1. Introduction
As one of the largest organs in the human body and a primary protein reservoir, skeletal muscle plays a vital role in locomotion and metabolic regulation [
1]. However, muscle integrity is frequently compromised by protein homeostasis imbalance, exemplified by sarcopenia-a pathological condition characterized by significant loss of skeletal muscle mass and strength. Sarcopenia arises from multifactorial aetiologies, including musculoskeletal trauma, ageing, diabetes mellitus, and neoplastic diseases [
2,
3], leading to severe reductions in patient independence and quality of life, while substantially increasing direct and indirect healthcare costs associated with disability. Glucocorticoids (GCs), widely prescribed for their anti-inflammatory and immunosuppressive properties [
4], are a known aetiological factor for sarcopenia when administered chronically or at supraphysiological doses [
5]. This condition may also manifest during endogenous GC overproduction under pathological conditions [
6]. Clinical reports indicate a 60% incidence of glucocorticoid-induced myopathy [
7]. Synthetic GCs, exemplified by dexamethasone (Dex), induce skeletal muscle atrophy through dual mechanisms: (1) suppression of muscle protein synthesis via inhibition of the Akt/mTOR signaling pathway [
8], and (2) acceleration of proteolytic catabolism mediated by the ubiquitin-proteasome system (UPS). The latter process is orchestrated by forkhead box (FoxO) transcription factors, which activate two muscle-specific E3 ubiquitin ligases—muscle RING finger 1 (MuRF-1) and atrogin-1 (also known as MAFbx)—to drive muscle wasting [
9]. While these pathogenic mechanisms have been extensively characterized, the molecular regulators that balance catabolic and anabolic pathways during GC-induced atrophy remain poorly understood, representing a critical knowledge gap in developing targeted therapies.
Mitochondrial dysfunction serves as a critical aetiological factor in muscle atrophy induced by disuse and pathological conditions [
10]. Peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), a master regulator of mitochondrial biogenesis, exhibits downregulated signalling in skeletal muscle mitochondrial dysfunction. Notably, activation of the PGC-1α pathway ameliorates muscle strength deficits and protein metabolism dysregulation [
11]. Sirtuin 1 (SIRT1), a member of the evolutionarily conserved NAD+-dependent histone deacetylase family [
12], plays a pivotal role in skeletal muscle remodelling. SIRT1 directly deacetylates and activates PGC-1α, thereby stimulating mitochondrial biogenesis in muscle tissues [
13]. This mechanistic linkage positions SIRT1 as a promising therapeutic target for addressing muscle dysfunction.
To date, physical activity remains the most clinically validated intervention for sarcopenia; however, its implementation is constrained in elderly and mobility-impaired populations. This limitation has spurred intense interest in developing exercise mimetics as therapeutic alternatives for sarcopenia [
14]. Irisin, a myokine secreted by myofibres during exercise-induced proteolytic cleavage of fibronectin type III domain-containing protein 5 (FNDC5) [
15], plays a critical role in muscle regeneration and homeostasis. Beyond promoting post-injury repair, irisin enhances metabolic regulation and muscular strength, establishing its status as a key mechanotransduction molecule bridging physical activity and metabolic health [
16]. Recent evidence demonstrates that irisin improves denervation-induced muscle injury by activating the IL-6 signalling pathway to enhance myogenesis and myoblast fusion, while simultaneously stimulating ERK1/2-dependent protein synthesis [
17]. These findings position Irisin as a promising therapeutic target for sarcopenia intervention.
In this study, we investigated the alterations of FNDC5/irisin in GC-induced sarcopenia and evaluated whether irisin-based intervention could ameliorate GC-associated muscle atrophy and mitochondrial dysfunction. Notably, our results demonstrated that protein levels of FNDC5/irisin in both skeletal muscle and circulation were significantly downregulated during muscle wasting. Intraperitoneal injection administration of recombinant irisin protein attenuated GC-induced muscle atrophy and mitochondrial abnormalities in murine models. Collectively, these findings establish irisin as a promising exercise mimetic alternative for sarcopenia treatment, targeting pathological muscle loss and functional decline.
2. Materials and Methods
2.1. Animals
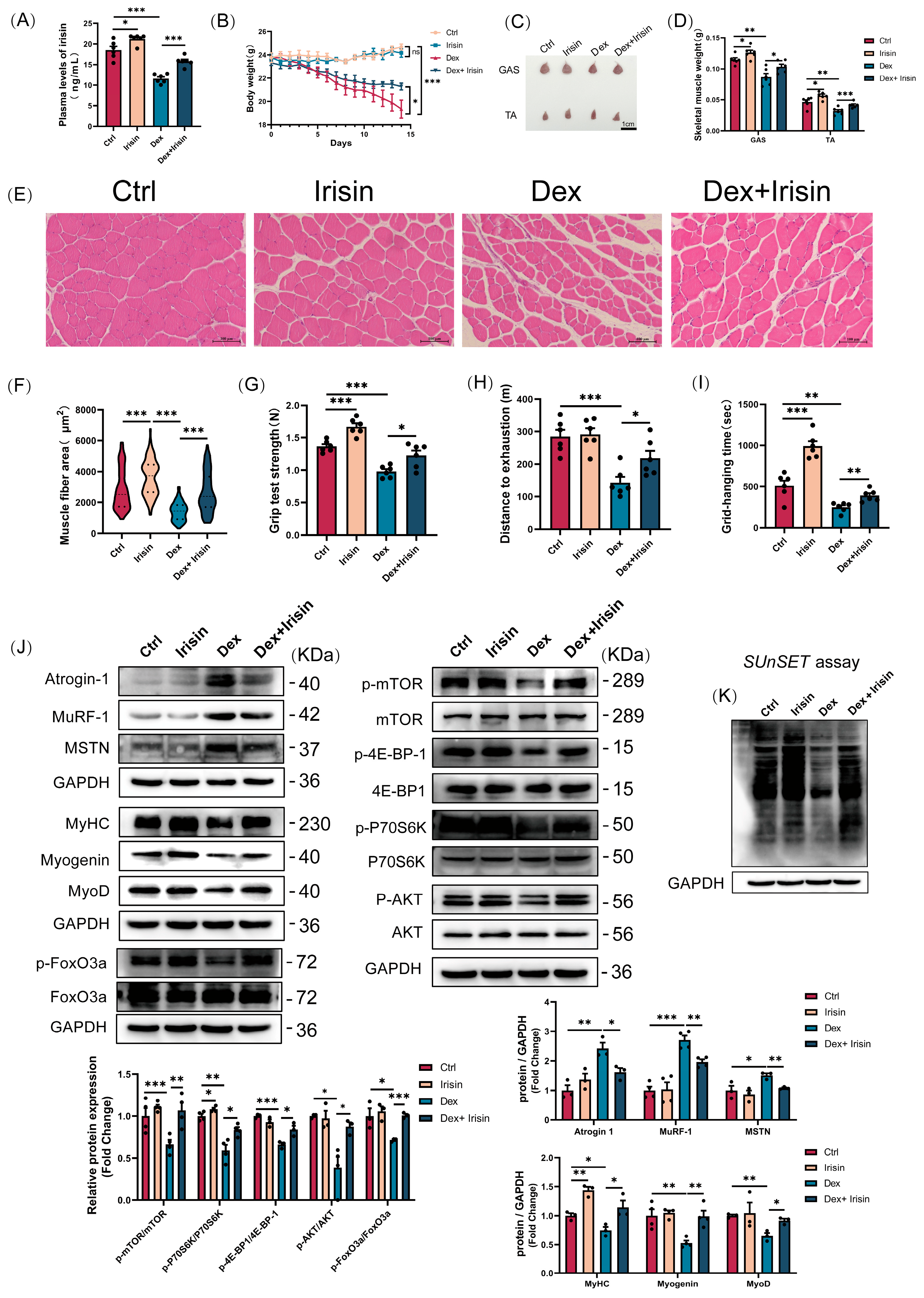
Male C57BL/6J mice (8 weeks old) were housed under controlled conditions (temperature: 22 ± 3 °C; humidity: 40–50%; 12 h light-dark cycle) with ad libitum access to food and water. Animals were randomly assigned to five groups (
n = 6 per group) and administered treatments as follows: (i) Control group: vehicle (PEG300 solution, i.p.); (ii) Irisin group: The irisin stock solution was prepared using sterile physiological saline as the solvent at a concentration of 500 μg/mL (2.5 mg/kg, i.p.); (iii) Dex group: The formulation of the dexamethasone stock solution comprises PEG300 at a concentration of 2.5 mg/mL (25 mg/kg, i.p.); (iv) Dex + Irisin group: dexamethasone (25 mg/kg, i.p.) + irisin (2.5 mg/kg, i.p.); (v) Dex + Irisin + EX-527 (SIRT1 inhibiting drug) group: dexamethasone (25 mg/kg, i.p.) + Irisin (2.5 mg/kg, i.p.) + EX-527 (10 mg/kg, i.p.). Based on previous studies [
16], Irisin was administered three times weekly, while dexamethasone and EX-527 were administered daily, over a total duration of 14 consecutive days. Body weight was recorded daily. Samples of blood, gastrocnemius (GAS), and tibialis anterior (TA) muscles were collected at 24 h following the last injection. Upon collection, the muscle tissues were immediately weighed and photographed, followed by fixation in 4% paraformaldehyde or flash-freezing in liquid nitrogen for subsequent analysis. All experimental manipulations were carried out in compliance with protocols authorized by the Animal Care Committee of Shanxi Agricultural University (SXAU-EAW2024M.ID.011005320).
2.2. Cell Culture and Differentiation
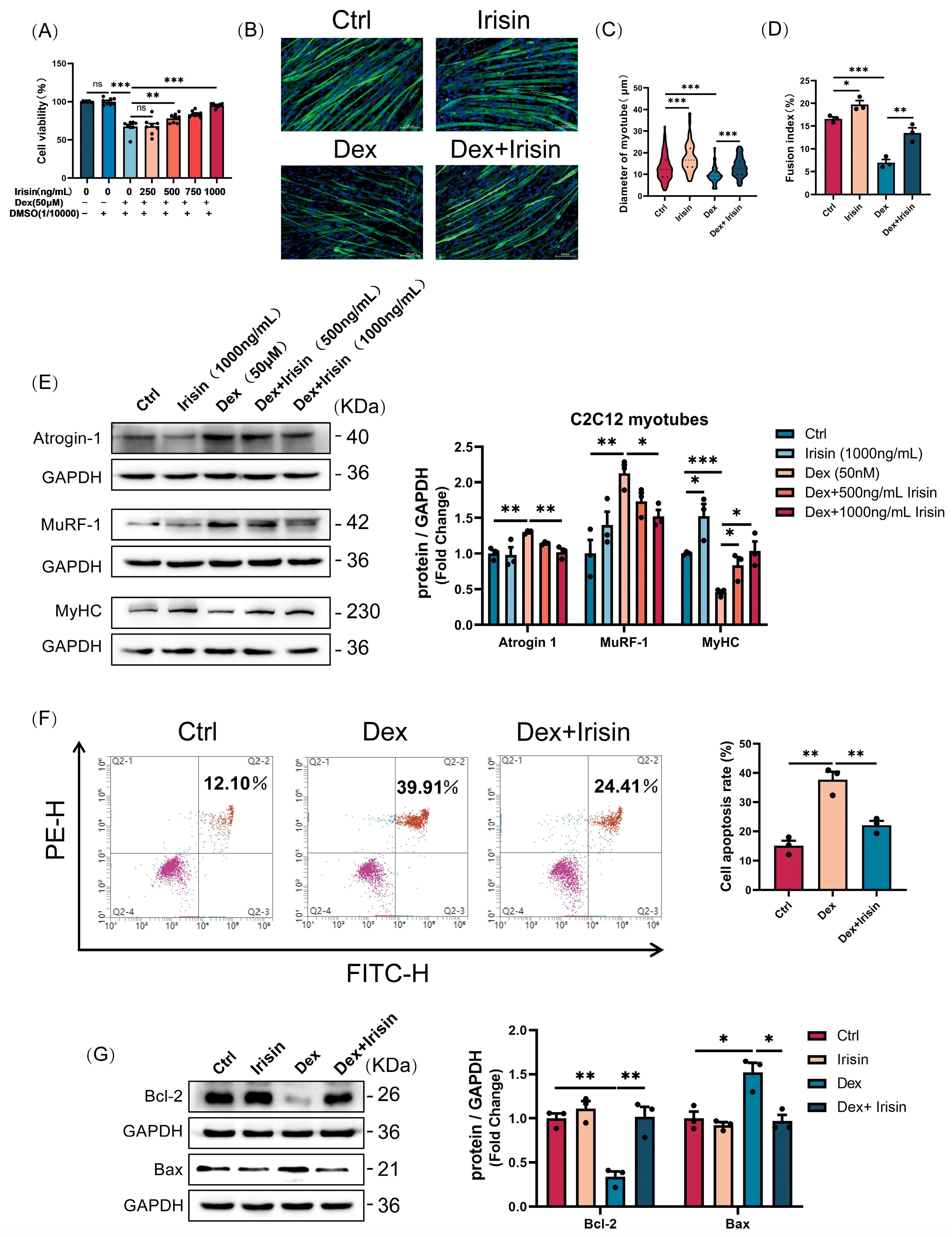
The mouse C2C12 myoblast cell line was acquired from the American Type Tissue Culture Collection (ATCC). Cells were maintained in high-glucose Dulbecco’s Modified Eagle Medium (BI, Beit HaEmek, Israel) supplemented with 10% fetal bovine serum (Sciencell, Carlsbad, CA, USA) at 37 °C in a humidified 5% CO2 atmosphere. Upon reaching 70% confluency, the growth medium was replaced with a differentiation medium consisting of DMEM and 2% horse serum to induce myotube formation. Cultures were maintained under this condition for 4 days, with medium replacement daily, to induce multinucleated myotube formation. To induce myotube atrophy, fully differentiated myotubes were treated with 50 μM Dex (MCE, Monmouth Junction, NJ, USA) for 24 h. R-irisin (Phoenix Pharmaceuticals, Burlingame, CA, USA) and EX-527 (MCE, Monmouth Junction, NJ, USA) were administered concomitantly with Dex during the atrophy induction phase.
2.3. Cell Viability Assay
C2C12 myotubes, fully differentiated following seeding at an initial density of 1 × 104 cells per well in 96-well plates, were subjected to a 24 h treatment with 50 μM dexamethasone (Dex) and varying concentrations of R-irisin (250, 500, 750, and 1000 ng/mL). Cell viability was subsequently assessed using a Cell Counting Kit-8 (CCK-8; YEASEN, Shanghai, China). Briefly, 10 μL of CCK-8 reagent was added to each well, followed by a 2 h incubation at 37 °C. The absorbance was then measured at 450 nm using a microplate reader (Molecular Devices, San Jose, CA, USA). The percentage of cell viability was calculated relative to the untreated control group using the formula: Cell Viability (%) = (As − A0)/(Ac − A0) × 100%, where As, A0, and Ac were the absorptions of test sample, back-ground, and control (DMSO), respectively.
2.4. Cellular Immunofluorescence Staining
Following fixation with 4% paraformaldehyde (15–20 min) and permeabilization with 0.1% Triton X-100 (10 min), the cells were blocked for 30 min at room temperature using 5% goat serum (Solarbio, Beijing, China). Subsequently, the cells were incubated overnight at 4 °C with a primary antibody targeting myosin heavy chain (MyHC). After extensive washing, a species-matched Alexa Fluor-conjugated secondary antibody was applied for 1 h at 37 °C. Nuclei were visualized by counterstaining with DAPI for 10 min at room temperature. Fluorescence images were acquired with a Eclipse Ts2R microscope (Nikon, Yokohama, Japan) and processed using the NIS-Elements software (v4.20).
2.5. Tissue Section Preparation and Hematoxylin and Eosin Stain (H&E)
Muscle tissues were fixed in 4% paraformaldehyde for 24 h, followed by graded ethanol dehydration and xylene clearing. Paraffin-embedded tissues were sectioned into 0.8 μm-thick slices using an automated rotary microtome (LEICA, Hesse, Germany). The sections were deparaffinized with xylene, rehydrated through graded ethanol series, and stained with haematoxylin and eosin. After mounting with neutral balsam (Solarbio, Beijing, China) images were captured and processed using a microscope (Nikon, Yokohama, Japan) equipped with NIS-Elements imaging software.
2.6. Western Blot Analyses
Protein extracts were prepared from muscle tissues or myotubes by homogenization in cold RIPA buffer (Beyotime Biotechnology, Shanghai, China) containing a cocktail of protease and phosphatase inhibitors. Following lysis on ice for 30 min and centrifugation at 13,000× g for 5 min at 4 °C, the supernatant was collected. Protein concentration was determined using a BCA assay kit (Beyotime, Shanghai, China). Equal amounts of protein were then resolved by SDS-PAGE and electrophoretically transferred onto PVDF membranes (Millipore, Burlington, MA, USA). The membranes were blocked with 5% skimmed milk in TBST for 1 h at room temperature before being incubated with primary antibodies overnight at 4 °C. After washing, the membranes were probed with appropriate HRP-conjugated secondary antibodies for 1 h at 37 °C. Signal detection was performed using enhanced chemiluminescence reagents (ABBKINE, Wuhan, China), and band intensities were captured with a ChemiDoc XRS+ imaging system (Bio-Rad, Hercules, CA, USA). Quantitative analysis was conducted using ImageJ software (v1.47, National Institutes of Health, Bethesda, MD, USA) after normalizing the band intensities to the endogenous control GAPDH.
2.7. Immunoprecipitation
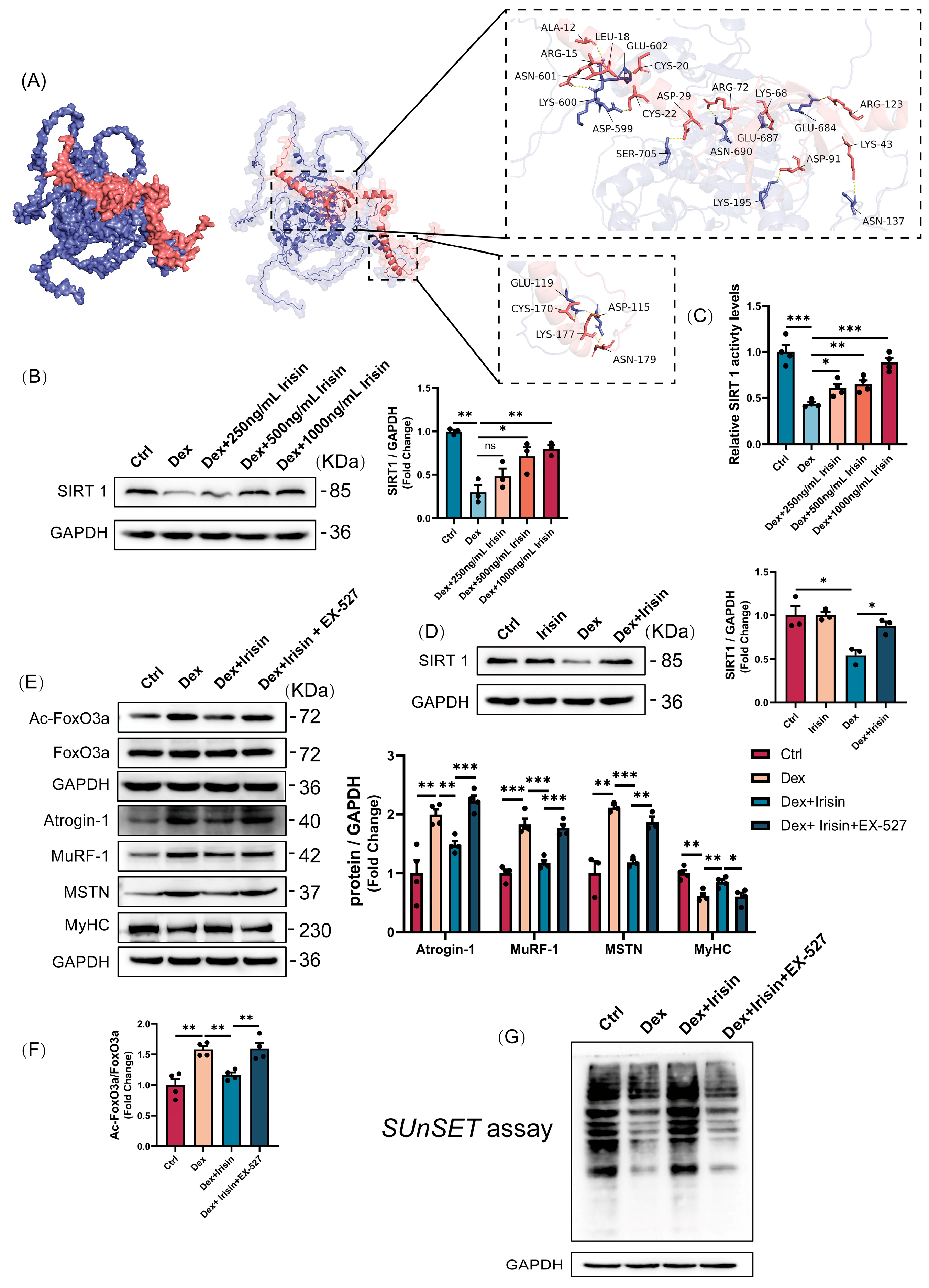
To distinguish the acetylated form of PGC-1α from its total protein levels, we conducted immunoprecipitation (IP) followed by Western blotting. In this procedure, cell lysates were incubated with anti-PGC-1α antibody and A/G agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4 °C under constant rotation. The beads were then pelleted and washed extensively with cold RIPA buffer. Bound proteins were eluted by heating in 5×SDS sample buffer and subjected to SDS-PAGE. Immunoblotting was subsequently carried out, employing an antibody against acetylated lysine to detect PGC-1α acetylation, with total PGC-1α serving as the internal control.
2.8. Cellular Mitochondrial Membrane Potential Assay
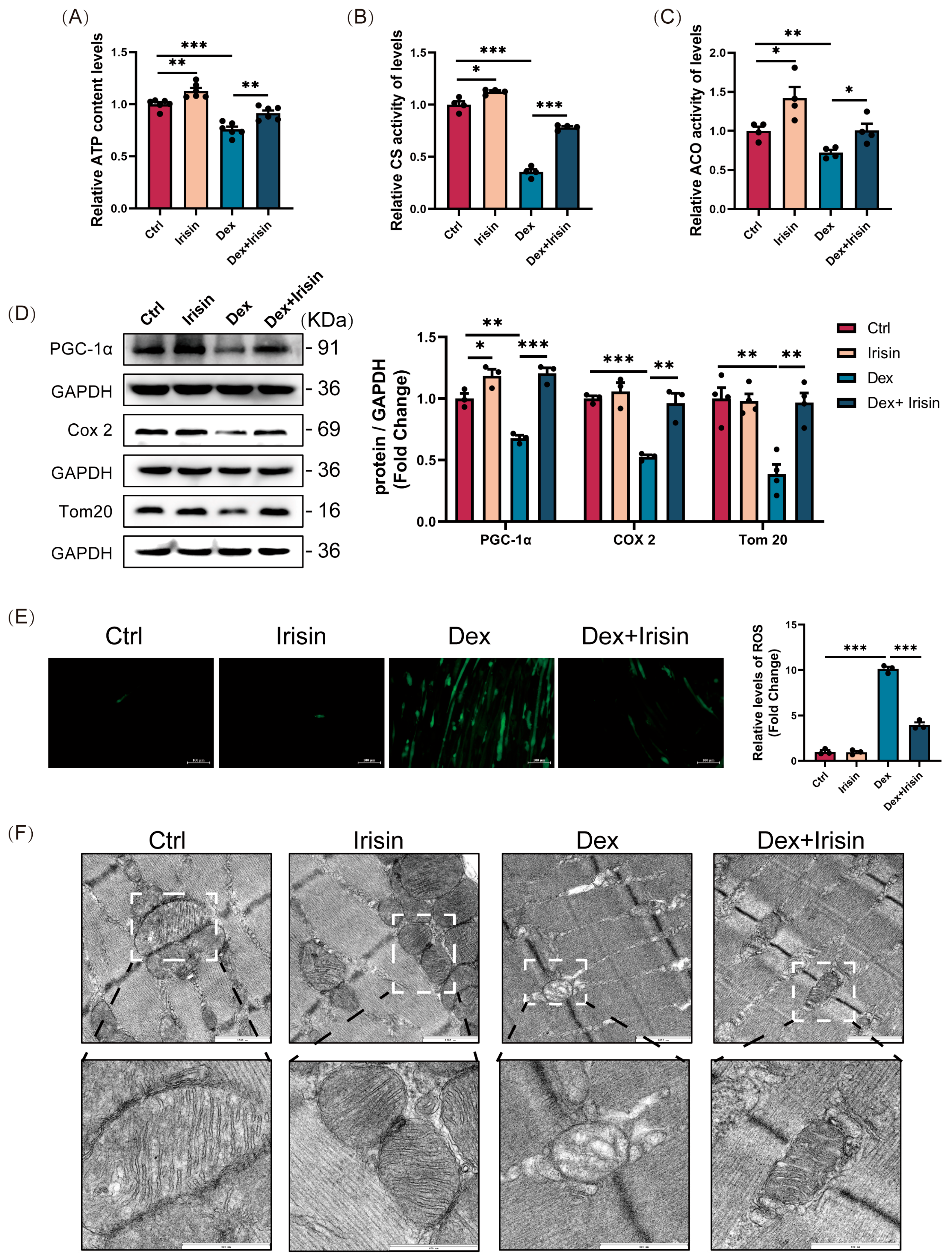
C2C12 myoblasts were seeded in confocal culture dishes and allowed to reach full differentiation. Differentiated myotubes were with dexamethasone (50 μM), R-irisin (1000 ng/mL), and the SIRT1 inhibitor EX-527 (20 μM) for 24 h. According to the manufacturer’s instructions, live cells were stained with JC-1 fluorescent dye (Beyotime Biotechnology, C2003S, China) at 37 °C for 20 min to visualize the level of mitochondrial membrane potential. Mitochondrial membrane potential was visualized by capturing fluorescence images using a confocal laser scanning microscopy (CLSM) system (Olympus, Yokohama, Japan).
2.9. Determination of ATP Content
ATP concentrations were measured following the instructions of a commercial detection kit (Beyotime Biotechnology, S0026, China). Muscle samples were homogenized in ice-cold lysis buffer and centrifuged (12,000× g, 5 min, 4 °C). The supernatant was then incubated with the ATP detection reagent, and luminescence was recorded on a FilterMax F5 microplate reader (Molecular Devices, San Jose, CA, USA). Sample ATP levels were interpolated from a standard curve.
2.10. Sirtuin1 Deacetylase Activity
SIRT1 deacetylase activity was quantified using a fluorometric assay kit (Elabscience®, Wuhan, China) following the manufacturer’s instructions. Briefly, 1 × 106 cells were homogenized in 0.2 mL physiological saline, centrifuged at 12,000× g for 5 min at 4 °C, and the supernatant was collected. The cell lysate was incubated with reaction buffer in a 96-well plate, and fluorescence intensity was monitored at excitation/emission wavelengths of 340/440 nm using a microplate reader (Molecular Devices, San Jose, CA, USA). Enzyme activity was normalized to total protein content.
2.11. Cellular Reactive Oxygen Species (ROS) Assay
Intracellular ROS levels were assessed using a commercially available ROS detection assay kit (Beyotime Biotechnology, Shanghai, China). C2C12 myoblasts were seeded in sterile cell culture dishes and allowed to reach full differentiation. Differentiated myotubes were incubated with the fluorescent probe DCFH-DA (dichlorofluorescin diacetate) at 37 °C for 20 min. Fluorescence intensity was quantified using a fluorescence microscope (Nikon, Yokohama, Japan) equipped with appropriate excitation/emission filters (Ex/Em = 488/525 nm).
2.12. Enzyme-Linked Immunosorbent Assay for Mouse Irisin
The concentration of irisin in plasma and cell supernatants from each mouse group was quantified using a species-specific enzyme-linked immunosorbent assay (ELISA) kit (Elabscience®, Wuhan, China) in strict accordance with the manufacturer’s instructions.
2.13. Skeletal Muscle Function Exploration
Exhaustion Time and Distance. Following a 2-day acclimation period, running performance was assessed as previously described [
18], Mice ran on a treadmill (SANS Biotechnology, Nanjing, China) with a 13% incline. The speed was increased by 5 cm/s every 2 min until exhaustion, which was designated when a mouse’s hindlimbs remained on the electric grid for >10 s. The total time to exhaustion and running distance were recorded.
Limb Grip Strength. Forelimb and hindlimb strength was measured using a dynamometer (SANS Biotechnology, Nanjing, China). Mice were allowed to grasp a horizontal grid and were pulled backward ten times. The peak force (in Newtons) applied before the mouse lost its grip was recorded for each trial. The average of ten measurements was normalized to the animal’s body weight.
Inverted Grid Test. Motor coordination and endurance were evaluated by placing mice on a wire grid, which was then inverted over a 2 s period. The grid was held 40–50 cm above a soft surface. The latency to fall was recorded as the hanging time.
2.14. Protein Synthesis by In Vitro SUnSET
The SUnSET assay was employed to assess the global protein synthesis rate. Briefly, puromycin (1 μg/mL) was administered to the culture medium 10 min before cell harvesting. The resulting cell extracts were then subjected to Western blot analysis using an anti-puromycin antibody (12D10).
2.15. Observations on Mitochondria in Gastrocnemius Muscle
Ultrastructural analysis of muscle tissue was performed by transmission electron microscopy (FEI, Hillsboro, OR, USA). Briefly, samples were fixed in 1% glutaraldehyde, sectioned, and mounted on copper grids for observation under the microscope equipped with a high-resolution camera.
2.16. Flow Cytometry Detection of Apoptosis
Cell apoptosis was assessed with an Annexin V-FITC/PI detection kit (ABBKINE, Wuhan, China) per the manufacturer’s protocol. In brief, 1 × 105 cells per sample were resuspended in binding buffer and stained with Annexin V-FITC and propidium iodide (PI) for 15 min at room temperature in the dark. Annexin V is a calcium-dependent phospholipid-binding protein that specifically recognizes and binds to phosphatidylserine (PS) externally exposed on the cell membrane. PI, a nucleic acid-binding dye, enters the cell and binds to nuclear DNA during the late stage of apoptosis when the cell membrane integrity is disrupted. The samples were then immediately analyzed on a BD LSRFortessa™ flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), configuring the FITC (Ex/Em: 488/520 nm) and PI (Ex/Em: 535/615 nm) channels. The resulting data were processed using FlowJo software (v10.8.1, BD Biosciences, Franklin Lakes, NJ, USA).
2.17. Statistical Analysis
All the statistical data are presented as the mean ± standard error of the mean (SEM). Comparisons between two groups were performed using a two-tailed Student’s t-test, and comparisons among multiple groups were performed using one-way analysis of variance (ANOVA) with Tukey’s post hoc test. A p value of less than 0.05 (p < 0.05) was considered to indicate statistical significance. All the data were drawn using GraphPad Prism 8.3.
4. Discussion
Sarcopenia, characterized by progressive skeletal muscle mass and strength loss, significantly impairs mobility, quality of life, and healthcare systems due to fall-related injuries, costly hospitalisations, and prolonged rehabilitation [
22]. Despite the established association with aging [
23], current therapeutic paradigms remain suboptimal. While resistance exercise remains the cornerstone intervention, its applicability is severely limited in sarcopenic populations with functional impairments. Pharmacological strategies targeting anabolic hormones (e.g., testosterone, growth hormone) exhibit inconsistent efficacy profiles, dose-limiting adverse effects, and marginal functional improvements [
24], underscoring the imperative for mechanism-driven therapeutic innovations.
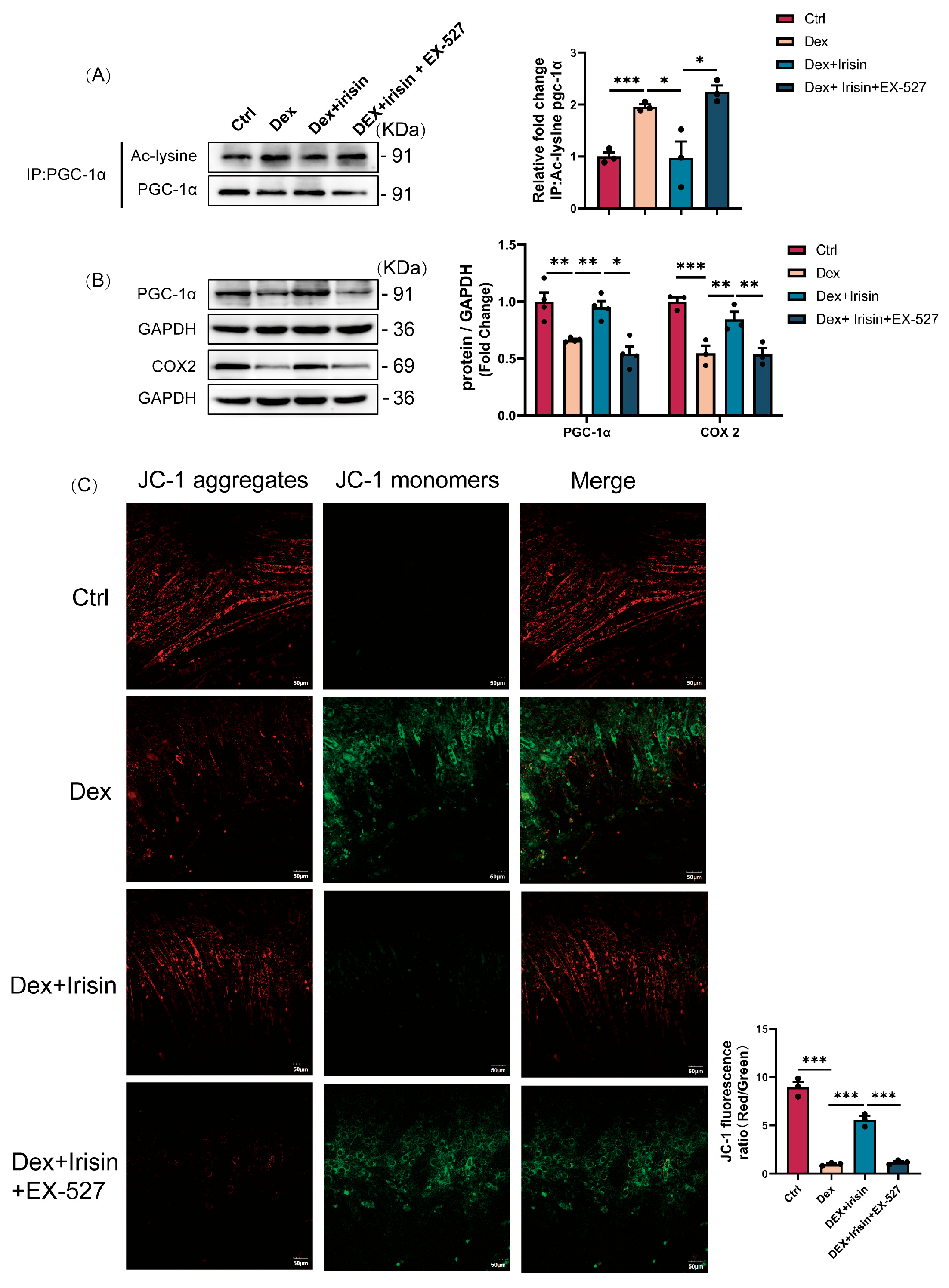
Our discovery of irisin’s dual regulatory role in proteostasis and mitochondrial homeostasis provides novel mechanistic insights into its therapeutic potential. The observed downregulation of circulating irisin in glucocorticoid-induced murine models, establishes a compelling correlative link between irisin deficiency and muscle wasting pathophysiology. Mechanistically, we delineate a SIRT1-dependent axis that synergistically suppresses catabolic pathways while enhancing anabolic signaling (
Figure 9). This regulatory cascade positions irisin as a master coordinator of skeletal muscle plasticity, bridging exercise mimetic effects with intracellular metabolic regulation.
The circulating levels of irisin have been identified as a biomarker for muscle mass and performance [
25]. For instance, aging [
26] and denervation-induced [
17] skeletal muscle loss and atrophy are associated with reduced irisin concentrations, potentially implicating irisin deficiency as a contributing factor to muscle wasting. In glucocorticoid-induced muscle atrophy, we observed a significant downregulation of FNDC5/irisin levels both in the circulation of mice in vivo and in ex vivo cultured C2C12 myotubes. This consistent reduction strongly suggests that irisin deficiency is a pathogenic factor driving glucocorticoid-induced muscle atrophy. Beyond its well-documented endocrine and paracrine roles in metabolic organs [
27], irisin exerts autocrine effects within muscle tissue. It upregulates growth-related genes in myocytes [
28], while enhancing myoblast proliferation and fusion [
17], thereby promoting muscle growth and rescuing dystrophic phenotypes in murine models of Duchenne muscular dystrophy [
29], denervation [
17], and hindlimb suspension [
30]. Our findings extend irisin’s therapeutic relevance to glucocorticoid-associated sarcopenia. Here, we demonstrate that irisin simultaneously promotes myotube development by upregulating MyHC, MyoD, and MyoG expression while suppressing atrophy-related proteins Atrogin-1, MuRF-1, and MSTN to counteract glucocorticoid-induced muscle wasting. Given irisin’s classification as an exercise mimetic [
31], we investigated its capacity to induce exercise-like phenotypes in murine muscle. Strikingly, irisin enhanced muscle strength in both healthy and atrophied murine models, evidenced by improved grip strength, grid-hanging performance, and increased treadmill endurance capacity. Subsequent cross-sectional area analysis revealed marked hypertrophic phenotypes in irisin-treated muscle tissues. Collectively, these observations align with characteristic adaptations induced by resistance exercise training.
SIRT1 critically regulates skeletal muscle remodelling through deacetylation-mediated modulation of FoxO transcriptional activity, which governs muscle mass and function [
32]. Here, we demonstrate that irisin enhances SIRT1 protein expression and deacetylase activity in a dose-dependent manner, thereby shifting the FoxO3a phosphorylation-acetylation equilibrium to suppress its nuclear translocation. This molecular mechanism provides a mechanistic basis for irisin-mediated inhibition of Atrogin-1 and MuRF1 expression. The Akt/mTOR axis, a central signalling pathway for skeletal muscle protein synthesis [
33], is restored by irisin to counteract glucocorticoid-induced protein synthesis defects. Puromycin incorporation assays revealed that irisin also enhances protein synthesis in normal myotubes, suggesting a broader anabolic role in muscle hypertrophy. Notably, irisin’s dual modulation of Akt/mTOR (enhancing translation initiation) and ubiquitin-proteasome system (reducing proteolysis) mirrors the physiological biphasic response to mechanical loading, wherein anabolic and anti-catabolic pathways are temporally segregated during muscle repair. This mechanistic duality positions irisin as a superior alternative to current therapies targeting single pathways, particularly in glucocorticoid-induced myopathy where dual-pathway dysregulation predominates.
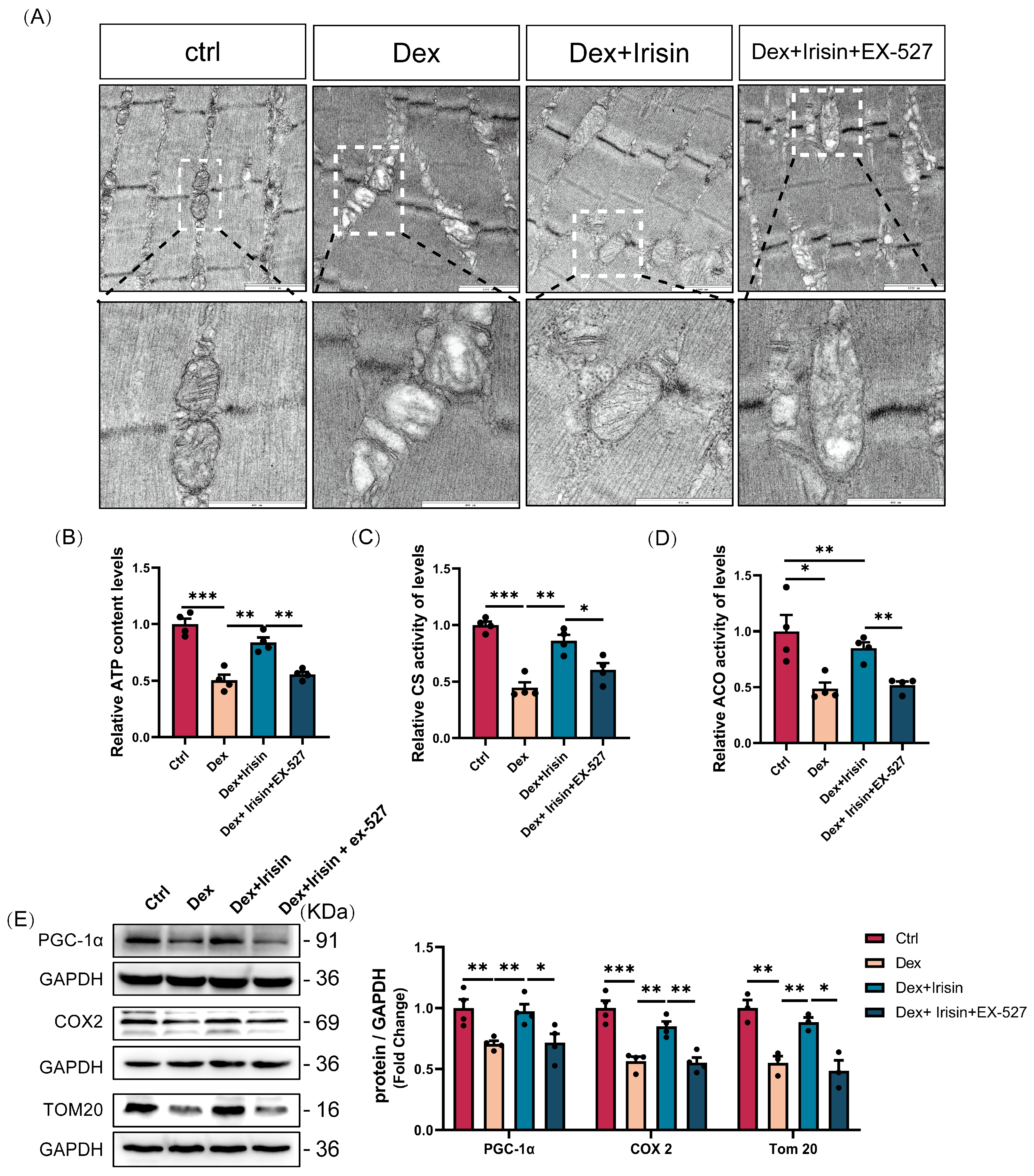
Beyond its role in proteostasis, irisin exerts profound effects on mitochondrial biogenesis and function, which are critical in glucocorticoid-induced muscle atrophy. PGC-1α, a master regulator of mitochondrial biogenesis and cellular energy metabolism, drives mitochondrial biogenesis by stimulating transcription factors nuclear respiratory factor 1 and 2 (NRF-1 and NRF-2), thereby increasing mitochondrial enzyme transcription [
34]. During muscle atrophy, mitochondrial degradation reduces mitochondrial mass and quantity, governed by mitophagy and mitochondrial fusion/fission dynamics [
35]. These mitochondrial quality control systems are essential for maintaining skeletal muscle mass by identifying and correcting mitochondrial dysfunction. Mitochondrial dysfunction triggers catabolic pathways that feedback to the nucleus to promote expression of muscle atrophy-related genes [
36]. Here, irisin enhances PGC-1α expression and reduces its acetylation status via SIRT1 activation, augmenting its transcriptional activity. This results in significant improvements in mitochondrial DNA synthesis, ATP production, and enzymatic activities of citrate synthase and aconitase, collectively restoring mitochondrial morphology and function. Normalization of mitochondrial parameters correlates with functional recovery, evidenced by improved grip strength and treadmill endurance in treated mice. This mitochondrial remodelling effect positions irisin as a central regulator of systemic energy homeostasis. By simultaneously enhancing mitochondrial biogenesis and protein synthesis, irisin creates a synergistic anabolic environment. This dual mechanism is particularly relevant in aged populations, where mitochondrial dysfunction serves as a primary driver of sarcopenia progression.