Distinct Intramuscular Extracellular Matrix Protein Responses to Exercise Training in COPD and Healthy Adults and Their Association with Muscle Remodeling
Highlights
- In COPD, exercise training induced distinct alterations in ECM protein expression related to tissue structure, cell adhesion, myogenesis, and necroptosis.
- These alterations differed from the adaptive remodeling observed in healthy controls.
- ECM proteins play a crucial role in regulating muscle fiber remodeling in response to exercise training.
- Impaired ECM remodeling may contribute to the reduced skeletal muscle adaptability seen in COPD patients, potentially limiting the effectiveness of exercise interventions.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Muscle Biopsy Morphological Analyses
2.4. Quantitative Real-Time PCR Analysis
2.5. Protein Extraction and Analysis
2.6. Statistical Analyses
3. Results
3.1. Myofiber Phenotypic Changes
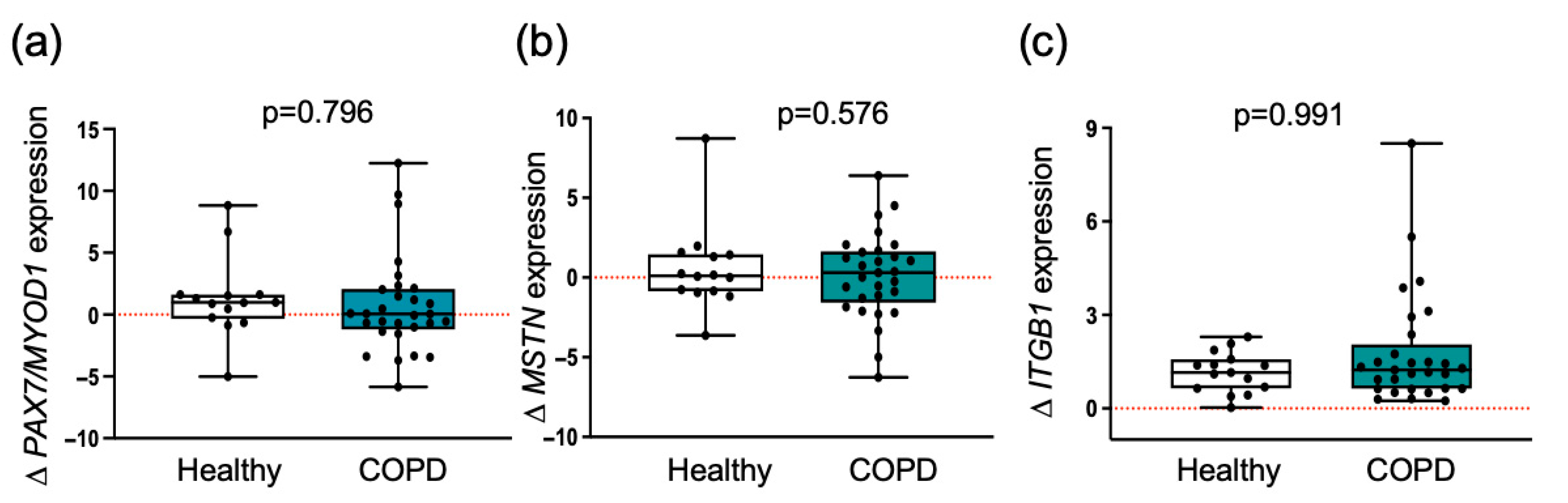
3.2. Myogenic Regulatory Molecules Are Similarly Induced by Exercise Training in Both Groups
| Healthy | COPD | |||||
|---|---|---|---|---|---|---|
| Individuals (n) | 14 | 29 | ||||
| Fiber type distribution (%) | ||||||
| Pre-training | Post-training | ∆ (post-pre) | Pre-training | Post-training | ∆ (post-pre) | |
| Type I % | 39.8 ± 4.6 | 44.5 ± 2.9 | 4.7 ± 3.4 | 33.0 ± 1.8 | 34.3 ± 2.6 † | 1.3 ± 2.2 ‡ |
| Type II % | 59.2 ± 5.9 | 57.6 ± 2.5 | −1.5 ± 4.1 | 65.9 ± 2.4 | 63.4 ± 2.8 | −2.6 ± 2.1 |
| Type IIa % | 38.9 ± 2.8 | 45.2 ± 2.5 # | 6.5 ± 2.3 | 52.0 ± 1.9 † | 54.9 ± 2.3 † | 3.9 ± 1.8 ‡ |
| Type IIx % | 20.2 ± 3.1 | 12.4 ± 1.6 # | −7.8 ± 2.6 | 15.5 ± 0.7 | 10.4 ± 0.5 # | −5.3 ± 0.8 |
| Cross-sectional area (CSA, μm2) | ||||||
| Pre-training | Post-training | %∆ (post-pre) | Pre-training | Post-training | %∆ (post-pre) | |
| Mean all fibers | 4582 ± 155.6 | 5214 ± 162.7 # | 13.6 ± 3.2 | 4100 ± 106.9 † | 4391 ± 129.3 #,† | 9.1 ± 1.9 ‡ |
| Type I | 3974 ± 146.8 | 4425 ± 207.8 # | 14.0 ± 6.6 | 4670 ± 171.9 † | 5049 ± 241.6 | 9.2 ± 3.5 |
| Type IIa | 5656 ± 205.8 | 6354 ± 174.9 # | 12.0 ± 4.0 | 4154 ± 138.7 † | 4460 ± 170.0 #,† | 11.8 ± 3.5 |
| Type IIx | 4176 ± 180.2 | 4813 ± 132.8 # | 14.6 ± 3.0 | 3339 ± 165.2 † | 3638 ± 155.6 #,† | 10.0 ± 3.7 ‡ |
| Capillary/fiber ratio | 2.02 ± 0.14 | 2.13 ± 0.13 | 13.2 ± 2.5 | 1.43 ± 0.07 † | 1.64 ± 0.07 #,† | 16.7 ± 4.3 ‡ |
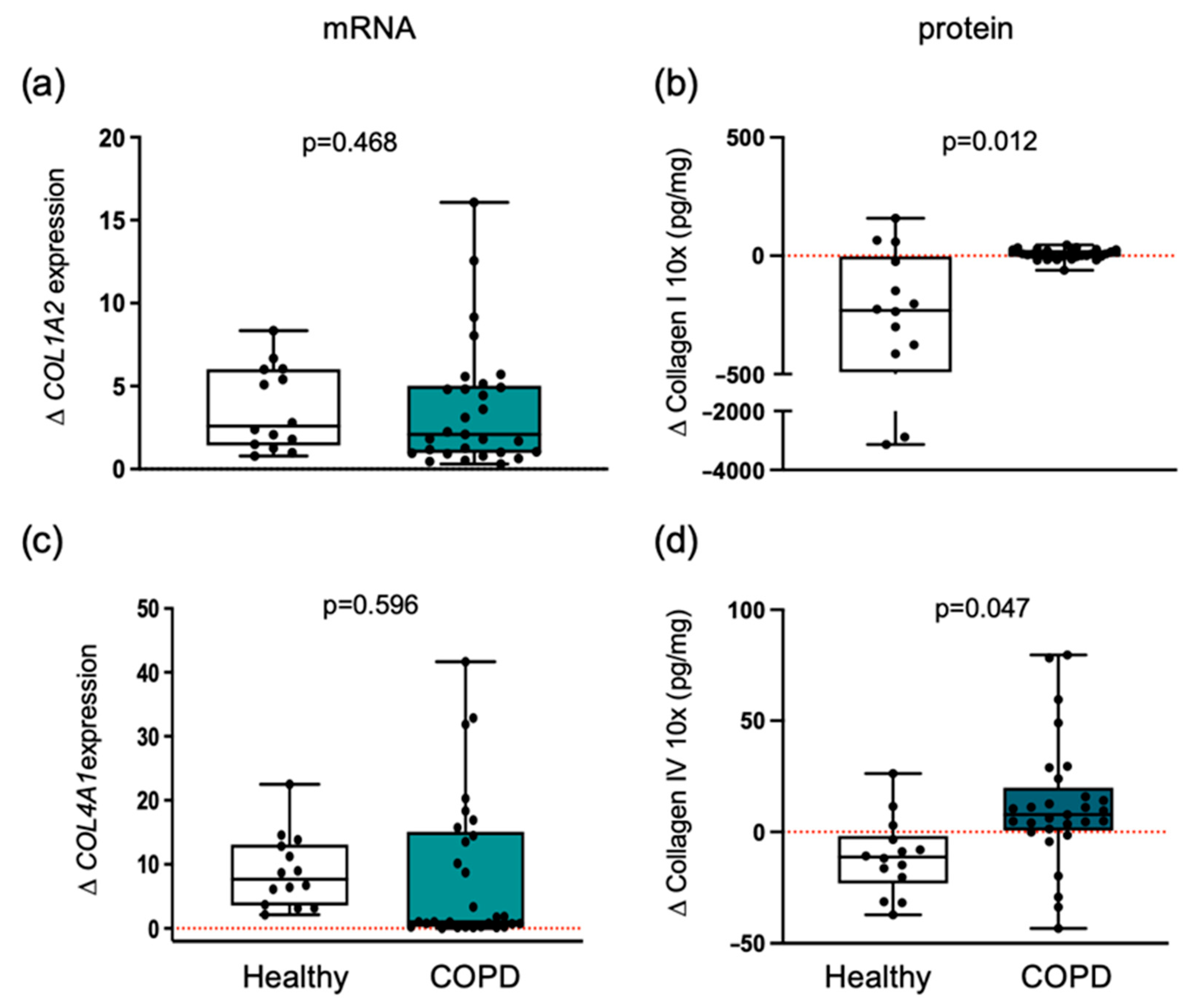
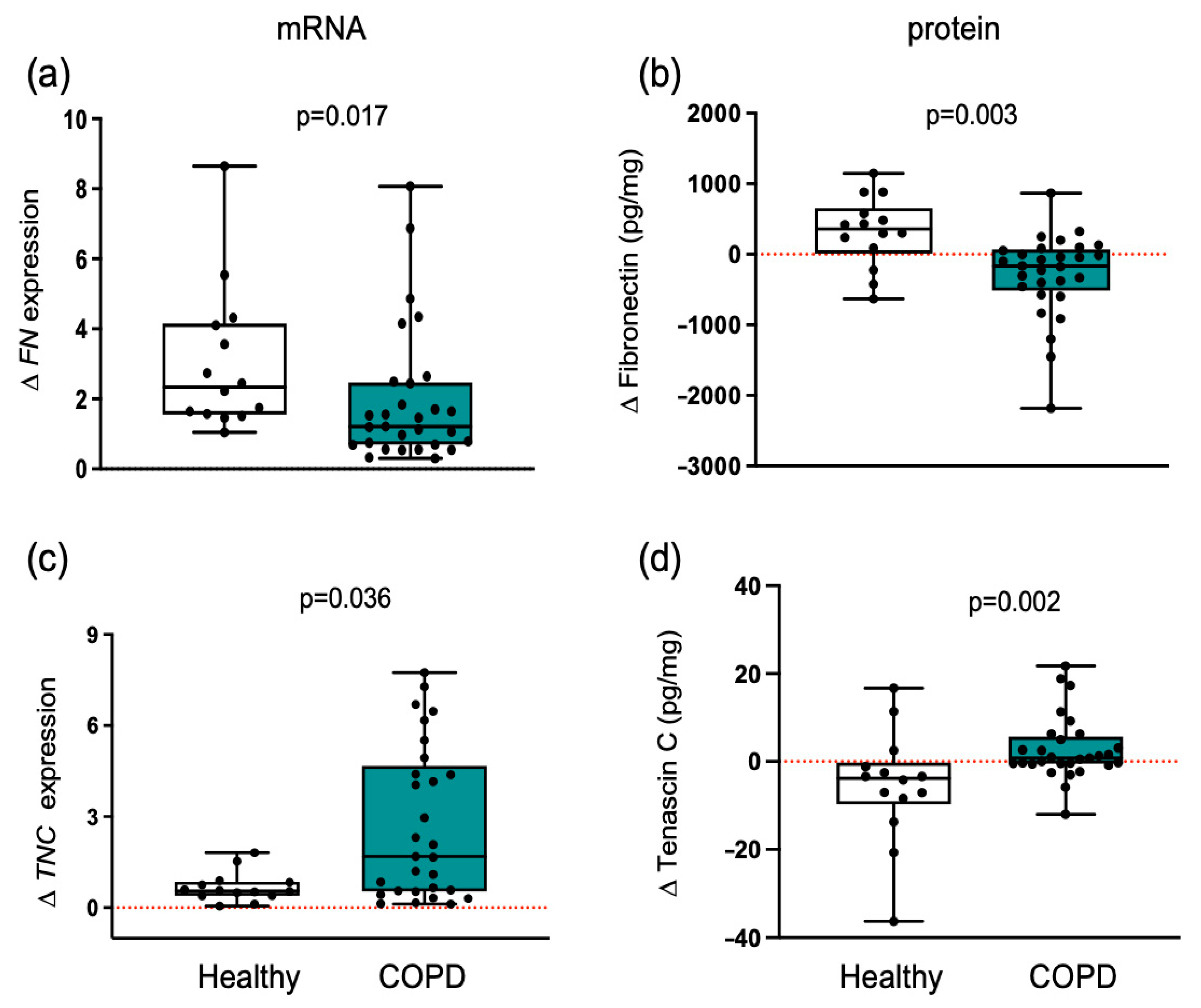
3.3. Collagen Differential Expression in COPD Compared to Healthy Individuals
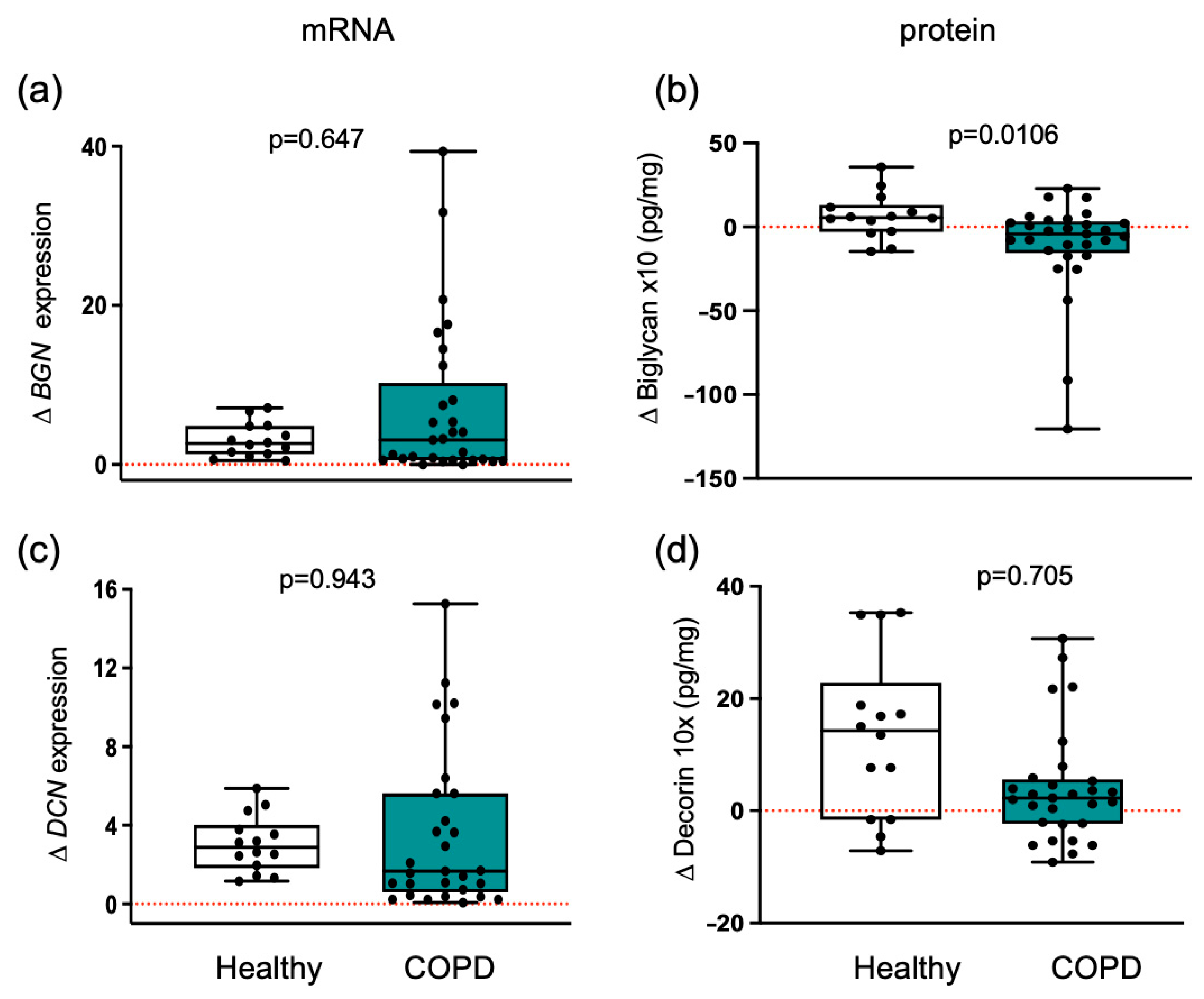
3.4. Myogenic Proteoglycans Are Downregulated in COPD Patients
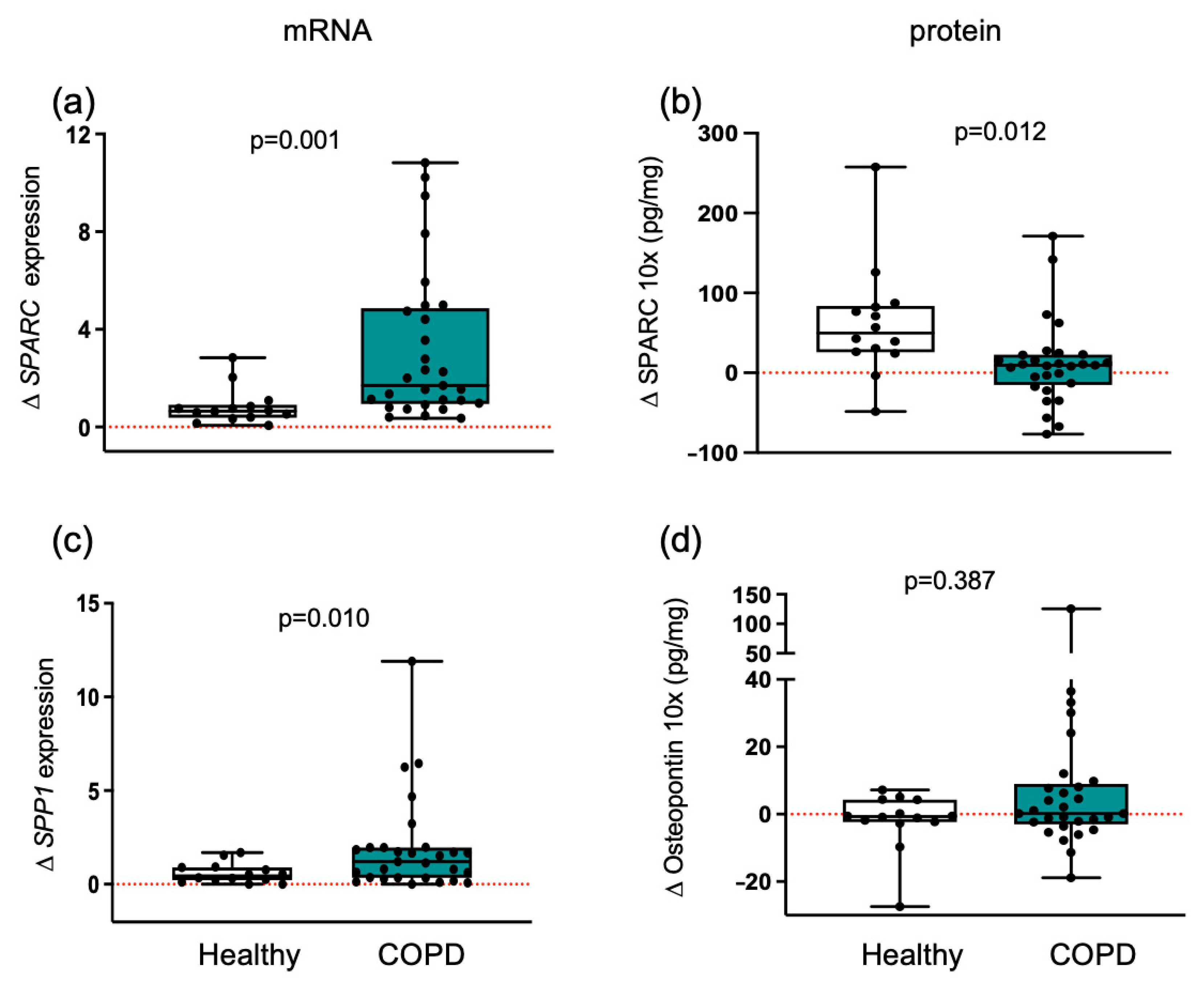
3.5. Matricellular Proteins Respond Differently to Training in Healthy and COPD Muscle
3.6. ECMs with Adhesive and De-Adhesive Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BGN | Byglican |
| COL1 | Collagen type I |
| COL4 | Collagen type IV |
| CSA | Cross-sectional area |
| DCN | Decorin |
| ECM | Extracellular matrix |
| FEV1 | Forced expiratory volume in 1 s |
| FN | Fibronectin |
| FVC | Forced vital capacity |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| MRF | Myogenic regulatory factor |
| MYOD1 | myogenic differentiation 1 |
| Pax7 | Paired box 7 gene |
| TNC | Tenascin C |
References
- Vogiatzis, I.; Simoes, D.C.M.; Stratakos, G.; Kourepini, E.; Terzis, G.; Manta, P.; Athanasopoulos, D.; Roussos, C.; Wagner, P.D.; Zakynthinos, S. Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur. Respir. J. 2010, 36, 301–310. [Google Scholar] [CrossRef]
- Henrot, P.; Dupin, I.; Schilfarth, P.; Esteves, P.; Blervaque, L.; Zysman, M.; Gouzi, F.; Hayot, M.; Pomiès, P.; Berger, P. Main Pathogenic Mechanisms and Recent Advances in COPD Peripheral Skeletal Muscle Wasting. Int. J. Mol. Sci. 2023, 24, 6454. [Google Scholar] [CrossRef]
- Granic, A.; Suetterlin, K.; Shavlakadze, T.; Grounds, M.D.; Sayer, A.A. Hallmarks of ageing in human skeletal muscle and implications for understanding the pathophysiology of sarcopenia in women and men. Clin. Sci. 2023, 137, 1721–1751. [Google Scholar] [CrossRef] [PubMed]
- Pavan, P.; Monti, E.; Bondi, M.; Fan, C.; Stecco, C.; Narici, M.; Reggiani, C.; Marcucci, L. Alterations of Extracellular Matrix Mechanical Properties Contribute to Age-Related Functional Impairment of Human Skeletal Muscles. Int. J. Mol. Sci. 2020, 21, 3992. [Google Scholar] [CrossRef]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix—What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Kritikaki, E.; Asterling, R.; Ward, L.; Padget, K.; Barreiro, E.; Simoes, D.C.M. Exercise Training-Induced Extracellular Matrix Protein Adaptation in Locomotor Muscles: A Systematic Review. Cells 2021, 10, 1022. [Google Scholar] [CrossRef] [PubMed]
- Mavropalias, G.; Boppart, M.; Usher, K.M.; Grounds, M.D.; Nosaka, K.; Blazevich, A.J. Exercise builds the scaffold of life: Muscle extracellular matrix biomarker responses to physical activity, inactivity, and aging. Biol. Rev. Camb. Philos. Soc. 2023, 98, 481–519. [Google Scholar] [CrossRef]
- Chang, J.; Garva, R.; Pickard, A.; Yeung, C.C.; Mallikarjun, V.; Swift, J.; Holmes, D.F.; Calverley, B.; Lu, Y.; Adamson, A.; et al. Circadian control of the secretory pathway maintains collagen homeostasis. Nat. Cell Biol. 2020, 22, 74–86. [Google Scholar] [CrossRef]
- Toyama, B.H.; Hetzer, M.W. Protein homeostasis: Live long, won’t prosper. Nat. Rev. Mol. Cell Biol. 2013, 14, 55–61. [Google Scholar] [CrossRef]
- Suzuki, A.; Yabu, A.; Nakamura, H. Advanced glycation end products in musculoskeletal system and disorders. Methods 2022, 203, 179–186. [Google Scholar] [CrossRef]
- Schuler, S.C.; Kirkpatrick, J.M.; Schmidt, M.; Santinha, D.; Koch, P.; Di Sanzo, S.; Cirri, E.; Hemberg, M.; Ori, A.; von Maltzahn, J. Extensive remodeling of the extracellular matrix during aging contributes to age-dependent impairments of muscle stem cell functionality. Cell Rep. 2021, 35, 109223. [Google Scholar] [CrossRef]
- Stearns-Reider, K.M.; D’Amore, A.; Beezhold, K.; Rothrauff, B.; Cavalli, L.; Wagner, W.R.; Vorp, D.A.; Tsamis, A.; Shinde, S.; Zhang, C.; et al. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell 2017, 16, 518–528. [Google Scholar] [CrossRef]
- Kritikaki, E.; Terzis, G.; Soundararajan, M.; Vogiatzis, I.; Simoes, D.C.M. Role of pulmonary rehabilitation in extracellular matrix protein expression in vastus lateralis muscle in atrophic and nonatrophic patients with COPD. ERJ Open Res. 2025, 11. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzis, I.; Terzis, G.; Nanas, S.; Stratakos, G.; Simoes, D.C.; Georgiadou, O.; Zakynthinos, S.; Roussos, C. Skeletal muscle adaptations to interval training in patients with advanced COPD. Chest 2005, 128, 3838–3845. [Google Scholar] [CrossRef] [PubMed]
- Tsitkanou, S.; Spengos, K.; Stasinaki, A.N.; Zaras, N.; Bogdanis, G.; Papadimas, G.; Terzis, G. Effects of high-intensity interval cycling performed after resistance training on muscle strength and hypertrophy. Scand. J. Med. Sci. Sports 2017, 27, 1317–1327. [Google Scholar] [CrossRef]
- Kritikaki, E.; Terzis, G.; Soundararajan, M.; Vogiatzis, I.; Simoes, D.C. Expression of intramuscular extracellular matrix proteins in vastus lateralis muscle fibres between atrophic and non-atrophic COPD. ERJ Open Res. 2024, 10, 00857–2023. [Google Scholar] [CrossRef]
- Englund, D.A.; Figueiredo, V.C.; Dungan, C.M.; Murach, K.A.; Peck, B.D.; Petrosino, J.M.; Brightwell, C.R.; Dupont, A.M.; Neal, A.C.; Fry, C.S.; et al. Satellite Cell Depletion Disrupts Transcriptional Coordination and Muscle Adaptation to Exercise. Function 2020, 2, zqaa033. [Google Scholar] [CrossRef]
- Goh, Q.; Millay, D.P. Requirement of myomaker-mediated stem cell fusion for skeletal muscle hypertrophy. eLife 2017, 6, e20007. [Google Scholar] [CrossRef]
- Rahman, N.I.A.; Lam, C.L.; Sulaiman, N.; Abdullah, N.A.H.; Nordin, F.; Ariffin, S.H.Z.; Yazid, M.D. PAX7, a Key for Myogenesis Modulation in Muscular Dystrophies through Multiple Signaling Pathways: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 13051. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Moon, J.S.; Park, S.Y.; Ho Lim, J.; Chun, H.J.; Qadri, A.F.; Hwang, Y.C.; Jan, A.T.; Ahmad, S.S.; et al. Myostatin and Its Regulation: A Comprehensive Review of Myostatin Inhibiting Strategies. Front. Physiol. 2022, 13, 876078. [Google Scholar] [CrossRef] [PubMed]
- Chiquet-Ehrismann, R.; Tucker, R.P. Tenascins and the importance of adhesion modulation. Cold Spring Harb. Perspect. Biol. 2011, 3, a004960. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, W.; Cai, G.; Ding, Y.; Wei, C.; Li, S.; Yang, Y.; Qin, J.; Liu, D.; Zhang, H.; et al. Myofiber necroptosis promotes muscle stem cell proliferation via releasing Tenascin-C during regeneration. Cell Res. 2020, 30, 1063–1077. [Google Scholar] [CrossRef]
- Molmen, K.S.; Almquist, N.W.; Skattebo, O. Effects of Exercise Training on Mitochondrial and Capillary Growth in Human Skeletal Muscle: A Systematic Review and Meta-Regression. Sports Med. 2025, 55, 115–144. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Brightwell, C.R.; Volpi, E.; Rasmussen, B.B.; Fry, C.S. Resistance exercise training promotes fiber type-specific myonuclear adaptations in older adults. J. Appl. Physiol. (1985) 2020, 128, 795–804. [Google Scholar] [CrossRef]
- Berrueta, L.; Muskaj, I.; Olenich, S.; Butler, T.; Badger, G.J.; Colas, R.A.; Spite, M.; Serhan, C.N.; Langevin, H.M. Stretching Impacts Inflammation Resolution in Connective Tissue. J. Cell. Physiol. 2016, 231, 1621–1627. [Google Scholar] [CrossRef]
- Martins, H.R.F.; Zotz, T.G.G.; Messa, S.P.; Capriglione, L.G.A.; Zotz, R.; Noronha, L.; Azevedo, M.L.V.; Gomes, A.R.S. Morphometric and Molecular Muscle Remodeling after Passive Stretching in Elderly Female Rats. Clinics 2020, 75, e1769. [Google Scholar] [CrossRef]
- Lukjanenko, L.; Jung, M.J.; Hegde, N.; Perruisseau-Carrier, C.; Migliavacca, E.; Rozo, M.; Karaz, S.; Jacot, G.; Schmidt, M.; Li, L.; et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat. Med. 2016, 22, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Rozo, M.; Li, L.; Fan, C.M. Targeting beta1-integrin signaling enhances regeneration in aged and dystrophic muscle in mice. Nat. Med. 2016, 22, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Boppart, M.D. Influence of exercise and aging on extracellular matrix composition in the skeletal muscle stem cell niche. J. Appl. Physiol. (1985) 2016, 121, 1053–1058. [Google Scholar] [CrossRef]
- Langberg, H.; Rosendal, L.; Kjaer, M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J. Physiol. 2001, 534, 297–302. [Google Scholar] [CrossRef]
| Characteristics | Healthy | COPD |
|---|---|---|
| Individuals (n) | 14 | 29 |
| Age (years) | 21.80 ± 0.60 | 65.68 ± 1.42 † |
| Weight (kg) | 74.20 ± 2.10 | 72.89 ± 2.62 |
| BMI (kg m−2) | 23.45 ± 0.58 | 25.94 ± 0.80 |
| FFM index (kg m−2) | 23.70 ± 0.67 | 17.76 ± 0.40 |
| FEV1 (L) | − | 1.13 ± 0.08 |
| FEV1 (% predicted) | − | 43.32 ± 3.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simoes, D.C.M.; Kritikaki, E.; Terzis, G.; Vogiatzis, I. Distinct Intramuscular Extracellular Matrix Protein Responses to Exercise Training in COPD and Healthy Adults and Their Association with Muscle Remodeling. Cells 2025, 14, 1656. https://doi.org/10.3390/cells14211656
Simoes DCM, Kritikaki E, Terzis G, Vogiatzis I. Distinct Intramuscular Extracellular Matrix Protein Responses to Exercise Training in COPD and Healthy Adults and Their Association with Muscle Remodeling. Cells. 2025; 14(21):1656. https://doi.org/10.3390/cells14211656
Chicago/Turabian StyleSimoes, Davina C. M., Efpraxia Kritikaki, Gerasimos Terzis, and Ioannis Vogiatzis. 2025. "Distinct Intramuscular Extracellular Matrix Protein Responses to Exercise Training in COPD and Healthy Adults and Their Association with Muscle Remodeling" Cells 14, no. 21: 1656. https://doi.org/10.3390/cells14211656
APA StyleSimoes, D. C. M., Kritikaki, E., Terzis, G., & Vogiatzis, I. (2025). Distinct Intramuscular Extracellular Matrix Protein Responses to Exercise Training in COPD and Healthy Adults and Their Association with Muscle Remodeling. Cells, 14(21), 1656. https://doi.org/10.3390/cells14211656








