaveric SLET | The overall success of allogeneic SLET was 83.3%. Successful outcomes at 1-year post-op were maintained in 87.5% of eyes in the living-related SLET group and 78.6% of eyes in the cadaveric group. Kaplan–Meier survival analysis: 5-year cumulative survival probability for 90± 4% of eyes in the living-related group and 82± 7% in the cadaveric group. |
| Factor | Molecular Mechanism | Clinical Implications |
|---|---|---|
| Hepatocyte growth factor (HGF) | Anti-fibrotic, decreases the expression of TGF-β and α-SMA; suppresses TNF-α, MIP-1, IL-6, and CD40. | Reduced corneal opacity and restored transparency with topical application in animal studies. |
| Soluble fms-like tyrosine kinase-1 (sFLT-1) | Anti-angiogenic, sequesters VEGF ligands, reduces VEGF receptor activation. | Unique among corneal MSCs, sFLT-1 reduces angiogenesis and supports corneal transparency in vitro and animal models. |
| Pigment epithelium-derived factor (PEDF) | Inhibits VEGF signaling via VEGFR-2 binding, γ-secretase cleavage, and VEGFR-1 phosphorylation alteration. | Inhibits corneal angiogenesis and maintains transparency through multiple pathways. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, A.; Goel, K.; Gour, A.; Sapra, M.; Sangwan, V.S.; Tripathi, R.; Tiwari, A. Unveiling the Molecular Mechanisms Underlying the Success of Simple Limbal Epithelial Transplantation (SLET). Cells 2025, 14, 200. https://doi.org/10.3390/cells14030200
Garg A, Goel K, Gour A, Sapra M, Sangwan VS, Tripathi R, Tiwari A. Unveiling the Molecular Mechanisms Underlying the Success of Simple Limbal Epithelial Transplantation (SLET). Cells. 2025; 14(3):200. https://doi.org/10.3390/cells14030200
Chicago/Turabian StyleGarg, Aastha, Kartik Goel, Abha Gour, Mehak Sapra, Virender Singh Sangwan, Ratnakar Tripathi, and Anil Tiwari. 2025. "Unveiling the Molecular Mechanisms Underlying the Success of Simple Limbal Epithelial Transplantation (SLET)" Cells 14, no. 3: 200. https://doi.org/10.3390/cells14030200
APA StyleGarg, A., Goel, K., Gour, A., Sapra, M., Sangwan, V. S., Tripathi, R., & Tiwari, A. (2025). Unveiling the Molecular Mechanisms Underlying the Success of Simple Limbal Epithelial Transplantation (SLET). Cells, 14(3), 200. https://doi.org/10.3390/cells14030200







