1. Introduction
Diabetes mellitus (DM) is a chronic complicated metabolic disorder characterized by hyperglycemia, which often results from defects in insulin secretion, insulin action, or both. According to the International Diabetes Federation, approximately 589 million adults were living with type 1 DM (T1DM) and type 2 (T2DM) in 2024; it estimates that by 2050, this number will increase to 853 million [
1]. More than 1.8 million children and adolescents are living with T1DM worldwide, and there is a continuous increase in the number of young patients with T1DM and T2DM [
1]. The increased incidence of T1DM has been associated with falling birth rates and compromised fertility [
2].
A large number of studies, both in diabetic men and animal models, indicate that DM causes male infertility via action at multiple levels including altered spermatogenesis, degenerative and apoptotic changes in the testes, altered glucose metabolism in Sertoli cells/blood testes barrier, reduced testosterone synthesis and secretion, ejaculatory dysfunction, and reduced libido [
3]. In men with chronic diabetes and obesity, late-onset hypogonadism syndrome is more popular. The real obstacles to conducting studies on human male are ethical reasons (e.g., nearly no testicular biopsies were available, therefore no systematic histopathology or molecular data from the human testis of patients with DM are available) [
4]. Therefore, the use of animal models has been crucial to provide more detailed molecular and cellular insights into the effect of hyperglycemia on the male reproductive system and fertility.
Many animal studies have employed streptozotocin (STZ)-induced diabetes, as STZ is the most prominent diabetogenic chemical that is widely used in experimental animals for creating animal models of type 1 and type 2 diabetes [
5,
6]. STZ is a naturally occurring compound, produced by the soil bacterium
Streptomyces achromogenes, that easily enters the pancreatic beta cells through glucose transporter-2, causing alkylation/damage of the DNA and subsequent cell death resulting in rapid hyperglycemia. STZ has been proven to be a better diabetogenic agent than alloxan, with a wider species effectiveness and greater reproducibility [
7]. Moreover, the STZ model mimics many of the acute and chronic complications of human diabetes and provides the established similarities of some of the structural, functional, and biochemical abnormalities to human disease. Hence, it is an appropriate model to assess the mechanisms of diabetes.
The mammalian testis is a complex multicellular organ, separated into two distinct compartments that carry out its principle functions. In the adult testis, spermatogenesis and sperm production occur within the seminiferous tubules, and androgen biosynthesis (steroidogenesis) occurs in Leydig cells located in the interstitium.
Two major events in testis development that occur during sexual maturation are the establishment of spermatogenesis and the development of the adult Leydig cell population responsible for androgen production. Spermatogenesis in mammals is a dynamic and highly regulated process that encompasses numerous proliferating and differentiating steps from spermatogonia to spermatozoa, resulting in the production of male gametes [
8].
Androgens are especially important for male sexual differentiation in fetal life, pubertal sexual maturation, and the maintenance of spermatogenesis in adulthood. The effects of androgens are mediated through the androgen receptor (AR), which binds testosterone (T) with high affinity. In the testis, AR is localized in peritubular cells, Leydig cells (LCs), and Sertoli cells (SCs) but not in germ cells (GCs). Preferential action of androgens at stages VII–VIII of the spermatogenic cycle of rats coincided with the maximal expression of AR protein in Sertoli cells, suggesting that androgen support for spermatogenesis is primarily mediated through Sertoli cells [
9]—a conclusion that is supported by knockout models for the selective ablation of AR in Sertoli cells (SCARKO mice) [
10].
Sertoli cells are involved in the regulation of spermatogenesis, providing nutritional support for germ cells. Glucose metabolism in Sertoli cells produces lactate for germ cells, which is crucial for spermatogenesis, and in particular, it is consumed by pachytene spermatocytes and round spermatids [
11]. Germ cells are highly reliant on carbohydrate metabolism as they need energy for their differentiation. However, metabolic stressors, such as diabetes, impair glucose transport and lactate production, compromising energy supply. Chronic hyperglycemia has been shown to increase glucose uptake and reduce lactate production by Sertoli cells, associated with decreased levels of testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) [
12].
Recent reviews have summarized a lot of data on the metabolic and signal pathways of DM action on male reproductive function. The main mechanism of DM is the induction of oxidative stress and inflammation, and in many papers, the role of molecules involved in these pathophysiological processes has been discussed in relation to semen pathology/sperm characteristics and male infertility and profiles of reproductive hormones [
12,
13,
14]. Less data have been published about the effect of DM on the cellular compositions of the testis and their functions. The investigations mainly used experimental models for T1DM induced in adulthood. A limited number of studies utilized neonatally induced DM as a model for T2DM, but the material taken for investigation was from adult animals. Two papers have been published about the effect of DM induced in neonatal or pubertal age (day 15) on developing testis, suggesting that more pronounced alterations occur in early-life induced DM compared with DM induced in adulthood [
15,
16].
The early postnatal period is crucial for the establishment of spermatogenesis—the resuming of the mitotic division of precursors germ cells on day 4.5 (pre-spermatogonia) gives rise to differentiated spermatogonia followed by the start of meiosis on day 12 in rats [
17]. In this respect, our interest was focused on the developmental effect of early DM induced neonatally on day 1 (NDM as it is applied to induce T2DM in adulthood), or prepubertally on day 10 (PDM). Hence, the aim of the present study was to follow the postnatal development of testicular germ and somatic cells (Leydig cells and Sertoli cells) under the condition of experimentally-induced NDM or PDM in relation to androgen production and action. The current work presents a comparative evaluation of the impact of two types of DM, providing new knowledge on the differential effects of early postnatal DM on developing testicular cell populations and the first wave of spermatogenesis. Identification of potential different changes in germ and somatic cells in PDM and NDM rats could contribute to understanding the different response of the testis to hyperglycemia depending on the time of its induction in early life.
2. Materials and Methods
2.1. Animal Model
The experimental protocol was performed in the Institute of Experimental Morphology, Pathology and Anthropology with Museum, Bulgarian Academy of Sciences according to the ARRIVE guidelines and EU Directive for animal experiments. The study was approved by the Bulgarian Agency for Food Safety, Approval number 282 from 24 September 2020.
Pregnant Wistar rats were purchased from the Experimental and Breeding Base for Laboratory Animals (EBBLA) (Slivnitza, Bulgaria). Mothers were separated into individual standard hard-bottom polypropylene cages, fed a standard diet, and had access to food and water ad libitum. After birth, the pups were i.p. injected with streptozotocin (STZ, Sigma-Aldrich, S0130, St. Louis, MO, USA) at a single dose of 100 mg/kg b.w. (dissolved in ice cold 0.1 M citrate buffer, pH 4.5) on day 1 to induce neonatal diabetes mellitus (NDM) or on day 10 to induce prepubertal diabetes mellitus (PDM) [
6,
15]. Control age-matched animals were injected with citrate buffer. Two days after STZ injection, blood glucose was measured using a glucometer (Accu-chek Performa, Roshe, Basel, Switzerland). Rats were considered diabetics when their blood glucose levels exceeded 12 mmol/L [
15].
At weaning (day 25), the male pups were separated from their mothers and left until the end of the experiment on day 45 or day 65 (for glucose and semen sampling). The experimental animals were divided into three groups (control, NDM and PDM) for the ages investigated. The rats were sacrificed (Small Animal Decapitator, Stoelting™ 51330, Wood Dale, IL, USA) under light anesthesia on postnatal days 25 (puberty) and 45 (post puberty/early adulthood). Blood was obtained after decapitation, and serum was stored at −80 °C for subsequent analysis. Both testes were excised, and the left testis was fixed in Bouin solution, dehydrated, and embedded in paraffin for further histological studies.
Sexually mature 65-day-old rats (control, NDM, and PDM groups) were used for the analysis of sperm concentration and sperm motility.
2.2. Measurement of Body and Testis Weight
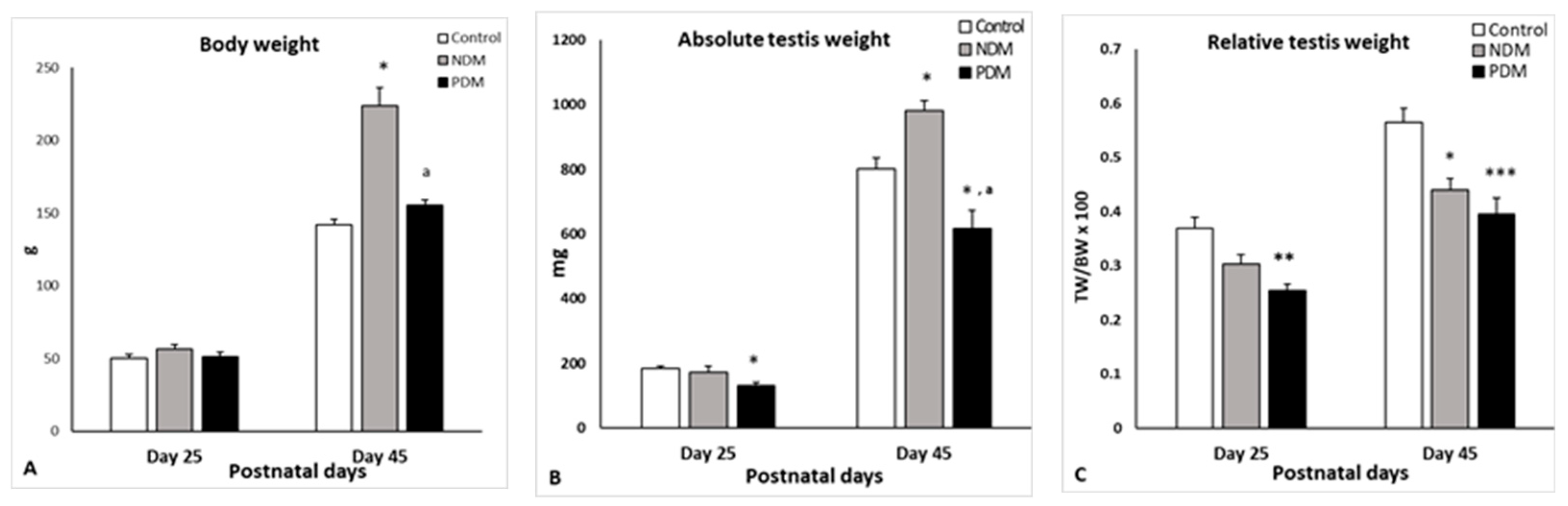
The body weight of the animals aged 25 and 45 days was measured before sacrifice (n = 10 in each experimental group). Testes of the control and experimental rats were excised, weighed, and the relative testis weight (gonado-somatic index) was calculated as a ratio of the average weight of both testes to body weight, multiplied by 100.
2.3. Measurement of Serum Glucose
Glucose levels (non-fasting on day 25 and fasting on days 45 and 65) were evaluated in the sera of control and diabetic animals (n = 10 in each experimental group) using commercial kits (Chema Diagnostica, Monsano, Italy) on a BA-88 biochemical analyzer (Mindray, Shenzhen, China).
2.4. Measurement of Serum Testosterone, Luteinizing Hormone, and Insulin
Serum levels of testosterone, luteinizing hormone (LH), and insulin (n = 10 in each experimental group) were evaluated by enzyme-linked immunosorbent assay (ELISA) according to the instructions of the kit manufacturer (Elabscience Biotechnology Co., Ltd., Wuhan, China). Competitive ELISA for testosterone (Cat. No. E-EL-0155) and sandwich-ELISA for luteinizing hormone (Cat. No. E-EL-R0026) and insulin (Cat. No. E-EL-R3034) were performed. The optical density was read at 450 nm on an ELISA Reader BioTek (BioTek Instruments, Winooski, VT, USA). Curve Expert Professional 2.7 software was used. The obtained values were multiplied by the dilution factor (×6 for LH and ×25 for insulin) to calculate the final concentration of the hormones. The concentration of testosterone and insulin is presented in ng/mL and for the luteinizing hormone in mlU/mL.
2.5. Stereological Analyses
For morphometric/stereological analyses of germ and Sertoli cells on days 25 and 45, Bouin fixed, 5 µm paraffin embedded tissue sections were stained with hematoxylin and eosin (H&E) (n = 6 in each experimental group). For Leydig cell enumeration, tissue sections were immunostained for 3β-hydroxysteroid dehydrogenase (3β-HSD), which is specific marker for steroid-producing cells. Testicular cell composition was estimated using standard stereological techniques involving the point counting of cell nuclei to determine the nuclear volume per testis, namely Sertoli cells, Leydig cells, and different maturational stages of germ cells, as previously described [
18]. In brief, cross-sections of the testes were examined using a 63× objective and a 121-point eyepiece graticule (Leica Microsystems, Wetzlar, Germany) fitted to a Zeiss AxioScope A1 microscope (Zeiss, Oberkochen, Germany). Applying a systematic sampling pattern from a random starting point, 32 microscopic fields (3872 points) were counted for each animal. Points falling over Leydig cells, Sertoli cells, or germ cell nuclei (including spermatogonia, spermatocytes, round and elongated spermatids), seminiferous epithelium, interstitium, and seminiferous tubule lumen were scored and expressed as relative (%) volume per testis. Values for percent nuclear volume were converted to absolute nuclear volume (ANV) per testis by reference to testis volume (=weight) because shrinkage was minimal. Cell nuclear volume can be equated to numbers of cells per testis, assuming no change in nuclear diameter of the target cell in the different experimental groups [
10,
18].
2.6. Immunohistochemistry (IHC) for Androgen Receptor (AR), 3β-Hydroxysteroid Dehydrogenase (3β-HSD), and Testicular Angiotensin Converting Enzyme (tACE)
Unless otherwise stated, all incubations were performed at room temperature. The deparaffinized and rehydrated 5 μm sections of the control and experimental testes from animals aged 25 and 45 days (n = 6 in each experimental group) were subjected to a temperature-induced antigen retrieval step in 0.01 M citrate buffer, pH 6.0—tACE, AR, and 3β-HSD. Endogenous peroxidase activity was blocked by immersing all sections in 3% (v/v) H2O2 in methanol for 30 min, followed by two 5-min washes in Tris-buffered saline (TBS). To block nonspecific binding sites, sections were incubated for 30 min with 10% blocking serum in TBS containing 5% bovine serum albumin (BSA): for AR, it was normal swine serum; for tACE and 3β-HSD—normal rabbit serum. After that, primary antibodies were added to the sections at appropriate dilutions in blocking serum and incubated overnight at 4 °C in a humidified chamber. Antibodies were applied as follows: rabbit polyclonal anti-androgen receptor (N-20; sc-816 Santa Cruz Biotechnology, Inc., Dallas, TX, USA), diluted 1:200; goat polyclonal anti-tACE (sc-12187, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), diluted 1:500; goat polyclonal anti-3β-HSD (P-18; sc-30820, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), diluted 1:500. After two 5-min washes in TBS, sections were incubated with biotinylated secondary antibodies as follows: for AR—swine anti-rabbit (dilution 1:500, DAKO Cytomation, Glostrup, Denmark); for tACE and 3β-HSD—rabbit anti-goat (Vector BA-5000) for 30 min. After two additional 5-min washes in TBS, sections were incubated for 30 min with avidin-biotin conjugated to horseradish peroxidase (ABC Reagent, Vector Laboratories Inc., Newark, CA, USA). Sections were washed twice in TBS, and immunostaining was developed using 3,3′-diaminobenzidine (liquid DAB; DAKO Corp., Glostrup, Denmark) The sections were counterstained with hematoxylin, dehydrated before mounting, and observed under a light microscope Zeiss AxioScope A1 (Carl Zeiss, Oberkochen, Germany).
Negative controls were run in parallel by omitting the primary antibody under the same conditions. For AR, the negative control was also conducted by pre-absorption of the primary antibody with peptide immunogen sc-816P.
2.7. Assessment of Spermatogenesis by Seminiferous Tubules with tACE Phenotype on Day 45
Completion of the cycle of the seminiferous epithelium was performed by the evaluation of the number of seminiferous tubules (STs) with normal tACE vs. altered phenotype at the early (I–IV), middle (VII–VIII), or late stages (IX–XIV) of the spermatogenic cycle (n = 6 animals in each group). For this purpose, the method for Johnson’s score [
19] was applied, and 100 ST were examined across different fields (covering the whole section) using Zeiss AxioStar Plus Microscope (Carl Zeiss, Oberkochen, Germany) at a magnification of ×200 (20× objective). Data are expressed as a percentage of normal or altered ST per all tubules from each stage group.
2.8. Quantification of Androgen Receptor Immunostaining Intensity
For quantification of the protein expression (intensity of AR immunostaining) in 25- and 45-day-old rats (n = 6 in each experimental group), we applied a systematic sampling pattern from a random starting point. Each second microscope field of testicular cross section (following vertical direction from the top to the bottom of the tissue cross section) was used to capture the microscope image at a magnification of ×400 (40× objective). Images were obtained through a Zeiss AxioScope A1 light microscope (Carl Zeiss, Oberkochen, Germany) equipped with an AxioCam ERC 5s-Zeiss digital camera (Carl Zeiss, Oberkochen, Germany).
ImageJ
For image analysis, RGB images with a resolution 2048 × 1536 pixels saved as .jpg were used. We processed the images using the open source software ImageJ 1.54p,
https://imagej.net/ij/ (accessed on 28 October 2025), and its version with multiple plugins—Fiji. The intensity of AR immunostaining was evaluated after color deconvolution using the IHC profile plugin with the selection of Vector “H DAB” and selection of Color_2. AR is localized in the nuclei of Sertoli, perutubular, and Leydig cells in the testis. Our regions of interest (ROIs) were the nuclei of Sertoli cells, which were manually outlined and added to ROI Manager, and the staining intensity was measured as the “mean gray value” parameter. In ImageJ, the pixel intensity values for any color range from 0 to 255, where 0 represents the darkest shade and 255 represents the lightest shade of the color [
20]. Based on this, the staining intensities were divided into three groups: strong (between 1 and 85), moderate to weak (between 86 and 170), and faint to negative (between 171 and 255). We presented the values as reciprocal staining intensity (RSI), where RSI = 255—mean gray value. Therefore, the AR staining intensity was defined as strong at RSI values between 171 and 255; moderate to weak between 169 and 86, and faint to negative when the RSI values were between 85 and 0.
For 25-day-old animals, STs were scored until 100 Sertoli cell nuclei per animal were measured.
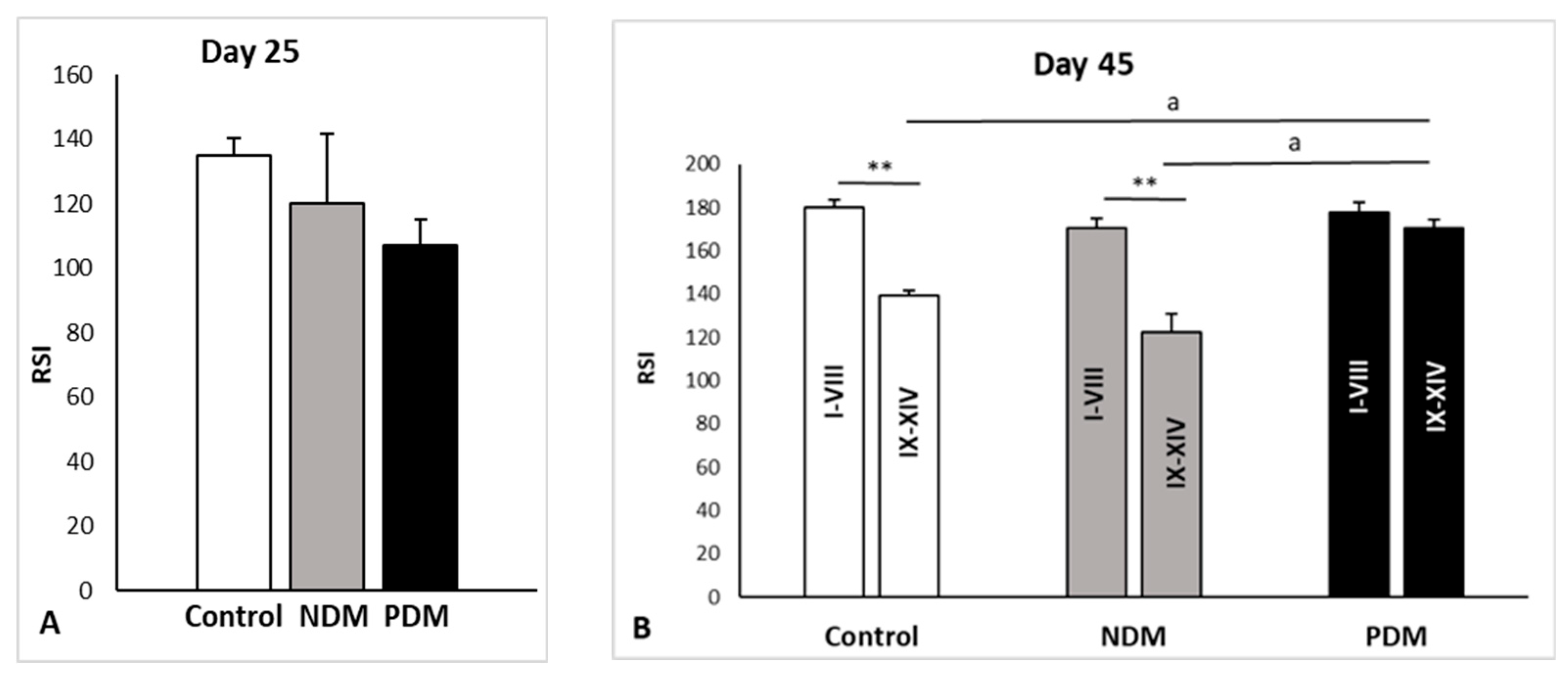
For each animal aged 45 days, STs from each of three stage groups (I–VI; VII–VIII; IX–XIV) were scored until 100 Sertoli cell nuclei per stage group were measured. In the control group, a comparison in intensity of AR immunostaining was conducted between stages I–VI (early) and VII–VIII (middle) and IX–XIV (late stages). The same approach was applied in NDM animals where the spermatogenic cycle was completed.
Due to incomplete spermatogenic cycle in 45-day-old PDM rats, where a partial or complete lack of elongating spermatids in stages I–VIII was established, it was not possible to distinguish the early (I–VI) from the middle (VII–VIII) stages. For this reason, in the PDM animals, a comparison in intensity of AR immunostaining was performed between stages I–VIII and the late stages (IX–XIV). Differences between experimental groups were evaluated by a comparison of the intensity of AR immunostaining between stages I–VIII and the late stages (IX–XIV).
2.9. Sperm Concentration and Sperm Motility
The sperm concentration and motility were evaluated by computer-assisted sperm analysis (SCA®, Sperm Class Analyzer, Microptic®, Barcelona, Spain). Briefly, the semen samples were collected from both cauda epididymides of 65-day-old control and diabetic rats (NDM and PDM) (n = 10 in each experimental group) and placed in pre-warmed (37 °C) HEPES solution (pH 7.4). After incubation at 37 °C for 20 min, 5 μL of each sample was transferred to a Leja 20 chamber (Leja Products B.V., Nieuw-Vennep, The Netherlands) and analyzed using a microscope (Nikon Eclipse E200, Nikon, Tokyo, Japan) with a 10× objective (Nikon 10×/0.25 Ph1 BM, Nikon) under negative phase contrast and a camera with high resolution (768 × 576 pixels). All samples were evaluated twice to determine the sperm concentration and motility (percentage of sperm moving faster than 10 µm/s).
2.10. Statistical Analysis
The obtained data were presented as the mean value ± standard error (SE) and 95% confidence interval for means. The Shapiro–Wilks test was used to test for the normality distribution of data in the animal experimental groups. The mean values were compared with one-way ANOVA including test of homogeneity of variances and Bonferroni or Games–Howell post hoc test, depending on whether equal variances were assumed or not assumed. The comparison of a pair of indicators in each group (two dependent samples) was used with the paired samples test (for AR expression). Statistical analysis was performed using IBM SPSS Statistics (v.25), where statistical significance was considered at p < 0.05.
4. Discussion
During the last two decades, although a large number of studies both on diabetic men and experimental diabetic animals have been published about the impact of DM on male reproduction, many of them have conflicting results. The prevailing notion is that DM alters spermatogenesis, sperm parameters, biosynthesis of testosterone and induces degenerative changes in the testis, which lead to sub-fertility or infertility. Although extensive research has been conducted on experimental models for the induction of DM in adulthood (sexually maturity), studies on the effect of hyperglycemia on immature animals are very limited.
The early postnatal period is known to be critical for the development of male germ cells, where after a prolonged resting period in fetal and neonatal life, the precursors of germ cells (pre-spermatogonia) resume mitotic division on day 4.5 in rats, giving rise to differentiated spermatogonia. The latter proceed to a series of consecutive divisions to enter the first prophase of meiotic division with the formation of primary spermatocytes occurring on day 12 in rats. Both major events are crucial for the establishment of spermatogenesis, and any interference during this time results in poor reproductive capacity and infertility [
17]. Aside from the initiative role of pituitary gonadotrophic hormones and androgen locally produced in the testis, insulin might also have a direct action on testicular cell composition. Germ cells are highly dependent on carbohydrates needed for energy homeostasis, being vulnerable to any disturbance in glucose metabolism [
12,
23].
By evaluating the impact of DM induced in early life, the current study provides new data suggesting that neonatal or prepubertally induced hyperglycemia might exert differential effects on testicular cell populations (germ and somatic cells) during the first wave of spermatogenesis, with a possible impact on semen quality.
Glucose levels, as measured on day 25, showed that the NDM rats were normoglycemic, as expected [see King, 2012 [
5] for neonatally induced T2DM], and later developed hyperglycemia. PDM animals were hyperglycemic on day 25 and then maintained their DM status until days 45 and 65 in tandem with insulin insufficiency, suggesting that PDM rats might represent the T1DM model. Our unpublished data on insulin resistance index support that NDM rats developed T2DM, exhibiting slightly decreased insulin levels and insulin resistance in contrast to PDM, which were insulin deficient and non-resistant.
In our model, 25-day-old control and experimental rats were deprived of food for 2 h, and for this reason, we considered their glucose was non-fasting as it was not according to the standard protocol for overnight fasting [
24]. According to Carper et al. [
25], 2 h of fasting is optimal to assess insulin tolerance in rodents (mice and rats), which have a faster metabolic rate than humans.
Day 25 in rats is considered as mid-puberty, and spermatogenesis proceeds to late pachytene-diplotene spermatocytes. Day 45 represents post-puberty/early adulthood as spermatogenesis is completed but without sperm ejaculation, and therefore animals are still sexually immature.
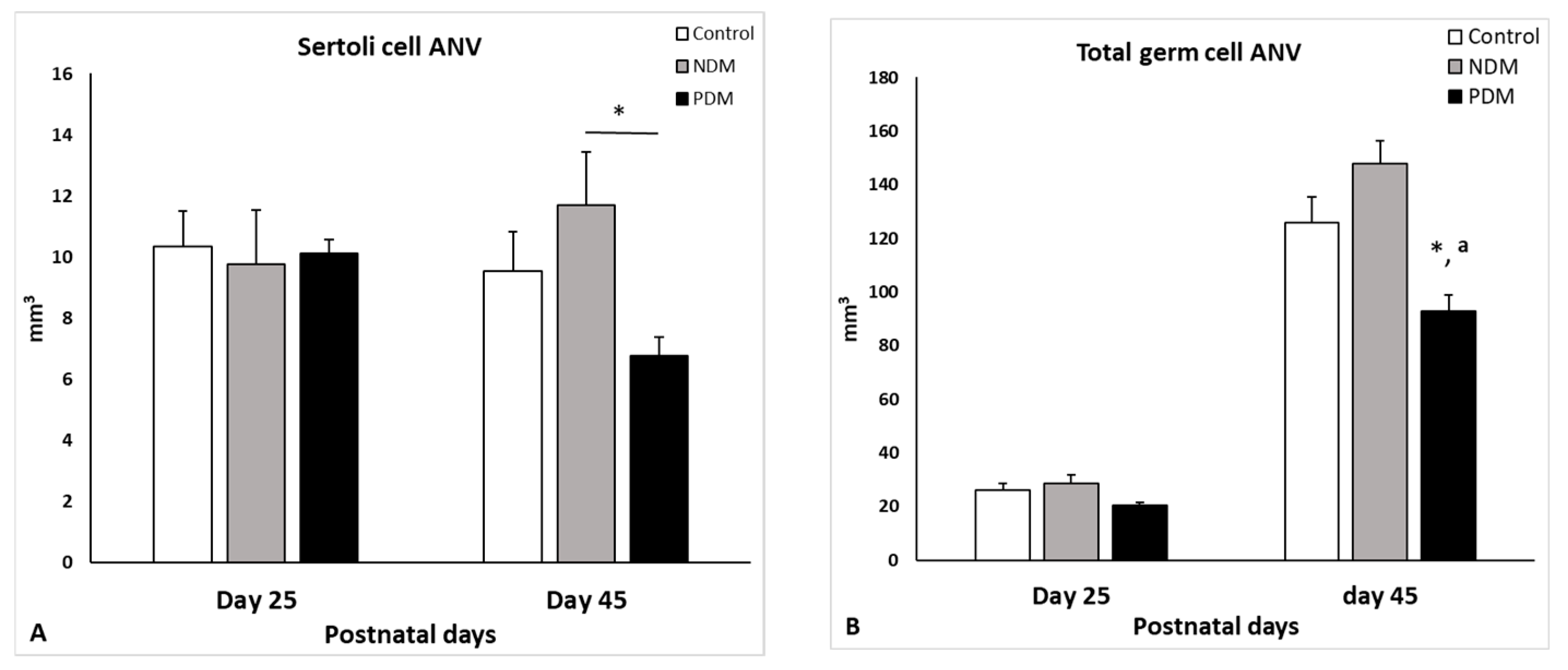
Quantification of total germ cell population on day 25 and day 45 revealed different effects of both types of DM. NDM resulted in a slight elevation of TGC-ANV at both ages by 9–18% compared to the controls while PDM caused a decrease in this parameter by app. 25% than the control. Limited data are available where the germ cell number was reduced in adult animals with T1DM or T2DM induced by different treatment protocols (high fat diet or nicotinamide plus STZ as well drinking of fructose) [
19,
26,
27,
28,
29]. In the few papers published on neonatally-induced DM, there are no data on testicular cell numbers. A comparative study by Barsiah et al. [
26] demonstrated a more pronounced reduction in germ and Sertoli cell number by T1DM than adult T2DM. Our new data for germ cell counts suggest different effects on spermatogenesis caused by both types of DM—PDM reduced TGC-ANV in contrast to NDM, which did not produce a negative effect.
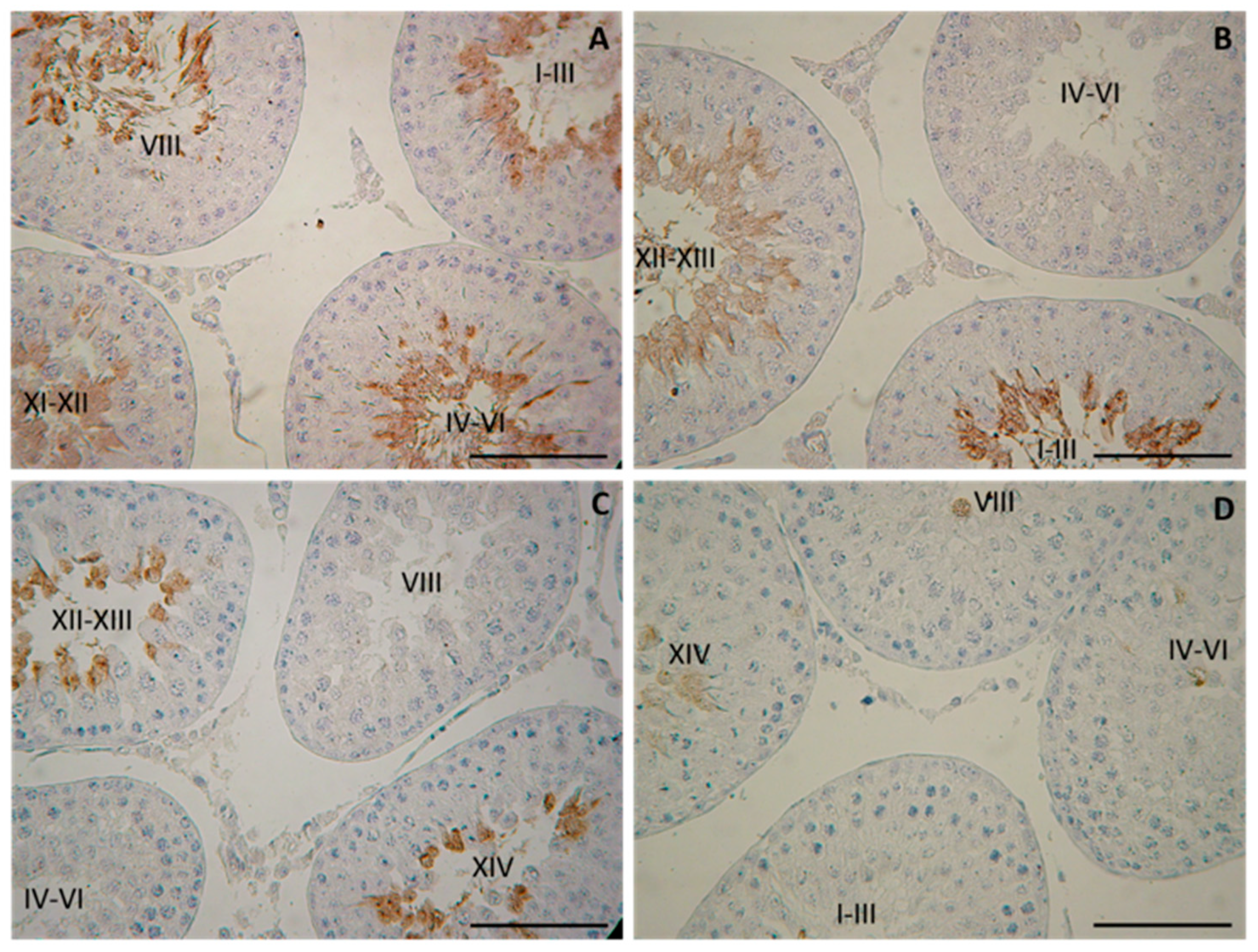
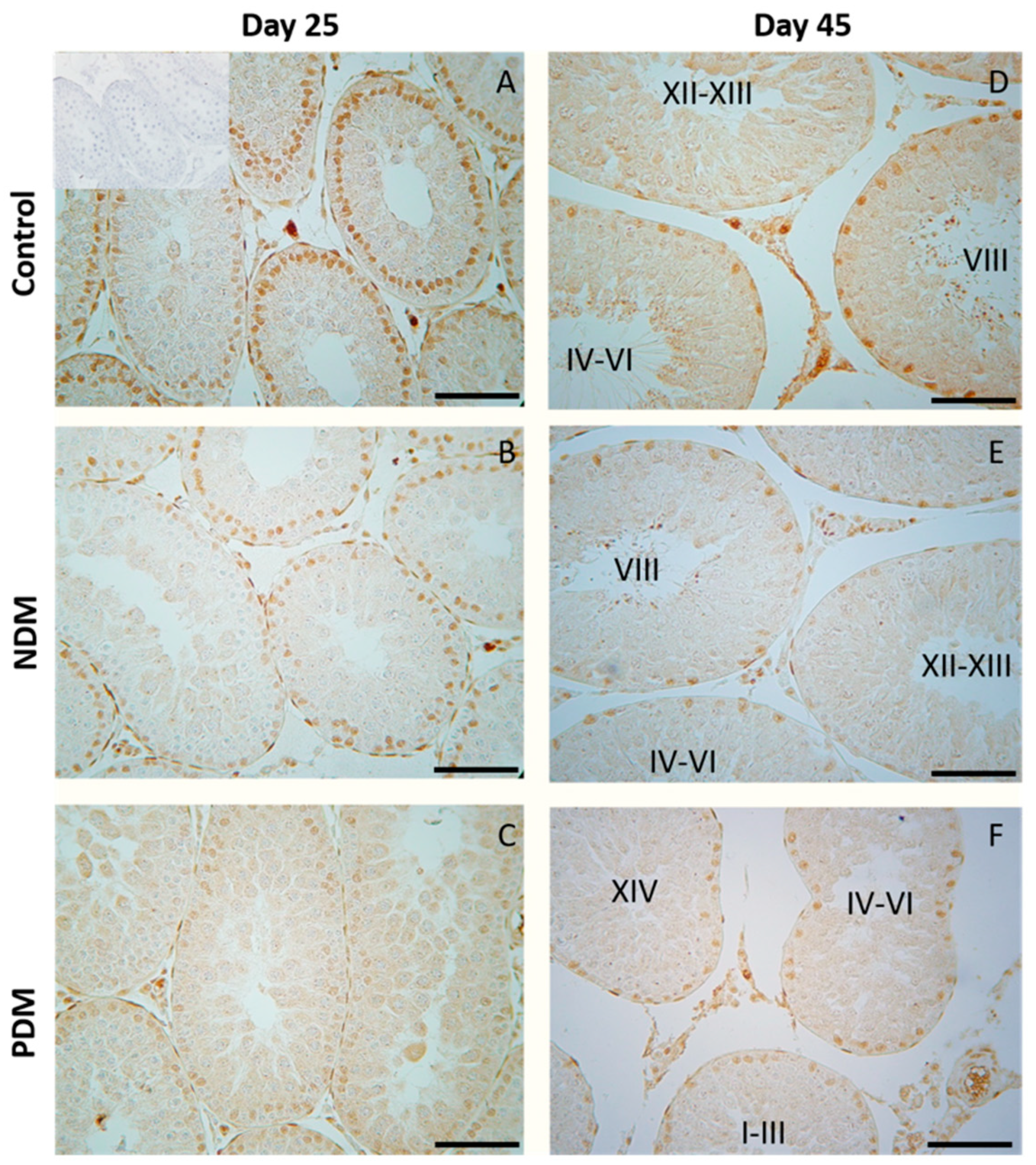
Evaluation of the proceeding of spermatogenesis in post-pubertal diabetic rats (45-day-old) demonstrated, for the first time, a delayed development of the late stages in spermatogenesis (elongated spermatids, visualized by tACE), in PDM but not in NDM rats. Quantification of the STs with altered tACE phenotype in PDM rats provided new evidence about the pattern of delayed spermatid development. The initial step of spermatid elongation, which occurs in late stages, was less altered compared to more advanced steps in early stages (I–VI). The STs in stages IV–VI were the most affected as none of them contained elongated spermatids. These data are indicative of the delayed completion of spermatogenesis due to impaired late steps of spermatid elongation. It seems that germ cells are more vulnerable to PDM than to NDM, which could be explained by different glucose profiles and different administration times of STZ. PDM was induced at the time of the active proliferation of spermatogonia before entering meiosis, while NDM was induced during the quiescent period (mitotic arrest) of precursor spermatogonia (pre-spermatogonia).
Many studies have indicated that apoptosis and oxidative stress, in tandem with inflammation, are responsible for germ cell loss in diabetic conditions (summarized in review articles) [
12,
13,
14]. Recently, we reported the increased protein expression of pro-apoptotic factor Bax, which was more pronounced in the PDM than NDM rats, and these data could provide an explanation for the decreased TGC-ANV in PDM animals [
30]. Moreover, the levels of some molecular markers for oxidative stress [3-nitrotyrosine (3-NT) and 4-hydroxynonenal (4-HNE)] were more elevated in PDM than in NDM, which was associated with an increased protein expression of the pro-inflammatory marker, tumor necrosis factor alpha (TNF-α) [
31].
To follow the consequences of impaired spermatogenesis on fertility, we evaluated the semen parameters (sperm count/concentration and sperm motility) on day 65 in both diabetic groups. The sperm count and sperm motility decreased to a similar extent in PDM and NDM. An extremely high number of round shaped abnormal undifferentiated cells were counted (11-fold increase) in NDM but not in PDM, which might be a reason for the decreased sperm parameters in NDM despite no obvious destructive changes in the testes of post-pubertal 45-day-old rats. Most papers have reported a decreased sperm concentration and motility in T1DM [
29,
32,
33,
34] and in T2DM [
27], but data from NDM in adulthood are contradictory—decreased semen parameters [
16] vs. increased sperm concentration [
35].
There is a general agreement that changes in absolute testis weight reflect those in TGC-ANV, and our results support this finding. Most of the data in the literature indicate lower absolute testis weight in adult rodents with T1DM and T2DM. Barsiah et al. [
26] reported decreased testis weight only in adult animals with T1DM but not in T2DM—a finding that fits our results regarding the different effect of PDM and NDM on the testis weight. We found higher absolute volumes of seminiferous epithelium (SE) and tubular lumen in 45-day-old NDM rats in contrast to lower values in PDM. There are discrepancies in the literature data regarding the testicular macro-parameters due to different treatment regimens. Some studies have reported a decrease in SE volume, ST, and lumen diameter in diabetic rats [
19], while other investigations found an elevation in their values [
26,
33].
Sertoli cells are essential for developing germ cells to sustain spermatogenesis by providing them with a unique microenvironment, physical support, growth factors, and appropriate nutrients including glucose. Any metabolic alteration in these cells caused by DM might be responsible for impaired spermatogenesis, resulting in compromised fertility. These cells have glucose sensing machinery that reacts to hormonal fluctuations, and several mechanisms operate to counteract hyper/hypoglycemic events [
23]. Extensive research has been conducted on the expression of molecular markers for oxidative stress and inflammation including innate immune response under the condition of hyperglycemia [
11,
36]. Less data are available on quantitative aspects of adult Sertoli cells in conditions of DM, but none are available for Sertoli cells of developing testis. Our data revealed that at pubertal age, NDM and PDM did not produce any significant changes in SC-ANV. A reduced number of SCs and GCs was reported in adult T1DM and T2DM associated with increased apoptosis [
19,
27,
29,
37]. A possible explanation for the slight elevation in SC-ANV in NDM post-pubertal rats in our study might be explained by the higher absolute testis weight. Increased Sertoli cell number was demonstrated by Tavares et al. [
38] in in vitro studies on neonatal mouse organ cultures as well on the TM4 Sertoli cell line treated by D-glucose. An important indicator for Sertoli cell support toward germ cells is the ratio of TGC-ANV–SC-ANV [
18], and in the current study, we did not find any evidence for altered Sertoli cell supportive function.
Sertoli cells have long been considered the prime candidates for the androgen regulation of spermatogenesis because of the specific expression of AR through the stages of seminiferous epithelium. The current study did not find any significant changes in AR expression in Sertoli cells in pubertal 25-day-old NDM and PDM rats. Later, at the post-pubertal stage when spermatogenesis was completed, the stage-specific pattern in Sertoli cells was not maintained in PDM. Instead, a uniform pattern of AR expression was seen as a result of increased expression in the late stages of the spermatogenic cycle. Such a phenomenon has been reported by us under other experimental conditions of hormonal manipulation such as androgen withdrawal [
22]. A possible compensatory mechanism of androgen signaling could be suggested, as it is essential for developing germ cells under the condition of compromised androgen production in PDM. According to Ballester et al. [
39], AR protein levels (measured by Western blot) were not changed in T1DM adult rats while Favaro et al. [
40] found a reduced expression of AR in prostate epithelium (reduced number of AR positive cells and protein levels in tissue homogenates). Our results on the quantification of AR protein expression provide new knowledge on altered androgen action in post-pubertal Sertoli cells by PDM but not by NDM. Such a finding might explain reduced TCG-ANV as a result of the delayed development of advanced stages of spermatogenesis (elongated spermatids).
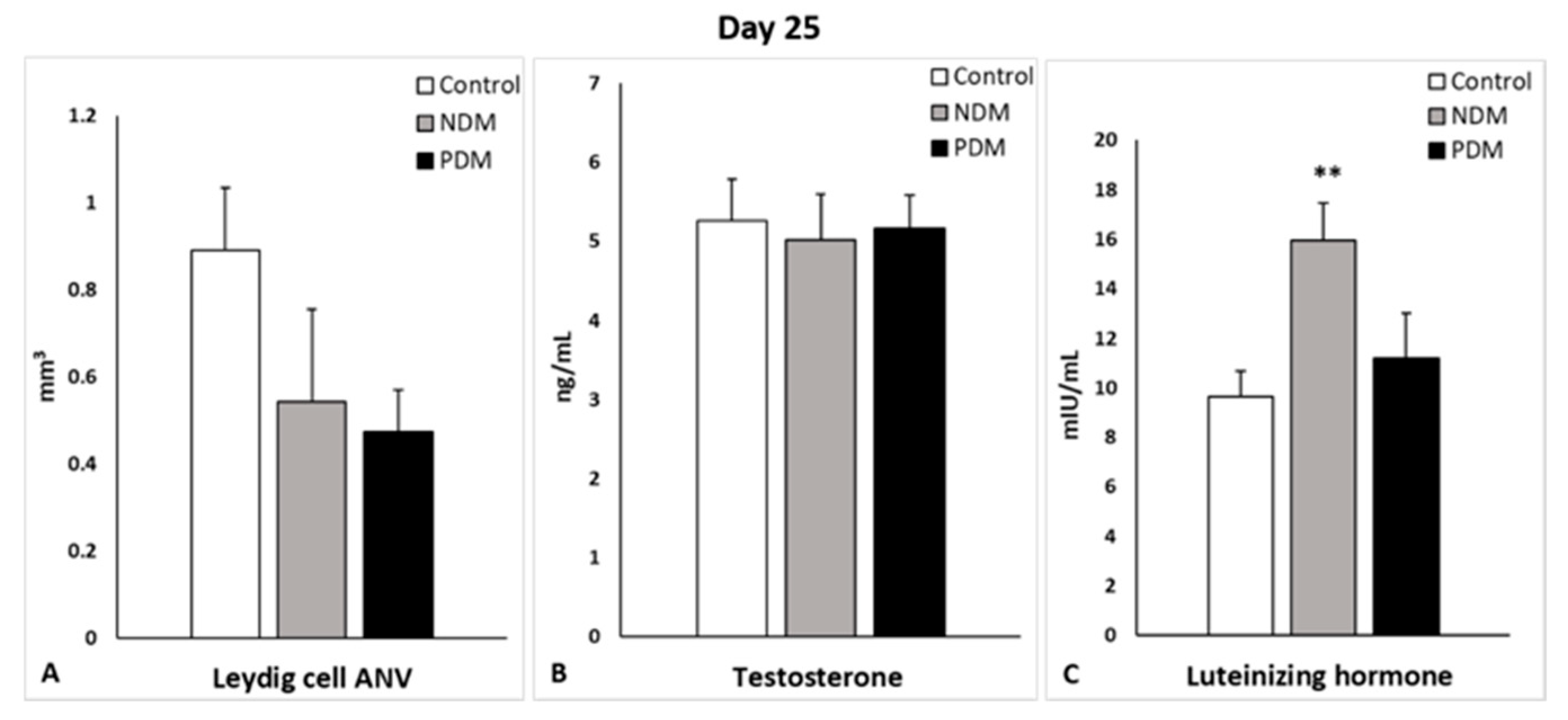
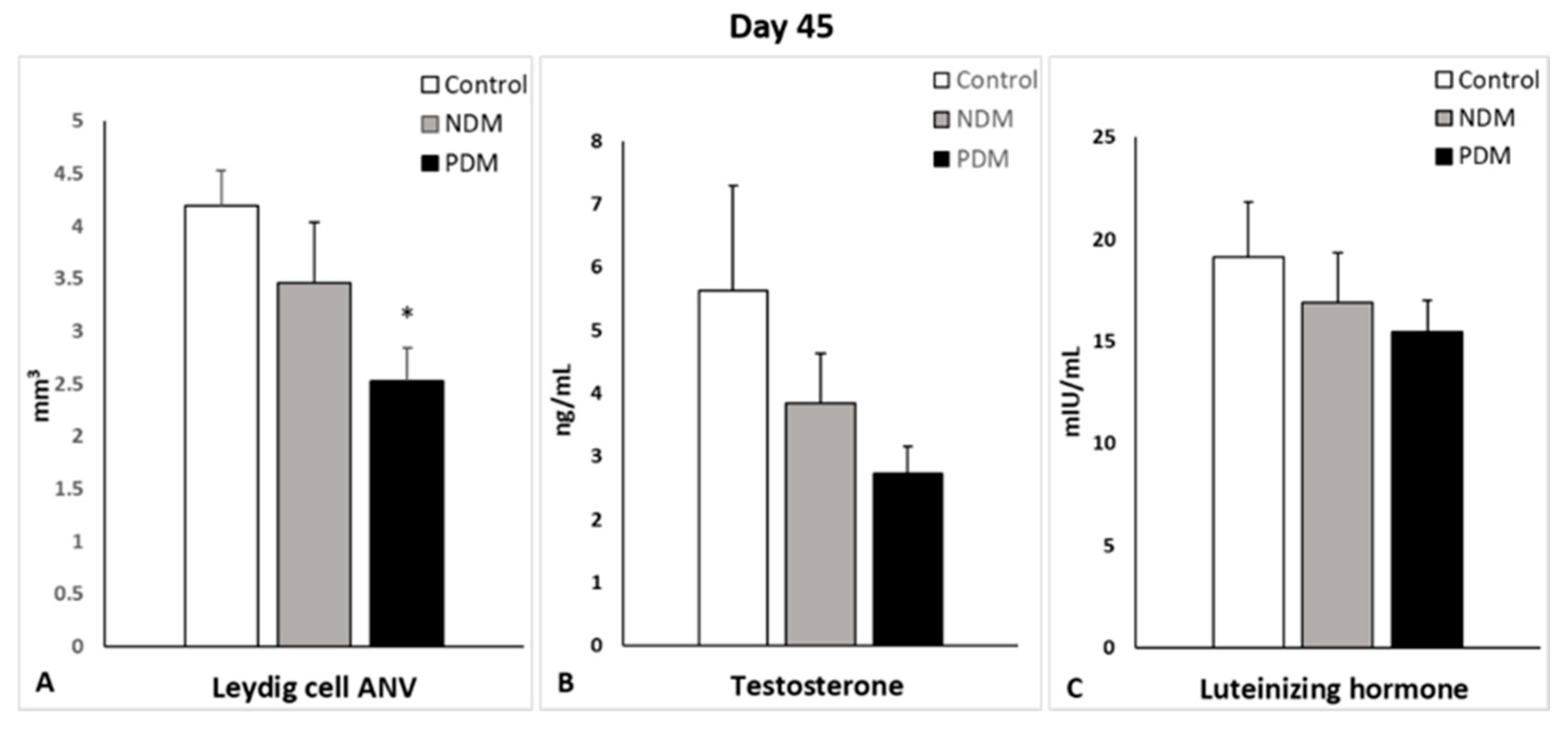
Leydig cells are known to drive spermatogenesis via the synthesis and secretion of testosterone after stimulation by pituitary luteinizing hormone. Testosterone, in turn, acts on Sertoli cells to establish a unique environment for the normal progression of germ cells through the spermatogenic cycle [
8]. Experimental manipulations for androgen deficiency have provided evidence for a reduced size of the adult Leydig cell population [
18]. After visualization by a specific LC marker (3β-HSD), we found decreased LC-ANV in pubertal and post-pubertal NDM and PDM rats compared to controls that was more pronounced in PDM. Intact testosterone production in both pubertal DM groups could be explained by an increased ratio of nuclear to cytoplasm volume, indicative that LCs are functionally more active in conditions of hyperglycemia. The elevation in LH levels in PDM on day 25 could be interpreted as a compensatory mechanism for providing stimuli to the LCs so that they can maintain normal testosterone production. At the end of puberty, the LC-ANV and testosterone production of LCs were less influenced by NDM compared to PDM, where the changes in testosterone concentration corresponded to that in LC-ANV. Our results for low levels of LH reduced the testosterone concentration on day 45, which is suggestive of a lack of a classical feedback mechanism. Young patients with type 2 diabetes have significantly lower plasma testosterone concentrations and “inappropriately” low LH and FSH concentrations with a very high prevalence of hypogonadotrophic hypogonadism [
41]. Data for decreased levels of testosterone, LH, and FSH in human males and in animal models were summarized by Maresch et al. (2018) [
4] and have also been reported in adult rats with alloxan-induced T1DM and T2DM (by fructose drink) [
24]. Our finding for reduced insulin concentrations in 45-day-old PDM could explain the lower level of LH and testosterone. Decreased testosterone and LH levels in adult diabetic rats resulted from the suppression of insulin, which was able to adversely affect Leydig cell proliferation and hormone production [
37,
39].
There are some discrepancies between the data on LC number and T biosynthesis in T1DM and T2DM diabetic models. Most of the studies demonstrated decreased number of LCs as well as reduced serum T levels [
19,
29,
37,
39]. In other studies, Leydig cell hypertrophy or hyperplasia and decreased T levels were reported [
15,
42]. In addition, low levels of testosterone and pituitary gonadotropic hormones (FSH and LH) were summarized in recent review articles [
12,
13,
43]. Downregulation of the expression of key genes of androgen biosynthesis is involved in the suppression of testosterone production in Leydig cells [
44,
45]. One paper reported different hormonal profiles between adult T1DM and T2DM [
26]—decreased serum gonadotrophins in T1DM but not in T2DM. These data support our results, suggesting that the ANV of adult LCs and their testosterone production is affected by PDM and altered to a lesser extent by NDM.