Desloratadine Induces TP53-Dependent Apoptosis in MCF-7 Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Treatment Protocol
2.3. Determination of IC50 Value
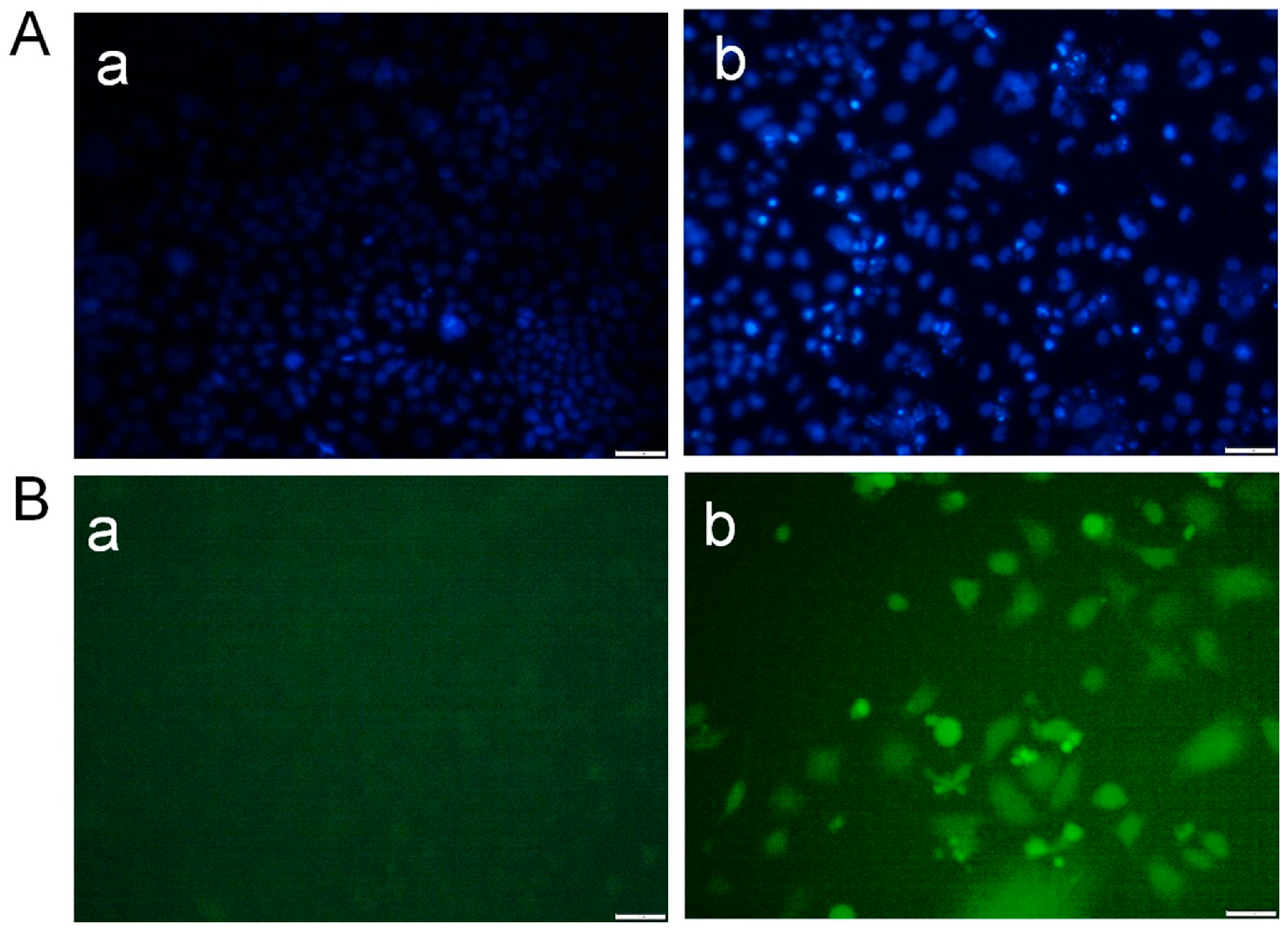
2.4. Morphological Study Using a Fluorescence Microscope
2.5. Observation of Reactive Oxygen Species (ROS) Changes
2.6. Detection of Early and Late Apoptosis by Fluorescence Microscopy and Flow Cytometry
2.7. Cell Cycle Analysis by Flow Cytometry
2.8. Gene Expression by Real-Time PCR
2.9. Involvement of Caspases in the Apoptosis Process
2.10. In Silico Study
2.11. CRISPR-Cas9 Knockout of TP53
2.12. Statistical Analysis
3. Results
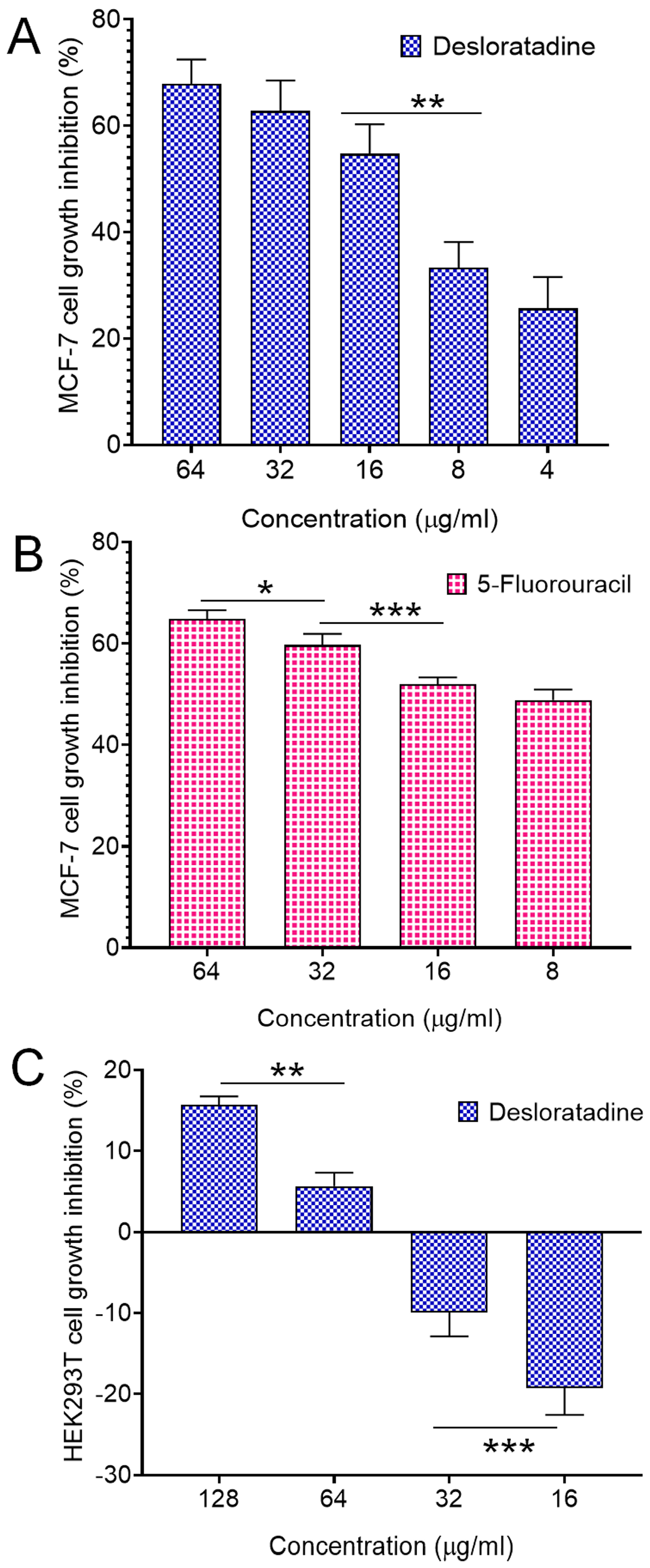
3.1. Desloratadine Inhibits MCF-7 Cell Growth and Induces Apoptotic Morphology
3.2. Desloratadine Induces ROS Generation and Detection of Early and Late Apoptosis
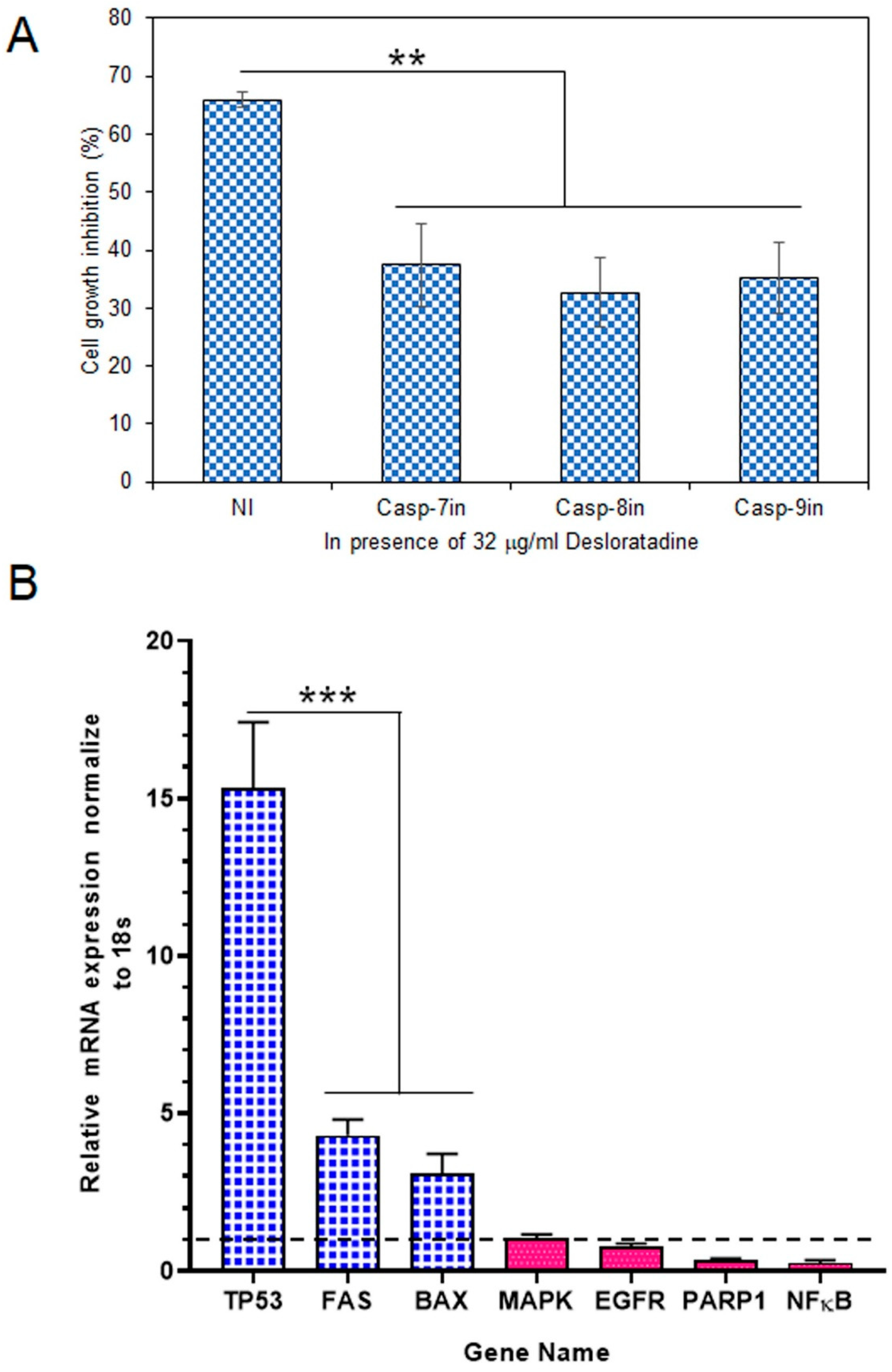
3.3. Effect of Caspase Inhibitors in the Apoptosis Process and Modulation of Apoptotic and Survival Gene Expression in MCF-7 Cells
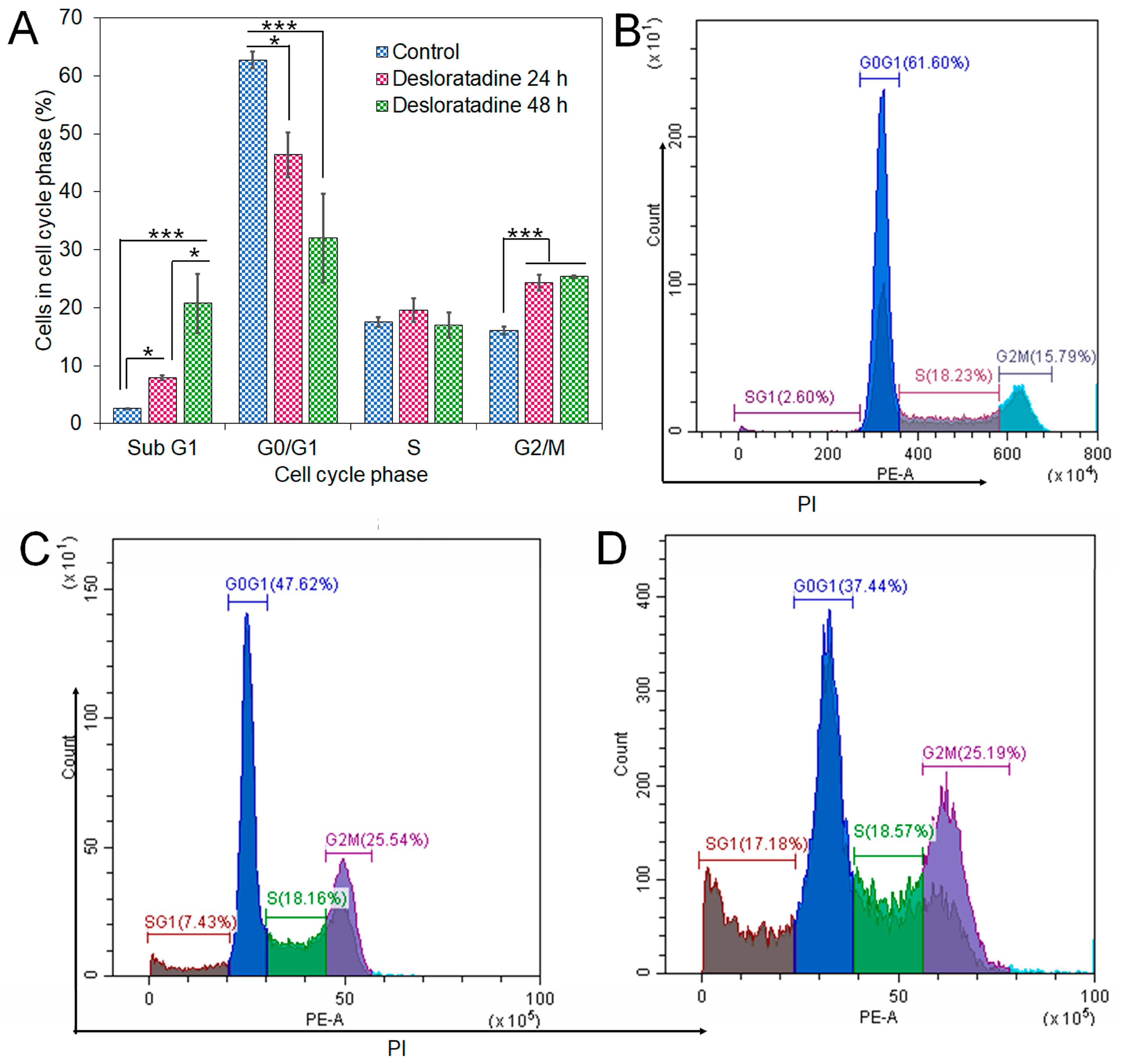
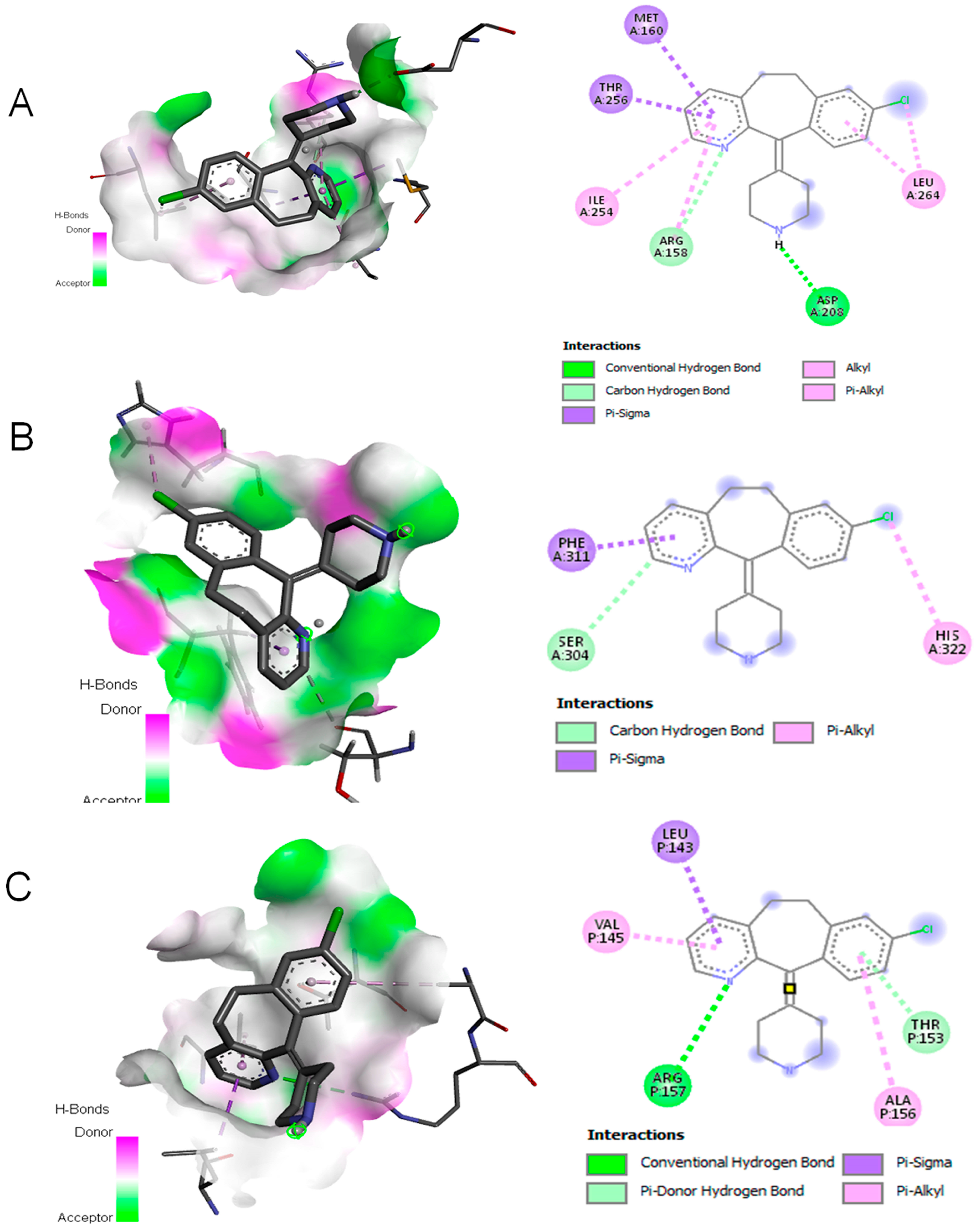
3.4. Desloratadine Induces Cell Cycle Arrest and Shows Strong Binding Affinity for Apoptotic Regulators
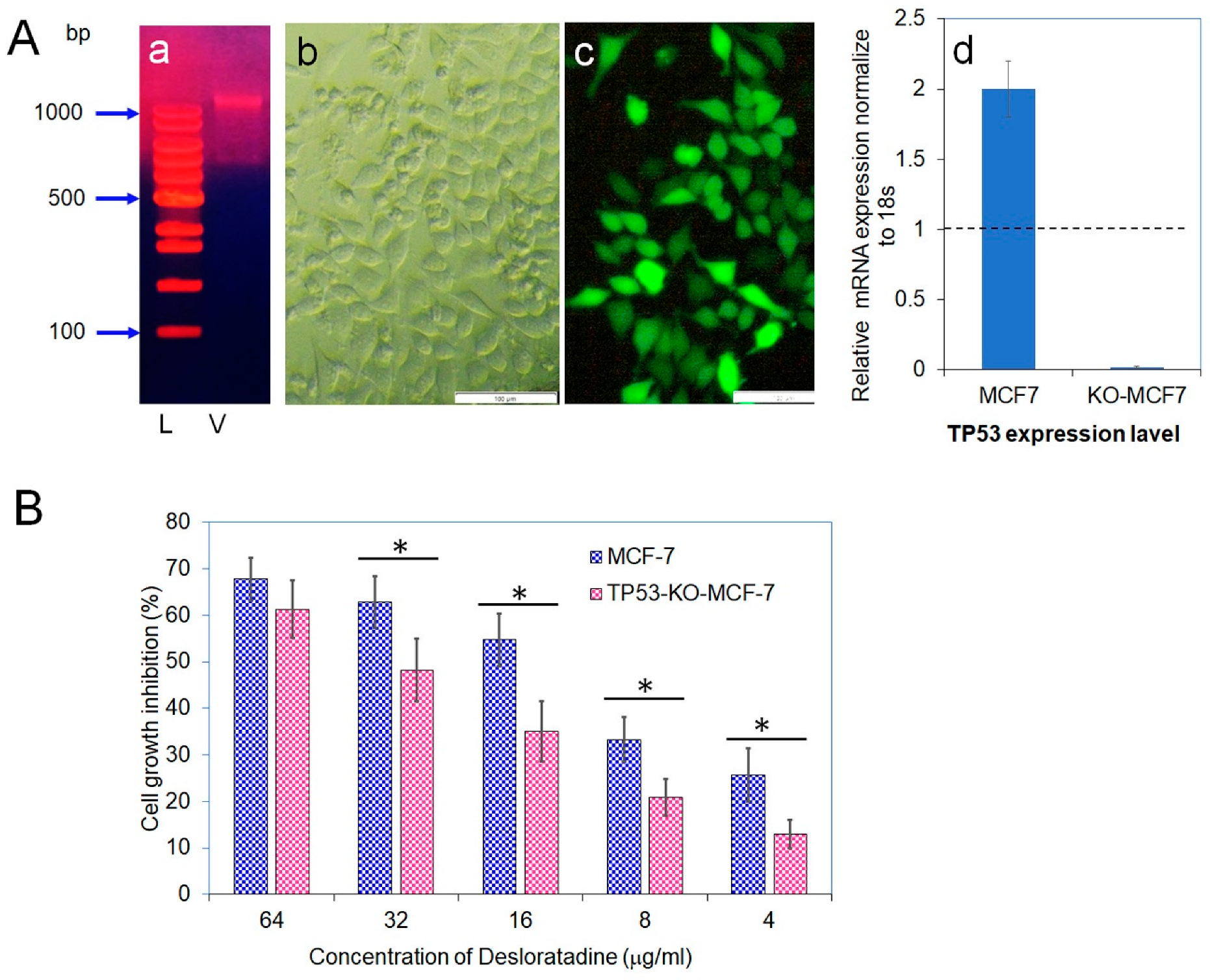
3.5. CRISPR-Cas9 Disruption of TP53 Confirms Its Role in Desloratadine-Mediated Apoptosis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MTT | (3-[4:5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide) |
| DMEM | Dulbecco’s Modified Eagle Medium |
| HEPES | (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) |
| FITC | Fluorescein isothiocyanate |
| IC50 | Half maximal inhibitory concentration |
| PBS | Phosphate-buffered saline |
| PARP1 | Poly(ADP-ribose) polymerase 1 |
| EGFR | Epidermal growth factor receptor |
| MAPK | Mitogen-Activated Protein Kinase |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| ERK | Extracellular Signal-Regulated Kinase |
| ANOVA | One-way analysis of variance |
| FBS | Fetal bovine serum |
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Ashburn, T.T.; Thor, K.B. Drug Repositioning: Identifying and Developing New Uses for Existing Drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Zhuang, Y.; Keith, W.K. Cell Cycle Arrest in Metformin Treated Breast Cancer Cells Involves Activation of AMPK, Downregulation of Cyclin D1, and Requires P27Kip1 or P21Cip1. J. Mol. Signal 2008, 3, 18. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Liu, Z.; Yang, K.; Lin, H.; Xiong, K. Non-Coding RNAs as Potential Targets in Metformin Therapy for Cancer. Cancer Cell Int. 2024, 24, 333. [Google Scholar] [CrossRef]
- Lu, W.; Lin, C.; Roberts, M.J.; Waud, W.R.; Piazza, G.A.; Li, Y. Niclosamide Suppresses Cancer Cell Growth by Inducing Wnt Co-Receptor LRP6 Degradation and Inhibiting the Wnt/β-Catenin Pathway. PLoS ONE 2011, 6, e29290. [Google Scholar] [CrossRef]
- Jiang, H.; Li, A.M.; Ye, J. The Magic Bullet: Niclosamide. Front. Oncol. 2022, 12, 1004978. [Google Scholar] [CrossRef]
- Gucalp, A.; Tolaney, S.; Isakoff, S.J.; Ingle, J.N.; Liu, M.C.; Carey, L.A.; Blackwell, K.; Rugo, H.; Nabell, L.; Forero, A.; et al. Phase II Trial of Bicalutamide in Patients with Androgen Receptor-Positive, Estrogen Receptor-Negative Metastatic Breast Cancer. Clin. Cancer Res. 2013, 19, 5505–5512. [Google Scholar] [CrossRef] [PubMed]
- Rhanine, Y.; Bonnefoi, H.; Goncalves, A.; Debled, M.; Le Moulec, S.; Bonichon, N.; Macgrogan, G.; Arnedos, M.; Dubroca-Dehez, B.; Grellety, T. Efficacy of Antiandrogens in Androgen Receptor-Positive Triple-Negative Metastatic Breast Cancer: Real-Life Data. Breast 2024, 73, 103667. [Google Scholar] [CrossRef]
- Canonica, G.W.; Blaiss, M. Antihistaminic, Anti-Inflammatory, and Antiallergic Properties of the Nonsedating Second-Generation Antihistamine Desloratadine: A Review of the Evidence. World Allergy Organ. J. 2011, 4, 47–53. [Google Scholar] [CrossRef]
- Murdoch, D.; Goa, K.L.; Keam, S.J. Desloratadine: An Update of Its Efficacy in the Management of Allergic Disorders. Drugs 2003, 63, 2051–2077. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.L.; Cho, J. Pathophysiological Roles of Histamine Receptors in Cancer Progression: Implications and Perspectives as Potential Molecular Targets. Biomolecules 2021, 11, 1232. [Google Scholar] [CrossRef]
- Bakker, R.A.; Schoonus, S.B.; Smit, M.J.; Timmerman, H.; Histamine, R.L. H(1)-Receptor Activation of Nuclear Factor-Kappa B: Roles for G Beta Gamma- and G Alpha(q/11)-Subunits in Constitutive and Agonist-Mediated Signaling. Mol. Pharmacol. 2001, 60, 1133–1142. [Google Scholar] [CrossRef]
- Fernández-Nogueira, P.; Noguera-Castells, A.; Fuster, G.; Recalde-Percaz, L.; Moragas, N.; López-Plana, A.; Enreig, E.; Jauregui, P.; Carbó, N.; Almendro, V.; et al. Histamine Receptor 1 Inhibition Enhances Antitumor Therapeutic Responses through Extracellular Signal-Regulated Kinase (ERK) Activation in Breast Cancer. Cancer Lett. 2018, 424, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Medina, V.; Croci, M.; Crescenti, E.; Mohamad, N.; Sanchez-Jiménez, F.; Massari, N.; Nuñez, M.; Cricco, G.; Martin, G.; Bergoc, R.; et al. The Role of Histamine in Human Mammary Carcinogenesis: H3 and H4 Receptors as Potential Therapeutic Targets for Breast Cancer Treatment. Cancer Biol. Ther. 2008, 7, 28–35. [Google Scholar] [CrossRef]
- Lamas, D.J.M.; Rivera, E.S.; Medina, V.A. Histamine H4 Receptor: Insights into a Potential Therapeutic Target in Breast Cancer. Front. Biosci.—Sch. 2015, 7, 1–9. [Google Scholar] [CrossRef]
- Medina, V.A.; Brenzoni, P.G.; Lamas, D.J.M.; Massari, N.; Mondillo, C.; Nunez, M.A.; Pignataro, O.; Rivera, E.S. Role of Histamine H4 Receptor in Breast Cancer Cell Proliferation. Front. Biosci. 2011, 3, 1042–1060. [Google Scholar] [CrossRef]
- Wu, R.L.; Anthes, J.C.; Kreutner, W.; Harris, A.G.; West, R.E., Jr. Desloratadine Inhibits Constitutive and Histamine-Stimulated Nuclear Factor-ΚB Activity Consistent with Inverse Agonism at the Histamine H1 Receptor. Int. Arch. Allergy Immunol. 2004, 135, 313–318. [Google Scholar] [CrossRef]
- Agrawal, D.K. Pharmacology and Clinical Efficacy of Desloratadine as an Anti-Allergic and Anti-Inflammatory Drug. Expert Opin. Investig. Drugs 2001, 10, 547–560. [Google Scholar] [CrossRef]
- Gupta, S.; Banfield, C.; Affrime, M.; Marco, A.; Cayen, M.; Herron, J.; Padhi, D. Desloratadine Demonstrates Dose Proportionality in Healthy Adults After Single Doses. Clin. Pharmacokinet. 2002, 41, 1–6. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Z.; Tavana, O.; Gu, W. Understanding the Complexity of P53 in a New Era of Tumor Suppression. Cancer Cell 2024, 42, 946–967. [Google Scholar] [CrossRef]
- Berger, C.E.; Qian, Y.; Liu, G.; Chen, H.; Chen, X. P53, a Target of Estrogen Receptor (ER) α, Modulates DNA Damage-Induced Growth Suppression in ER-Positive Breast Cancer Cells. J. Biol. Chem. 2012, 287, 30117–30127. [Google Scholar] [CrossRef] [PubMed]
- Sebaugh, J.L. Guidelines for Accurate EC50/IC50 Estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Al Khzem, A.H.; Gomaa, M.S.; Alturki, M.S.; Tawfeeq, N.; Sarafroz, M.; Alonaizi, S.M.; Al Faran, A.; Alrumaihi, L.A.; Alansari, F.A.; Alghamdi, A.A. Drug Repurposing for Cancer Treatment: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 12441. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, T.; Sarkar, S.; Ghosh, S.S. Harnessing Drug Repurposing to Combat Breast Cancer by Targeting Altered Metabolism and Epithelial-to-Mesenchymal Transition Pathways. ACS Pharmacol. Transl. Sci. 2024, 7, 3780–3794. [Google Scholar] [CrossRef]
- Verbaanderd, C.; Maes, H.; Schaaf, M.B.; Sukhatme, V.P.; Pantziarka, P.; Sukhatme, V.; Agostinis, P.; Bouche, G. Repurposing Drugs in Oncology (ReDO)—Chloroquine and Hydroxychloroquine as Anti-Cancer Agents. Ecancermedicalscience 2017, 11, 781. [Google Scholar] [CrossRef]
- Guo, D.; Liu, Z.; Zhou, J.; Ke, C.; Li, D. Significance of Programmed Cell Death Pathways in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 9947. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting Apoptosis in Cancer Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Kanneganti, T.D. Caspase-7: A Protease Involved in Apoptosis and Inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 21–24. [Google Scholar] [CrossRef]
- Desroches, A.; Denault, J.B. Caspase-7 Uses RNA to Enhance Proteolysis of Poly(ADP-Ribose) Polymerase 1 and Other RNA-Binding Proteins. Proc. Natl. Acad. Sci. USA 2019, 116, 21521–21528. [Google Scholar] [CrossRef]
- Kabir, S.R.; Islam, F.; Asaduzzaman, A.K.M. Biogenic Silver/Silver Chloride Nanoparticles Inhibit Human Cancer Cells Proliferation in Vitro and Ehrlich Ascites Carcinoma Cells Growth in Vivo. Sci. Rep. 2022, 12, 8909. [Google Scholar] [CrossRef]
- Nogueira, V.; Hay, N. Molecular Pathways: Reactive Oxygen Species Homeostasis in Cancer Cells and Implications for Cancer Therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.R.; Islam, J.; Ahamed, M.S.; Alam, M.T. Asparagus Racemosus and Geodorum Densiflorum Lectins Induce Apoptosis in Cancer Cells by Altering Proteins and Genes Expression. Int. J. Biol. Macromol. 2021, 191, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, K.; Pathak, C. Cellular Dynamics of Fas-Associated Death Domain in the Regulation of Cancer and Inflammation. Int. J. Mol. Sci. 2024, 25, 3228. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, Z.; Liu, K. Unraveling the Complexity of STAT3 in Cancer: Molecular Understanding and Drug Discovery. J. Exp. Clin. Cancer Res. 2024, 43, 23. [Google Scholar] [CrossRef]
- Gilmore, T.D. NF-ΚB and Human Cancer: What Have We Learned over the Past 35 Years? Biomedicines 2021, 9, 889. [Google Scholar] [CrossRef]
- Vidicevic-Novakovic, S.; Stanojevic, Z.; Tomonjic, N.; Karapandza, K.; Zekovic, J.; Martinovic, T.; Grujicic, D.; Ilic, R.; Raicevic, S.; Tasic, J.; et al. Proapoptotic and Proautophagy Effect of H1-Receptor Antagonist Desloratadine in Human Glioblastoma Cell Lines. Med. Oncol. 2023, 40, 241. [Google Scholar] [CrossRef]
- Jung, J.H.; Lee, H.; Kim, J.H.; Sim, D.Y.; Ahn, H.; Kim, B.; Chang, S.; Kim, S.H. P53-Dependent Apoptotic Effect of Puromycin via Binding of Ribosomal Protein L5 and L11 to Mdm2 and Its Combination Effect with Rita or Doxorubicin. Cancers 2019, 11, 582. [Google Scholar] [CrossRef] [PubMed]

| 18s rRNA | F | GTAACCCGTTGAACCCCATT |
| R | CCATCCAATCGGTAGTAGCG | |
| BAX | F | CATATAACCCCGTCAACGCAG |
| R | GCAGCCGCCACAAACATAC | |
| PARP-1 | F | GGCCTCGGTGGATGGAATG |
| R | GCAAACTAACCCGGATAGTCTCT | |
| EGFR | F | AGGCACGAGTAACAAGCTCAC |
| R | ATGAGGACATAACCAGCCACC | |
| MAPK4 | F | CGGTGTCAATGGTTTGGTGC |
| R | GACGATGTTGTCGTGGTCCA | |
| TP53 | F | GCCCAACAACACCAGCTCCT |
| R | CCTGGGCATCCTTGAGTTCC | |
| STAT3 | F | CAGCAGCTTGACACACGGTA |
| R | AAACACCAAAGTGGCATGTGA | |
| FAS | F | AGCTTGGTCTAGAGTGAAAA |
| R | GAGGCAGAATCATGAGATAT | |
| BCL2 | F | AGTTATCGGCTTCAGTGGTCT |
| R | CTGCCCGCTTCCTAGCTTG | |
| NF-κB | F | GAATTGCTGCTTCGGAATGGA |
| R | CATGCGGGCATCTACCTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabir, S.R.; Abdullah, T.; Azam, G.; Molla, T.H.; Ali, H.; Miah, M.; Alam, M.T.; Miah, S. Desloratadine Induces TP53-Dependent Apoptosis in MCF-7 Breast Cancer Cells. Cells 2025, 14, 1725. https://doi.org/10.3390/cells14211725
Kabir SR, Abdullah T, Azam G, Molla TH, Ali H, Miah M, Alam MT, Miah S. Desloratadine Induces TP53-Dependent Apoptosis in MCF-7 Breast Cancer Cells. Cells. 2025; 14(21):1725. https://doi.org/10.3390/cells14211725
Chicago/Turabian StyleKabir, Syed Rashel, Taufique Abdullah, Gausul Azam, Tamzid Hossain Molla, Hasan Ali, Mojnu Miah, Mohammad Taufiq Alam, and Sayem Miah. 2025. "Desloratadine Induces TP53-Dependent Apoptosis in MCF-7 Breast Cancer Cells" Cells 14, no. 21: 1725. https://doi.org/10.3390/cells14211725
APA StyleKabir, S. R., Abdullah, T., Azam, G., Molla, T. H., Ali, H., Miah, M., Alam, M. T., & Miah, S. (2025). Desloratadine Induces TP53-Dependent Apoptosis in MCF-7 Breast Cancer Cells. Cells, 14(21), 1725. https://doi.org/10.3390/cells14211725








