The Proteomic Landscape of CTNNB1 Mutated Low-Grade Early-Stage Endometrial Carcinomas
Highlights
- Shotgun proteomics reveal that low-grade early-stage endometrial carcinomas with CTNNB1 mutations show dysregulated pathways associated with keratinization, immune dysregulation, and altered calcium homeostasis.
- CTNNB1 mutations are associated with an immunosuppressive microenvironment and potentially harbor dysregulation in calcium-dependent WNT signaling.
- Aggressive behavior observed in CTNNB1 mutated endometrial carcinomas can be explained by their capacity of downregulating the immune system, the potential activation of WNT signaling through non-canonical pathways, and increased cell plasticity.
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and CTNNB1 Mutation Determination
2.2. Protein Extraction and Processing
2.3. TMT Labeling and LC-MS
2.4. Validation Cohort
2.5. Statistical Analysis
2.6. Quantitative PCR Analysis
2.7. Tissue-Based Analyses
3. Results
3.1. Patient Characteristics of Discovery and Validation Cohorts
3.2. Protein Quantification and Gene Set Enrichment Analysis
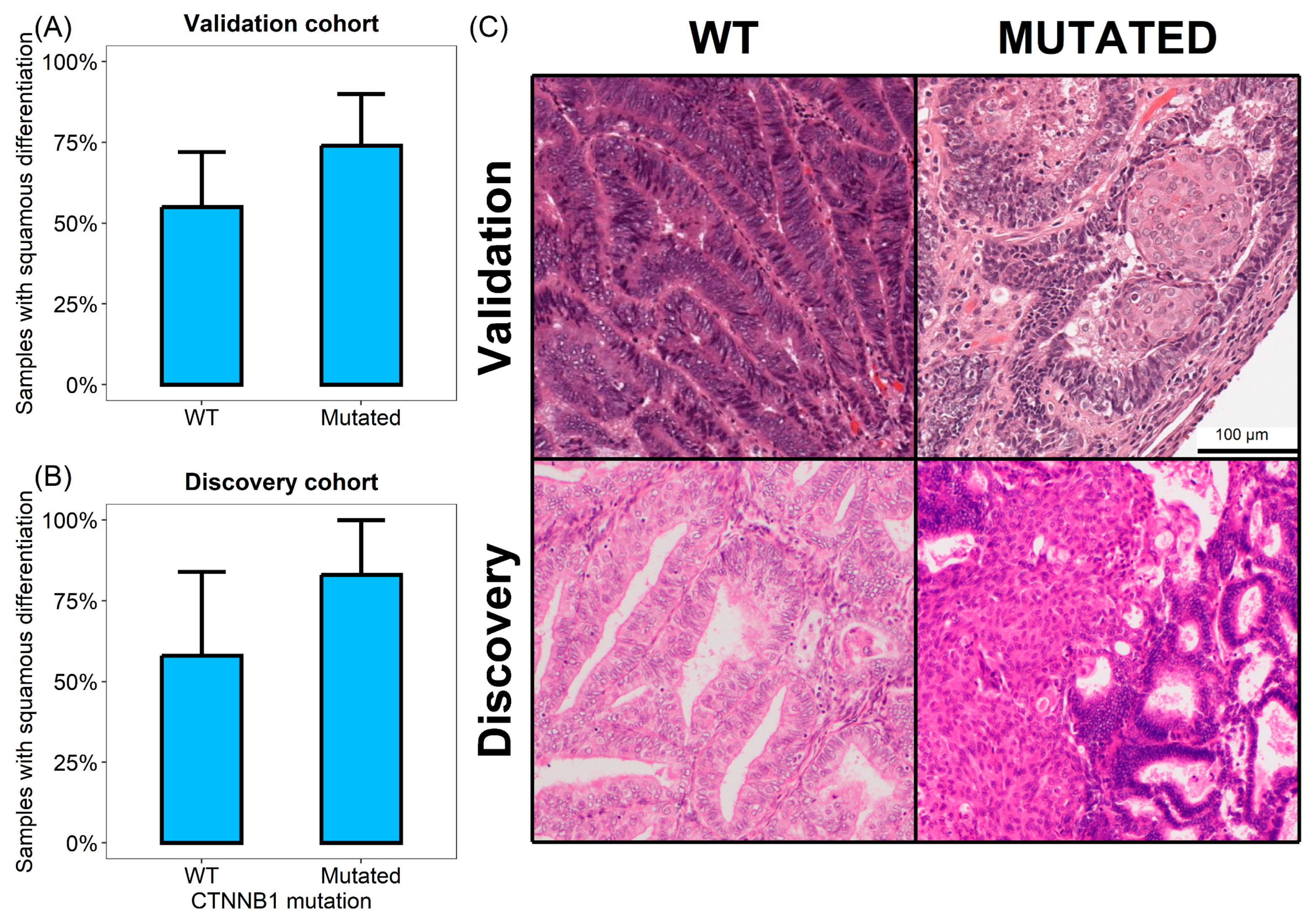
3.3. CTNNB1 Mutated Tumors Show Increased Squamous Differentiation Capabilities
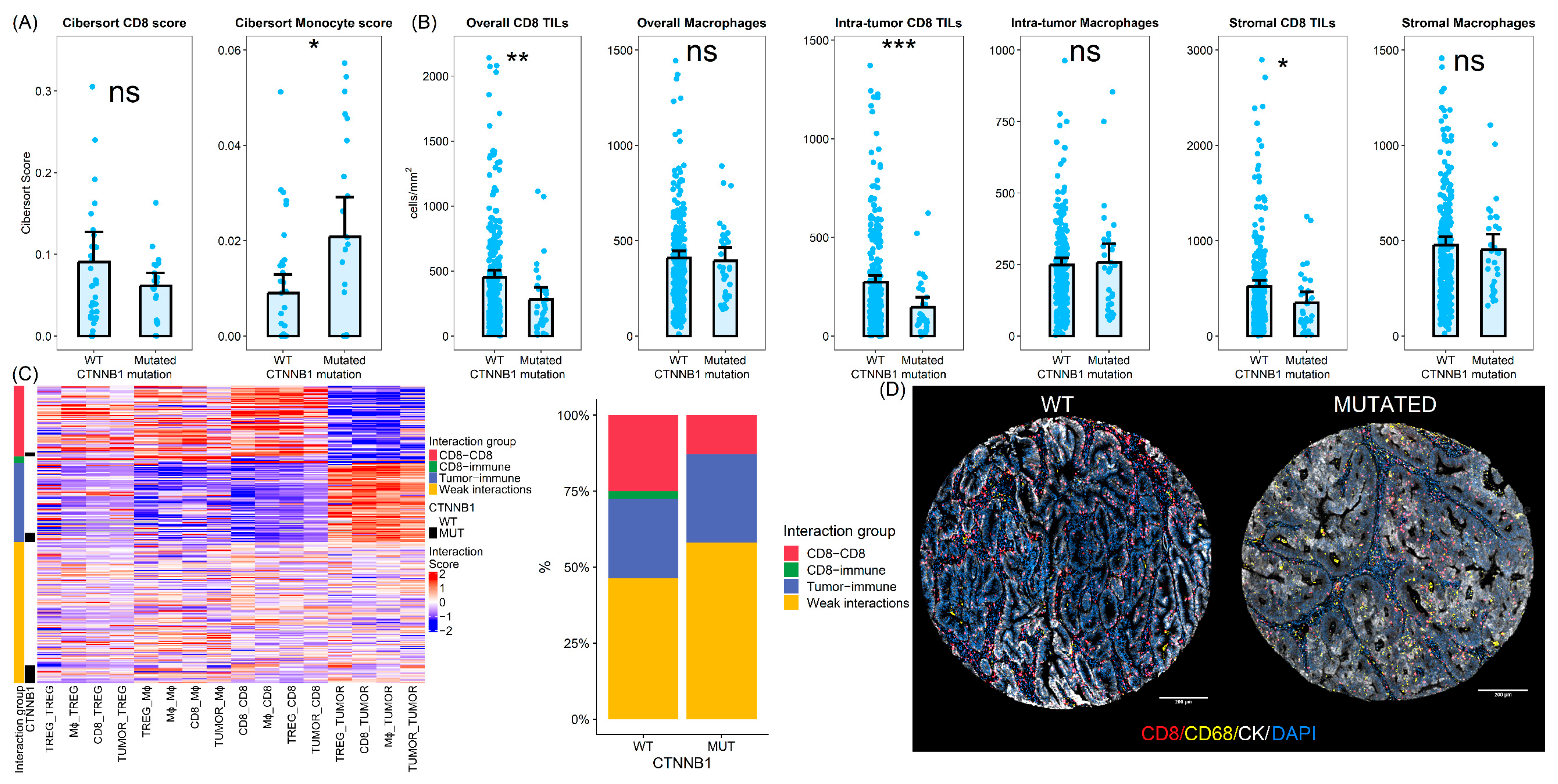
3.4. CTNNB1 Mutated Tumors Show Differences in the Tumor Immune Microenvironment
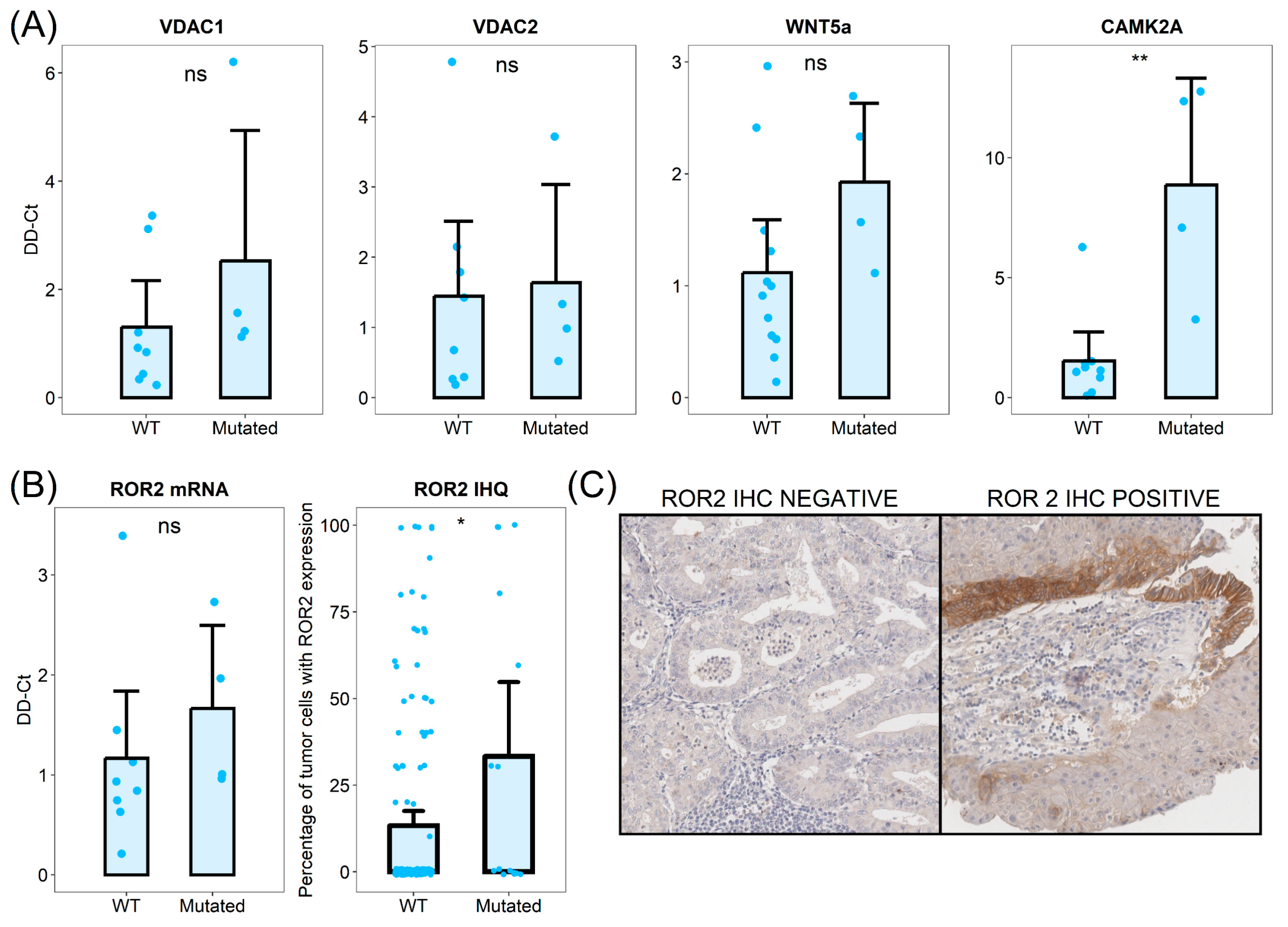
3.5. CTNNB1 Mutated Tumors Show Dysregulation of Intracellular Calcium and Calcium-Dependent Wnt Pathway Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TMA | Tissue MicroArray |
| GO | Gene Ontology |
| GSEA | Gene Set Enrichment Analysis |
| MIF | Multiplex Immuno Fluorescence |
| TILs | Tumor-Infiltrating Lymphocytes |
| WT | Wild-Type |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Merry, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today (accessed on 3 August 2023).
- Jeppesen, M.M.; Jensen, P.T.; Gilså Hansen, D.; Iachina, M.; Mogensen, O. The nature of early-stage endometrial cancer recurrence—A national cohort study. Eur. J. Cancer 2016, 69, 51–60. [Google Scholar] [CrossRef]
- Levine, D.; The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Kommoss, S.; McConechy, M.K.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Grevenkamp, F.; Karnezis, A.; Yang, W.; et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef]
- Trovik, J.; Wik, E.; Werner, H.M.J.; Krakstad, C.; Helland, H.; Vandenput, I.; Njolstad, T.S.; Stefansson, I.M.; Marcickiewicz, J.; Tingulstad, S.; et al. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicentre trial. Eur. J. Cancer 2013, 49, 3431–3441. [Google Scholar] [CrossRef] [PubMed]
- Asano, H.; Hatanaka, K.C.; Matsuoka, R.; Dong, P.; Mitamura, T.; Konno, Y.; Kato, T.; Kobayashi, N.; Ihira, K.; Nozaki, A.; et al. L1CAM Predicts Adverse Outcomes in Patients with Endometrial Cancer Undergoing Full Lymphadenectomy and Adjuvant Chemotherapy. Ann. Surg. Oncol. 2020, 27, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Ramon-Patino, J.L.; Ruz-Caracuel, I.; Heredia-Soto, V.; Garcia de la Calle, L.E.; Zagidullin, B.; Wang, Y.; Berjon, A.; Lopez-Janeiro, A.; Miguel, M.; Escudero, J.; et al. Prognosis Stratification Tools in Early-Stage Endometrial Cancer: Could We Improve Their Accuracy? Cancers 2022, 14, 912. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; Huvila, J.; Chiu, D.; Thompson, E.F.; Scott, S.; Salvador, S.; Vicus, D.; Helpman, L.; Gotlieb, W.; Kean, S.; et al. Grade and Estrogen Receptor Expression Identify a Subset of No Specific Molecular Profile Endometrial Carcinomas at a Very Low Risk of Disease-Specific Death. Mod. Pathol. 2023, 36, 100085. [Google Scholar] [CrossRef]
- Costigan, D.C.; Dong, F.; Nucci, M.R.; Howitt, B.E. Clinicopathologic and Immunohistochemical Correlates of CTNNB1 Mutated Endometrial Endometrioid Carcinoma. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2020, 39, 119–127. [Google Scholar] [CrossRef]
- Ruz-Caracuel, I.; López-Janeiro, Á.; Heredia-Soto, V.; Ramón-Patino, J.L.; Yébenes, L.; Berjón, A.; Hernández, A.; Gallego, A.; Ruiz, P.; Redondo, A.; et al. Clinicopathological features and prognostic significance of CTNNB1 mutation in low-grade, early-stage endometrial endometrioid carcinoma. Virchows Arch. Int. J. Pathol. 2021, 479, 1167–1176. [Google Scholar] [CrossRef]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef]
- McMellen, A.; Woodruff, E.R.; Corr, B.R.; Bitler, B.G.; Moroney, M.R. Wnt Signaling in Gynecologic Malignancies. Int. J. Mol. Sci. 2020, 21, 4272. [Google Scholar] [CrossRef]
- Wu, X.; Que, H.; Li, Q.; Wei, X. Wnt/β-catenin mediated signaling pathways in cancer: Recent advances, and applications in cancer therapy. Mol. Cancer 2025, 24, 171. [Google Scholar] [CrossRef]
- Luke, J.J.; Bao, R.; Sweis, R.F.; Spranger, S.; Gajewski, T.F. WNT/β-catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin. Cancer Res. 2019, 25, 3074–3083. [Google Scholar] [CrossRef]
- Xue, W.; Yang, L.; Chen, C.; Ashrafizadeh, M.; Tian, Y.; Sun, R. Wnt/β-catenin-driven EMT regulation in human cancers. Cell Mol. Life Sci. CMLS 2024, 81, 79. [Google Scholar] [CrossRef]
- He, K.; Gan, W.J. Wnt/β-Catenin Signaling Pathway in the Development and Progression of Colorectal Cancer. Cancer Manag. Res. 2023, 15, 435–448. [Google Scholar] [CrossRef]
- Xie, J.; Huang, L.; Lu, Y.G.; Zheng, D.L. Roles of the Wnt Signaling Pathway in Head and Neck Squamous Cell Carcinoma. Front. Mol. Biosci. 2021, 7, 590912. [Google Scholar] [CrossRef]
- Nakatani, Y.; Masudo, K.; Miyagi, Y.; Inayama, Y.; Kawano, N.; Tanaka, Y.; Kato, K.; Ito, T.; Kitamura, H.; Nagashima, Y.; et al. Aberrant nuclear localization and gene mutation of beta-catenin in low-grade adenocarcinoma of fetal lung type: Up-regulation of the Wnt signaling pathway may be a common denominator for the development of tumors that form morules. Mod. Pathol. Off. J. U S Can. Acad. Pathol. Inc. 2002, 15, 617–624. [Google Scholar]
- Lawrie, L.C.; Fothergill, J.E.; Murray, G.I. Spot the differences: Proteomics in cancer research. Lancet Oncol. 2001, 2, 270–277. [Google Scholar] [CrossRef]
- Dou, Y.; Kawaler, E.A.; Cui Zhou, D.; Gritsenko, M.A.; Huang, C.; Blumenberg, L.; Karpova, A.; Petyuk, V.A.; Savage, S.R.; Satpathy, S.; et al. Proteogenomic Characterization of Endometrial Carcinoma. Cell 2020, 180, 729–748.e26. [Google Scholar] [CrossRef] [PubMed]
- López-Janeiro, Á.; Ruz-Caracuel, I.; Ramón-Patino, J.L.; De Los Ríos, V.; Villalba Esparza, M.; Berjón, A.; Yébenes, L.; Hernández, A.; Masetto, I.; Kadioglu, E.; et al. Proteomic Analysis of Low-Grade, Early-Stage Endometrial Carcinoma Reveals New Dysregulated Pathways Associated with Cell Death and Cell Signaling. Cancers 2021, 13, 794. [Google Scholar] [CrossRef] [PubMed]
- Montero-Calle, A.; López-Janeiro, Á.; Mendes, M.L.; Perez-Hernandez, D.; Echevarría, I.; Ruz-Caracuel, I.; Heredia-Soto, V.; Mendiola, M.; Hardisson, D.; Argüeso, P.; et al. In-depth quantitative proteomics analysis revealed C1GALT1 depletion in ECC-1 cells mimics an aggressive endometrial cancer phenotype observed in cancer patients with low C1GALT1 expression. Cell. Oncol. Dordr. Neth. 2023, 46, 697–715. [Google Scholar] [CrossRef]
- Montero-Calle, A.; Garranzo-Asensio, M.; Poves, C.; Sanz, R.; Dziakova, J.; Peláez-García, A.; Ríos, V.d.L.; Martinez-Useros, J.; Fernández-Aceñero, M.J.; Barderas, R. In-Depth Proteomic Analysis of Paraffin-Embedded Tissue Samples from Colorectal Cancer Patients Revealed TXNDC17 and SLC8A1 as Key Proteins Associated with the Disease. J. Proteome Res. 2024, 23, 4802–4820. [Google Scholar] [CrossRef]
- Zecha, J.; Satpathy, S.; Kanashova, T.; Avanessian, S.C.; Kane, M.H.; Clauser, K.R.; Mertins, P.; Carr, S.A.; Kuster, B. TMT Labeling for the Masses: A Robust and Cost-efficient, In-solution Labeling Approach. Mol. Cell. Proteom. 2019, 18, 1468–1478. [Google Scholar] [CrossRef]
- Montero-Calle, A.; Coronel, R.; Garranzo-Asensio, M.; Solís-Fernández, G.; Rábano, A.; de Los Ríos, V.; Fernández-Aceñero, M.J.; Mendes, M.L.; Martínez-Useros, J.; Megías, D.; et al. Proteomics analysis of prefrontal cortex of Alzheimer’s disease patients revealed dysregulated proteins in the disease and novel proteins associated with amyloid-β pathology. Cell Mol. Life Sci. CMLS 2023, 80, 141. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bandla, C.; Kundu, D.J.; Kamatchinathan, S.; Bai, J.; Hewapathirana, S.; John, N.S.; Prakash, A.; Walzer, M.; Wang, S.; et al. The PRIDE database at 20 years: 2025 update. Nucleic Acids Res. 2025, 53, D543–D553. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: Analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef]
- Romani, C.; Calza, S.; Todeschini, P.; Tassi, R.A.; Zanotti, L.; Bandiera, E.; Sartori, E.; Pecorelli, S.; Ravaggi, A.; Santin, A.D.; et al. Identification of optimal reference genes for gene expression normalization in a wide cohort of endometrioid endometrial carcinoma tissues. PLoS ONE 2014, 9, e113781. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- López-Janeiro, Á.; Villalba-Esparza, M.; Brizzi, M.E.; Jiménez-Sánchez, D.; Ruz-Caracuel, I.; Kadioglu, E.; Masetto, I.; Goubert, V.; Garcia-Ros, D.; Melero, I.; et al. The association between the tumor immune microenvironments and clinical outcome in low-grade, early-stage endometrial cancer patients. J. Pathol. 2022, 258, 426–436. [Google Scholar] [CrossRef]
- Cancer Imaging Archive. Available online: https://www.cancerimagingarchive.net/collection/cptac-ucec (accessed on 10 August 2022).
- Frederic Delhoume. Very Large Image Viewer. Available online: https://github.com/delhoume/vliv (accessed on 10 August 2022).
- Meljen, V.T.; Mittenzwei, R.; Wong, J.; Puechl, A.; Whitaker, R.; Broadwater, G.; Hall, A.H.M.; Bean, S.M.; Bentley, R.C.; Elvin, J.A.; et al. Endometrial Adenocarcinomas With No Specific Molecular Profile: Morphologic Features and Molecular Alterations of “Copy-number Low” Tumors. Int. J. Gynecol. Pathol. 2021, 40, 587–596. [Google Scholar] [CrossRef]
- Niu, S.; Lucas, E.; Molberg, K.; Strickland, A.; Wang, Y.; Carrick, K.; Rivera-Colon, G.; Gwin, K.; SoRelle, J.A.; Castrillon, D.H.; et al. Morules But Not Squamous Differentiation are a Reliable Indicator of CTNNB1 (β-catenin) Mutations in Endometrial Carcinoma and Precancers. Am. J. Surg. Pathol. 2022, 46, 1447–1455. [Google Scholar] [CrossRef]
- Mitra, A.P.; Bartsch, C.C.; Bartsch, G.; Miranda, G.; Skinner, E.C.; Daneshmand, S. Does presence of squamous and glandular differentiation in urothelial carcinoma of the bladder at cystectomy portend poor prognosis? An intensive case-control analysis. Urol. Oncol. 2014, 32, 117–127. [Google Scholar] [CrossRef]
- Li, H.S.; Chen, Y.; Zhang, M.Y.; Cheng, K.; Zhou, Y.W.; Liu, J.Y. Increased proportion of the squamous cell carcinoma component is associated with worse survival in resected gastric adenosquamous carcinoma: A STROBE compliant cohort study. Medicine 2020, 99, e21980. [Google Scholar] [CrossRef]
- Andrade DAPde da Silva, V.D.; Matsushita Gde, M.; de Lima, M.A.; Vieira Mde, A.; Andrade, C.E.M.C.; Schmidt, R.L.; dos Reis, R. Squamous differentiation portends poor prognosis in low and intermediate-risk endometrioid endometrial cancer. PLoS ONE 2019, 14, e0220086. [Google Scholar]
- Aslan, K.; Öz, M.; Ersak, B.; Müftüoğlu, H.K.; Moraloğlu Tekin, Ö.; Meydanli, M.M. The prognostic value of squamous differentiation in endometrioid type endometrial cancer: A matched analysis. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2022, 42, 494–500. [Google Scholar] [CrossRef] [PubMed]
- van Weverwijk, A.; de Visser, K.E. Mechanisms driving the immunoregulatory function of cancer cells. Nat. Rev. Cancer 2023, 23, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Kühl, M. The WNT/Calcium pathway: Biochemical mediators, tools and future requirements. Front. Biosci. 2004, 9, 967. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.E.; Qian Ma, S.S.; Quadir, A.; Ward, R.L. The dual role of the novel Wnt receptor tyrosine kinase, ROR2, in human carcinogenesis. Int. J. Cancer 2013, 133, 779–787. [Google Scholar] [CrossRef]
- Quezada, M.J.; Lopez-Bergami, P. The signaling pathways activated by ROR1 in cancer. Cell Signal. 2023, 104, 110588. [Google Scholar] [CrossRef]
- Thrasivoulou, C.; Millar, M.; Ahmed, A. Activation of intracellular calcium by multiple Wnt ligands and translocation of β-catenin into the nucleus: A convergent model of Wnt/Ca2+ and Wnt/β-catenin pathways. J. Biol. Chem. 2013, 288, 35651–35659. [Google Scholar] [CrossRef]
- Parrish, M.L.; Osborne-Frazier, M.L.; Broaddus, R.R.; Gladden, A.B. Differential Localization of β-Catenin Protein in CTNNB1 Mutant Endometrial Cancers Results in Distinct Transcriptional Profiles. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc. 2025, 38, 100791. [Google Scholar] [CrossRef]
- Fatima, I.; Barman, S.; Rai, R.; Thiel, K.W.; Chandra, V. Targeting Wnt Signaling in Endometrial Cancer. Cancers 2021, 13, 2351. [Google Scholar] [CrossRef]
- Yuan, S.; Sun, R.; Shi, H.; Chapman, N.M.; Hu, H.; Guy, C.; Rankin, S.; Kc, A.; Palacios, G.; Meng, X.; et al. VDAC2 loss elicits tumour destruction and inflammation for cancer therapy. Nature 2025, 640, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Balgley, B.M.; Guo, T.; Zhao, K.; Fang, X.; Tavassoli, F.A.; Lee, C.S. Evaluation of archival time on shotgun proteomics of formalin-fixed and paraffin-embedded tissues. J. Proteome Res. 2009, 8, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.M.S.; Sykes, E.K.; Rukhaya, J.; Anees, A.; Zhong, Q.; Jackson, C.; Panizza, B.J.; Reddel, R.R.; Balleine, R.L.; Hains, P.G.; et al. The effect of storage time and temperature on the proteomic analysis of FFPE tissue sections. Clin. Proteom. 2025, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Hood, B.L.; Conrads, T.P.; Veenstra, T.D. Mass spectrometric analysis of formalin-fixed paraffin-embedded tissue: Unlocking the proteome within. Proteomics 2006, 6, 4106–4114. [Google Scholar] [CrossRef]

| Discovery Cohort | Validation Cohort | ||||
|---|---|---|---|---|---|
| CTNNB1 WT | CTNNB1 MUT | CTNNB1 WT | CTNNB1 MUT | ||
| n | 12 | 6 | 37 (62%) | 23 (38%) | |
| Age (y, mean, p25–p75) | 63 (58–67) | 73 (69–78) | 65 (61–69) | 59 (55–65) | |
| FIGO 2018 (n, %) | FIGO IA | 5 (42%) | 2 (33%) | 25 (68%) | 19 (83%) |
| FIGO IB | 7 (58%) | 3 (50%) | 8 (22%) | 3 (13%) | |
| FIGO II | 0 | 1 (17%) | 3 (8%) | 1 (4%) | |
| FIGO I NOS | 0 | 0 | 1 (3%) | 0 | |
| Grade (n, %) | Grade 1 | 9 (75%) | 4 (67%) | 15 (41%) | 16 (70%) |
| Grade 2 | 3 (25%) | 2 (33%) | 22 (59%) | 7 (30%) | |
| LVI (n, %) | Absent | 8 (67%) | 3 (50%) | 31 (84%) | 20 (87%) |
| Present | 4 (33%) | 3 (50%) | 6 (16%) | 3 (13%) | |
| MMR (IHC status) | Proficient | 12 (100%) | 6 (100%) | 4 (11%) | 13 (55%) |
| Deficient | 0 | 0 | 6 (16%) | 0 | |
| NA | 0 | 0 | 27 (73%) | 10 (45%) | |
| POLE | WT | 9 (75%) | 3 (50%) | 23 (100%) | 37 (100%) |
| Mutated | 0 | 0 | 0 | 0 | |
| NA | 3 (25%) | 3 (50%) | 0 | 0 | |
| RELAPSE (n, %) | Local | 2 (17%) | 3 (50%) | ||
| Distant, nodal | 4 (33%) | 3 (50%) | |||
| NA | 0 | 0 | 23 (100%) | 37 (100%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Janeiro, A.; Brizzi, E.; Ruz-Caracuel, I.; Alexandru, R.; de Andrea, C.; Berjón, A.; Yebenes, L.; Mendiola, M.; Heredia-Soto, V.; Montero-Calle, A.; et al. The Proteomic Landscape of CTNNB1 Mutated Low-Grade Early-Stage Endometrial Carcinomas. Cells 2025, 14, 1676. https://doi.org/10.3390/cells14211676
Lopez-Janeiro A, Brizzi E, Ruz-Caracuel I, Alexandru R, de Andrea C, Berjón A, Yebenes L, Mendiola M, Heredia-Soto V, Montero-Calle A, et al. The Proteomic Landscape of CTNNB1 Mutated Low-Grade Early-Stage Endometrial Carcinomas. Cells. 2025; 14(21):1676. https://doi.org/10.3390/cells14211676
Chicago/Turabian StyleLopez-Janeiro, Alvaro, Emilia Brizzi, Ignacio Ruz-Caracuel, Raluca Alexandru, Carlos de Andrea, Alberto Berjón, Laura Yebenes, Marta Mendiola, Victoria Heredia-Soto, Ana Montero-Calle, and et al. 2025. "The Proteomic Landscape of CTNNB1 Mutated Low-Grade Early-Stage Endometrial Carcinomas" Cells 14, no. 21: 1676. https://doi.org/10.3390/cells14211676
APA StyleLopez-Janeiro, A., Brizzi, E., Ruz-Caracuel, I., Alexandru, R., de Andrea, C., Berjón, A., Yebenes, L., Mendiola, M., Heredia-Soto, V., Montero-Calle, A., Barderas, R., de los Rios, V., Redondo, A., Pelaez-Garcia, A., & Hardisson, D. (2025). The Proteomic Landscape of CTNNB1 Mutated Low-Grade Early-Stage Endometrial Carcinomas. Cells, 14(21), 1676. https://doi.org/10.3390/cells14211676









