Cyclic Nucleotide Phosphodiesterase Families as Targets to Treat Pulmonary Arterial Hypertension: Beyond PDE5 Inhibitors?
Abstract
1. Pulmonary Arterial Hypertension
1.1. Introduction
1.2. Definition, Classification, and Pathophysiology of PAH
1.3. Current Therapeutic Options
2. Cyclic Nucleotide Pathways as Therapeutic Targets in PAH
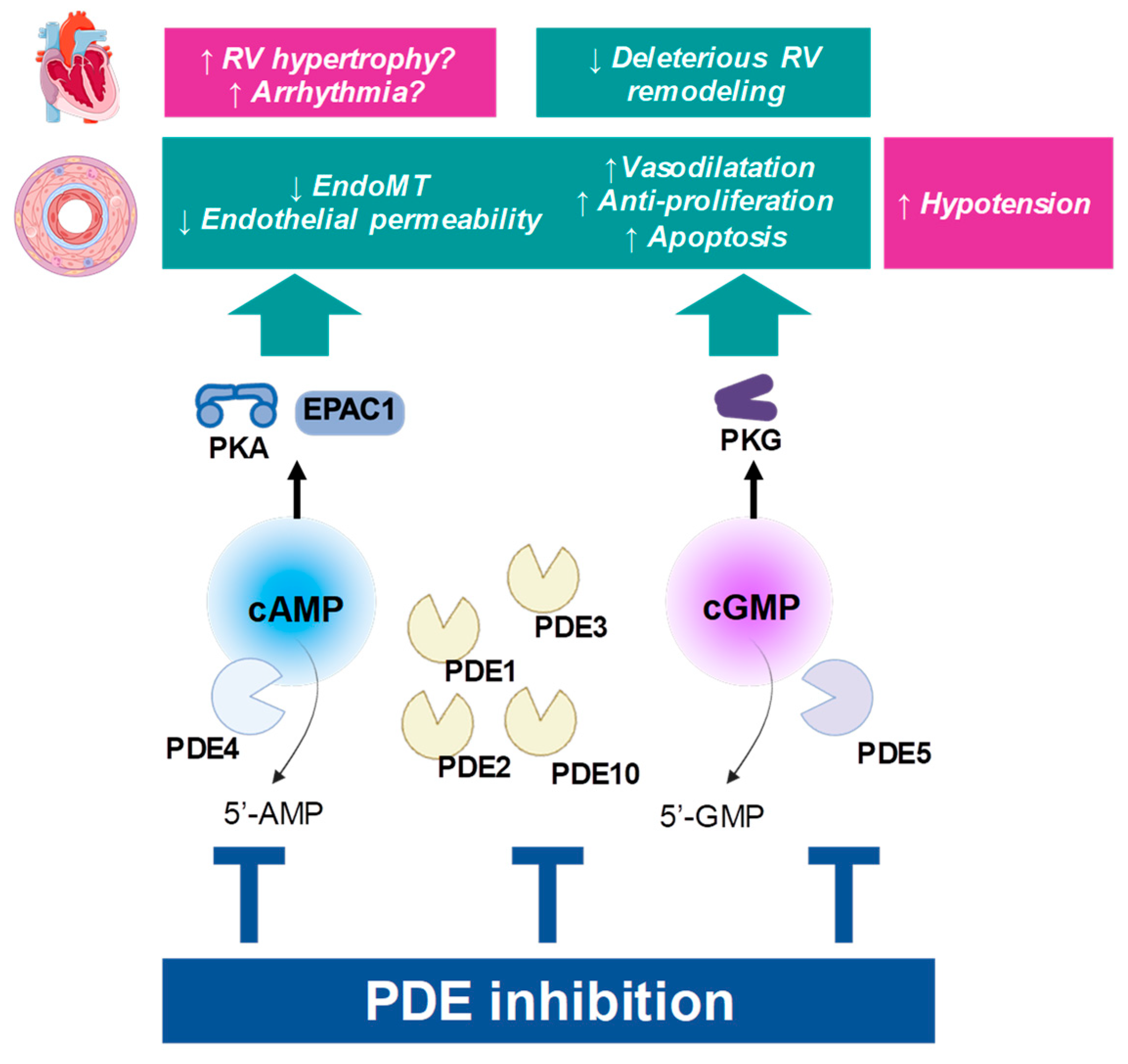
2.1. General Roles of Cyclic Nucleotide Pathways in Circulation
2.2. Molecular Determinants of the cAMP and cGMP Pathway
2.2.1. Synthesis of cGMP and cAMP
cGMP
cAMP
2.2.2. Hydrolysis of cGMP and cAMP by PDEs
| PDE | Gene | Km (µM) [38] | Substrate Selectivity and Salient Features | Pulmonary Artery Activity/Expression | Inhibitors (IC50, nM) |
|---|---|---|---|---|---|
| PDE1 | PDE1A | cAMP: 73–120 cGMP: 2.6–5 | dual substrate Ca2+/calmodulin-regulated | Vessel: (+) [39,40,41,42] VSMC: (+) [43,44,45] (PDE1C highly expressed in proliferative phenotype) [43] EC: ND | 8MM-IBMX (NS) vinpocetine: 14,000 |
| PDE1B | cAMP: 10–24 cGMP: 1.2–5.9 | ||||
| PDE1C | cAMP: 0.3–1.2 cGMP: 0.6–2.2 | ||||
| PDE2 | PDE2A | cAMP: 30–50 cGMP: 10–30 | dual substrate cGMP-stimulated | Vessel: (+) [39,40,42,46,47] VSMC: (+) [44] EC: (+) [48] | EHNA: 800 Bay 60-7550: 4.7 |
| PDE3 | PDE3A PDE3B | cAMP: 0.02–0.15 cGMP: 0.18 | dual substrate cGMP-inhibited | Vessel: (++) [39,40,42] VSMC: (+) [44,49] EC: (+) [48] | cilostamide: 25–50 cilostazol: 200 milrinone: 150 |
| PDE4 | PDE4A PDE4B PDE4C PDE4D | cAMP: 2.9–10 | cAMP-selective | Vessel: (++) [39,40,42] VSMC: (++) [44] EC: (+) [48] | rolipram:1000 cilomilast: 70–120 roflumilast: 0.4–0.6 |
| PDE5 | PDE5A | cGMP: 1–6.2 | cGMP-binding, cGMP-selective | Vessel: (+++) [39,40,42] VSMC: (+++) [43,49,50] EC: ND | zaprinast: 500–700 sildenafil: 5 vardenafil: 1 tadalafil: 5 |
| PDE6 | PDE6A | cGMP: 15–17 | cGMP-selective, photoreceptor | not expressed [34] | ND |
| PDE7 | PDE7A PDE7B | cAMP: 0.1–0.2 | cAMP-selective | VSMC: (+) (mRNA) [51,52] EC: ND | rolipram-insensitive |
| PDE8 | PDE8A PDE8B | cAMP: 0.04–0.06 | cAMP-selective | VSMC: (+) (human mRNA) [52]; (−) (rat mRNA) [51] EC: ND | ND |
| PDE9 | PDE9A | cGMP: 0.17–0.39 | cGMP-selective | VSMC: (±) (mRNA) [51,52] EC: ND | Bay 73-6691: 55 PF-04447943: 2.8 |
| PDE10 | PDE10A | cAMP: 0.26 cGMP: 7.2 | cAMP-inhibited, dual substrate | VSMC: (+) (mRNA) [52]; (−) (rat mRNA) [51]; (immunoreactivity) [53] EC: ND | papaverine: 36 |
| PDE11 | PDE11A | cAMP: 1.04–5.7 cGMP: 0.52–4.2 | dual substrate | VSMC: (+) (mRNA) [52] EC: ND | ND |
2.3. General Modulation of cAMP and cGMP Pathways in PAH
2.3.1. Alterations of cAMP and cGMP Levels in PAH
2.3.2. Therapies to Stimulate the cGMP Pathway in PAH
Promoting the NO–sGC Axis
Promoting the NP System
2.3.3. Therapies to Stimulate the cAMP Pathway in PAH
3. Exploring PDE Families in Pulmonary Arteries and Relevance in PAH
3.1. Overview of PDE Families
3.2. Phosphodiesterase 1 (PDE1)
3.2.1. Enzymatic Properties
3.2.2. Expression Pattern
3.2.3. Functional Role and Therapeutic Potential
3.2.4. Perspectives and Limitations
3.3. Phosphodiesterase 2 (PDE2)
3.3.1. Enzymatic Properties
3.3.2. Expression Pattern
3.3.3. Functional Role and Therapeutic Potential
3.3.4. Perspectives and Limitations
3.4. Phosphodiesterase 3 (PDE3)
3.4.1. Enzymatic Properties
3.4.2. Expression Pattern
3.4.3. Functional Role and Therapeutic Potential
3.4.4. Perspectives and Limitations
3.5. Phosphodiesterase 4 (PDE4)
3.5.1. Enzymatic Properties
3.5.2. Expression Pattern
3.5.3. Functional Role and Therapeutic Potential
3.5.4. Perspectives and Limitations
3.6. Phosphodiesterase 5 (PDE5)
3.6.1. Enzymatic Properties
3.6.2. Expression Pattern
3.6.3. Functional Role and Therapeutic Potential
3.6.4. Perspectives and Limitations
3.7. Phosphodiesterase 9 (PDE9)
3.7.1. Enzymatic Properties
3.7.2. Expression Pattern
3.7.3. Functional Role and Therapeutic Potential
3.7.4. Perspectives and Limitations
3.8. Phosphodiesterase 10 (PDE10)
3.8.1. Enzymatic Properties
3.8.2. Expression Pattern
3.8.3. Functional Role and Therapeutic Potential
3.8.4. Perspectives and Limitations
3.9. Other PDEs
4. Future Directions and Limitations
4.1. Potential Systemic Adverse Effects of PDE Inhibition (e.g., Hypotension, Cardiac Effects)
4.2. Challenges in Developing Isoform- or Cell-Type-Selective PDE Inhibitors
4.2.1. Identification of Relevant PDE Isoforms and Subcellular Complexes
4.2.2. Toward High-Resolution Targeting?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | adenylyl cyclase |
| BMPR2 | bone morphogenetic protein receptor 2 |
| cAMP | 3′, 5′ cyclic adenosine monophosphate |
| cGMP | 3′, 5′ cyclic guanosine monophosphate |
| CHx | chronic hypoxia |
| EC | endothelial cell |
| EndoMT | endothelial-to-mesenchymal transition |
| eNOS | endothelial nitric oxide synthase |
| EPAC1 | exchange protein activated by cAMP 1 |
| ET-1 | endothelin-1 |
| iPAH | idiopathic pulmonary arterial hypertension |
| MCT | monocrotaline |
| mPAP | mean pulmonary arterial pressure |
| MRP4 | multidrug resistance-associated protein 4 |
| NO | nitric oxide |
| PA | pulmonary artery |
| pGC | particulate guanylyl cyclase |
| PGI2 | prostacyclin |
| PKA | cAMP-activated protein kinase |
| PKG | cGMP-activated protein kinase |
| PTGIS | PGI2 synthase |
| PH | pulmonary hypertension |
| PAH | pulmonary arterial hypertension |
| PAEC | pulmonary arterial endothelial cell |
| PASMC | pulmonary arterial smooth muscle cell |
| PDE | phosphodiesterase |
| PDE5i | phosphodiesterase type-5 inhibitors |
| RV | right ventricle |
| sGC | soluble guanylyl cyclase |
| SMC | smooth muscle cell |
| SuHx | Sugen5416– chronic hypoxia |
| TxA2 | thromboxane A2 |
References
- Leber, L.; Beaudet, A.; Muller, A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: Identification of the most accurate estimates from a systematic literature review. Pulm. Circ. 2021, 11, 2045894020977300. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Badesch, D.B.; Ghofrani, H.A.; Gibbs, J.S.R.; Gomberg-Maitland, M.; McLaughlin, V.V.; Preston, I.R.; Souza, R.; Waxman, A.B.; Grunig, E.; et al. Phase 3 Trial of Sotatercept for Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2023, 388, 1478–1490. [Google Scholar] [CrossRef]
- Sharma, M.; Paudyal, V.; Syed, S.K.; Thapa, R.; Kassam, N.; Surani, S. Management of Pulmonary Arterial Hypertension: Current Strategies and Future Prospects. Life 2025, 15, 430. [Google Scholar] [CrossRef]
- Chin, K.M.; Gaine, S.P.; Gerges, C.; Jing, Z.C.; Mathai, S.C.; Tamura, Y.; McLaughlin, V.V.; Sitbon, O. Treatment algorithm for pulmonary arterial hypertension. Eur. Respir. J. 2024, 64, 2401325. [Google Scholar] [CrossRef]
- Kelly, M.P.; Nikolaev, V.O.; Gobejishvili, L.; Lugnier, C.; Hesslinger, C.; Nickolaus, P.; Kass, D.A.; Pereira de Vasconcelos, W.; Fischmeister, R.; Brocke, S.; et al. Cyclic nucleotide phosphodiesterases as drug targets. Pharmacol. Rev. 2025, 77, 100042. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart. J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, M.; Guignabert, C.; Humbert, M.; Nicolls, M.R. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ. Res. 2014, 115, 165–175. [Google Scholar] [CrossRef] [PubMed]
- D’Alonzo, G.E.; Barst, R.J.; Ayres, S.M.; Bergofsky, E.H.; Brundage, B.H.; Detre, K.M.; Fishman, A.P.; Goldring, R.M.; Groves, B.M.; Kernis, J.T.; et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann. Intern. Med. 1991, 115, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Ghofrani, H.A. The molecular targets of approved treatments for pulmonary arterial hypertension. Thorax 2016, 71, 73–83. [Google Scholar] [CrossRef]
- Benza, R.L.; Miller, D.P.; Barst, R.J.; Badesch, D.B.; Frost, A.E.; McGoon, M.D. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012, 142, 448–456. [Google Scholar] [CrossRef]
- Boucly, A.; Savale, L.; Jais, X.; Bauer, F.; Bergot, E.; Bertoletti, L.; Beurnier, A.; Bourdin, A.; Bouvaist, H.; Bulifon, S.; et al. Association between Initial Treatment Strategy and Long-Term Survival in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2021, 204, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Chambers, D.C.; Cherikh, W.S.; Harhay, M.O.; Hayes, D., Jr.; Hsich, E.; Khush, K.K.; Meiser, B.; Potena, L.; Rossano, J.W.; Toll, A.E.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: Donor and recipient size match. J. Heart Lung Transpl. 2019, 38, 1042–1055. [Google Scholar] [CrossRef]
- Kolaitis, N.A. Lung Transplantation for Pulmonary Arterial Hypertension. Chest 2023, 164, 992–1006. [Google Scholar] [CrossRef]
- Guignabert, C.; Humbert, M. Targeting transforming growth factor-β receptors in pulmonary hypertension. Eur. Respir. J. 2021, 57, 2002341. [Google Scholar] [CrossRef]
- Chen, C.N.; Watson, G.; Zhao, L. Cyclic guanosine monophosphate signalling pathway in pulmonary arterial hypertension. Vasc. Pharmacol. 2013, 58, 211–218. [Google Scholar] [CrossRef]
- Sassi, Y.; Hulot, J.S. Pulmonary hypertension: Novel pathways and emerging therapies inhibitors of cGMP and cAMP metabolism. Handb. Exp. Pharmacol. 2013, 218, 513–529. [Google Scholar] [CrossRef]
- Francis, S.H.; Busch, J.L.; Corbin, J.D.; Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef]
- Surapisitchat, J.; Beavo, J.A. Regulation of endothelial barrier function by cyclic nucleotides: The role of phosphodiesterases. In Handb Exp Pharmacol; Springer: Berlin/Heidelberg, Germany, 2011; pp. 193–210. [Google Scholar]
- Manoury, B.; Idres, S.; Leblais, V.; Fischmeister, R. Ion channels as effectors of cyclic nucleotide pathways: Functional relevance for arterial tone regulation. Pharmacol. Ther. 2020, 209, 107499. [Google Scholar] [CrossRef]
- Vina, D.; Seoane, N.; Vasquez, E.C.; Campos-Toimil, M. cAMP Compartmentalization in Cerebrovascular Endothelial Cells: New Therapeutic Opportunities in Alzheimer’s Disease. Cells 2021, 10, 1951. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Newby, A.C.; Bond, M. Ending Restenosis: Inhibition of Vascular Smooth Muscle Cell Proliferation by cAMP. Cells 2019, 8, 1447. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Al-Lamki, R.S.; Wu, C.; Weiss, A.; Berk, J.; Schermuly, R.T.; Morrell, N.W. Sildenafil potentiates bone morphogenetic protein signaling in pulmonary arterial smooth muscle cells and in experimental pulmonary hypertension. Arter. Thromb. Vasc. Biol. 2013, 33, 34–42. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Al-Lamki, R.S.; Southwood, M.; Zhao, J.; Lever, A.M.; Grimminger, F.; Schermuly, R.T.; Morrell, N.W. Smad-dependent and smad-independent induction of id1 by prostacyclin analogues inhibits proliferation of pulmonary artery smooth muscle cells in vitro and in vivo. Circ. Res. 2010, 107, 252–262. [Google Scholar] [CrossRef]
- Kamel, R.; Leroy, J.; Vandecasteele, G.; Fischmeister, R. Cyclic nucleotide phosphodiesterases as therapeutic targets in cardiac hypertrophy and heart failure. Nat. Rev. Cardiol. 2022, 20, 90–108. [Google Scholar] [CrossRef]
- Benza, R.L.; Grunig, E.; Sandner, P.; Stasch, J.P.; Simonneau, G. The nitric oxide-soluble guanylate cyclase-cGMP pathway in pulmonary hypertension: From PDE5 to soluble guanylate cyclase. Eur. Respir. Rev. 2024, 33, 230183. [Google Scholar] [CrossRef]
- Kuhn, M. Molecular Physiology of Membrane Guanylyl Cyclase Receptors. Physiol. Rev. 2016, 96, 751–804. [Google Scholar] [CrossRef]
- Bobin, P.; Belacel-Ouari, M.; Bedioune, I.; Zhang, L.; Leroy, J.; Leblais, V.; Fischmeister, R.; Vandecasteele, G. Cyclic nucleotide phosphodiesterases in heart and vessels: A therapeutic perspective. Arch. Cardiovasc. Dis. 2016, 109, 431–443. [Google Scholar] [CrossRef]
- Baliga, R.S.; Macallister, R.J.; Hobbs, A.J. Vasoactive peptides and the pathogenesis of pulmonary hypertension: Role and potential therapeutic application. Handb. Exp. Pharmacol. 2013, 218, 477–511. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.; Cooper, D.M. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol. Rev. 2007, 87, 965–1010. [Google Scholar] [CrossRef]
- Jourdan, K.B.; Mason, N.A.; Long, L.; Philips, P.G.; Wilkins, M.R.; Morrell, N.W. Characterization of adenylyl cyclase isoforms in rat peripheral pulmonary arteries. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 280, L1359–L1369. [Google Scholar] [CrossRef] [PubMed]
- El-Haroun, H.; Bradbury, D.; Clayton, A.; Knox, A.J. Interleukin-1beta, transforming growth factor-beta1, and bradykinin attenuate cyclic AMP production by human pulmonary artery smooth muscle cells in response to prostacyclin analogues and prostaglandin E2 by cyclooxygenase-2 induction and downregulation of adenylyl cyclase isoforms 1, 2, and 4. Circ. Res. 2004, 94, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Mathie, A.; Peters, J.A. Guide to Receptors and Channels (GRAC), 5th edition. Br. J. Pharmacol. 2011, 164 (Suppl. S1), S1–S324. [Google Scholar] [CrossRef]
- Keravis, T.; Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: Benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br. J. Pharmacol. 2012, 165, 1288–1305. [Google Scholar] [CrossRef]
- Francis, S.H.; Blount, M.A.; Corbin, J.D. Mammalian cyclic nucleotide phosphodiesterases: Molecular mechanisms and physiological functions. Physiol. Rev. 2011, 91, 651–690. [Google Scholar] [CrossRef] [PubMed]
- Baillie, G.S.; Tejeda, G.S.; Kelly, M.P. Therapeutic targeting of 3’,5’-cyclic nucleotide phosphodiesterases: Inhibition and beyond. Nat. Rev. Drug Discov. 2019, 18, 770–796. [Google Scholar] [CrossRef] [PubMed]
- Sassi, Y.; Lipskaia, L.; Vandecasteele, G.; Nikolaev, V.O.; Hatem, S.N.; Cohen Aubart, F.; Russel, F.G.; Mougenot, N.; Vrignaud, C.; Lechat, P.; et al. Multidrug resistance-associated protein 4 regulates cAMP-dependent signaling pathways and controls human and rat SMC proliferation. J. Clin. Investig. 2008, 118, 2747–2757. [Google Scholar] [CrossRef]
- Hara, Y.; Sassi, Y.; Guibert, C.; Gambaryan, N.; Dorfmuller, P.; Eddahibi, S.; Lompre, A.M.; Humbert, M.; Hulot, J.S. Inhibition of MRP4 prevents and reverses pulmonary hypertension in mice. J. Clin. Investig. 2011, 121, 2888–2897. [Google Scholar] [CrossRef]
- Omori, K.; Kotera, J. Overview of PDEs and their regulation. Circ. Res. 2007, 100, 309–327. [Google Scholar] [CrossRef]
- Maclean, M.R.; Johnston, E.D.; McCulloch, K.M.; Pooley, L.; Houslay, M.D.; Sweeney, G. Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: Changes in pulmonary hypertension. J. Pharmacol. Exp. Ther. 1997, 283, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Tenor, H.; Dent, G.; Schudt, C.; Na-kashima, M.; Magnussen, H. Identification of PDE isozymes in human pulmonary artery and effect of selective PDE inhibitors. Am. J. Physiol. 1994, 266, 536–543. [Google Scholar] [CrossRef]
- Crosswhite, P.; Sun, Z. Inhibition of phosphodiesterase-1 attenuates cold-induced pulmonary hypertension. Hypertension 2013, 61, 585–592. [Google Scholar] [CrossRef]
- Pauvert, O.; Salvail, D.; Rousseau, E.; Lugnier, C.; Marthan, R.; Savineau, J.P. Characterisation of cyclic nucleotide phosphodiesterase isoforms in the media layer of the main pulmonary artery. Biochem. Pharmacol. 2002, 63, 1763–1772. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Pullamsetti, S.S.; Kwapiszewska, G.; Dumitrascu, R.; Tian, X.; Weissmann, N.; Ghofrani, H.A.; Kaulen, C.; Dunkern, T.; Schudt, C.; et al. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: Target for reverse-remodeling therapy. Circulation 2007, 115, 2331–2339. [Google Scholar] [CrossRef]
- Growcott, E.J.; Spink, K.G.; Ren, X.; Afzal, S.; Banner, K.H.; Wharton, J. Phosphodiesterase type 4 expression and anti-proliferative effects in human pulmonary artery smooth muscle cells. Respir. Res. 2006, 7, 9. [Google Scholar] [CrossRef]
- Kimura, M.; Tamura, Y.; Guignabert, C.; Takei, M.; Kosaki, K.; Tanabe, N.; Tatsumi, K.; Saji, T.; Satoh, T.; Kataoka, M.; et al. A genome-wide association analysis identifies PDE1A|DNAJC10 locus on chromosome 2 associated with idiopathic pulmonary arterial hypertension in a Japanese population. Oncotarget 2017, 8, 74917–74926. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haynes, J., Jr.; Killilea, D.W.; Peterson, P.D.; Thompson, W.J. Erythro-9-(2-hydroxy-3-nonyl)adenine inhibits cyclic-3’,5’-guanosine monophosphate-stimulated phosphodiesterase to reverse hypoxic pulmonary vasoconstriction in the perfused rat lung. J. Pharmacol. Exp. Ther. 1996, 276, 752–757. [Google Scholar] [CrossRef]
- Bubb, K.J.; Trinder, S.L.; Baliga, R.S.; Patel, J.; Clapp, L.H.; MacAllister, R.J.; Hobbs, A.J. Inhibition of Phosphodiesterase 2 Augments cGMP and cAMP Signaling to Ameliorate Pulmonary Hypertension. Circulation 2014, 130, 496–507. [Google Scholar] [CrossRef]
- Suttorp, N.; Weber, U.; Welsch, T.; Schudt, C. Role of phosphodiesterases in the regulation of endothelial permeability in vitro. J. Clin. Investig. 1993, 91, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Murray, F.; MacLean, M.R.; Pyne, N.J. Increased expression of the cGMP-inhibited cAMP-specific (PDE3) and cGMP binding cGMP-specific (PDE5) phosphodiesterases in models of pulmonary hypertension. Br. J. Pharmacol. 2002, 137, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Sebkhi, A.; Strange, J.W.; Phillips, S.C.; Wharton, J.; Wilkins, M.R. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation 2003, 107, 3230–3235. [Google Scholar] [CrossRef]
- Phillips, P.G.; Long, L.; Wilkins, M.R.; Morrell, N.W. cAMP phosphodiesterase inhibitors potentiate effects of prostacyclin analogs in hypoxic pulmonary vascular remodeling. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 288, L103–L115. [Google Scholar] [CrossRef][Green Version]
- Murray, F.; Patel, H.H.; Suda, R.Y.; Zhang, S.; Thistlethwaite, P.A.; Yuan, J.X.; Insel, P.A. Expression and activity of cAMP phosphodiesterase isoforms in pulmonary artery smooth muscle cells from patients with pulmonary hypertension: Role for PDE1. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L294–L303. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Vroom, C.; Ghofrani, H.A.; Weissmann, N.; Bieniek, E.; Grimminger, F.; Seeger, W.; Schermuly, R.T.; Pullamsetti, S.S. Phosphodiesterase 10A upregulation contributes to pulmonary vascular remodeling. PLoS ONE 2011, 6, e18136. [Google Scholar] [CrossRef] [PubMed]
- MacLean, M.R.; Sweeney, G.; Baird, M.; McCulloch, K.M.; Houslay, M.; Morecroft, I. 5-Hydroxytryptamine receptors mediating vasoconstriction in pulmonary arteries from control and pulmonary hypertensive rats. Br. J. Pharmacol. 1996, 119, 917–930. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Bhatia, V.; Hinton, M.; Singh, N.; Khan, M.W.; Chelikani, P.; Dakshinamurti, S. Adenylyl Cyclase Isoform 6 in the Pulmonary Artery Is Inhibited by Hypoxia via Cysteine Nitrosylation. Am. J. Respir. Cell Mol. Biol. 2024, 72, 82–96. [Google Scholar] [CrossRef]
- Cohen, A.H.; Hanson, K.; Morris, K.; Fouty, B.; McMurty, I.F.; Clarke, W.; Rodman, D.M. Inhibition of cyclic 3’-5’-guanosine monophosphate-specific phosphodiesterase selectively vasodilates the pulmonary circulation in chronically hypoxic rats. J. Clin. Investig. 1996, 97, 172–179. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Stasch, J.P.; Pullamsetti, S.S.; Middendorff, R.; Muller, D.; Schluter, K.D.; Dingendorf, A.; Hackemack, S.; Kolosionek, E.; Kaulen, C.; et al. Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur. Respir. J. 2008, 32, 881–891. [Google Scholar] [CrossRef]
- Champion, H.C.; Bivalacqua, T.J.; Greenberg, S.S.; Giles, T.D.; Hyman, A.L.; Kadowitz, P.J. Adenoviral gene transfer of endothelial nitric-oxide synthase (eNOS) partially restores normal pulmonary arterial pressure in eNOS-deficient mice. Proc. Natl. Acad. Sci. USA 2002, 99, 13248–13253. [Google Scholar] [CrossRef]
- Grego, A.; Fernandes, C.; Fonseca, I.; Dias-Neto, M.; Costa, R.; Leite-Moreira, A.; Oliveira, S.M.; Trindade, F.; Nogueira-Ferreira, R. Endothelial dysfunction in cardiovascular diseases: Mechanisms and in vitro models. Mol. Cell Biochem. 2025, 480, 4671–4695. [Google Scholar] [CrossRef]
- Giaid, A.; Saleh, D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1995, 333, 214–221. [Google Scholar] [CrossRef]
- Cella, G.; Bellotto, F.; Tona, F.; Sbarai, A.; Mazzaro, G.; Motta, G.; Fareed, J. Plasma markers of endothelial dysfunction in pulmonary hypertension. Chest 2001, 120, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Girgis, R.E.; Champion, H.C.; Diette, G.B.; Johns, R.A.; Permutt, S.; Sylvester, J.T. Decreased exhaled nitric oxide in pulmonary arterial hypertension: Response to bosentan therapy. Am. J. Respir. Crit. Care Med. 2005, 172, 352–357. [Google Scholar] [CrossRef]
- Pepke-Zaba, J.; Higenbottam, T.W.; Dinh-Xuan, A.T.; Stone, D.; Wallwork, J. Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet 1991, 338, 1173–1174. [Google Scholar] [CrossRef]
- Turanlahti, M.; Pesonen, E.; Pohjavuori, M.; Lassus, P.; Fyhrquist, F.; Andersson, S. Plasma cyclic guanosine monophosphate reflecting the severity of persistent pulmonary hypertension of the newborn. Biol. Neonate 2001, 80, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D., Jr.; Lang, P.; Bigatello, L.M.; Vlahakes, G.J.; Zapol, W.M. Inhaled nitric oxide in congenital heart disease. Circulation 1993, 87, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Beghetti, M.; Habre, W.; Friedli, B.; Berner, M. Continuous low dose inhaled nitric oxide for treatment of severe pulmonary hypertension after cardiac surgery in paediatric patients. Br. Heart J. 1995, 73, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.A.; Galie, N.; Grimminger, F.; Grunig, E.; Humbert, M.; Jing, Z.C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef]
- Sandner, P.; Zimmer, D.P.; Milne, G.T.; Follmann, M.; Hobbs, A.; Stasch, J.P. Soluble Guanylate Cyclase Stimulators and Activators. Handb. Exp. Pharmacol. 2021, 264, 355–394. [Google Scholar] [CrossRef]
- Casserly, B.; Klinger, J.R. Brain natriuretic peptide in pulmonary arterial hypertension: Biomarker and potential therapeutic agent. Drug Des. Dev. Ther. 2009, 3, 269–287. [Google Scholar] [CrossRef]
- Klinger, J.R.; Warburton, R.R.; Pietras, L.A.; Smithies, O.; Swift, R.; Hill, N.S. Genetic disruption of atrial natriuretic peptide causes pulmonary hypertension in normoxic and hypoxic mice. Am. J. Physiol. 1999, 276, L868–L874. [Google Scholar] [CrossRef]
- Zhao, L.; Long, L.; Morrell, N.W.; Wilkins, M.R. NPR-A-Deficient mice show increased susceptibility to hypoxia-induced pulmonary hypertension. Circulation 1999, 99, 605–607. [Google Scholar] [CrossRef]
- Klinger, J.R.; Thaker, S.; Houtchens, J.; Preston, I.R.; Hill, N.S.; Farber, H.W. Pulmonary hemodynamic responses to brain natriuretic peptide and sildenafil in patients with pulmonary arterial hypertension. Chest 2006, 129, 417–425. [Google Scholar] [CrossRef]
- Michaels, A.D.; Chatterjee, K.; De Marco, T. Effects of intravenous nesiritide on pulmonary vascular hemodynamics in pulmonary hypertension. J. Card. Fail. 2005, 11, 425–431. [Google Scholar] [CrossRef]
- Baliga, R.S.; Zhao, L.; Madhani, M.; Lopez-Torondel, B.; Visintin, C.; Selwood, D.; Wilkins, M.R.; MacAllister, R.J.; Hobbs, A.J. Synergy between natriuretic peptides and phosphodiesterase 5 inhibitors ameliorates pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2008, 178, 861–869. [Google Scholar] [CrossRef]
- Clements, R.T.; Vang, A.; Fernandez-Nicolas, A.; Kue, N.R.; Mancini, T.J.; Morrison, A.R.; Mallem, K.; McCullough, D.J.; Choudhary, G. Treatment of Pulmonary Hypertension with Angiotensin II Receptor Blocker and Neprilysin Inhibitor Sacubitril/Valsartan. Circ. Heart Fail. 2019, 12, e005819. [Google Scholar] [CrossRef]
- Hobbs, A.J.; Moyes, A.J.; Baliga, R.S.; Ghedia, D.; Ochiel, R.; Sylvestre, Y.; Dore, C.J.; Chowdhury, K.; Maclagan, K.; Quartly, H.L.; et al. Neprilysin inhibition for pulmonary arterial hypertension: A randomized, double-blind, placebo-controlled, proof-of-concept trial. Br. J. Pharmacol. 2019, 176, 1251–1267. [Google Scholar] [CrossRef] [PubMed]
- Sonnweber, T.; Pizzini, A.; Nairz, M.; Weiss, G.; Tancevski, I. Arachidonic Acid Metabolites in Cardiovascular and Metabolic Diseases. Int. J. Mol. Sci. 2018, 19, 3285. [Google Scholar] [CrossRef] [PubMed]
- Adatia, I.; Barrow, S.E.; Stratton, P.D.; Miall-Allen, V.M.; Ritter, J.M.; Haworth, S.G. Thromboxane A2 and prostacyclin biosynthesis in children and adolescents with pulmonary vascular disease. Circulation 1993, 88, 2117–2122. [Google Scholar] [CrossRef]
- Christman, B.W.; McPherson, C.D.; Newman, J.H.; King, G.A.; Bernard, G.R.; Groves, B.M.; Loyd, J.E. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N. Engl. J. Med. 1992, 327, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Cool, C.D.; Geraci, M.W.; Wang, J.; Abman, S.H.; Wright, L.; Badesch, D.; Voelkel, N.F. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1999, 159, 1925–1932. [Google Scholar] [CrossRef]
- Shaul, P.W.; Kinane, B.; Farrar, M.A.; Buja, L.M.; Magness, R.R. Prostacyclin production and mediation of adenylate cyclase activity in the pulmonary artery. Alterations after prolonged hypoxia in the rat. J. Clin. Investig. 1991, 88, 447–455. [Google Scholar] [CrossRef]
- Higenbottam, T.; Wheeldon, D.; Wells, F.; Wallwork, J. Long-term treatment of primary pulmonary hypertension with continuous intravenous epoprostenol (prostacyclin). Lancet 1984, 1, 1046–1047. [Google Scholar] [CrossRef]
- Barst, R.J.; Rubin, L.J.; Long, W.A.; McGoon, M.D.; Rich, S.; Badesch, D.B.; Groves, B.M.; Tapson, V.F.; Bourge, R.C.; Brundage, B.H.; et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N. Engl. J. Med. 1996, 334, 296–301. [Google Scholar] [CrossRef]
- McLaughlin, V.V.; Shillington, A.; Rich, S. Survival in primary pulmonary hypertension: The impact of epoprostenol therapy. Circulation 2002, 106, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Zolty, R. Pulmonary arterial hypertension specific therapy: The old and the new. Pharmacol. Ther. 2020, 214, 107576. [Google Scholar] [CrossRef]
- Kuwano, K.; Hashino, A.; Asaki, T.; Hamamoto, T.; Yamada, T.; Okubo, K.; Kuwabara, K. 2-[4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy]-N-(methylsulfonyl)acetam ide (NS-304), an orally available and long-acting prostacyclin receptor agonist prodrug. J. Pharmacol. Exp. Ther. 2007, 322, 1181–1188. [Google Scholar] [CrossRef]
- Simonneau, G.; Torbicki, A.; Hoeper, M.M.; Delcroix, M.; Karlocai, K.; Galie, N.; Degano, B.; Bonderman, D.; Kurzyna, M.; Efficace, M.; et al. Selexipag: An oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. Eur. Respir. J. 2012, 40, 874–880. [Google Scholar] [CrossRef]
- Sitbon, O.; Channick, R.; Chin, K.M.; Frey, A.; Gaine, S.; Galie, N.; Ghofrani, H.A.; Hoeper, M.M.; Lang, I.M.; Preiss, R.; et al. Selexipag for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2015, 373, 2522–2533. [Google Scholar] [CrossRef] [PubMed]
- Wharton, J.; Strange, J.W.; Moller, G.M.; Growcott, E.J.; Ren, X.; Franklyn, A.P.; Phillips, S.C.; Wilkins, M.R. Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am. J. Respir. Crit. Care Med. 2005, 172, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Murray, F.; Maclean, M.R.; Insel, P.A. Role of phosphodiesterases in adult-onset pulmonary arterial hypertension. In Handb Exp Pharmacol; Springer: Berlin/Heidelberg, Germany, 2011; pp. 279–305. [Google Scholar]
- Chang, L.T.; Sun, C.K.; Sheu, J.J.; Chiang, C.H.; Youssef, A.A.; Lee, F.Y.; Wu, C.J.; Yip, H.K. Cilostazol therapy attenuates monocrotaline-induced pulmonary arterial hypertension in rat model. Circ. J. 2008, 72, 825–831. [Google Scholar] [CrossRef]
- Ito, T.; Zhang, E.; Omori, A.; Kabwe, J.; Kawai, M.; Maruyama, J.; Okada, A.; Yokochi, A.; Sawada, H.; Mitani, Y.; et al. Model difference in the effect of cilostazol on the development of experimental pulmonary hypertension in rats. BMC Pulm. Med. 2021, 21, 377. [Google Scholar] [CrossRef]
- Xing, Y.; Hou, Y.; Fan, T.; Gao, R.; Feng, X.; Li, B.; Pang, J.; Guo, W.; Shu, T.; Li, J.; et al. Endothelial phosphodiesterase 4B inactivation ameliorates endothelial-to-mesenchymal transition and pulmonary hypertension. Acta Pharm. Sin. B 2024, 14, 1726–1741. [Google Scholar] [CrossRef]
- Izikki, M.; Raffestin, B.; Klar, J.; Hatzelmann, A.; Marx, D.; Tenor, H.; Zadigue, P.; Adnot, S.; Eddahibi, S. Effects of roflumilast, a phosphodiesterase-4 inhibitor, on hypoxia- and monocrotaline-induced pulmonary hypertension in rats. J. Pharmacol. Exp. Ther. 2009, 330, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Schermuly, R.T.; Kreisselmeier, K.P.; Ghofrani, H.A.; Yilmaz, H.; Butrous, G.; Ermert, L.; Ermert, M.; Weissmann, N.; Rose, F.; Guenther, A.; et al. Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am. J. Respir. Crit. Care Med. 2004, 169, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mason, N.A.; Morrell, N.W.; Kojonazarov, B.; Sadykov, A.; Maripov, A.; Mirrakhimov, M.M.; Aldashev, A.; Wilkins, M.R. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation 2001, 104, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mason, N.A.; Strange, J.W.; Walker, H.; Wilkins, M.R. Beneficial effects of phosphodiesterase 5 inhibition in pulmonary hypertension are influenced by natriuretic Peptide activity. Circulation 2003, 107, 234–237. [Google Scholar] [CrossRef]
- Li, A.; Zhu, Z.; He, Y.; Dong, Q.; Tang, D.; Chen, Z.; Huang, W. DDCI-01, a novel long acting phospdiesterase-5 inhibitor, attenuated monocrotaline-induced pulmonary hypertension in rats. Pulm. Circ. 2020, 10, 2045894020939842. [Google Scholar] [CrossRef]
- Zhang, B.; Zou, Z.K.; Cai, J.F.; Tan, W.M.; Chen, J.W.; Li, W.E.; Liang, J.N.; Wu, W.P.; Wang, G.; Ruan, X.H.; et al. Discovery and Optimization of Dihydroquinolin-2(1H)-ones as Novel Highly Selective and Orally Bioavailable Phosphodiesterase 5 Inhibitors for the Treatment of Pulmonary Arterial Hypertension. J. Med. Chem. 2024, 67, 22134–22144. [Google Scholar] [CrossRef]
- Murray, F.; MacLean, M.R.; Pyne, N.J. An assessment of the role of the inhibitory gamma subunit of the retinal cyclic GMP phosphodiesterase and its effect on the p42/p44 mitogen-activated protein kinase pathway in animal and cellular models of pulmonary hypertension. Br. J. Pharmacol. 2003, 138, 1313–1319. [Google Scholar] [CrossRef]
- Kolb, T.M.; Johnston, L.; Damarla, M.; Kass, D.A.; Hassoun, P.M. PDE9A deficiency does not prevent chronic-hypoxic pulmonary hypertension in mice. Physiol. Rep. 2021, 9, e15057. [Google Scholar] [CrossRef]
- Bender, A.T.; Beavo, J.A. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol. Rev. 2006, 58, 488–520. [Google Scholar] [CrossRef]
- Roks, A.J.M. Phosphodiesterase-1 in the cardiovascular system. Cell Signal 2022, 92, 110251. [Google Scholar] [CrossRef]
- Pauvert, O.; Lugnier, C.; Keravis, T.; Marthan, R.; Rousseau, E.; Savineau, J.P. Effect of sildenafil on cyclic nucleotide phosphodiesterase activity, vascular tone and calcium signaling in rat pulmonary artery. Br. J. Pharmacol. 2003, 139, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Nagel, D.J.; Zhou, Q.; Cygnar, K.D.; Zhao, H.; Li, F.; Pi, X.; Knight, P.A.; Yan, C. Role of cAMP-phosphodiesterase 1C signaling in regulating growth factor receptor stability, vascular smooth muscle cell growth, migration, and neointimal hyperplasia. Circ. Res. 2015, 116, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Rybalkin, S.D.; Rybalkina, I.; Beavo, J.A.; Bornfeldt, K.E. Cyclic nucleotide phosphodiesterase 1C promotes human arterial smooth muscle cell proliferation. Circ. Res. 2002, 90, 151–157. [Google Scholar] [CrossRef]
- Evgenov, O.V.; Busch, C.J.; Evgenov, N.V.; Liu, R.; Petersen, B.; Falkowski, G.E.; Petho, B.; Vas, A.; Bloch, K.D.; Zapol, W.M.; et al. Inhibition of phosphodiesterase 1 augments the pulmonary vasodilator response to inhaled nitric oxide in awake lambs with acute pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L723–L729. [Google Scholar] [CrossRef]
- Ahn, H.S.; Crim, W.; Romano, M.; Sybertz, E.; Pitts, B. Effects of selective inhibitors on cyclic nucleotide phosphodiesterases of rabbit aorta. Biochem. Pharmacol. 1989, 38, 3331–3339. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.N.; Miller, J.R. Methylxanthine inhibitors of phosphodiesterases. Methods Enzym. 1988, 159, 489–496. [Google Scholar] [CrossRef]
- Bonoczk, P.; Gulyas, B.; Adam-Vizi, V.; Nemes, A.; Karpati, E.; Kiss, B.; Kapas, M.; Szantay, C.; Koncz, I.; Zelles, T.; et al. Role of sodium channel inhibition in neuroprotection: Effect of vinpocetine. Brain Res. Bull. 2000, 53, 245–254. [Google Scholar] [CrossRef]
- Jeon, K.I.; Xu, X.; Aizawa, T.; Lim, J.H.; Jono, H.; Kwon, D.S.; Abe, J.; Berk, B.C.; Li, J.D.; Yan, C. Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism. Proc. Natl. Acad. Sci. USA 2010, 107, 9795–9800. [Google Scholar] [CrossRef]
- Yan, C.; Zhao, A.Z.; Bentley, J.K.; Beavo, J.A. The calmodulin-dependent phosphodiesterase gene PDE1C encodes several functionally different splice variants in a tissue-specific manner. J. Biol. Chem. 1996, 271, 25699–25706. [Google Scholar] [CrossRef]
- Laursen, M.; Beck, L.; Kehler, J.; Christoffersen, C.T.; Bundgaard, C.; Mogensen, S.; Mow, T.J.; Pinilla, E.; Knudsen, J.S.; Hedegaard, E.R.; et al. Novel selective PDE type 1 inhibitors cause vasodilatation and lower blood pressure in rats. Br. J. Pharmacol. 2017, 174, 2563–2575. [Google Scholar] [CrossRef]
- Le, M.L.; Jiang, M.Y.; Han, C.; Yang, Y.Y.; Wu, Y. PDE1 inhibitors: A review of the recent patent literature (2008-present). Expert. Opin. Ther. Pat. 2022, 32, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Nadur, N.F.; de Azevedo, L.L.; Caruso, L.; Graebin, C.S.; Lacerda, R.B.; Kummerle, A.E. The long and winding road of designing phosphodiesterase inhibitors for the treatment of heart failure. Eur. J. Med. Chem. 2020, 212, 113123. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.B.; Katugampola, S.; Able, S.; Napier, C.; Harding, S.E. Profiling of cAMP and cGMP phosphodiesterases in isolated ventricular cardiomyocytes from human hearts: Comparison with rat and guinea pig. Life Sci. 2012, 90, 328–336. [Google Scholar] [CrossRef]
- Beavo, J.A.; Francis, S.H.; Houslay, K.F. Cyclic Nucleotide Phosphodiesterases in Health and Disease; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Sadek, M.S.; Cachorro, E.; El-Armouche, A.; Kammerer, S. Therapeutic Implications for PDE2 and cGMP/cAMP Mediated Crosstalk in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 7462. [Google Scholar] [CrossRef]
- Martinez, S.E.; Wu, A.Y.; Glavas, N.A.; Tang, X.B.; Turley, S.; Hol, W.G.; Beavo, J.A. The two GAF domains in phosphodiesterase 2A have distinct roles in dimerization and in cGMP binding. Proc. Natl. Acad. Sci. USA 2002, 99, 13260–13265. [Google Scholar] [CrossRef]
- Wu, A.Y.; Tang, X.B.; Martinez, S.E.; Ikeda, K.; Beavo, J.A. Molecular determinants for cyclic nucleotide binding to the regulatory domains of phosphodiesterase 2A. J. Biol. Chem. 2004, 279, 37928–37938. [Google Scholar] [CrossRef]
- Wang, L.; Hubert, F.; Idres, S.; Belacel-Ouari, M.; Domergue, V.; Domenichini, S.; Lefebvre, F.; Mika, D.; Fischmeister, R.; Leblais, V.; et al. Phosphodiesterases type 2, 3 and 4 promote vascular tone in mesenteric arteries from rats with heart failure. Eur. J. Pharmacol. 2023, 944, 175562. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, K.; Hensley, K.; Florio, V.A.; Wolda, S.L. Differential expression of the cyclic GMP-stimulated phosphodiesterase PDE2A in human venous and capillary endothelial cells. J. Histochem. Cytochem. 1999, 47, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Podzuweit, T.; Nennstiel, P.; Muller, A. Isozyme selective inhibition of cGMP-stimulated cyclic nucleotide phosphodiesterases by erythro-9-(2-hydroxy-3-nonyl) adenine. Cell Signal 1995, 7, 733–738. [Google Scholar] [CrossRef]
- Mery, P.F.; Pavoine, C.; Pecker, F.; Fischmeister, R. Erythro-9-(2-hydroxy-3-nonyl)adenine inhibits cyclic GMP-stimulated phosphodiesterase in isolated cardiac myocytes. Mol. Pharmacol. 1995, 48, 121–130. [Google Scholar] [CrossRef]
- Boess, F.G.; Hendrix, M.; van der Staay, F.J.; Erb, C.; Schreiber, R.; van Staveren, W.; de Vente, J.; Prickaerts, J.; Blokland, A.; Koenig, G. Inhibition of phosphodiesterase 2 increases neuronal cGMP, synaptic plasticity and memory performance. Neuropharmacology 2004, 47, 1081–1092. [Google Scholar] [CrossRef]
- Seybold, J.; Thomas, D.; Witzenrath, M.; Boral, S.; Hocke, A.C.; Burger, A.; Hatzelmann, A.; Tenor, H.; Schudt, C.; Krull, M.; et al. Tumor necrosis factor-alpha-dependent expression of phosphodiesterase 2: Role in endothelial hyperpermeability. Blood 2005, 105, 3569–3576. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.R.; Verde, I.; Cooper, D.M.; Fischmeister, R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation 2006, 113, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Degerman, E.; Belfrage, P.; Manganiello, V.C. Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3). J. Biol. Chem. 1997, 272, 6823–6826. [Google Scholar] [CrossRef]
- Sudo, T.; Tachibana, K.; Toga, K.; Tochizawa, S.; Inoue, Y.; Kimura, Y.; Hidaka, H. Potent effects of novel anti-platelet aggregatory cilostamide analogues on recombinant cyclic nucleotide phosphodiesterase isozyme activity. Biochem. Pharmacol. 2000, 59, 347–356. [Google Scholar] [CrossRef]
- Bardou, M.; Goirand, F.; Marchand, S.; Rouget, C.; Devillier, P.; Dumas, J.P.; Morcillo, E.J.; Rochette, L.; Dumas, M. Hypoxic vasoconstriction of rat main pulmonary artery: Role of endogenous nitric oxide, potassium channels, and phosphodiesterase inhibition. J. Cardiovasc. Pharmacol. 2001, 38, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.S.; Smith, C.J.; Taylor, A.M.; Rhoades, R.A. Phosphodiesterase inhibition improves agonist-induced relaxation of hypertensive pulmonary arteries. J. Pharmacol. Exp. Ther. 1997, 282, 1650–1657. [Google Scholar] [CrossRef]
- Clarke, W.R.; Uezono, S.; Chambers, A.; Doepfner, P. The type III phosphodiesterase inhibitor milrinone and type V PDE inhibitor dipyridamole individually and synergistically reduce elevated pulmonary vascular resistance. Pulm. Pharmacol. 1994, 7, 81–89. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Ghofrani, H.A.; Enke, B.; Weissmann, N.; Grimminger, F.; Seeger, W.; Schudt, C.; Walmrath, D. Low-dose systemic phosphodiesterase inhibitors amplify the pulmonary vasodilatory response to inhaled prostacyclin in experimental pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1999, 160, 1500–1506. [Google Scholar] [CrossRef]
- Chen, E.P.; Bittner, H.B.; Davis, R.D., Jr.; Van Trigt, P., 3rd. Milrinone improves pulmonary hemodynamics and right ventricular function in chronic pulmonary hypertension. Ann. Thorac. Surg. 1997, 63, 814–821. [Google Scholar] [CrossRef]
- Pullamsetti, S.S.; Savai, R.; Schaefer, M.B.; Wilhelm, J.; Ghofrani, H.A.; Weissmann, N.; Schudt, C.; Fleming, I.; Mayer, K.; Leiper, J.; et al. cAMP phosphodiesterase inhibitors increases nitric oxide production by modulating dimethylarginine dimethylaminohydrolases. Circulation 2011, 123, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Matot, I.; Gozal, Y. Pulmonary responses to selective phosphodiesterase-5 and phosphodiesterase-3 inhibitors. Chest 2004, 125, 644–651. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- James, A.T.; Corcoran, J.D.; McNamara, P.J.; Franklin, O.; El-Khuffash, A.F. The effect of milrinone on right and left ventricular function when used as a rescue therapy for term infants with pulmonary hypertension. Cardiol. Young 2016, 26, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Bassler, D.; Choong, K.; McNamara, P.; Kirpalani, H. Neonatal persistent pulmonary hypertension treated with milrinone: Four case reports. Biol. Neonate 2006, 89, 1–5. [Google Scholar] [CrossRef]
- Mika, D.; Bobin, P.; Lindner, M.; Boet, A.; Hodzic, A.; Lefebvre, F.; Lechene, P.; Sadoune, M.; Samuel, J.L.; Algalarrondo, V.; et al. Synergic PDE3 and PDE4 control intracellular cAMP and cardiac excitation-contraction coupling in a porcine model. J. Mol. Cell Cardiol. 2019, 133, 57–66. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Krupnik, E.; Tenor, H.; Schudt, C.; Weissmann, N.; Rose, F.; Grimminger, F.; Seeger, W.; Walmrath, D.; Ghofrani, H.A. Coaerosolization of phosphodiesterase inhibitors markedly enhances the pulmonary vasodilatory response to inhaled iloprost in experimental pulmonary hypertension. Maintenance of lung selectivity. Am. J. Respir. Crit. Care Med. 2001, 164, 1694–1700. [Google Scholar] [CrossRef]
- Elmi-Sarabi, M.; Jarry, S.; Couture, E.J.; Haddad, F.; Cogan, J.; Sweatt, A.J.; Rousseau-Saine, N.; Beaubien-Souligny, W.; Fortier, A.; Denault, A.Y. Pulmonary Vasodilator Response of Combined Inhaled Epoprostenol and Inhaled Milrinone in Cardiac Surgical Patients. Anesth. Analg. 2023, 136, 282–294. [Google Scholar] [CrossRef]
- Fertig, B.A.; Baillie, G.S. PDE4-Mediated cAMP Signalling. J. Cardiovasc. Dev. Dis. 2018, 5, 8. [Google Scholar] [CrossRef]
- Rich, T.C.; Tse, T.E.; Rohan, J.G.; Schaack, J.; Karpen, J.W. In vivo assessment of local phosphodiesterase activity using tailored cyclic nucleotide-gated channels as cAMP sensors. J. Gen. Physiol. 2001, 118, 63–78. [Google Scholar] [CrossRef]
- Richeldi, L.; Azuma, A.; Cottin, V.; Kreuter, M.; Maher, T.M.; Martinez, F.J.; Oldham, J.M.; Valenzuela, C.; Clerisme-Beaty, E.; Gordat, M.; et al. Nerandomilast in Patients with Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2025, 392, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.M.; Assassi, S.; Azuma, A.; Cottin, V.; Hoffmann-Vold, A.M.; Kreuter, M.; Oldham, J.M.; Richeldi, L.; Valenzuela, C.; Wijsenbeek, M.S.; et al. Nerandomilast in Patients with Progressive Pulmonary Fibrosis. N. Engl. J. Med. 2025, 392, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- Schermuly, R.T.; Kreisselmeier, K.P.; Ghofrani, H.A.; Samidurai, A.; Pullamsetti, S.; Weissmann, N.; Schudt, C.; Ermert, L.; Seeger, W.; Grimminger, F. Antiremodeling effects of iloprost and the dual-selective phosphodiesterase 3/4 inhibitor tolafentrine in chronic experimental pulmonary hypertension. Circ. Res. 2004, 94, 1101–1108. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Rose, F.; Schermuly, R.T.; Olschewski, H.; Wiedemann, R.; Weissmann, N.; Schudt, C.; Tenor, H.; Seeger, W.; Grimminger, F. Amplification of the pulmonary vasodilatory response to inhaled iloprost by subthreshold phosphodiesterase types 3 and 4 inhibition in severe pulmonary hypertension. Crit. Care Med. 2002, 30, 2489–2492. [Google Scholar] [CrossRef]
- Liu, S.F.; Nambiar Veetil, N.; Li, Q.; Kucherenko, M.M.; Knosalla, C.; Kuebler, W.M. Pulmonary hypertension: Linking inflammation and pulmonary arterial stiffening. Front. Immunol. 2022, 13, 959209. [Google Scholar] [CrossRef]
- Huertas, A.; Tu, L.; Humbert, M.; Guignabert, C. Chronic inflammation within the vascular wall in pulmonary arterial hypertension: More than a spectator. Cardiovasc. Res. 2020, 116, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J.D.; Turko, I.V.; Beasley, A.; Francis, S.H. Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. Eur. J. Biochem. 2000, 267, 2760–2767. [Google Scholar] [CrossRef]
- Francis, S.H.; Bessay, E.P.; Kotera, J.; Grimes, K.A.; Liu, L.; Thompson, W.J.; Corbin, J.D. Phosphorylation of isolated human phosphodiesterase-5 regulatory domain induces an apparent conformational change and increases cGMP binding affinity. J. Biol. Chem. 2002, 277, 47581–47587. [Google Scholar] [CrossRef]
- Zoraghi, R.; Bessay, E.P.; Corbin, J.D.; Francis, S.H. Structural and functional features in human PDE5A1 regulatory domain that provide for allosteric cGMP binding, dimerization, and regulation. J. Biol. Chem. 2005, 280, 12051–12063. [Google Scholar] [CrossRef][Green Version]
- Corbin, J.D.; Beasley, A.; Blount, M.A.; Francis, S.H. High lung PDE5: A strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem. Biophys. Res. Commun. 2005, 334, 930–938. [Google Scholar] [CrossRef]
- Nagendran, J.; Archer, S.L.; Soliman, D.; Gurtu, V.; Moudgil, R.; Haromy, A.; St Aubin, C.; Webster, L.; Rebeyka, I.M.; Ross, D.B.; et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation 2007, 116, 238–248. [Google Scholar] [CrossRef]
- Mauricio, M.D.; Segarra, G.; Medina, P.; Aldasoro, M.; Martinez-Leon, J.B.; Vila, J.M. Relaxation and cyclic GMP levels in response to sildenafil in human pulmonary arteries from donors. Eur. J. Pharmacol. 2006, 530, 259–262. [Google Scholar] [CrossRef]
- Kang, K.K.; Ahn, G.J.; Sohn, Y.S.; Ahn, B.O.; Kim, W.B. DA-8159, a new PDE5 inhibitor, attenuates the development of compensatory right ventricular hypertrophy in a rat model of pulmonary hypertension. J. Int. Med. Res. 2003, 31, 517–528. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.Y.; Guan, Q. Oral sildenafil prevents and reverses the development of pulmonary hypertension in monocrotaline-treated rats. Interact. Cardiovasc. Thorac. Surg. 2007, 6, 608–613. [Google Scholar] [CrossRef]
- Sauzeau, V.; Rolli-Derkinderen, M.; Lehoux, S.; Loirand, G.; Pacaud, P. Sildenafil prevents change in RhoA expression induced by chronic hypoxia in rat pulmonary artery. Circ. Res. 2003, 93, 630–637. [Google Scholar] [CrossRef]
- Michelakis, E.D.; Tymchak, W.; Noga, M.; Webster, L.; Wu, X.C.; Lien, D.; Wang, S.H.; Modry, D.; Archer, S.L. Long-term treatment with oral sildenafil is safe and improves functional capacity and hemodynamics in patients with pulmonary arterial hypertension. Circulation 2003, 108, 2066–2069. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Ghofrani, H.A.; Torbicki, A.; Barst, R.J.; Rubin, L.J.; Badesch, D.; Fleming, T.; Parpia, T.; Burgess, G.; Branzi, A.; et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2005, 353, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Brundage, B.H.; Ghofrani, H.A.; Oudiz, R.J.; Simonneau, G.; Safdar, Z.; Shapiro, S.; White, R.J.; Chan, M.; Beardsworth, A.; et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009, 119, 2894–2903. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.C.; Yu, Z.X.; Shen, J.Y.; Wu, B.X.; Xu, K.F.; Zhu, X.Y.; Pan, L.; Zhang, Z.L.; Liu, X.Q.; Zhang, Y.S.; et al. Vardenafil in pulmonary arterial hypertension: A randomized, double-blind, placebo-controlled study. Am. J. Respir. Crit. Care Med. 2011, 183, 1723–1729. [Google Scholar] [CrossRef]
- Barnes, H.; Brown, Z.; Burns, A.; Williams, T. Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database Syst. Rev. 2019, 1, CD012621. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Chaumais, M.C.; Savale, L.; Natali, D.; Price, L.C.; Jais, X.; Humbert, M.; Simonneau, G.; Sitbon, O. Phosphodiesterase type 5 inhibitors in pulmonary arterial hypertension. Adv. Ther. 2009, 26, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int. J. Impot. Res. 2004, 16 (Suppl. S1), S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Lorigo, M.; Oliveira, N.; Cairrao, E. PDE-Mediated Cyclic Nucleotide Compartmentation in Vascular Smooth Muscle Cells: From Basic to a Clinical Perspective. J. Cardiovasc. Dev. Dis. 2021, 9, 4. [Google Scholar] [CrossRef]
- Guilluy, C.; Sauzeau, V.; Rolli-Derkinderen, M.; Guerin, P.; Sagan, C.; Pacaud, P.; Loirand, G. Inhibition of RhoA/Rho kinase pathway is involved in the beneficial effect of sildenafil on pulmonary hypertension. Br. J. Pharmacol. 2005, 146, 1010–1018. [Google Scholar] [CrossRef]
- Li, S.; Pan, Y.; Ke, R.; Xie, X.; Zhai, C.; Shi, W.; Wang, J.; Yan, X.; Chai, L.; Wang, Q.; et al. Inhibition of phosphodiesterase-5 suppresses calcineurin/NFAT- mediated TRPC6 expression in pulmonary artery smooth muscle cells. Sci. Rep. 2017, 7, 6088. [Google Scholar] [CrossRef]
- Galie, N.; Muller, K.; Scalise, A.V.; Grunig, E. PATENT PLUS: A blinded, randomised and extension study of riociguat plus sildenafil in pulmonary arterial hypertension. Eur. Respir. J. 2015, 45, 1314–1322. [Google Scholar] [CrossRef]
- Souza, R.; Kawut, S.M. What is new about Rio? Eur. Respir. J. 2015, 45, 1211–1213. [Google Scholar] [CrossRef]
- Li, Q.; Huang, S.S.; Zhang, D.C.; Zhang, W.Y.; Mao, Y.M.; Chen, R.; Jing, Z.C. Evaluation of Safety and Pharmacokinetics of DDCI-01, a Phosphodiesterase Type 5 Inhibitor, in Healthy Participants. Clin. Pharmacokinet. 2025, 64, 573–583. [Google Scholar] [CrossRef]
- Soderling, S.H.; Bayuga, S.J.; Beavo, J.A. Identification and Characterization of a Novel Family of Cyclic Nucleotide Phosphodiesterases. J. Biol. Chem. 1998, 273, 15553–15558. [Google Scholar] [CrossRef] [PubMed]
- Guipponi, M.; Scott, H.S.; Kudoh, J.; Kawasaki, K.; Shibuya, K.; Shintani, A.; Asakawa, S.; Chen, H.; Lalioti, M.D.; Rossier, C.; et al. Identification and characterization of a novel cyclic nucleotide phosphodiesterase gene (PDE9A) that maps to 21q22.3: Alternative splicing of mRNA transcripts, genomic structure and sequence. Hum. Genet. 1998, 103, 386–392. [Google Scholar] [CrossRef]
- Fisher, D.A.; Smith, J.F.; Pillar, J.S.; Denis, S.H.S.; Cheng, J.B.C. Isolation and Characterization of PDE9A, a Novel Human cGMP-specific Phosphodiesterase. J. Biol. Chem. 1998, 273, 15559–15564. [Google Scholar] [CrossRef]
- Patel, N.S.; Klett, J.; Pilarzyk, K.; Lee, D.I.; Kass, D.; Menniti, F.S.; Kelly, M.P. Identification of new PDE9A isoforms and how their expression and subcellular compartmentalization in the brain change across the life span. Neurobiol. Aging 2018, 65, 217–234. [Google Scholar] [CrossRef]
- Rentero, C.; Monfort, A.; Puigdomenech, P. Identification and distribution of different mRNA variants produced by differential splicing in the human phosphodiesterase 9A gene. Biochem. Biophys. Res. Commun. 2003, 301, 686–692. [Google Scholar] [CrossRef]
- Lee, D.I.; Zhu, G.; Sasaki, T.; Cho, G.S.; Hamdani, N.; Holewinski, R.; Jo, S.H.; Danner, T.; Zhang, M.; Rainer, P.P.; et al. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature 2015, 519, 472–476. [Google Scholar] [CrossRef]
- van der Horst, I.W.; Morgan, B.; Eaton, F.; Reiss, I.; Tibboel, D.; Thebaud, B. Expression and function of phosphodiesterases in nitrofen-induced congenital diaphragmatic hernia in rats. Pediatr. Pulmonol. 2010, 45, 320–325. [Google Scholar] [CrossRef]
- Almeida, C.B.; Scheiermann, C.; Jang, J.E.; Prophete, C.; Costa, F.F.; Conran, N.; Frenette, P.S. Hydroxyurea and a cGMP-amplifying agent have immediate benefits on acute vaso-occlusive events in sickle cell disease mice. Blood 2012, 120, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Heckman, P.R.; Wouters, C.; Prickaerts, J. Phosphodiesterase inhibitors as a target for cognition enhancement in aging and Alzheimer’s disease: A translational overview. Curr. Pharm. Des. 2015, 21, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, R.J.; Chapin, D.S.; Christoffersen, C.; Freeman, J.; Fonseca, K.R.; Geoghegan, K.F.; Grimwood, S.; Guanowsky, V.; Hajos, M.; Harms, J.F.; et al. Phosphodiesterase 9A regulates central cGMP and modulates responses to cholinergic and monoaminergic perturbation in vivo. J. Pharmacol. Exp. Ther. 2012, 341, 396–409. [Google Scholar] [CrossRef]
- Hutson, P.H.; Finger, E.N.; Magliaro, B.C.; Smith, S.M.; Converso, A.; Sanderson, P.E.; Mullins, D.; Hyde, L.A.; Eschle, B.K.; Turnbull, Z.; et al. The selective phosphodiesterase 9 (PDE9) inhibitor PF-04447943 (6-[(3S,4S)-4-methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl]-1-(tetrahydro-2H-py ran-4-yl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one) enhances synaptic plasticity and cognitive function in rodents. Neuropharmacology 2011, 61, 665–676. [Google Scholar] [CrossRef]
- Wunder, F.; Tersteegen, A.; Rebmann, A.; Erb, C.; Fahrig, T.; Hendrix, M. Characterization of the first potent and selective PDE9 inhibitor using a cGMP reporter cell line. Mol. Pharmacol. 2005, 68, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.A.; Aronovitz, M.J.; Liu, P.; Martin, G.L.; Tam, K.; Pande, S.; Karas, R.H.; Bloomfield, D.M.; Mendelsohn, M.E.; Blanton, R.M. CRD-733, a Novel PDE9 (Phosphodiesterase 9) Inhibitor, Reverses Pressure Overload-Induced Heart Failure. Circ. Heart Fail. 2021, 14, e007300. [Google Scholar] [CrossRef] [PubMed]
- Rosenbrock, H.; Giovannini, R.; Schanzle, G.; Koros, E.; Runge, F.; Fuchs, H.; Marti, A.; Reymann, K.G.; Schroder, U.H.; Fedele, E.; et al. The Novel Phosphodiesterase 9A Inhibitor BI 409306 Increases Cyclic Guanosine Monophosphate Levels in the Brain, Promotes Synaptic Plasticity, and Enhances Memory Function in Rodents. J. Pharmacol. Exp. Ther. 2019, 371, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Buncha, V.; Fopiano, K.A.; Lang, L.; Ilatovskaya, D.V.; Verin, A.; Bagi, Z. Phosphodiesterase 9A inhibition improves aging-related increase in pulmonary vascular resistance in mice. Geroscience 2024, 46, 5191–5202. [Google Scholar] [CrossRef]
- Scott, N.J.A.; Prickett, T.C.R.; Charles, C.J.; Frampton, C.M.; Richards, A.M.; Rademaker, M.T. Augmentation of Natriuretic Peptide Bioactivity via Combined Inhibition of Neprilysin and Phosphodiesterase-9 in Heart Failure. JACC Heart Fail. 2022, 11, 227–239. [Google Scholar] [CrossRef]
- Scott, N.J.A.; Rademaker, M.T.; Charles, C.J.; Espiner, E.A.; Richards, A.M. Hemodynamic, Hormonal, and Renal Actions of Phosphodiesterase-9 Inhibition in Experimental Heart Failure. J. Am. Coll. Cardiol. 2019, 74, 889–901. [Google Scholar] [CrossRef]
- Rademaker, M.T.; Scott, N.J.A.; Charles, C.J.; Richards, A.M. Combined Inhibition of Phosphodiesterases 5 and 9 in Experimental Heart Failure. JACC Heart Fail. 2023, 12, 100–113. [Google Scholar] [CrossRef]
- Claffey, M.M.; Helal, C.J.; Verhoest, P.R.; Kang, Z.; Fors, K.S.; Jung, S.; Zhong, J.; Bundesmann, M.W.; Hou, X.; Lui, S.; et al. Application of structure-based drug design and parallel chemistry to identify selective, brain penetrant, in vivo active phosphodiesterase 9A inhibitors. J. Med. Chem. 2012, 55, 9055–9068. [Google Scholar] [CrossRef]
- Zhang, L.; Bouadjel, K.; Manoury, B.; Vandecasteele, G.; Fischmeister, R.; Leblais, V. Cyclic nucleotide signalling compartmentation by PDEs in cultured vascular smooth muscle cells. Br. J. Pharmacol. 2019, 176, 1780–1792. [Google Scholar] [CrossRef]
- Charnigo, R.J.; Beidler, D.; Rybin, D.; Pittman, D.D.; Tan, B.; Howard, J.; Michelson, A.D.; Frelinger, A.L., III; Clarke, N. PF-04447943, a Phosphodiesterase 9A Inhibitor, in Stable Sickle Cell Disease Patients: A Phase Ib Randomized, Placebo-Controlled Study. Clin. Transl. Sci. 2019, 12, 180–188. [Google Scholar] [CrossRef]
- Moschetti, V.; Boland, K.; Feifel, U.; Hoch, A.; Zimdahl-Gelling, H.; Sand, M. First-in-human study assessing safety, tolerability and pharmacokinetics of BI 409306, a selective phosphodiesterase 9A inhibitor, in healthy males. Br. J. Clin. Pharmacol. 2016, 82, 1315–1324. [Google Scholar] [CrossRef]
- Jager, R.; Russwurm, C.; Schwede, F.; Genieser, H.G.; Koesling, D.; Russwurm, M. Activation of PDE10 and PDE11 phosphodiesterases. J. Biol. Chem. 2012, 287, 1210–1219. [Google Scholar] [CrossRef]
- Gross-Langenhoff, M.; Hofbauer, K.; Weber, J.; Schultz, A.; Schultz, J.E. cAMP is a ligand for the tandem GAF domain of human phosphodiesterase 10 and cGMP for the tandem GAF domain of phosphodiesterase 11. J. Biol. Chem. 2006, 281, 2841–2846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, X.; Xu, J.; Rothfuss, J.; Mach, R.H.; Tu, Z. Synthesis and in vitro evaluation of new analogues as inhibitors for phosphodiesterase 10A. Eur. J. Med. Chem. 2011, 46, 3986–3995. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, S.; Zhou, Q.; Zhang, C.; Gao, Y.; Wang, H.; Li, Z.; Wu, D.; Wu, Y.; Huang, Y.Y.; et al. Discovery of highly selective and orally available benzimidazole-based phosphodiesterase 10 inhibitors with improved solubility and pharmacokinetic properties for treatment of pulmonary arterial hypertension. Acta Pharm. Sin. B 2020, 10, 2339–2347. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Yu, Y.F.; Zhang, C.; Chen, Y.; Zhou, Q.; Li, Z.; Zhou, S.; Li, Z.; Guo, L.; Wu, D.; et al. Validation of Phosphodiesterase-10 as a Novel Target for Pulmonary Arterial Hypertension via Highly Selective and Subnanomolar Inhibitors. J. Med. Chem. 2019, 62, 3707–3721. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arroyo, J.; Saleem, S.J.; Mizuno, S.; Syed, A.A.; Bogaard, H.J.; Abbate, A.; Taraseviciene-Stewart, L.; Sung, Y.; Kraskauskas, D.; Farkas, D.; et al. A brief overview of mouse models of pulmonary arterial hypertension: Problems and prospects. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L977–L991. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Lighthouse, J.K.; Mickelsen, D.M.; Wu, J.; Yao, P.; Small, E.M.; Yan, C. A Novel Role of Cyclic Nucleotide Phosphodiesterase 10A in Pathological Cardiac Remodeling and Dysfunction. Circulation 2020, 141, 217–233. [Google Scholar] [CrossRef]
- Schmidt, C.J.; Chapin, D.S.; Cianfrogna, J.; Corman, M.L.; Hajos, M.; Harms, J.F.; Hoffman, W.E.; Lebel, L.A.; McCarthy, S.A.; Nelson, F.R.; et al. Preclinical characterization of selective phosphodiesterase 10A inhibitors: A new therapeutic approach to the treatment of schizophrenia. J. Pharmacol. Exp. Ther. 2008, 325, 681–690. [Google Scholar] [CrossRef]
- Molina, C.E.; Johnson, D.M.; Mehel, H.; Spatjens, R.L.; Mika, D.; Algalarrondo, V.; Slimane, Z.H.; Lechene, P.; Abi-Gerges, N.; van der Linde, H.J.; et al. Interventricular differences in beta-adrenergic responses in the canine heart: Role of phosphodiesterases. J. Am. Heart Assoc. 2014, 3, e000858. [Google Scholar] [CrossRef] [PubMed]
- Ghigo, A.; Mika, D. cAMP/PKA signaling compartmentalization in cardiomyocytes: Lessons from FRET-based biosensors. J. Mol. Cell Cardiol. 2019, 131, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Abou-Saleh, H.; Kameno, Y.; Marei, I.; de Nucci, G.; Ahmetaj-Shala, B.; Shala, F.; Kirkby, N.S.; Jennings, L.; Al-Ansari, D.E.; et al. Studies on metal-organic framework (MOF) nanomedicine preparations of sildenafil for the future treatment of pulmonary arterial hypertension. Sci. Rep. 2021, 11, 4336. [Google Scholar] [CrossRef] [PubMed]

| GROUP 1. Pulmonary Arterial Hypertension (PAH) | ||
| 1.1 Idiopathic | ||
| 1.1.1 Not responding to vasoreactivity testing | ||
| 1.1.2 Responding to vasoreactivity testing | ||
| 1.2 Heritable: mutations of genes: BMPR2, EIF2AK4, ACVRL1, ENG… | ||
| 1.3 Associated with drugs and toxins (e.g., fenfluramine, dasatinib) | ||
| 1.4 Associated with: | ||
| 1.4.1 Connective tissue disease | ||
| 1.4.2 HIV infection | ||
| 1.4.3 Portal hypertension | ||
| 1.4.4 Congenital heart disease | ||
| 1.4.5 Schistosomiasis | ||
| 1.5 PAH with features of venous/capillary (PVOD/PCH) involvement | ||
| 1.6 Persistent PH of the newborn | ||
| GROUP 2. PH associated with left heart disease | ||
| GROUP 3. PH associated with lung diseases and/or hypoxia | ||
| GROUP 4. PH associated with pulmonary artery obstructions | ||
| GROUP 5. PH with unclear and/or multifactorial mechanisms | ||
| PDEs | Main Tissue Expression | Compound (Brand Name, Year of Approval) | Indication and Status |
|---|---|---|---|
| PDE1 | brain, smooth muscle, heart, testis | vinpocetine (N.A.) | cerebral vascular disorders and memory impairment; sold as an over-the-counter supplement. |
| PDE2 | adrenal cortex, brain, heart | - | - |
| PDE3 | heart, smooth muscle, adipose tissue, platelets | cilostazol (PLETAL, 1999) | intermittent claudication; second-line therapy |
| milrinone (PRIMACOR, 1987) | congestive heart failure; mainly used in surgery and critical care units for hemodynamic support. | ||
| amrinone (INOCOR, 1984) | congestive heart failure; no longer used | ||
| enoximone (N.A.) | congestive heart failure; limited | ||
| anagrelide (AGRYLIN, 1997) | thrombocythaemia; second-line therapy | ||
| PDE4 | ubiquitous | roflumilast (DALIRESP, 2011) | chronic obstructive pulmonary disease; add-on therapy |
| (ZORYVE, 2022) | plaque psoriasis; topical form in dermatology | ||
| apremilast (OTEZLA, 2014) | psoriasis and psoriatic disorders; Behçet’s disease | ||
| crisaborole (EUCRISA, 2016) | moderate atopic dermatitis (patients >2 years old) | ||
| drotaverine (N.A.) | functional bowel disorders: antispasmodics used worldwide | ||
| PDE5 | smooth muscle, platelets, cerebellum | sildenafil (VIAGRA, 1998) | erectile dysfunction |
| (REVATIO, 2005) | PAH | ||
| vardenafil (LEVITRA, 2003) (STAXYN, 2010) | erectile dysfunction | ||
| tadalafil (CIALIS, 2003) | erectile dysfunction, benign prostatic hyperplasia | ||
| (ADCIRCA, 2009) | PAH | ||
| avanafil (STENDRA, 2012) | erectile dysfunction | ||
| PDE6 | retina | - | - |
| PDE7 | skeletal muscle, immune cells, and brain | - | - |
| PDE8 | immune cells, liver, kidney, testis, thyroid | - | - |
| PDE9 | brain, kidney | - | - |
| PDE10 | brain, testis | papaverine (1938) | visceral and vascular spasm, and erectile dysfunction; not a first-line medication |
| PDE11 | prostate, testis, skeletal muscle |
| PDE | Expression in Patients (RNA Level, Unless Specified) | Effects of Inhibitors on PA Vascular Reactivity | Efficacy of PDE Inhibition in Animal Models | |||||
|---|---|---|---|---|---|---|---|---|
| Animal Model | Change in Expression | Inhibitor Used In Vivo | Key Findings | Mechanistic Insights | Reference | |||
| PDE1 | ↑ PDE1A (++), RNA, and protein [43,45,52] ↑ PDE1C (+++), RNA, and protein [41,43,52] | 8-MM-IBMX: dilates hypoxic rat PA more than normoxic rat PA [43] | rat MCT | ↑ PDE1A | 8-MM-IBMX | effective (curative protocol) | Schermuly et al., 2007 [43] | |
| mouse CHx | ↑ PDE1A | |||||||
| rat cold-induced PAH | ↑ PDE1C | 8-MM-IBMX | effective (curative protocol) | decrease in macrophage infiltration | Crosswhite and Sun, 2013 [41] | |||
| PDE2 | ↓ [47] ↑ (+) [52] | Bay 60-7750 and EHNA have vasorelaxant effects in rat PA and perfused lung | mouse CHx and bleomycin | ↓ PDE2 [52] (hypoxic rat PA) | Bay 60-7550 | effective (preventive protocol) effective in combination with other therapies (curative protocol) | increase in GMP and cAMP signalling | Bubb et al., 20l4 [47] |
| PDE3 | ↑ PDE3B (+++), RNA, and protein [52] ↑ PDE3A (+) [52] | cilostamide attenuates acute hypoxic vasoconstriction; motapizone dose-dependently relaxes human PA [40] | rat CHx | cilostamide | ineffective alone effective in combination with iloprost or rolipram (preventive) | Phillips et al., 2005 [51] | ||
| rat MCT | cilostazol | effective (preventive and curative protocols) | Chang et al., 2008 [91]; Ito et al., 2021 [92] | |||||
| rat CHx | cilostazol | ineffective (preventive) | Ito et al., 2021 [92] | |||||
| PDE4 | PDE4A-D were unchanged in PASMCs [52] ↑ (++) PDE4B in lung, RNA, and protein [93] | rolipram did not relax human PA [40]; rolipram relaxes rat PA [42] | rat CHx | rolipram | ineffective alone effective in combination with iloprost or cilostamide | Phillips et al., 2005 [51] | ||
| rat MCT and CHx | roflumilast | effective (preventive protocols in MCT and CHx rat PA) effective (curative protocol in MCT rat PAH) | reduces interleukin-6 and monocyte chemotactic protein-1 | Izikki et al., 2009 [94] | ||||
| mouse SuHx | ↑ PDE4B in the lung; ↑ PDE4B in PA ECs (under Hx) | roflumilast | effective (preventive protocol) | promotes EndoMT by attenuating the PKA-CREB-BMPR2 axis | Xing et al., 2024 [93] | |||
| PDE4B global KO and EC-specific KO | ||||||||
| rat SuHx | ↑ PDE4B in lung | BI 1015550 | effective (curative protocol) | |||||
| PDE5 | ↑ PDE5A, RNA, and protein [52,89] | zaprinast relaxes human PA dose-dependently [40] E4021, sildenafil decreases hypoxic rat PA pressure [50,56] | rat CHx | PDE5 ↑ (IHC) | sildenafil | effective (preventive and curative protocol) | Sebkhi et al., 2013 [50] Baliga et al., 2008 [74] | |
| rat MCT | sildenafil | effective (curative protocol) | decreases MMP-2 and MMP-9 | Schermuly et al., 2004 [95] | ||||
| mouse CHx | sildenafil | effective (preventive protocol) | Zhao et al., 2001 [96]; Zhao et al., 2003 [97] | |||||
| rat MCT | DDCI | effective (preventive protocol) | Li et al., 2020 [98] | |||||
| rat MCT | dihydroquinolin-2(1H)-ones | effective (preventive protocol) | Zhang et al., 2024 [99] | |||||
| PDE6 | ↑ PDE “γ” under hypoxia [100] | rat CHx | ↑ PDE “γ” under Hx [100] | Not documented | ||||
| PDE7 | ↑ (+) [52] | Not documented | ||||||
| PDE8 | unchanged [52] | Not documented | ||||||
| PDE9 | unchanged [52] | mouse CHx | genetic ablation | ineffective | Kolb et al., 2021 [101] | |||
| PDE10 | unchanged [52] | rat MCT | PDE10 ↑ | papaverine | effective (curative protocol) | Tian et al., 2011 [53] | ||
| PDE11 | unchanged [52] | not documented | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Fischmeister, R.; Manoury, B. Cyclic Nucleotide Phosphodiesterase Families as Targets to Treat Pulmonary Arterial Hypertension: Beyond PDE5 Inhibitors? Cells 2025, 14, 1670. https://doi.org/10.3390/cells14211670
Wang L, Fischmeister R, Manoury B. Cyclic Nucleotide Phosphodiesterase Families as Targets to Treat Pulmonary Arterial Hypertension: Beyond PDE5 Inhibitors? Cells. 2025; 14(21):1670. https://doi.org/10.3390/cells14211670
Chicago/Turabian StyleWang, Liting, Rodolphe Fischmeister, and Boris Manoury. 2025. "Cyclic Nucleotide Phosphodiesterase Families as Targets to Treat Pulmonary Arterial Hypertension: Beyond PDE5 Inhibitors?" Cells 14, no. 21: 1670. https://doi.org/10.3390/cells14211670
APA StyleWang, L., Fischmeister, R., & Manoury, B. (2025). Cyclic Nucleotide Phosphodiesterase Families as Targets to Treat Pulmonary Arterial Hypertension: Beyond PDE5 Inhibitors? Cells, 14(21), 1670. https://doi.org/10.3390/cells14211670








