Sequencing Cellular Therapies in the Management of Follicular Lymphoma
Highlights
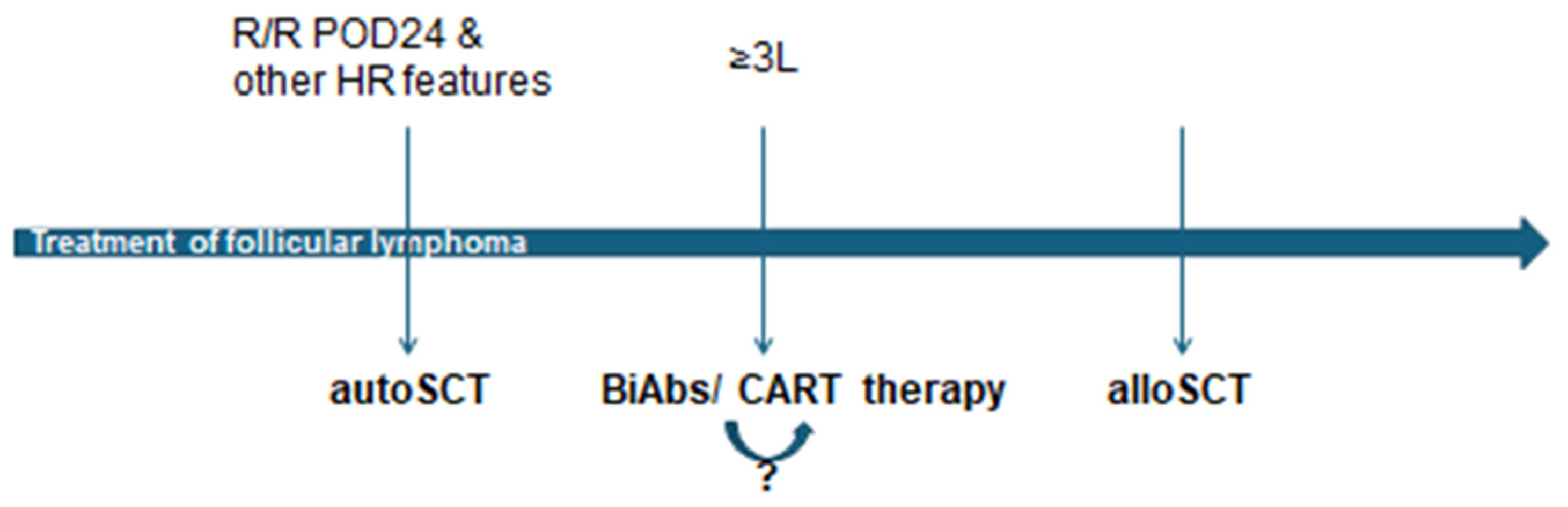
- This article thoroughly reviews the current landscape and optimal sequencing of advanced cellular therapies, including autologous stem cell transplantation, allogeneic stem cell transplantation, and CAR T-cell therapy, for managing relapsed/refractory follicular lymphoma.
- It highlights the significant efficacy of CAR T-cell therapy in achieving high response rates and durable remissions, potentially serving as a bridge between transplant modalities, and discusses the role of newer bispecific antibodies as convenient off-the-shelf options.
- 3.
- The evolving treatment paradigm for follicular lymphoma necessitates highly individualized decisions that consider patient characteristics, disease features, and the complex interplay of various cellular and novel immunotherapies.
- 4.
- Further research into predictive biomarkers, refined treatment algorithms, and the impact of sequential therapies (e.g., bispecific antibodies before CAR-T) is crucial to optimizing patient outcomes and integrating these powerful new options effectively into clinical practice.
Abstract
1. Introduction
2. The Importance of Autologous Stem Cell Transplantation
3. Allogeneic Stem Cell Transplantation: A Curative Option?
4. CAR-T Cell Therapy: A Bridge Between Transplants?
4.1. Major CAR-T Trials in Follicular Lymphoma
4.2. CAR-T Between AutoSCT and AlloSCT
5. The Role of Bispecific Antibodies
| Bispecific Antibody | Study Name/ Design | Patient Population | ORR | CRR | Median Follow-Up | Key Safety Findings |
|---|---|---|---|---|---|---|
| Mosunetuzumab [29] a CD3 × CD20 bispecific antibody | Phase 2, single-arm | R/R FL with ≥2 prior lines, including anti-CD20 and alkylating agent | High objective responses (specific ORR not given, but CRR is 60.0%) | 60.0% | 18.3 months | Favorable safety profile. CRS: 44% (G1-2: 42%, G3: 1%, G4: 1%). No treatment-related Grade 5 AEs. |
| Epcoritamab [24] a CD3 × CD20 bispecific antibody | EPCORE NHL-1, Phase 1–2, multicohort, single-arm | Multiply R/R CD20+ FL with ≥2 prior lines of therapy | 82.0% (105/128) | 63% | Relatively short; additional follow-up planned | Manageable safety profile. Neutropenia: 25% (G3-4). CRS: 65% (G1-2), 2% (G3). ICANS: 6%. Optimized dosing reduced CRS to 49% (no G3/4). |
| Odronextamab [32] a CD3 × CD20 bispecific antibody | ELM-2 Phase II study | R/R FL after ≥2 prior lines of systemic therapy | 80.0% | 73.4% | 20.1 months | Generally manageable safety profile. CRS: 56% (G ≥ 3: 1.7%). Neutropenia: 39%. Pyrexia: 38%. |
5.1. Impact of Prior Bispecific Antibody Treatment on CAR-T Outcome
5.2. Comparing MAIC Analyses: Lisocabtagene Maraleucel and Axicabtagene Ciloleucel Versus Mosunetuzumab, and Cost-Effectiveness
6. Comparing the Pros and Cons of Bispecific Antibodies and CART Therapy in Follicular Lymphoma
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clemente, M.B.; Collazo-Lorduy, A.; Yanguas-Casás, N.; Calvo, V.; Provencio, M. Unveiling the Role of the Tumor Microenvironment in the Treatment of Follicular Lymphoma. Cancers 2022, 14, 2158. [Google Scholar] [CrossRef] [PubMed]
- Méndez, M.; Torrente, M.; Provencio, M. Follicular Lymphomas and Their Transformation: Past and Current Research. Expert Rev. Hematol. 2017, 10, 515. [Google Scholar] [CrossRef]
- Lossos, I.S.; Gascoyne, R.D. Transformation of Follicular Lymphoma. Best Pract. Res. Clin. Haematol. 2011, 24, 147. [Google Scholar] [CrossRef]
- Maeshima, A.M. Histologic Transformation of Follicular Lymphoma: Pathologists’ Viewpoint. J. Clin. Exp. Hematop. 2023, 63, 12. [Google Scholar] [CrossRef]
- Kumar, E.; Okosun, J. Follicular Lymphoma: Current Therapeutic Landscape and Future Prospects. Hematol. Oncol. 2025, 43, e70070. [Google Scholar] [CrossRef]
- Caridà, G.; Martino, E.A.; Bruzzese, A.; Caracciolo, D.; Labanca, C.; Mendicino, F.; Lucia, E.; Olivito, V.; Rossi, T.; Neri, A.; et al. Relapsed/Refractory Follicular Lymphoma: Current Advances and Emerging Perspectives. Eur. J. Haematol. 2025, 114, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Morabito, F.; Martino, E.A.; Nizzoli, M.E.; Talami, A.; Pozzi, S.; Martino, M.; Neri, A.; Gentile, M. Comparative Analysis of Bispecific Antibodies and CAR T-Cell Therapy in Follicular Lymphoma. Eur. J. Haematol. 2024, 114, 4–16. [Google Scholar] [CrossRef]
- Russler-Germain, D.A.; Bartlett, N.L. Sequencing Bispecific Antibodies and CAR T Cells for FL. Hematology 2024, 2024, 310. [Google Scholar] [CrossRef]
- Lopedote, P.; Shadman, M. Targeted Treatment of Relapsed or Refractory Follicular Lymphoma: Focus on the Therapeutic Potential of Mosunetuzumab. Cancer Manag. Res. 2023, 15, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Salles, G. How Do I Sequence Therapy for Follicular Lymphoma? Hematology 2020, 2020, 287. [Google Scholar] [CrossRef]
- Iqbal, M.; Kumar, A.; Dreger, P.; Chavez, J.G.; Sauter, C.S.; Sureda, A.M.; Bachanová, V.; Maziarz, R.T.; Dreyling, M.; Smith, S.M.; et al. Clinical Practice Recommendations for Hematopoietic Cell Transplantation and Cellular Therapies in Follicular Lymphoma: A Collaborative Effort on Behalf of the American Society for Transplantation and Cellular Therapy and the European Society for Blood and Marrow Transplantation. Transplant. Cell. Ther. 2024, 30, 832. [Google Scholar] [CrossRef] [PubMed]
- Tomblyn, M.; Ewell, M.; Bredeson, C.; Kahl, B.S.; Goodman, S.; Horowitz, M.M.; Vose, J.M.; Negrin, R.S.; Laport, G.G. Autologous versus Reduced-Intensity Allogeneic Hematopoietic Cell Transplantation for Patients with Chemosensitive Follicular Non-Hodgkin Lymphoma beyond First Complete Response or First Partial Response. Biol. Blood Marrow Transplant. 2010, 17, 1051. [Google Scholar] [CrossRef] [PubMed]
- Jurinović, V.; Metzner, B.; Pfreundschuh, M.; Schmitz, N.; Wandt, H.; Keller, U.; Dreger, P.; Dreyling, M.; Hiddemann, W.; Unterhalt, M.; et al. Autologous Stem Cell Transplantation for Patients with Early Progression of Follicular Lymphoma: A Follow-Up Study of 2 Randomized Trials from the German Low Grade Lymphoma Study Group. Biol. Blood Marrow Transplant. 2018, 24, 1172. [Google Scholar] [CrossRef]
- Casulo, C.; Friedberg, J.W.; Ahn, K.W.; Flowers, C.R.; DiGilio, A.; Smith, S.M.; Ahmed, S.; Inwards, D.J.; Aljurf, M.; Chen, A.I.; et al. Autologous Transplantation in Follicular Lymphoma with Early Therapy Failure: A National LymphoCare Study and Center for International Blood and Marrow Transplant Research Analysis. Biol. Blood Marrow Transplant. 2017, 24, 1163. [Google Scholar] [CrossRef]
- Casulo, C.; Barr, P.M. How I Treat Early-Relapsing Follicular Lymphoma. Blood 2019, 133, 1540. [Google Scholar] [CrossRef]
- Rodgers, T.D.; Casulo, C.; Boissard, F.; Launonen, A.; Parreira, J.; Cartron, G. Early Relapse in First-Line Follicular Lymphoma: A Review of the Clinical Implications and Available Mitigation and Management Strategies. Oncol. Ther. 2021, 9, 329. [Google Scholar] [CrossRef]
- Kenyeres, A.; Kiss, E.; Simon, Z.; Illes, A.; Jona, A. Age and Lymphocyte/Monocyte Ratio as Prognostic Factors for Autologous Transplantation in the Treatment of Patients with Follicular Lymphoma. J. Int. Med. Res. 2024, 52, 03000605231221012. [Google Scholar] [CrossRef]
- Robinson, S.; Boumendil, A.; Finel, H.; Schouten, H.C.; Ehninger, G.; Maertens, J.; Crawley, C.; Rambaldi, A.; Russell, N.H.; Anders, W.; et al. Reduced Intensity Allogeneic Stem Cell Transplantation for Follicular Lymphoma Relapsing after an Autologous Transplant Achieves Durable Long-Term Disease Control: An Analysis from the Lymphoma Working Party of the EBMT. Ann. Oncol. 2016, 27, 1088. [Google Scholar] [CrossRef]
- Lenz, G. Cellular Therapy in Follicular Lymphoma: Autologous Stem Cell Transplantation, Allogeneic Stem Cell Transplantation, and Chimeric Antigen Receptor T-Cell Therapy. Hematol./Oncol. Clin. 2020, 34, 701–714. [Google Scholar]
- Robinson, S.; Canals, C.; Luang, J.J.; Tilly, H.; Crawley, C.; Cahn, J.; Pohlreich, D.; Gouill, S.L.; Gilleece, M.; Milpied, N.; et al. The Outcome of Reduced Intensity Allogeneic Stem Cell Transplantation and Autologous Stem Cell Transplantation When Performed as a First Transplant Strategy in Relapsed Follicular Lymphoma: An Analysis from the Lymphoma Working Party of the EBMT. Bone Marrow Transplant. 2013, 48, 1409. [Google Scholar] [CrossRef] [PubMed]
- Stem Cells and Cloning: Advances and Applications. Stem Cells Cloning Adv. Appl. 2020. [CrossRef]
- Snowden, J.A.; Sánchez-Ortega, I.; Corbacioglu, S.; Basak, G.; Chabannon, C.; de la Cámara, R.; Dolstra, H.; Duarte, R.F.; Glaß, B.; Greco, R.; et al. Indications for Haematopoietic Cell Transplantation for Haematological Diseases, Solid Tumours and Immune Disorders: Current Practice in Europe, 2022. Bone Marrow Transplant. 2022, 57, 1217. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Muñoz, J.; Trotman, J. Current and Future Therapies for Follicular Lymphoma. Exp. Hematol. Oncol. 2024, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Linton, K.; Vitolo, U.; Jurczak, W.; Lugtenburg, P.J.; Gyan, E.; Sureda, A.; Christensen, J.H.; Hess, B.T.; Tilly, H.; Córdoba, R.; et al. Epcoritamab Monotherapy in Patients with Relapsed or Refractory Follicular Lymphoma (EPCORE NHL-1): A Phase 2 Cohort of a Single-Arm, Multicentre Study. Lancet Haematol. 2024, 11, e593–e605. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Chávez, J.C.; Sehgal, A.R.; Epperla, N.; Ulrickson, M.L.; Bachy, E.; Munshi, P.N.; Casulo, C.; Maloney, D.G.; de Vos, S.; et al. Three-Year Follow-up Analysis of Axicabtagene Ciloleucel in Relapsed/Refractory Indolent Non-Hodgkin Lymphoma (ZUMA-5). Blood 2023, 143, 496. [Google Scholar] [CrossRef]
- Dreyling, M.; Fowler, N.; Dickinson, M.; Martínez-López, J.; Kolstad, A.; Butler, J.; Ghosh, M.; Popplewell, L.; Chávez, J.C.; Bachy, E.; et al. Durable Response after Tisagenlecleucel in Adults with Relapsed/Refractory Follicular Lymphoma: ELARA Trial Update. Blood 2024, 143, 1713. [Google Scholar] [CrossRef]
- Morschhauser, F.; Dahiya, S.; Palomba, M.L.; Martin Garcia-Sancho, A.; Ortega, J.L.R.; Kuruvilla, J.; Jäger, U.; Cartron, G.; Izutsu, K.; Dreyling, M.; et al. Lisocabtagene Maraleucel in Follicular Lymphoma: The Phase 2 TRANSCEND FL Study. Nat. Med. 2024, 30, 2199. [Google Scholar] [CrossRef] [PubMed]
- Nastoupil, L.J.; Bonner, A.; Wang, P.; Almuallem, L.; Desai, J.; Farazi, T.A.; Kumar, J.; Dahiya, S. Matching-Adjusted Indirect Comparison of Efficacy and Safety of Lisocabtagene Maraleucel and Mosunetuzumab for the Treatment of Third-Line or Later Relapsed or Refractory Follicular Lymphoma. Exp. Hematol. Oncol. 2025, 14, 30. [Google Scholar] [CrossRef]
- Budde, L.E.; Sehn, L.H.; Matasar, M.J.; Schuster, S.J.; Assouline, S.; Giri, P.; Kuruvilla, J.; Canales, M.; Dietrich, S.; Fay, K.; et al. Safety and Efficacy of Mosunetuzumab, a Bispecific Antibody, in Patients with Relapsed or Refractory Follicular Lymphoma: A Single-Arm, Multicentre, Phase 2 Study. Lancet Oncol. 2022, 23, 1055. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Bartlett, N.L.; Matasar, M.J.; Schuster, S.J.; Assouline, S.; Giri, P.; Kuruvilla, J.; Shadman, M.; Cheah, C.Y.; Dietrich, S.; et al. Long-Term 3-Year Follow-up of Mosunetuzumab in Relapsed or Refractory Follicular Lymphoma after ≥2 Prior Therapies. Blood 2024, 145, 708–719. [Google Scholar] [CrossRef]
- Linton, K.; Vose, J.M.; Jurczak, W.; Lugtenburg, P.J.; Gyan, E.; Chávez, J.C.; Sureda, A.; Christensen, J.H.; Tilly, H.; Córdoba, R.; et al. Epcoritamab Monotherapy Demonstrates Deep and Durable Responses at 3-Year Follow-Up in Patients with Relapsed/Refractory Follicular Lymphoma. Hematol. Oncol. 2025, 43, e240_70094. [Google Scholar] [CrossRef]
- Kim, T.M.; Taszner, M.; Novelli, S.; Cho, S.; Villasboas, J.C.; Merli, M.; Ubieto, A.J.; Tessoulin, B.; Poon, L.M.; Tucker, D.; et al. Safety and Efficacy of Odronextamab in Patients with Relapsed or Refractory Follicular Lymphoma. Ann. Oncol. 2024, 35, 1039. [Google Scholar] [CrossRef]
- Danilov, A.V.; Kambhampati, S.; Linton, K.; Cumings, K.; Chirikov, V.; Mutebi, A.; Chawla, S.B.; Chhibber, A.; Navarro, F.R.; Gonçalves, F.M.; et al. Indirect Comparison of Epcoritamab vs Chemoimmunotherapy, Mosunetuzumab, or Odronextamab in Follicular Lymphoma. Blood Adv. 2025, 9, 3754. [Google Scholar] [CrossRef] [PubMed]
- Nastoupil, L.J.; Bonner, A.; Wang, P.; Almuallem, L.; Desai, J.; Fasan, O.; Farazi, T.; Kumar, J.; Dahiya, S. Matching-Adjusted Indirect Comparison (MAIC) of Effcacy and Safety of Lisocabtagene Maraleucel (Liso-Cel) and Mosunetuzumab for the Treatment (Tx) of Third Line or Later (3L+) Relapsed or Refractory (R/R) Follicular Lymphoma (FL). Blood 2023, 142, 2338. [Google Scholar] [CrossRef]
- Ray, M.D.; Kanters, S.; Beygi, S.; Best, T.; Wulff, J.; Limbrick-Oldfield, E.; Patel, A.R.; Oluwole, O.O. Matching-Adjusted Indirect Comparisons of Axicabtagene Ciloleucel to Mosunetuzumab for the Treatment of Relapsed/Refractory Follicular Lymphoma. Transplant. Cell. Ther. 2024, 30, 885-e1. [Google Scholar] [CrossRef]
- Oluwole, O.O.; Ray, M.D.; Zur, R.M.; Little, A.; Ferrufino, C.P.; Doble, B.; Patel, A.R.; Bilir, P. Cost-Effectiveness of Axicabtagene Ciloleucel Versus Mosunetuzumab in Relapsed/Refractory Follicular Lymphoma in the US. Blood 2023, 142, 5082. [Google Scholar] [CrossRef]
- Sun, L.; Romancik, J.T. The Development and Application of Bispecific Antibodies in B-Cell Non-Hodgkin Lymphoma. J. Pers. Med. 2025, 15, 51. [Google Scholar] [CrossRef]
- Omer, M.H.; Shafqat, A.; Ahmad, O.; Alkattan, K.; Yaqinuddin, A.; Damlaj, M. Bispecific Antibodies in Hematological Malignancies: A Scoping Review. Cancers 2023, 15, 4550. [Google Scholar] [CrossRef]
- Jacobs, R.; Jacobson, C.A. The Treatment of Follicular Lymphoma with CD19-Directed Chimeric Antigen Receptor T-Cell Therapy. Front. Oncol. 2024, 14, 1384600. [Google Scholar] [CrossRef]
- Cappell, K.M.; Kochenderfer, J.N. Long-Term Outcomes Following CAR T Cell Therapy: What We Know so Far. Nat. Rev. Clin. Oncol. 2023, 20, 359. [Google Scholar] [CrossRef]
| Trial | CAR-T Product | Patient Population | Overall Response Rate | Complete Remission Rate | Key Findings |
|---|---|---|---|---|---|
| ZUMA-5 [25] | Axicabtagene Ciloleucel (axi-cel) directed against the CD19 antigen | Relapsed/Refractory (R/R) indolent Non-Hodgkin Lymphoma (iNHL), including follicular lymphoma and marginal zone lymphoma, after ≥2 lines of therapy | 94% (Primary analysis: 92%) | 80% (Primary analysis: 74%) | Demonstrated continued durable responses after 3 years, with very few relapses beyond 2 years. Median progression-free survival in FL was 40.2 months. Elevated baseline total metabolic tumor volume and recent prior bendamustine use may affect durable remissions. |
| ELARA [26] | Tisagenlecleucel directed against the CD19 antigen | R/R Follicular Lymphoma (grades 1–3A) after ≥2 lines of prior therapy or after autologous stem cell transplantation (auto-SCT) | 86% | 69% | Showed highly durable efficacy and a favorable safety profile with a median follow-up of 29 months 24-month PFS rate was 57.4%. No new safety signals or treatment-related deaths were reported. Low levels of tumor-infiltrating LAG3 + CD3+ exhausted T cells and higher baseline levels of naive CD8+ T cells were associated with improved outcomes. |
| TRANSCEND FL [27] | Lisocabtagene Maraleucel (liso-cel) directed against the CD19 antigen | R/R Follicular Lymphoma, including second-line (2L) patients who all had progression of disease within 24 months from diagnosis % (L+), 96% (2L) | 97% | 94% (3L+), 100% (2L) | Showed promising results for both 2L and 3L+ R/R FL; minimal residual disease negativity was observed. Cytokine release syndrome occurred in 58% of patients (Grade ≥ 3, 1%); neurological events occurred in 15% of patients (Grade ≥ 3, 2%). The patient population was relatively young, consistent with other phase 2 studies for axi-cel and tisagenlecleucel. |
| Bispecific Antibodies | CART Therapy |
|---|---|
| off the shelf | currently approved products are autologous (require apheresis) |
| no risk of manufacturing failure | potential for manufacturing failures |
| no lymphodepletion | requires lymphodepletion |
| lower incidence and severity of CRS/ICANS | increased incidence of high-grade CRS/ICANS |
| requires multiple doses | one and done administration |
| high incidence of infections, need for IVIG support | high incidence of infections, need for IVIG support |
| dose interruption allowed to manage toxicities |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóna, Á.; Illés, Á. Sequencing Cellular Therapies in the Management of Follicular Lymphoma. Cells 2025, 14, 1671. https://doi.org/10.3390/cells14211671
Jóna Á, Illés Á. Sequencing Cellular Therapies in the Management of Follicular Lymphoma. Cells. 2025; 14(21):1671. https://doi.org/10.3390/cells14211671
Chicago/Turabian StyleJóna, Ádám, and Árpád Illés. 2025. "Sequencing Cellular Therapies in the Management of Follicular Lymphoma" Cells 14, no. 21: 1671. https://doi.org/10.3390/cells14211671
APA StyleJóna, Á., & Illés, Á. (2025). Sequencing Cellular Therapies in the Management of Follicular Lymphoma. Cells, 14(21), 1671. https://doi.org/10.3390/cells14211671





