Alterations in Circulating T-Cell Subsets with Gut-Homing/Residency Phenotypes Associated with HIV-1 Status and Subclinical Atherosclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Blood Collection and Phenotypic Analysis
2.3. CellEngine Flow Cytometry Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical and Laboratory Characteristics of the Study Participants
3.2. Immune Profile Alterations Indicative of HIV and Subclinical CVD Status
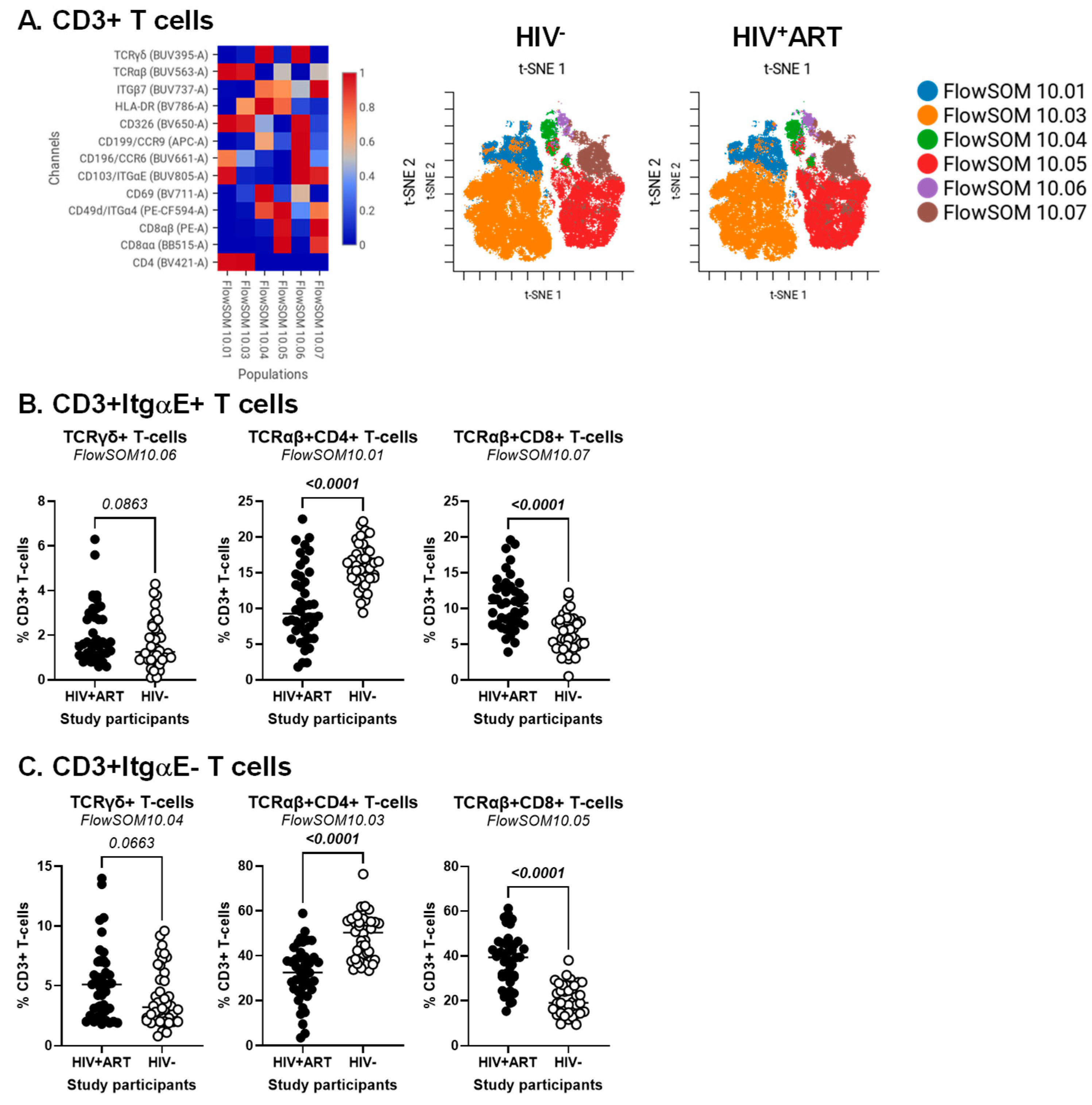
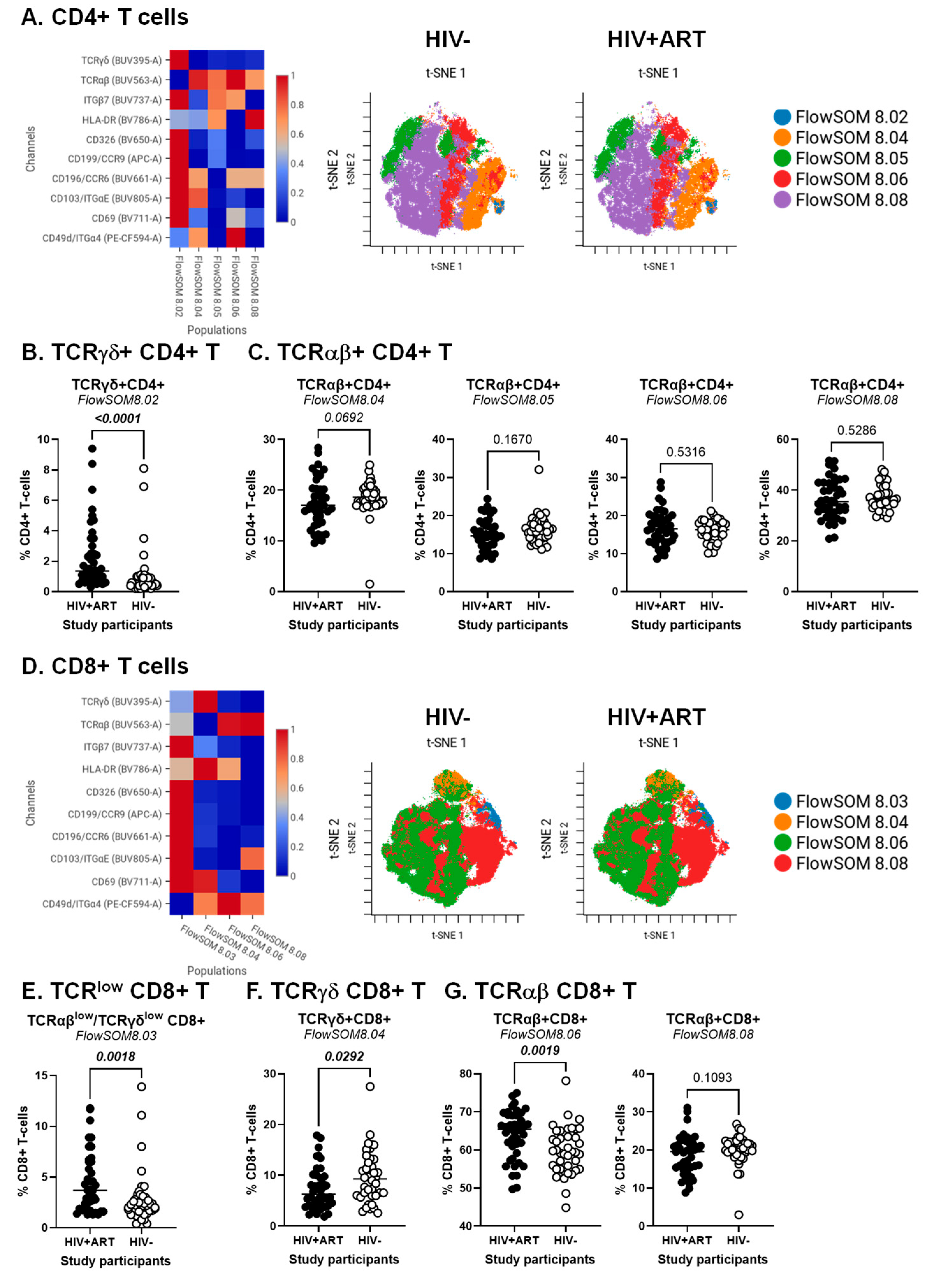
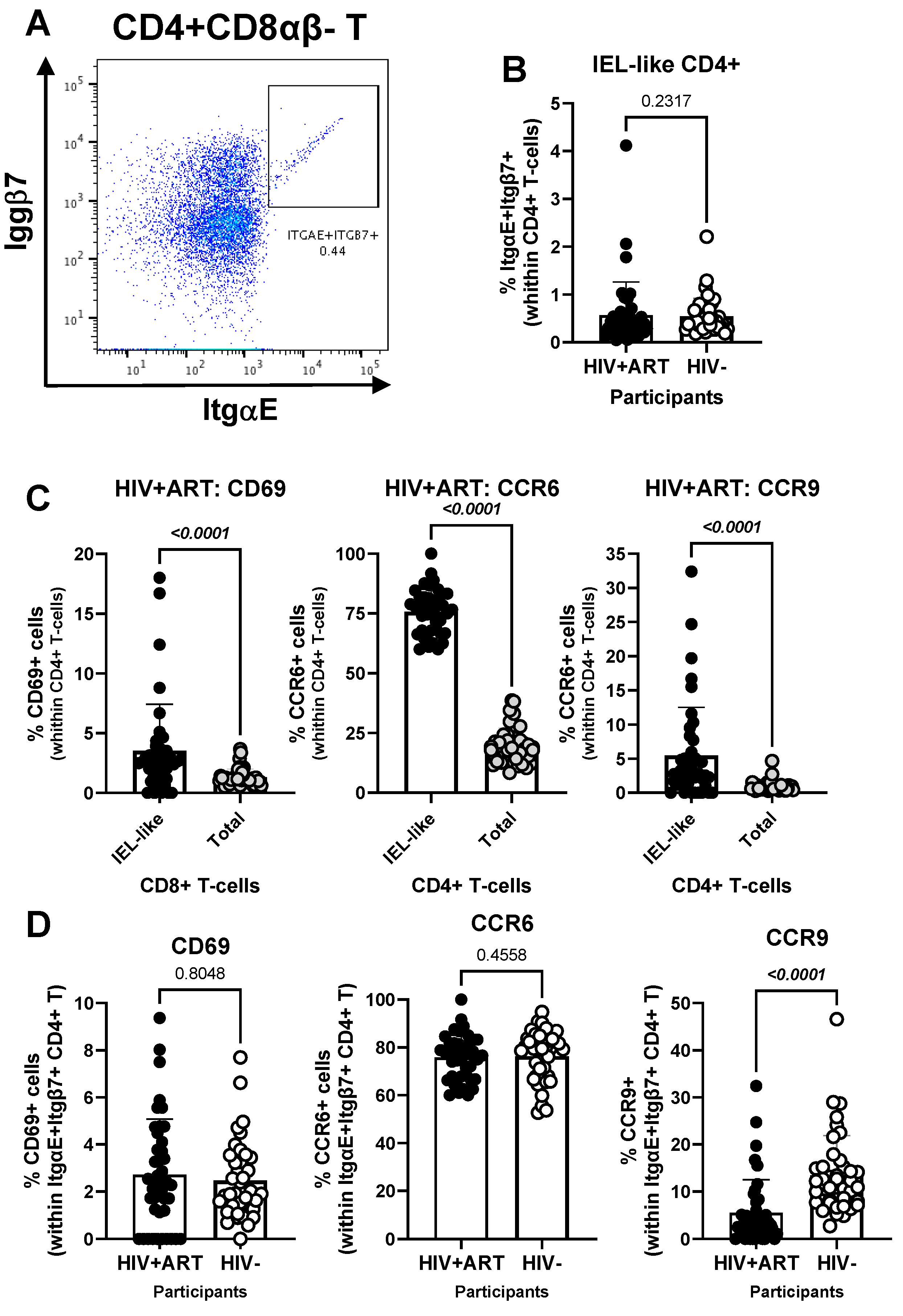
3.3. CellEngine Identification of CD4+ and CD8+ T-Cell Subsets with Gut-Homing Phenotype and Altered Frequency and Activation Status During ART-Treated HIV Infection
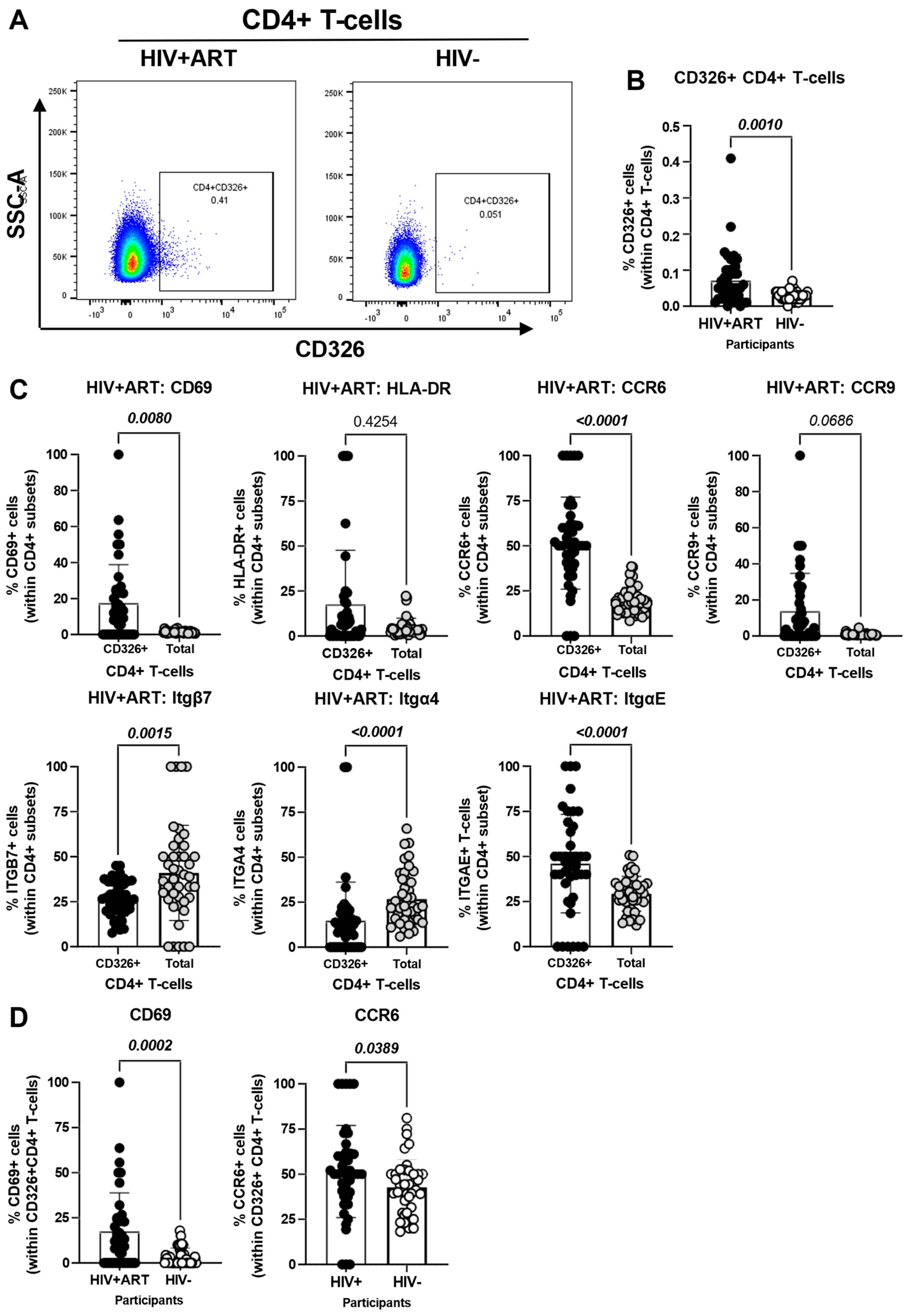
3.4. Increased Frequencies of CD4+ T-Cells with a Gut-Homing/Residency CD326+CD69+CCR6+ Phenotype in HIV+ vs. HIV− Participants
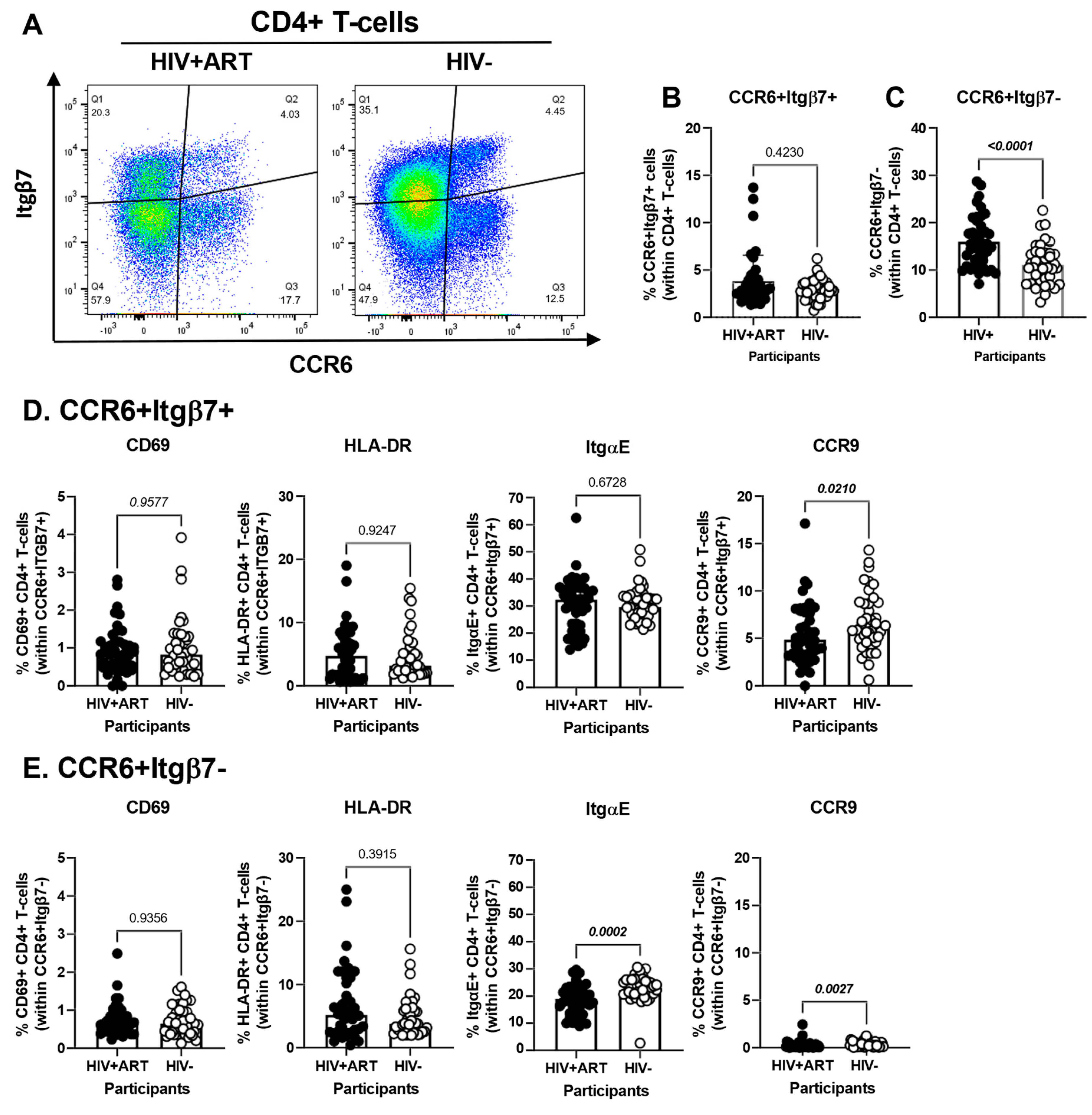
3.5. Increased Frequencies of CCR6+ItgB7− CD4+ T-Cells in the Peripheral Blood of HIV+ vs. HIV− Participants
3.6. Decreased Frequencies and Gut-Homing Potential of CD8+ IEL-like T-Cells in the Peripheral Blood of HIV+ vs. HIV− Participants
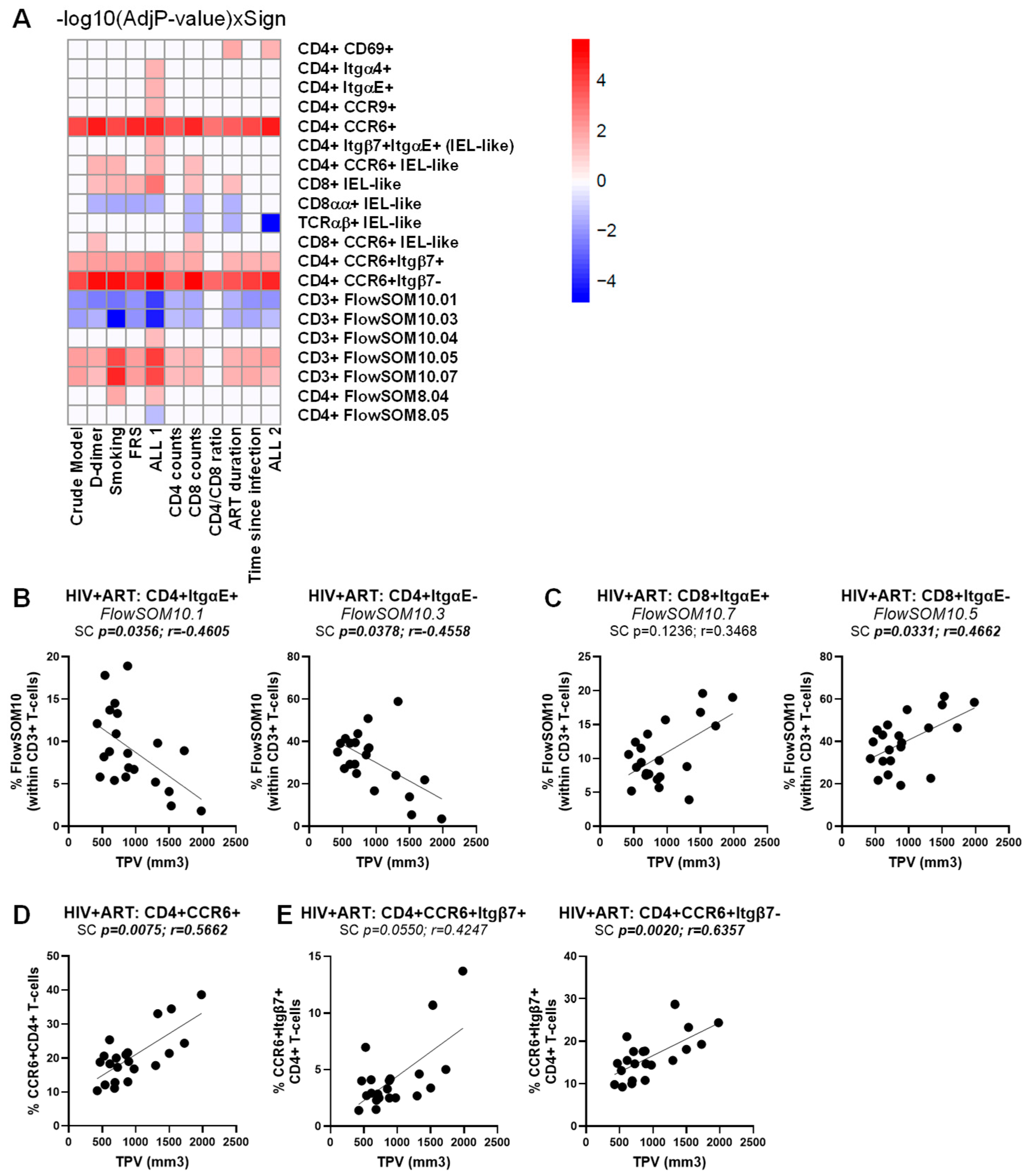
3.7. Multivariate Logistic Regression Analyses Identify Immune Subpopulations That Predict the HIV Status and the CVD Risk in ART-Treated PWH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | Antiretroviral therapy |
| BMI | Body mass index |
| CCTA | Computed Coronary Tomography Angiography |
| CHACS | Canadian HIV and aging cohort study |
| CVD | Cardiovascular disease |
| EpCAM | Epithelial cell adhesion molecule |
| FRS | Framingham risk score |
| GALT | Gut-associated lymphoid tissues |
| GLM | Generalized linear model |
| HIV-1 | Human immunodeficiency virus type 1 |
| IEC | Intestinal epithelial cells |
| IEL | Intraepithelial lymphocyte |
| IQR | Interquartile range |
| Itg | Integrin |
| PBMC | Peripheral blood mononucleated cells |
| PWH | People with HIV |
| SD | Standard deviation |
| SOM | Self-organizing map |
| TPV | Total plaque volume |
| t-SNE | t-Distributed Stochastic Neighbour Embedding |
References
- WHO. HIV Data and Statistics. 2023. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics (accessed on 15 September 2025).
- Barre-Sinoussi, F.; Ross, A.L.; Delfraissy, J.F. Past, present and future: 30 years of HIV research. Nat. Rev. Microbiol. 2013, 11, 877–883. [Google Scholar] [CrossRef]
- Deeks, S.G.; Archin, N.; Cannon, P.; Collins, S.; Jones, R.B.; de Jong, M.; Lambotte, O.; Lamplough, R.; Ndung’u, T.; Sugarman, J.; et al. Research priorities for an HIV cure: International AIDS Society Global Scientific Strategy 2021. Nat. Med. 2021, 27, 2085–2098. [Google Scholar] [CrossRef]
- Siliciano, J.D.; Siliciano, R.F. In Vivo Dynamics of the Latent Reservoir for HIV-1: New Insights and Implications for Cure. Annu. Rev. Pathol. 2022, 17, 271–294. [Google Scholar] [CrossRef]
- McMyn, N.F.; Varriale, J.; Fray, E.J.; Zitzmann, C.; MacLeod, H.; Lai, J.; Singhal, A.; Moskovljevic, M.; Garcia, M.A.; Lopez, B.M.; et al. The latent reservoir of inducible, infectious HIV-1 does not decrease despite decades of antiretroviral therapy. J. Clin. Invest. 2023, 133, e171554. [Google Scholar] [CrossRef]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef]
- Akusjarvi, S.S.; Neogi, U. Biological Aging in People Living with HIV on Successful Antiretroviral Therapy: Do They Age Faster? Curr. HIV/AIDS Rep. 2023, 20, 42–50. [Google Scholar] [CrossRef]
- Perkins, M.V.; Joseph, S.B.; Dittmer, D.P.; Mackman, N. Cardiovascular Disease and Thrombosis in HIV Infection. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 175–191. [Google Scholar] [CrossRef]
- Mudd, J.C.; Brenchley, J.M. Gut Mucosal Barrier Dysfunction, Microbial Dysbiosis, and Their Role in HIV-1 Disease Progression. J. Infect. Dis. 2016, 214 (Suppl. S2), S58–S66. [Google Scholar] [CrossRef]
- Agrati, C.; De Biasi, S.; Fidanza, L.; Gibellini, L.; Nasi, M.; Pinti, M.; Cossarizza, A. The importance of advanced cytometry in defining new immune cell types and functions relevant for the immunopathogenesis of HIV infection. AIDS 2020, 34, 2169–2185. [Google Scholar] [CrossRef]
- Paiardini, M.; Muller-Trutwin, M. HIV-associated chronic immune activation. Immunol. Rev. 2013, 254, 78–101. [Google Scholar] [CrossRef]
- Rousseau, R.K.; Szadkowski, L.; Kovacs, C.M.; Saikali, M.F.; Nadeem, R.; Malazogu, F.; Huibner, S.; Cummins, C.L.; Kaul, R.; Walmsley, S.L. Activation and gut-homing of peripheral T cells in HIV immunologic non-responders despite long term viral suppression. PLoS ONE 2021, 16, e0254149. [Google Scholar] [CrossRef]
- Cibrian, D.; Sanchez-Madrid, F. CD69: From activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef]
- Walsh, D.A.; Borges da Silva, H.; Beura, L.K.; Peng, C.; Hamilton, S.E.; Masopust, D.; Jameson, S.C. The Functional Requirement for CD69 in Establishment of Resident Memory CD8(+) T Cells Varies with Tissue Location. J. Immunol. 2019, 203, 946–955. [Google Scholar] [CrossRef]
- Moretti, S.; Schietroma, I.; Sberna, G.; Maggiorella, M.T.; Sernicola, L.; Farcomeni, S.; Giovanetti, M.; Ciccozzi, M.; Borsetti, A. HIV-1-Host Interaction in Gut-Associated Lymphoid Tissue (GALT): Effects on Local Environment and Comorbidities. Int. J. Mol. Sci. 2023, 24, 12193. [Google Scholar] [CrossRef]
- Veazey, R.S. Intestinal CD4 Depletion in HIV / SIV Infection. Curr. Immunol. Rev. 2019, 15, 76–91. [Google Scholar] [CrossRef]
- Busman-Sahay, K.; Starke, C.E.; Nekorchuk, M.D.; Estes, J.D. Eliminating HIV reservoirs for a cure: The issue is in the tissue. Curr. Opin. HIV AIDS 2021, 16, 200–208. [Google Scholar] [CrossRef]
- Chomont, N.; El-Far, M.; Ancuta, P.; Trautmann, L.; Procopio, F.A.; Yassine-Diab, B.; Boucher, G.; Boulassel, M.R.; Ghattas, G.; Brenchley, J.M.; et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009, 15, 893–900. [Google Scholar] [CrossRef]
- McBride, J.A.; Striker, R. Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 2017, 13, e1006624. [Google Scholar] [CrossRef]
- Lu, W.; Mehraj, V.; Vyboh, K.; Cao, W.; Li, T.; Routy, J.P. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J. Int. AIDS Soc. 2015, 18, 20052. [Google Scholar] [CrossRef]
- Grossman, Z.; Meier-Schellersheim, M.; Paul, W.E.; Picker, L.J. Pathogenesis of HIV infection: What the virus spares is as important as what it destroys. Nat. Med. 2006, 12, 289–295. [Google Scholar] [CrossRef]
- Thompson, C.G.; Gay, C.L.; Kashuba, A.D.M. HIV Persistence in Gut-Associated Lymphoid Tissues: Pharmacological Challenges and Opportunities. AIDS Res. Hum. Retroviruses 2017, 33, 513–523. [Google Scholar] [CrossRef]
- El-Far, M.; Tremblay, C.L. Gut microbial diversity in HIV infection post combined antiretroviral therapy: A key target for prevention of cardiovascular disease. Curr. Opin. HIV AIDS 2018, 13, 38–44. [Google Scholar]
- So-Armah, K.; Benjamin, L.A.; Bloomfield, G.S.; Feinstein, M.J.; Hsue, P.; Njuguna, B.; Freiberg, M.S. HIV and cardiovascular disease. Lancet HIV 2020, 7, e279–e293. [Google Scholar] [CrossRef]
- Lewis, C.V.; Taylor, W.R. Intestinal barrier dysfunction as a therapeutic target for cardiovascular disease. Am. J. Physiol.-Heart Circ. Physiol. 2020, 319, H1227–H1233. [Google Scholar] [CrossRef]
- Turcotte, I.; El-Far, M.; Sadouni, M.; Chartrand-Lefebvre, C.; Filali-Mouhim, A.; Fromentin, R.; Chamberland, A.; Jenabian, M.A.; Baril, J.G.; Trottier, B.; et al. Association Between the Development of Subclinical Cardiovascular Disease and Human Immunodeficiency Virus (HIV) Reservoir Markers in People with HIV on Suppressive Antiretroviral Therapy. Clin. Infect. Dis. 2023, 76, 1318–1321. [Google Scholar] [CrossRef]
- Ramani, H.; Gosselin, A.; Bunet, R.; Jenabian, M.A.; Sylla, M.; Pagliuzza, A.; Chartrand-Lefebvre, C.; Routy, J.P.; Goulet, J.P.; Thomas, R.; et al. IL-32 Drives the Differentiation of Cardiotropic CD4+ T Cells Carrying HIV DNA in People with HIV. J. Infect. Dis. 2024, 229, 1277–1289. [Google Scholar] [CrossRef]
- Guenin-Mace, L.; Konieczny, P.; Naik, S. Immune-Epithelial Cross Talk in Regeneration and Repair. Annu. Rev. Immunol. 2023, 41, 207–228. [Google Scholar] [CrossRef]
- Mahapatro, M.; Erkert, L.; Becker, C. Cytokine-Mediated Crosstalk between Immune Cells and Epithelial Cells in the Gut. Cells 2021, 10, 111. [Google Scholar] [CrossRef]
- Wacleche, V.S.; Landay, A.; Routy, J.P.; Ancuta, P. The Th17 Lineage: From Barrier Surfaces Homeostasis to Autoimmunity, Cancer, and HIV-1 Pathogenesis. Viruses 2017, 9, 303. [Google Scholar] [CrossRef]
- Planas, D.; Routy, J.P.; Ancuta, P. New Th17-specific therapeutic strategies for HIV remission. Curr. Opin. HIV AIDS 2019, 14, 85–92. [Google Scholar] [CrossRef]
- Fert, A.; Raymond Marchand, L.; Wiche Salinas, T.R.; Ancuta, P. Targeting Th17 cells in HIV-1 remission/cure interventions. Trends Immunol. 2022, 43, 580–594. [Google Scholar] [CrossRef]
- Buckner, J.H.; Harrison, O.J. Th17 cells: From gut homeostasis to CNS pathogenesis. Trends Immunol. 2022, 43, 167–169. [Google Scholar] [CrossRef]
- Kim, C.J.; McKinnon, L.R.; Kovacs, C.; Kandel, G.; Huibner, S.; Chege, D.; Shahabi, K.; Benko, E.; Loutfy, M.; Ostrowski, M.; et al. Mucosal Th17 cell function is altered during HIV infection and is an independent predictor of systemic immune activation. J. Immunol. 2013, 191, 2164–2173. [Google Scholar] [CrossRef]
- Arthos, J.; Cicala, C.; Nawaz, F.; Byrareddy, S.N.; Villinger, F.; Santangelo, P.J.; Ansari, A.A.; Fauci, A.S. The Role of Integrin alpha4beta7 in HIV Pathogenesis and Treatment. Curr. HIV/AIDS Rep. 2018, 15, 127–135. [Google Scholar] [CrossRef]
- Pollock, J.; Kaul, R. How integral is the alpha4beta7 integrin to HIV transmission? eBioMedicine 2021, 63, 103148. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, C.; Li, Y.; Liu, Z.; Wang, J.; Zhang, Y.; Yan, Z.; Zhang, Y.; Li, G.; Chen, J. Distinct chemokines selectively induce HIV-1 gp120-integrin alpha4beta7 binding via triggering conformer-specific activation of alpha4beta7. Signal Transduct. Target. Ther. 2021, 6, 265. [Google Scholar] [CrossRef]
- Sivro, A.; Schuetz, A.; Sheward, D.; Joag, V.; Yegorov, S.; Liebenberg, L.J.; Yende-Zuma, N.; Stalker, A.; Mwatelah, R.S.; Selhorst, P.; et al. Integrin alpha4beta7 expression on peripheral blood CD4(+) T cells predicts HIV acquisition and disease progression outcomes. Sci. Transl. Med. 2018, 10, eaam6354. [Google Scholar] [CrossRef] [PubMed]
- Mavigner, M.; Cazabat, M.; Dubois, M.; L’Faqihi, F.E.; Requena, M.; Pasquier, C.; Klopp, P.; Amar, J.; Alric, L.; Barange, K.; et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. J. Clin. Invest. 2012, 122, 62–69. [Google Scholar] [CrossRef]
- Loiseau, C.; Requena, M.; Mavigner, M.; Cazabat, M.; Carrere, N.; Suc, B.; Barange, K.; Alric, L.; Marchou, B.; Massip, P.; et al. CCR6 regulatory T cells blunt the restoration of gut Th17 cells along the CCR6-CCL20 axis in treated HIV-1-infected individuals. Mucosal Immunol. 2016, 9, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Nayrac, M.; Requena, M.; Loiseau, C.; Cazabat, M.; Suc, B.; Carrere, N.; Barange, K.; Alric, L.; Martin-Blondel, G.; Izopet, J.; et al. Th22 cells are efficiently recruited in the gut by CCL28 as an alternative to CCL20 but do not compensate for the loss of Th17 cells in treated HIV-1-infected individuals. Mucosal Immunol. 2021, 14, 219–228. [Google Scholar] [CrossRef]
- Sumida, H. Dynamics and clinical significance of intestinal intraepithelial lymphocytes. Immunol. Med. 2019, 42, 117–123. [Google Scholar] [CrossRef]
- Olivares-Villagomez, D.; Van Kaer, L. Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier. Trends Immunol. 2018, 39, 264–275. [Google Scholar] [CrossRef]
- Gui, Y.; Cheng, H.; Zhou, J.; Xu, H.; Han, J.; Zhang, D. Development and function of natural TCR(+) CD8alphaalpha(+) intraepithelial lymphocytes. Front. Immunol. 2022, 13, 1059042. [Google Scholar] [CrossRef]
- Hu, M.D.; Jia, L.; Edelblum, K.L. Policing the intestinal epithelial barrier: Innate immune functions of intraepithelial lymphocytes. Curr. Pathobiol. Rep. 2018, 6, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, A.; Mucida, D.; Bilate, A.M. Intraepithelial Lymphocytes of the Intestine. Annu. Rev. Immunol. 2024, 42, 289–316. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.M.; Witherden, D.A.; Havran, W.L. gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 2017, 17, 733–745. [Google Scholar] [CrossRef]
- Van Kaer, L.; Olivares-Villagomez, D. Development, Homeostasis, and Functions of Intestinal Intraepithelial Lymphocytes. J. Immunol. 2018, 200, 2235–2244. [Google Scholar] [CrossRef]
- Yukl, S.A.; Khan, S.; Chen, T.H.; Trapecar, M.; Wu, F.; Xie, G.; Telwatte, S.; Fulop, D.; Pico, A.R.; Laird, G.M.; et al. Shared Mechanisms Govern HIV Transcriptional Suppression in Circulating CD103(+) and Gut CD4(+) T Cells. J. Virol. 2020, 95. [Google Scholar] [CrossRef]
- Kiniry, B.E.; Li, S.; Ganesh, A.; Hunt, P.W.; Somsouk, M.; Skinner, P.J.; Deeks, S.G.; Shacklett, B.L. Detection of HIV-1-specific gastrointestinal tissue resident CD8(+) T-cells in chronic infection. Mucosal Immunol. 2018, 11, 909–920. [Google Scholar] [CrossRef]
- Snelson, M.; Muralitharan, R.R.; Liu, C.F.; Marko, L.; Forslund, S.K.; Marques, F.Z.; Tang, W.H.W. Gut-Heart Axis: The Role of Gut Microbiota and Metabolites in Heart Failure. Circ. Res. 2025, 136, 1382–1406. [Google Scholar] [CrossRef] [PubMed]
- Troseid, M.; Andersen, G.O.; Broch, K.; Hov, J.R. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. eBioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef]
- Giguere, K.; Chartrand-Lefebvre, C.; Baril, J.G.; Conway, B.; El-Far, M.; Falutz, J.; Harris, M.; Jenabian, M.A.; Leipsic, J.; Loutfy, M.; et al. Baseline characteristics of a prospective cohort study of aging and cardiovascular diseases among people living with HIV. HIV Med. 2023, 24, 1210–1221. [Google Scholar] [CrossRef]
- Wiche Salinas, T.R.; Zhang, Y.; Gosselin, A.; Rosario, N.F.; El-Far, M.; Filali-Mouhim, A.; Routy, J.P.; Chartrand-Lefebvre, C.; Landay, A.L.; Durand, M.; et al. Alterations in Th17 Cells and Non-Classical Monocytes as a Signature of Subclinical Coronary Artery Atherosclerosis during ART-Treated HIV-1 Infection. Cells 2024, 13, 157. [Google Scholar] [CrossRef]
- Chen, Z.; Boldeanu, I.; Nepveu, S.; Durand, M.; Chin, A.S.; Kauffmann, C.; Mansour, S.; Soulez, G.; Tremblay, C.; Chartrand-Lefebvre, C. In vivo coronary artery plaque assessment with computed tomography angiography: Is there an impact of iterative reconstruction on plaque volume and attenuation metrics? Acta Radiol. 2017, 58, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Chartrand-Lefebvre, C.; Baril, J.G.; Trottier, S.; Trottier, B.; Harris, M.; Walmsley, S.; Conway, B.; Wong, A.; Routy, J.P.; et al. The Canadian HIV and aging cohort study—Determinants of increased risk of cardio-vascular diseases in HIV-infected individuals: Rationale and study protocol. BMC Infect. Dis. 2017, 17, 611. [Google Scholar] [CrossRef] [PubMed]
- Boldeanu, I.; Sadouni, M.; Mansour, S.; Baril, J.G.; Trottier, B.; Soulez, G.; Chin, A.S.; Leipsic, J.; Tremblay, C.; Durand, M.; et al. Prevalence and Characterization of Subclinical Coronary Atherosclerotic Plaque with CT among Individuals with HIV: Results from the Canadian HIV and Aging Cohort Study. Radiology 2021, 299, 571–580. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing (Version 4.4.1); R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 15 September 2025).
- Gullaksen, S.; Funck, K.L.; Laugesen, E.; Hansen, T.K.; Dey, D.; Poulsen, P.L. Volumes of coronary plaque disease in relation to body mass index, waist circumference, truncal fat mass and epicardial adipose tissue in patients with type 2 diabetes mellitus and controls. Diab Vasc. Dis. Res. 2019, 16, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Schlesselman, J.J.; Stolley, P.D. Case-Control Studies: Design, Conduct, Analysis; Oxford University Press: Oxford, UK, 1982; pp. 144–147. [Google Scholar]
- Kenward, M.G.; Roger, J.H. The use of baseline covariates in crossover studies. Biostatistics 2010, 11, 1–17. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Rothan, C.; Yero, A.; Shi, T.; Farnos, O.; Chartrand-Lefebvre, C.; El-Far, M.; Costiniuk, C.T.; Tsoukas, C.; Tremblay, C.; Durand, M.; et al. Antiretroviral therapy-treated HIV-infected adults with coronary artery disease are characterized by a distinctive regulatory T-cell signature. AIDS 2021, 35, 1003–1014. [Google Scholar] [CrossRef]
- Agace, W.W.; Higgins, J.M.; Sadasivan, B.; Brenner, M.B.; Parker, C.M. T-lymphocyte-epithelial-cell interactions: Integrin alpha(E)(CD103)beta(7), LEEP-CAM and chemokines. Curr. Opin. Cell Biol. 2000, 12, 563–568. [Google Scholar] [CrossRef]
- Huang, S.H.; Ren, Y.; Thomas, A.S.; Chan, D.; Mueller, S.; Ward, A.R.; Patel, S.; Bollard, C.M.; Cruz, C.R.; Karandish, S.; et al. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8+ T cells. J. Clin. Invest. 2018, 128, 876–889. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, W.; Nie, Y.; Yang, Y.; Chen, G.; Huang, L.; Wu, H.; Lei, Y.; Chen, L.; Hu, Q.; et al. EpCAM Is Essential to Maintaining the Immune Homeostasis of Intestines via Keeping the Expression of pIgR in the Intestinal Epithelium of Mice. Front. Immunol. 2022, 13, 843378. [Google Scholar] [CrossRef] [PubMed]
- Yahyazadeh Mashhadi, S.M.; Kazemimanesh, M.; Arashkia, A.; Azadmanesh, K.; Meshkat, Z.; Golichenari, B.; Sahebkar, A. Shedding light on the EpCAM: An overview. J. Cell. Physiol. 2019, 234, 12569–12580. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabieres, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef]
- Han, D.; Li, R.; Shi, J.; Tan, P.; Zhang, R.; Li, J. Liquid biopsy for infectious diseases: A focus on microbial cell-free DNA sequencing. Theranostics 2020, 10, 5501–5513. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Khoo, B.L. Liquid biopsy technologies for hematological diseases. Med. Res. Rev. 2021, 41, 246–274. [Google Scholar] [CrossRef]
- Hegazy, A.N.; West, N.R.; Stubbington, M.J.T.; Wendt, E.; Suijker, K.I.M.; Datsi, A.; This, S.; Danne, C.; Campion, S.; Duncan, S.H.; et al. Circulating and Tissue-Resident CD4+ T Cells with Reactivity to Intestinal Microbiota Are Abundant in Healthy Individuals and Function Is Altered During Inflammation. Gastroenterology 2017, 153, 1320–1337.e16. [Google Scholar] [CrossRef] [PubMed]
- Samat, A.A.K.; van der Geest, J.; Vastert, S.J.; van Loosdregt, J.; van Wijk, F. Tissue-Resident Memory T Cells in Chronic Inflammation-Local Cells with Systemic Effects? Cells 2021, 10, 409. [Google Scholar] [CrossRef]
- Asawa, S.; Nuesch, M.; Gvozdenovic, A.; Aceto, N. Circulating tumour cells in gastrointestinal cancers: Food for thought? Br. J. Cancer 2023, 128, 1981–1990. [Google Scholar] [CrossRef]
- Monteiro, P.; Gosselin, A.; Wacleche, V.S.; El-Far, M.; Said, E.A.; Kared, H.; Grandvaux, N.; Boulassel, M.R.; Routy, J.P.; Ancuta, P. Memory CCR6+ CD4+ T cells are preferential targets for productive HIV type 1 infection regardless of their expression of integrin beta7. J. Immunol. 2011, 186, 4618–4630. [Google Scholar] [CrossRef]
- Folsom, A.R.; Gottesman, R.F.; Appiah, D.; Shahar, E.; Mosley, T.H. Plasma d-Dimer and Incident Ischemic Stroke and Coronary Heart Disease: The Atherosclerosis Risk in Communities Study. Stroke 2016, 47, 18–23. [Google Scholar] [CrossRef]
- Tibaut, M.; Caprnda, M.; Kubatka, P.; Sinkovic, A.; Valentova, V.; Filipova, S.; Gazdikova, K.; Gaspar, L.; Mozos, I.; Egom, E.E.; et al. Markers of Atherosclerosis: Part 1—Serological Markers. Heart Lung Circ. 2019, 28, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yang, Y.; Yang, F.; Liu, S.; Zhu, Z.; Lei, Z.; Guo, J. Functions of EpCAM in physiological processes and diseases (Review). Int. J. Mol. Med. 2018, 42, 1771–1785. [Google Scholar] [CrossRef]
- Planas, D.; Pagliuzza, A.; Ponte, R.; Fert, A.; Marchand, L.R.; Massanella, M.; Gosselin, A.; Mehraj, V.; Dupuy, F.P.; Isnard, S.; et al. LILAC pilot study: Effects of metformin on mTOR activation and HIV reservoir persistence during antiretroviral therapy. eBioMedicine 2021, 65, 103270. [Google Scholar] [CrossRef]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef]
- Nakayama, M.; Hori, A.; Toyoura, S.; Yamaguchi, S.I. Shaping of T Cell Functions by Trogocytosis. Cells 2021, 10, 1155. [Google Scholar] [CrossRef] [PubMed]
- Schriek, P.; Villadangos, J.A. Trogocytosis and cross-dressing in antigen presentation. Curr. Opin. Immunol. 2023, 83, 102331. [Google Scholar] [CrossRef] [PubMed]
- Vo, D.N.; Leventoux, N.; Campos-Mora, M.; Gimenez, S.; Corbeau, P.; Villalba, M. NK Cells Acquire CCR5 and CXCR4 by Trogocytosis in People Living with HIV-1. Vaccines 2022, 10, 688. [Google Scholar] [CrossRef]
- Couturier, J.; Suliburk, J.W.; Brown, J.M.; Luke, D.J.; Agarwal, N.; Yu, X.; Nguyen, C.; Iyer, D.; Kozinetz, C.A.; Overbeek, P.A.; et al. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. AIDS 2015, 29, 667–674. [Google Scholar] [CrossRef]
- Wanjalla, C.N.; McDonnell, W.J.; Barnett, L.; Simmons, J.D.; Furch, B.D.; Lima, M.C.; Woodward, B.O.; Fan, R.; Fei, Y.; Baker, P.G.; et al. Adipose Tissue in Persons with HIV Is Enriched for CD4(+) T Effector Memory and T Effector Memory RA(+) Cells, Which Show Higher CD69 Expression and CD57, CX3CR1, GPR56 Co-expression with Increasing Glucose Intolerance. Front. Immunol. 2019, 10, 408. [Google Scholar] [CrossRef]
- Wang, X.; Xu, H.; Alvarez, X.; Pahar, B.; Moroney-Rasmussen, T.; Lackner, A.A.; Veazey, R.S. Distinct expression patterns of CD69 in mucosal and systemic lymphoid tissues in primary SIV infection of rhesus macaques. PLoS ONE 2011, 6, e27207. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Bergsbaken, T.; Edelblum, K.L. The multifunctional nature of CD103 (alphaEbeta7 integrin) signaling in tissue-resident lymphocytes. Am. J. Physiol. Cell Physiol. 2022, 323, C1161–C1167. [Google Scholar] [CrossRef]
- Vimonpatranon, S.; Goes, L.R.; Chan, A.; Licavoli, I.; McMurry, J.; Wertz, S.R.; Arakelyan, A.; Huang, D.; Jiang, A.; Huang, C.; et al. MAdCAM-1 costimulation in the presence of retinoic acid and TGF-beta promotes HIV infection and differentiation of CD4+ T cells into CCR5+ TRM-like cells. PLoS Pathog. 2023, 19, e1011209. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, M.; Yang, Z.; Lu, C.; Wang, Q.; Wang, H.; Deng, C.; Liu, Y.; Yang, Y. The Roles of CCR9/CCL25 in Inflammation and Inflammation-Associated Diseases. Front. Cell Dev. Biol. 2021, 9, 686548. [Google Scholar] [CrossRef]
- Mayassi, T.; Barreiro, L.B.; Rossjohn, J.; Jabri, B. A multilayered immune system through the lens of unconventional T cells. Nature 2021, 595, 501–510. [Google Scholar] [CrossRef]
- Ziegler, H.; Welker, C.; Sterk, M.; Haarer, J.; Rammensee, H.G.; Handgretinger, R.; Schilbach, K. Human Peripheral CD4(+) Vdelta1(+) gammadeltaT Cells Can Develop into alphabetaT Cells. Front. Immunol. 2014, 5, 645. [Google Scholar] [CrossRef]
- Li, H.; Pauza, C.D. HIV envelope-mediated, CCR5/alpha4beta7-dependent killing of CD4-negative gammadelta T cells which are lost during progression to AIDS. Blood 2011, 118, 5824–5831. [Google Scholar] [CrossRef]
- Pauza, C.D.; Poonia, B.; Li, H.; Cairo, C.; Chaudhry, S. gammadelta T Cells in HIV Disease: Past, Present, and Future. Front. Immunol. 2014, 5, 687. [Google Scholar]
- Walker, E.M.; Slisarenko, N.; Gerrets, G.L.; Grasperge, B.F.; Mattison, J.A.; Kissinger, P.J.; Welsh, D.A.; Veazey, R.S.; Jazwinski, S.M.; Rout, N. Dysregulation of IL-17/IL-22 Effector Functions in Blood and Gut Mucosal Gamma Delta T Cells Correlates with Increase in Circulating Leaky Gut and Inflammatory Markers During cART-Treated Chronic SIV Infection in Macaques. Front. Immunol. 2021, 12, 647398. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.S.; Korner, H. CCR6/CCL20 chemokine axis in human immunodeficiency virus immunity and pathogenesis. J. Gen. Virol. 2017, 98, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Khoury, G.; Fromentin, R.; Solomon, A.; Chomont, N.; Sinclair, E.; Milush, J.M.; Hartogensis, W.; Bacchetti, P.; Roche, M.; et al. Human Immunodeficiency Virus (HIV)-Infected CCR6+ Rectal CD4+ T Cells and HIV Persistence on Antiretroviral Therapy. J. Infect. Dis. 2020, 221, 744–755. [Google Scholar] [CrossRef]
- Gosselin, A.; Wiche Salinas, T.R.; Planas, D.; Wacleche, V.S.; Zhang, Y.; Fromentin, R.; Chomont, N.; Cohen, E.A.; Shacklett, B.; Mehraj, V.; et al. HIV persists in CCR6+ CD4+ T cells from colon and blood during antiretroviral therapy. AIDS 2017, 31, 35–48. [Google Scholar] [CrossRef]
- Khoury, G.; Anderson, J.L.; Fromentin, R.; Hartogenesis, W.; Smith, M.Z.; Bacchetti, P.; Hecht, F.M.; Chomont, N.; Cameron, P.U.; Deeks, S.G.; et al. Persistence of integrated HIV DNA in CXCR3 + CCR6 + memory CD4+ T cells in HIV-infected individuals on antiretroviral therapy. AIDS 2016, 30, 1511–1520. [Google Scholar] [CrossRef]
- Mattapallil, J.J.; Reay, E.; Dandekar, S. An early expansion of CD8alphabeta T cells, but depletion of resident CD8alphaalpha T cells, occurs in the intestinal epithelium during primary simian immunodeficiency virus infection. AIDS 2000, 14, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Konno, A.; Okada, K.; Mizuno, K.; Nishida, M.; Nagaoki, S.; Toma, T.; Uehara, T.; Ohta, K.; Kasahara, Y.; Seki, H.; et al. CD8alpha alpha memory effector T cells descend directly from clonally expanded CD8alpha +beta high TCRalpha beta T cells in vivo. Blood 2002, 100, 4090–4097. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Qiu, Y.; Yang, H. Intestinal intraepithelial lymphocytes: Maintainers of intestinal immune tolerance and regulators of intestinal immunity. J. Leukoc. Biol. 2021, 109, 339–347. [Google Scholar] [CrossRef]
- Rock, A.E.; Russell, M.L.; Triant, V.A. Balancing polypharmacy and comorbidity management: Cardiovascular health. Curr. Opin. HIV AIDS 2025, 20, 409–415. [Google Scholar] [CrossRef]
- Das Adhikari, U.; Froehle, L.M.; Pipkin, A.N.; Baharlou, H.; Linder, A.H.; Shah, P.; Hussey, A.; Zhang, Q.; Nyquist, S.; Khwaled, S.; et al. Immunometabolic defects of CD8+ T cells disrupt gut barrier integrity in people with HIV. Cell 2025, 188, 5666–5679.e19. [Google Scholar] [CrossRef]

| HIV+ (n = 42) | HIV− (n = 40) | p-Value | |
|---|---|---|---|
| Demographics and clinical information | |||
| Age (years) # | 54.1 (42.2–67.8) | 54.5 (33.6–72.2) | 0.8086 |
| Male & | 40 (95%) | 34 (85%) | 0.1504 |
| BMI (kg/m2) # | 24.1 (19.10–34.50) | 26.35 (19.70–44.00) | 0.0052 |
| Framingham Score # | 9 (3–30) | 10 (2–18) | 0.3396 |
| Smoking & Never Current Smoker Former Smoker | 11 (26.1%) 17 (40.4%) 13 (30.9%) | 18 (45.0%) 10 (25.0%) 12 (30.0%) | 0.1826 |
| Statin treatment & | 11 (26.0%) | 5 (12.5%) | 0.0580 |
| Coronary Artery Disease Parameters | |||
| TPV (mm3) # All participants TPV+ participants | 526.3 (0.0–1981.0; n = 42) 851 (425–1,981; n = 21) | 37.3 (0.0–2253; n = 40) 182 (34–2,253; n = 20) | 0.0617 0.0002 |
| Laboratory Parameters | |||
| D-Dimer (μg/L) # | 310 (170–1130) | 287.0 (170–649) | 0.8127 |
| Fibrinogen (G/L) # | 3.05 (2.1–6.1) | 2.90 (2.1–4.2) | 0.5735 |
| LDL (mmol/L) # | 2.55 (0.8–4.6) | 3.1 (1.6–6.9) | 0.0452 |
| HDL (mmol/L) # | 1.2 (0.8–2.2) | 1.3 (0.7–1.9) | 0.1973 |
| Triglycerides (mmol/L) # | 1.65 (0.6–6.5) | 1.2 (0.6–6.4) | 0.2154 |
| TPV+ (n = 24) | TPV− (n = 16) | p-Value | |
|---|---|---|---|
| Demographics and clinical information | |||
| Age (years) # | 53.9 (42.2–64.1) | 54.3 (42.2–62.1) | 0.6573 |
| Male & | 23 (95.8%) | 15 (93.7%) | >0.9999 |
| BMI (kg/m2) # | 22.9 (19.1–34.5) | 25.5 (21.0–28.2) | 0.1320 |
| Framingham Score # | 9.0 (4–30) | 8 (3–18) | 0.1404 |
| Smoking & Never Smoked Current Smoker Former Smoker N/A | 4 (16%) 14 (58%) 6 (25%) 0 (0%) | 5 (31%) 3 (18%) 7 (43%) 1 (6%) | 0.0666 |
| Statin treatment & | 6 (25%) | 5 (31%) | 0.7275 |
| Laboratory Parameters | |||
| D-Dimer (ug/L) # | 340 (170–720) | 275.0 (170–1130) | 0.3029 |
| Fibrinogen (G/L) # | 3.2 (2.1–6.1) | 2.8 (2.1–3.7) | 0.0827 |
| LDL (mmol/L) # | 2.4 (0.8–4.3) | 2.8 (1.9–4.6) | 0.2972 |
| HDL (mmol/L) # | 1.1 (0.8–2.2) | 1.2 (0.8–2.2) | 0.7596 |
| Triglycerides (mmol/L) # | 1.7 (0.6–6.5) | 1.5 (0.8–3.2) | 0.2033 |
| Serology | |||
| Co-infection CMV # | 20 (83%) | 12 (75%) | 0.5227 |
| HIV disease parameters | |||
| Duration of HIV (years) # | 20.6 (5.7–30.8) | 17.7 (3.8–29.4) | 0.2363 |
| Duration of ART (years) # | 16.6 (3.3–24.8) | 13.6 (0.0–25.0) | 0.0706 |
| Nadir CD4 (xE9/L) # | 235 (20–556) | 190 (10–750) | 0.7889 |
| CD4 (%) # | 31.0 (3–46) | 35.0 (13–57) | 0.2250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira Gabriel, E.; Dias, J.; Filali-Mouhim, A.; Caballero, R.E.; Wiche Salinas, T.R.; Nayrac, M.; Chartrand-Lefebvre, C.; Routy, J.-P.; Durand, M.; El-Far, M.; et al. Alterations in Circulating T-Cell Subsets with Gut-Homing/Residency Phenotypes Associated with HIV-1 Status and Subclinical Atherosclerosis. Cells 2025, 14, 1732. https://doi.org/10.3390/cells14211732
Moreira Gabriel E, Dias J, Filali-Mouhim A, Caballero RE, Wiche Salinas TR, Nayrac M, Chartrand-Lefebvre C, Routy J-P, Durand M, El-Far M, et al. Alterations in Circulating T-Cell Subsets with Gut-Homing/Residency Phenotypes Associated with HIV-1 Status and Subclinical Atherosclerosis. Cells. 2025; 14(21):1732. https://doi.org/10.3390/cells14211732
Chicago/Turabian StyleMoreira Gabriel, Etiene, Jonathan Dias, Abdelali Filali-Mouhim, Ramon Edwin Caballero, Tomas Raul Wiche Salinas, Manon Nayrac, Carl Chartrand-Lefebvre, Jean-Pierre Routy, Madeleine Durand, Mohamed El-Far, and et al. 2025. "Alterations in Circulating T-Cell Subsets with Gut-Homing/Residency Phenotypes Associated with HIV-1 Status and Subclinical Atherosclerosis" Cells 14, no. 21: 1732. https://doi.org/10.3390/cells14211732
APA StyleMoreira Gabriel, E., Dias, J., Filali-Mouhim, A., Caballero, R. E., Wiche Salinas, T. R., Nayrac, M., Chartrand-Lefebvre, C., Routy, J.-P., Durand, M., El-Far, M., Tremblay, C., & Ancuta, P. (2025). Alterations in Circulating T-Cell Subsets with Gut-Homing/Residency Phenotypes Associated with HIV-1 Status and Subclinical Atherosclerosis. Cells, 14(21), 1732. https://doi.org/10.3390/cells14211732






