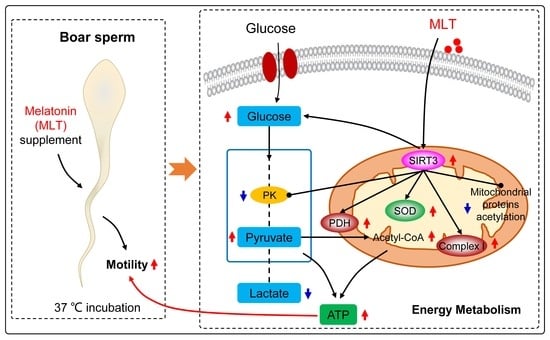
SIRT3 Mediates Coordination Between Energy Metabolism and SOD Activity in Melatonin-Enhanced Boar Sperm Motility
Highlights
- SIRT3 mediates the coordinated enhancement of energy metabolism and SOD activity by MLT in boar sperm.
- This dual regulation enhances metabolic flux and ATP synthesis, thereby boosting sperm motility.
- Targeting SIRT3 emerges as a promising strategy to ameliorate boar fertility.
- The MLT-SIRT3 axis offers novel mechanistic insights into human male infertility.
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Treatments
2.2. Mitochondria Isolation
2.3. Glucose, Lactate, and Pyruvate Assay
2.4. Acetyl-CoA Measurement
2.5. NAD+/NADH Ratio Assay
2.6. Measurement of Mitochondria Membrane Potential (MMP)
2.7. Mitochondrial Complex I Activity Measurement
2.8. The Activities of Pyruvate Kinase (PK) and SOD Measurement
2.9. ATP Content Measurement
2.10. Western Blot Assay
2.11. Statistical Analysis
3. Results
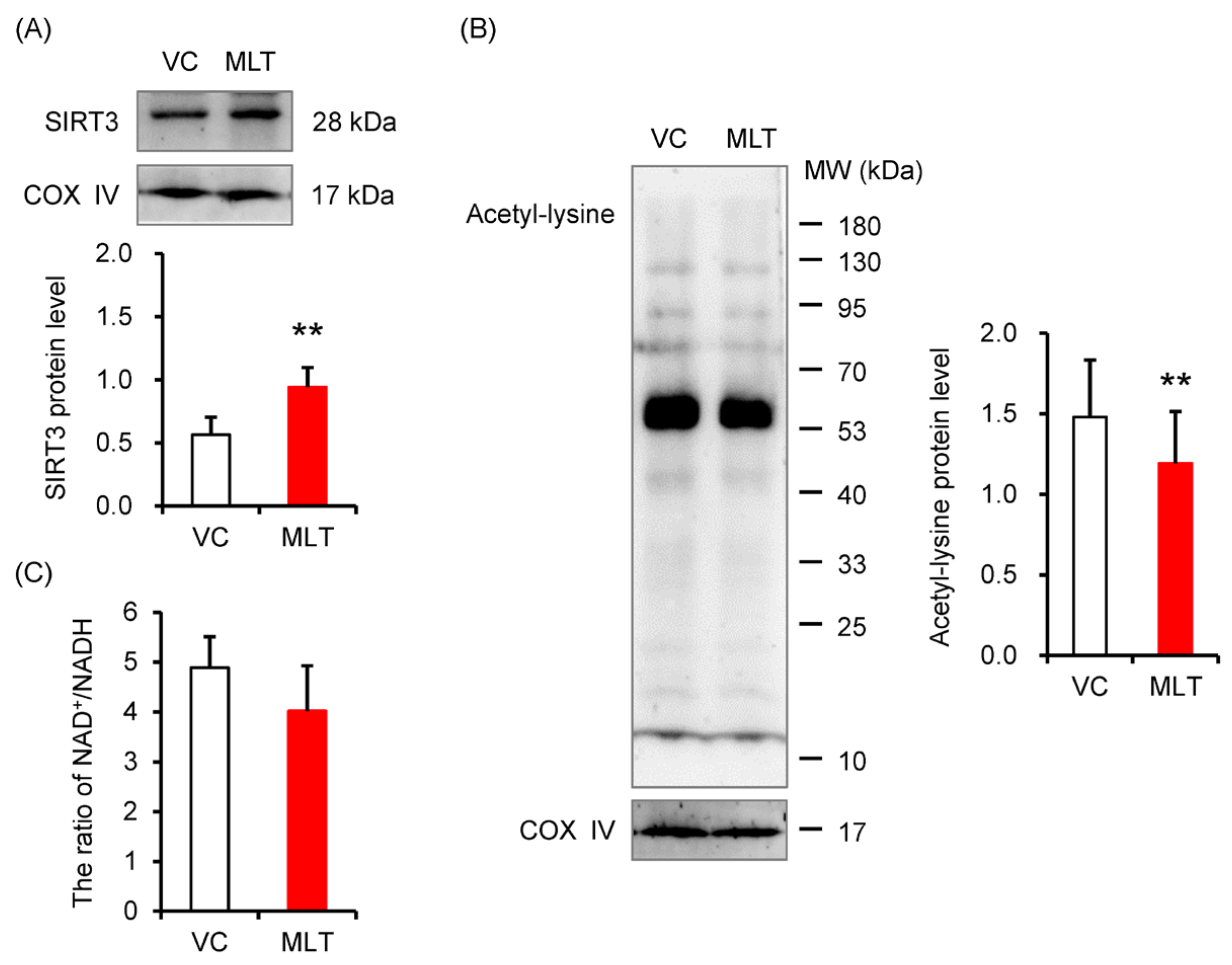
3.1. The Levels of SIRT3 and Acetylation of Mitochondrial Proteins
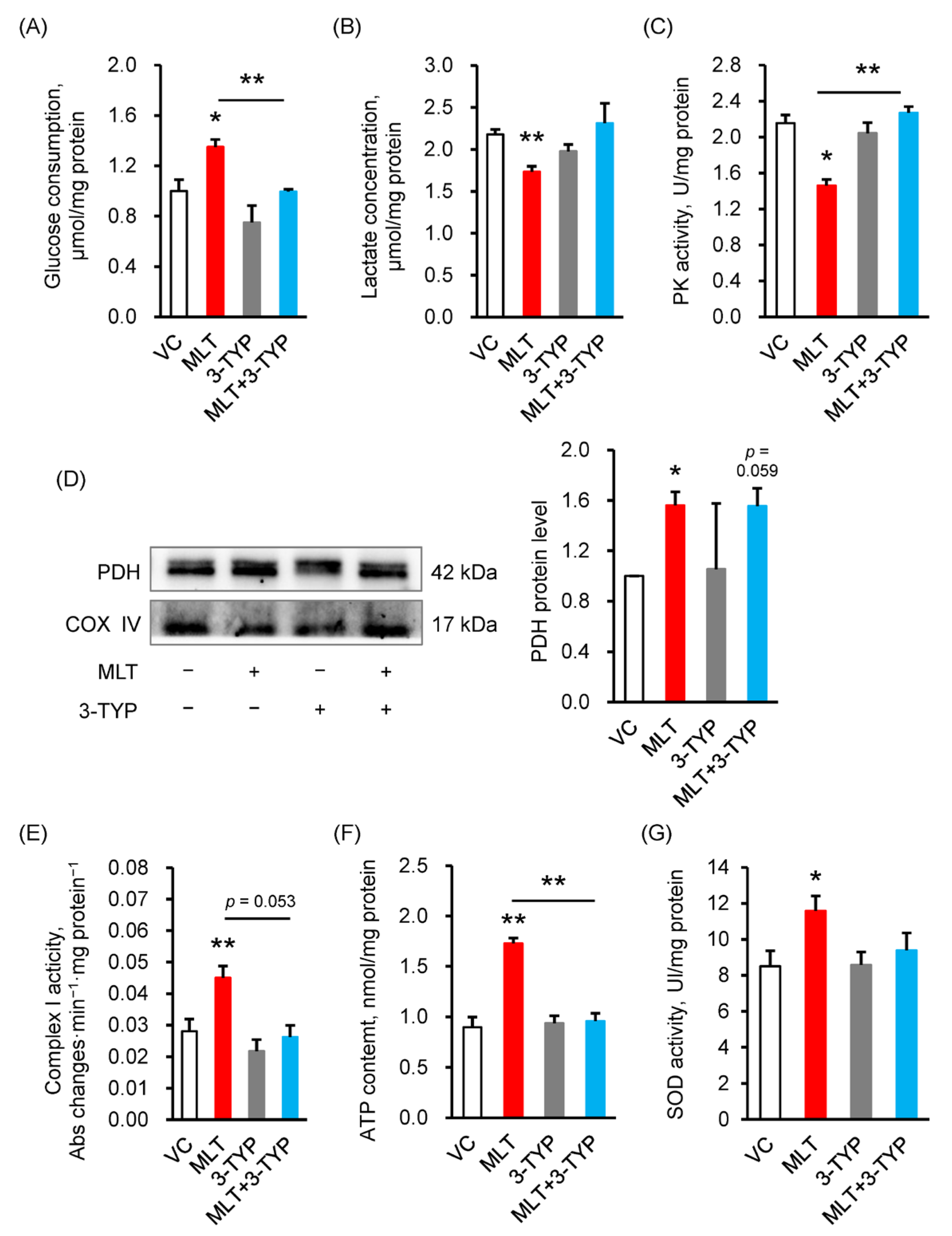
3.2. MLT Enhanced the Metabolic Flux in Boar Sperm
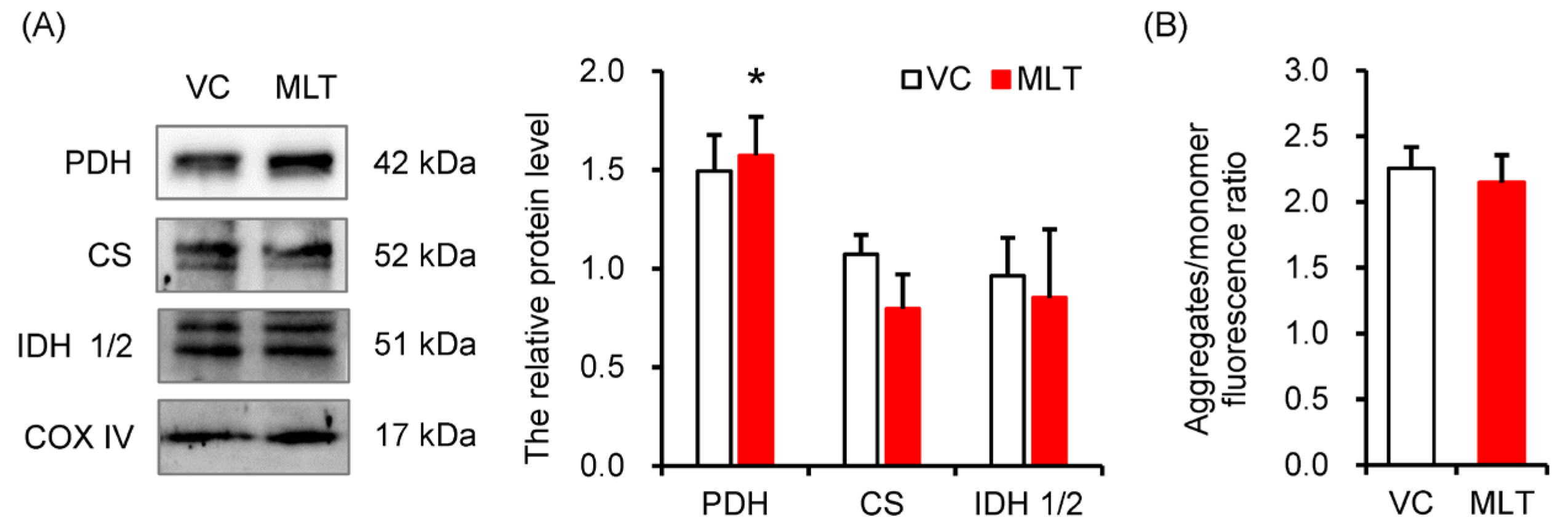
3.3. MLT Modulated the Protein Expression of Key Metabolic Enzymes
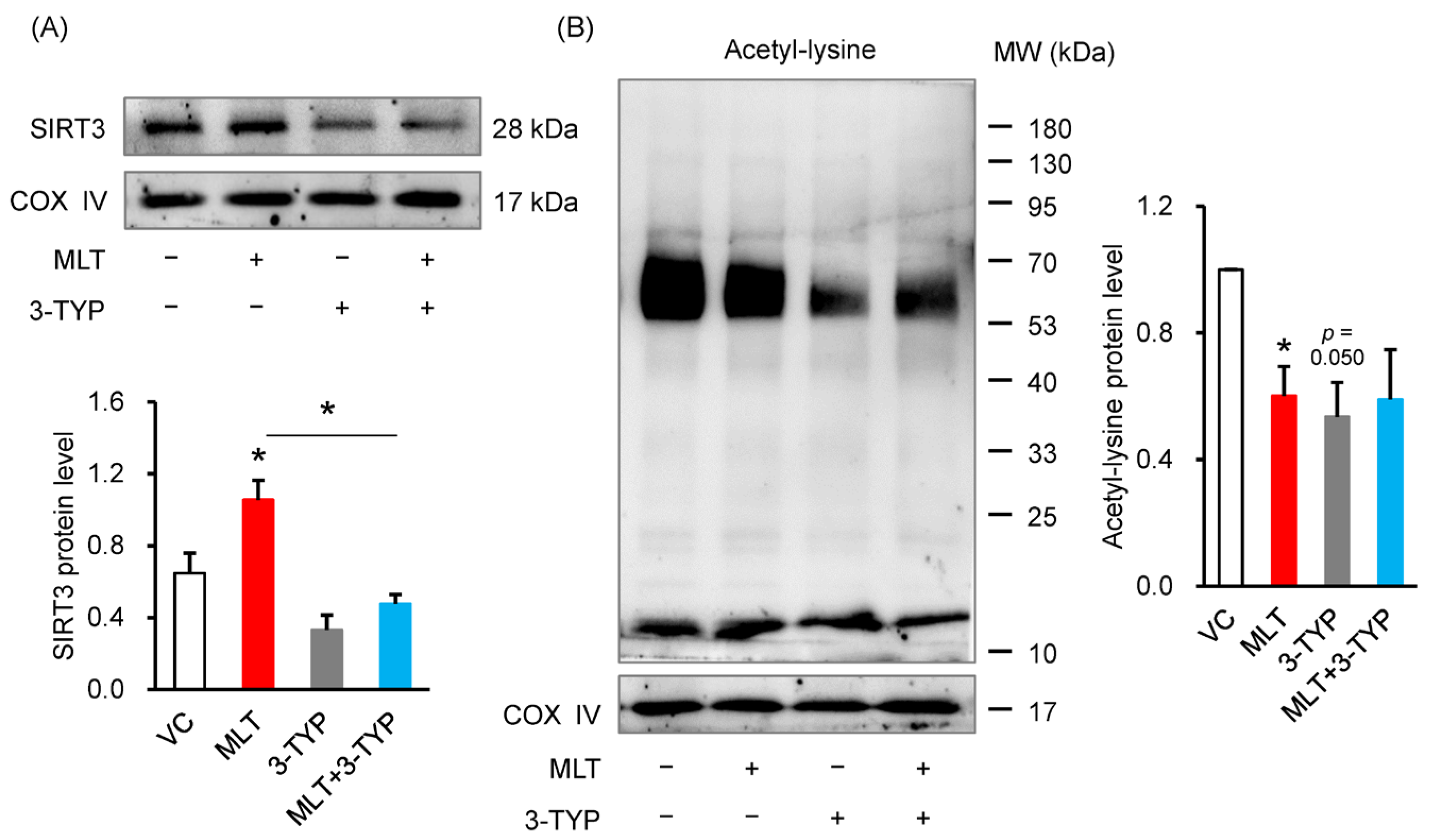
3.4. 3-TYP Abolished the MLT-Increased SIRT3 Level and MLT-Suppressed Acetylation Level
3.5. Inhibition of the SIRT3 Signaling Pathway Reversed the MLT-Induced ATP Synthesis of Sperm
3.6. Inhibition of the SIRT3 Signaling Pathway Reversed the MLT-Induced Sperm Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| CASA | Computer-assisted sperm analysis system |
| COX IV | Cytochrome-c oxidase subunit IV |
| CS | Citrate synthase |
| DMSO | Dimethyl sulfoxide |
| GLUT | Glucose transporter |
| IDH 1/2 | Isocitrate dehydrogenase |
| LDH | Lactate dehydrogenase |
| MLT | Melatonin |
| MMP | Mitochondria membrane potential |
| NAD+ | Nicotinamide adenine dinucleotide |
| NADH | Reduced NAD+ |
| OXPHOS | Oxidative phosphorylation |
| PDH | Pyruvate dehydrogenase |
| PK | Pyruvate kinase |
| SIRT3 | Sirtuin 3 |
| SOD | Superoxide dismutase |
| TCA | Tricarboxylic acid |
| VC | Vehicle control |
References
- Zou, C.X.; Yang, Z.M. Evaluation on sperm quality of freshly ejaculated boar semen during in vitro storage under different temperatures. Theriogenology 2000, 53, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.H.; Chao, H.T.; Chen, H.W.; Hwang, T.I.; Liao, T.L.; Wei, Y.H. Increase of oxidative stress in human sperm with lower motility. Fertil. Steril. 2008, 89, 1183–1190. [Google Scholar] [CrossRef]
- Vyt, P.; Maes, D.; Quinten, C.; Rijsselaere, T.; Deley, W.; Aarts, M.; de Kruif, A.; Soom, A. Detailed motility evaluation of boar semen and its predictive value for reproductive performance in sows. Vlaams Diergeneeskd. Tijdschr. 2008, 77, 291–298. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Agarwal, A.; Mohanty, G.; van der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [CrossRef]
- Marin, S.; Chiang, K.; Bassilian, S.; Lee, W.-N.P.; Boros, L.G.; Fernández-Novell, J.M.; Centelles, J.J.; Medrano, A.; Rodriguez-Gil, J.E.; Cascante, M. Metabolic strategy of boar spermatozoa revealed by a metabolomic characterization. FEBS Lett. 2003, 554, 342–346. [Google Scholar] [CrossRef]
- Nevo, A.C.; Polge, C.; Frederick, G. Aerobic and anaerobic metabolism of boar spermatozoa in relation to their motility. Reproduction 1970, 22, 109–118. [Google Scholar] [CrossRef][Green Version]
- Nakada, K.; Sato, A.; Yoshida, K.; Morita, T.; Tanaka, H.; Inoue, S.-I.; Yonekawa, H.; Hayashi, J.-I. Mitochondria-related male infertility. Proc. Natl. Acad. Sci. USA 2006, 103, 15148–15153. [Google Scholar] [CrossRef]
- John, J.C.S.; John, B.S. Sperm mitochondrial DNA. In Sperm Chromatin: Biological and Clinical Applications in Male Infertility and Assisted Reproduction, 1st ed.; Zini, A., Agarwal, A., Eds.; Springer: New York, NY, USA, 2011; pp. 81–94. [Google Scholar]
- Pfeiffer, T.; Schuster, S.; Bonhoeffer, S. Cooperation and competition in the evolution of ATP-producing pathways. Science 2001, 292, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Jane Rogers, B.; Yanagimachi, R. Retardation of guinea pig sperm acrosome reaction by glucose: The possible importance of pyruvate and lactate metabolism in capacitation and the acrosome reaction. Biol. Reprod. 1975, 13, 568–575. [Google Scholar] [CrossRef]
- Dziekońska, A.; Fraser, L.; Strzeżek, J. Effect of different storage temperatures on the metabolic activity of spermatozoa following liquid storage of boar semen. J. Anim. Feed Sci. 2009, 18, 638–649. [Google Scholar] [CrossRef]
- Amaral, A. Energy metabolism in mammalian sperm motility. WIREs Mech. Dis. 2022, 14, e1569. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef]
- Lantier, L.; Williams, A.S.; Williams, I.M.; Yang, K.K.; Bracy, D.P.; Goelzer, M.; James, F.D.; Gius, D.; Wasserman, D.H. SIRT3 is crucial for maintaining skeletal muscle insulin action and protects against severe insulin resistance in high-fat–fed mice. Diabetes 2015, 64, 3081–3092. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.-H.; Kim, H.-S.; Song, S.; Lee, I.H.; Liu, J.; Vassilopoulos, A.; Deng, C.-X.; Finkel, T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. USA 2008, 105, 14447–14452. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, Z.; Liu, N.; Zhang, L.; Gao, Z.; Sun, X.; Yu, M.; Wu, J.; Yang, F.; Zhao, Y.; et al. Exogenous H2S switches cardiac energy substrate metabolism by regulating SIRT3 expression in db/db mice. J. Mol. Med. 2018, 96, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Lombard, D.B.; Alt, F.W.; Cheng, H.-L.; Bunkenborg, J.; Streeper, R.S.; Mostoslavsky, R.; Kim, J.; Yancopoulos, G.; Valenzuela, D.; Murphy, A.; et al. Mammalian Sir2 Homolog SIRT3 Regulates Global Mitochondrial Lysine Acetylation. Mol. Cell. Biol. 2007, 27, 8807–8814. [Google Scholar] [CrossRef]
- Zhang, H.; Chai, J.; Cao, C.; Wang, X.; Pang, W. Supplementing Boar Diet with Nicotinamide Mononucleotide Improves Sperm Quality Probably through the Activation of the SIRT3 Signaling Pathway. Antioxidants 2024, 13, 507. [Google Scholar] [CrossRef]
- Gholami, M.; Saki, G.; Hemadi, M.; Khodadadi, A.; Mohammadi-Asl, J. Melatonin improves spermatogonial stem cells transplantation efficiency in azoospermic mice. Iran. J. Basic. Med. Sci. 2014, 17, 93–99. [Google Scholar]
- Barranco, I.; Casao, A.; Perez-Patiño, C.; Parrilla, I.; Muiño-Blanco, T.; Martinez, E.A.; Cebrian-Perez, J.A.; Roca, J. Profile and reproductive roles of seminal plasma melatonin of boar ejaculates used in artificial insemination programs1. J. Anim. Sci. 2017, 95, 1660–1668. [Google Scholar] [CrossRef]
- Yang, D.; Wang, C.; Lu, W.; Tian, X.; Sun, Y.; Peng, H. Beneficial effects of melatonin on boar sperm motility and kinematics are mediated by MT1 receptor. Theriogenology 2024, 226, 95–103. [Google Scholar] [CrossRef]
- Pezo, F.; Zambrano, F.; Uribe, P.; Moya, C.; de Andrade, A.F.C.; Risopatron, J.; Yeste, M.; Burgos, R.A.; Sanchez, R. Oxidative and nitrosative stress in frozen-thawed pig spermatozoa. I: Protective effect of melatonin and butylhydroxytoluene on sperm function. Res. Vet. Sci. 2021, 136, 143–150. [Google Scholar] [CrossRef]
- Ofosu, J.; Qazi, I.H.; Fang, Y.; Zhou, G. Use of melatonin in sperm cryopreservation of farm animals: A brief review. Anim. Reprod. Sci. 2021, 233, 106850. [Google Scholar] [CrossRef]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [PubMed]
- Owino, S.; Buonfiglio, D.D.C.; Tchio, C.; Tosini, G. Melatonin Signaling a Key Regulator of Glucose Homeostasis and Energy Metabolism. Front. Endocrinol. 2019, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.-C.; Zhang, J.-P.; Huo, Y.-N.; Xue, H.-Y.; Wang, W.; Zhang, J.-J.; Wang, X.-Z. Melatonin alleviates the heat stress-induced impairment of Sertoli cells by reprogramming glucose metabolism. J. Pineal Res. 2022, 73, e12819. [Google Scholar] [CrossRef]
- Lu, N.; Jiang, X.; Zhang, C.; Li, B.; Tu, W.; Lei, H.; Yao, W.; Xia, D. Melatonin mediates via melatonin receptor 1 in a temperature-dependent manner regulating ATP metabolism and antioxidative enzyme activity of boar spermatozoa in vitro. Theriogenology 2022, 188, 1–12. [Google Scholar] [CrossRef]
- Pi, H.; Xu, S.; Reiter, R.J.; Guo, P.; Zhang, L.; Li, Y.; Li, M.; Cao, Z.; Tian, L.; Xie, J. SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy 2015, 11, 1037–1051. [Google Scholar] [CrossRef]
- Gong, Y.; Guo, H.; Zhang, Z.; Zhou, H.; Zhao, R.; He, B. Heat Stress Reduces Sperm Motility via Activation of Glycogen Synthase Kinase-3α and Inhibition of Mitochondrial Protein Import. Front. Physiol. 2017, 8, 718. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, P.; Huang, Y.; Li, M.; Zhang, X.; Ding, C.; Feng, J.; Zhang, Z.; Zhang, X.; Gao, Y.; et al. Glycerol kinase-like proteins cooperate with Pld6 in regulating sperm mitochondrial sheath formation and male fertility. Cell Discov. 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Ohashi, K.; Otaka, N.; Kawanishi, H.; Takikawa, T.; Fang, L.; Takahara, K.; Tatsumi, M.; Ishihama, S.; Takefuji, M.; et al. Myonectin protects against skeletal muscle dysfunction in male mice through activation of AMPK/PGC1α pathway. Nat. Commun. 2023, 14, 4675. [Google Scholar] [CrossRef]
- Brydon, L.; Petit, L.; Delagrange, P.; Strosberg, A.D.; Jockers, R. Functional Expression of MT2 (Mel1b) Melatonin Receptors in Human PAZ6 Adipocytes. Endocrinology 2001, 142, 4264–4271. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.S.; Martins, A.D.; Rato, L.; Silva, B.M.; Oliveira, P.F.; Alves, M.G. Melatonin alters the glycolytic profile of Sertoli cells: Implications for male fertility. Mol. Hum. Reprod. 2014, 20, 1067–1076. [Google Scholar] [CrossRef]
- Fang, Y.; Zhao, C.; Xiang, H.; Zhao, X.; Zhong, R. Melatonin Inhibits Formation of Mitochondrial Permeability Transition Pores and Improves Oxidative Phosphorylation of Frozen-Thawed Ram Sperm. Front. Endocrinol. 2020, 10, 896. [Google Scholar] [CrossRef]
- Medrano, A.; Fernández-Novell, J.M.; Ramió, L.; Alvarez, J.; Goldberg, E.; Montserrat Rivera, M.; Guinovart, J.J.; Rigau, T.; Rodríguez-Gil, J.E. Utilization of citrate and lactate through a lactate dehydrogenase and ATP-regulated pathway in boar spermatozoa. Mol. Reprod. Dev. 2006, 73, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Ng, C.P.; Jones, O.; Fung, T.S.; Ryu, K.W.; Li, D.; Thompson, C.B. Lactate activates the mitochondrial electron transport chain independently of its metabolism. Mol. Cell 2023, 83, 3904–3920.e3907. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.E.; Kulkarni, S.; Wang, H.; Lu, J.; Prochownik, E.V. Genetic Dissociation of Glycolysis and the TCA Cycle Affects Neither Normal nor Neoplastic Proliferation. Cancer Res. 2022, 82, 944, Erratum in Cancer Res. 2017, 77, 5795–5807. [Google Scholar] [CrossRef]
- Gray, L.R.; Tompkins, S.C.; Taylor, E.B. Regulation of pyruvate metabolism and human disease. Cell. Mol. Life Sci. 2014, 71, 2577–2604. [Google Scholar] [CrossRef]
- Prieto, O.B.; Algieri, C.; Spinaci, M.; Trombetti, F.; Nesci, S.; Bucci, D. Cell bioenergetics and ATP production of boar spermatozoa. Theriogenology 2023, 210, 162–168. [Google Scholar] [CrossRef]
- Brooks, D.E.; Mann, T. Pyruvate Metabolism in Boar Spermatozoa. Reproduction 1973, 34, 105–119. [Google Scholar] [CrossRef][Green Version]
- Hereng, T.H.; Elgstøen, K.B.P.; Cederkvist, F.H.; Eide, L.; Jahnsen, T.; Skålhegg, B.S.; Rosendal, K.R. Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa. Hum. Reprod. 2011, 26, 3249–3263. [Google Scholar] [CrossRef]
- Kumar, N. Sperm mitochondria, the driving force behind human spermatozoa activities: Its functions and dysfunctions-a narrative review. Curr. Mol. Med. 2023, 23, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Braga, P.C.; Rebelo, I.; Oliveira, P.F.; Alves, M.G. Mitochondria quality control and male fertility. Biology 2023, 12, 827. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Yang, C.; Xiong, H.; Lin, Y.; Yao, J.; Li, H.; Xie, L.; Zhao, W.; Yao, Y.; et al. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science 2010, 327, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Seto, E. Lysine acetylation: Codified crosstalk with other posttranslational modifications. Mol. Cell 2008, 31, 449–461. [Google Scholar] [CrossRef]
- Anderson, K.A.; Hirschey, M.D. Mitochondrial protein acetylation regulates metabolism. Essays Biochem. 2012, 52, 23–35. [Google Scholar]
- Kim, S.C.; Sprung, R.; Chen, Y.; Xu, Y.; Ball, H.; Pei, J.; Cheng, T.; Kho, Y.; Xiao, H.; Xiao, L. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell 2006, 23, 607–618. [Google Scholar] [CrossRef]
- Baeza, J.; Smallegan, M.J.; Denu, J.M. Site-Specific Reactivity of Nonenzymatic Lysine Acetylation. Acs Chem. Biol. 2015, 10, 122–128. [Google Scholar] [CrossRef]
- Newman, J.C.; He, W.; Verdin, E. Mitochondrial Protein Acylation and Intermediary Metabolism: Regulation by Sirtuins and Implications for Metabolic Disease. J. Biol. Chem. 2012, 287, 42436–42443. [Google Scholar] [CrossRef]
- Fernandes, J.; Weddle, A.; Kinter, C.S.; Humphries, K.M.; Kinter, M. Lysine Acetylation Activates Mitochondrial Aconitase in the Heart. Biochemistry 2015, 54, 4008. [Google Scholar] [CrossRef]
- Song, C.; Fu, B.; Zhang, J.; Zhao, J.; Yuan, M.; Peng, W.; Zhang, Y.; Wu, H. Sodium fluoride induces nephrotoxicity via oxidative stress-regulated mitochondrial SIRT3 signaling pathway. Sci. Rep. 2018, 8, 7737, Erratum in Sci. Rep. 2017, 7, 672. [Google Scholar] [CrossRef] [PubMed]
- Imanaka, S.; Shigetomi, H.; Kobayashi, H. Reprogramming of glucose metabolism of cumulus cells and oocytes and its therapeutic significance. Reprod. Sci. 2022, 29, 653–667. [Google Scholar] [CrossRef]
- Zu, Y.; Chen, X.-F.; Li, Q.; Zhang, S.-T.; Si, L.-N. PGC-1α activates SIRT3 to modulate cell proliferation and glycolytic metabolism in breast cancer. Neoplasma 2021, 68, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Lannuzel, A.; Michel, P.P.; Höglinger, G.U.; Champy, P.; Jousset, A.; Medja, F.; Lombès, A.; Darios, F.; Gleye, C.; Laurens, A.; et al. The mitochondrial complex I inhibitor annonacin is toxic to mesencephalic dopaminergic neurons by impairment of energy metabolism. Neuroscience 2003, 121, 287–296. [Google Scholar] [CrossRef]
- Plaza Davila, M.; Martin Muñoz, P.; Tapia, J.A.; Ortega Ferrusola, C.; Balao da Silva, C.C.; Peña, F.J. Inhibition of Mitochondrial Complex I Leads to Decreased Motility and Membrane Integrity Related to Increased Hydrogen Peroxide and Reduced ATP Production, while the Inhibition of Glycolysis Has Less Impact on Sperm Motility. PLoS ONE 2015, 10, e0138777. [Google Scholar] [CrossRef]
- Han, L.; Wang, H.; Li, L.; Li, X.; Ge, J.; Reiter, R.J.; Wang, Q. Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT 3-SOD 2-dependent pathway. J. Pineal Res. 2017, 63, e12431. [Google Scholar] [CrossRef]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie Restriction Reduces Oxidative Stress by SIRT3-Mediated SOD2 Activation. Cell Metab. 2010, 12, 662–667. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, X.; Bojan, N.; Cao, C.; Chai, J.; Pang, W. Nicotinamide mononucleotide enhances porcine sperm quality by activating the SIRT3-SOD2/ROS pathway and promoting oxidative phosphorylation. Anim. Reprod. Sci. 2025, 275, 107797. [Google Scholar] [CrossRef]
- Shamsi, M.B.; Venkatesh, S.; Tanwar, M.; Talwar, P.; Sharma, R.K.; Dhawan, A.; Kumar, R.; Gupta, N.P.; Malhotra, N.; Singh, N.; et al. DNA integrity and semen quality in men with low seminal antioxidant levels. Mutat. Res. 2009, 665, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Koziorowska-Gilun, M.; Koziorowski, M.; Fraser, L.; Strzeżek, J. Antioxidant defence system of boar cauda epididymidal spermatozoa and reproductive tract fluids. Reprod. Domest. Anim. 2011, 46, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Park, S.-H.; Ozden, O.; Kim, H.-S.; Jiang, H.; Vassilopoulos, A.; Spitz, D.R.; Gius, D. Exploring the electrostatic repulsion model in the role of Sirt3 in directing MnSOD acetylation status and enzymatic activity. Free Radic. Biol. Med. 2012, 53, 828–833. [Google Scholar] [CrossRef]
- Hirschey, M.D.; Shimazu, T.; Goetzman, E.; Jing, E.; Schwer, B.; Lombard, D.B.; Grueter, C.A.; Harris, C.; Biddinger, S.; Ilkayeva, O.R.; et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010, 464, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.; Zhang, J.; Luo, X.; Du, Q.; Chang, L.; Zhao, X.; Huang, Y.; Tong, D. Porcine parvovirus infection impairs progesterone production in luteal cells through mitogen-activated protein kinases, p53, and mitochondria-mediated apoptosis. Biol. Reprod. 2018, 98, 558–569. [Google Scholar] [CrossRef]
- Brownstein, A.J.; Ganesan, S.; Summers, C.M.; Pearce, S.; Hale, B.J.; Ross, J.W.; Gabler, N.; Seibert, J.T.; Rhoads, R.P.; Baumgard, L.H.; et al. Heat stress causes dysfunctional autophagy in oxidative skeletal muscle. Physiol. Rep. 2017, 5, e13317. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Jiang, B.; Cohen, R.A.; Zang, M. Activation of sterol regulatory element binding protein and NLRP3 inflammasome in atherosclerotic lesion development in diabetic pigs. PLoS One 2013, 8, e67532. [Google Scholar] [CrossRef]
- Liu, L.; Lu, H.; Loor, J.J.; Aboragah, A.; Du, X.; He, J.; Peng, T.; Su, J.; Wang, Z.; Liu, G.; et al. Sirtuin 3 inhibits nuclear factor-κB signaling activated by a fatty acid challenge in bovine mammary epithelial cells. J. Dairy Sci. 2021, 104, 12871–12880. [Google Scholar] [CrossRef]
- Liu, L.; Xing, D.; Du, X.; Peng, T.; McFadden, J.W.; Wen, L.; Lei, H.; Dong, W.; Liu, G.; Wang, Z.; et al. Sirtuin 3 improves fatty acid metabolism in response to high nonesterified fatty acids in calf hepatocytes by modulating gene expression. J. Dairy Sci. 2020, 103, 6557–6568. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Su, F.; Chen, Y.; Wang, T.; Ali, W.; Jin, H.; Xiong, L.; Ma, Y.; Liu, Z.; Zou, H. Co-exposure to PVC microplastics and cadmium induces oxidative stress and fibrosis in duck pancreas. Sci. Total Environ. 2024, 927, 172395. [Google Scholar] [CrossRef]
- Wang, J.; Quan, R.; He, X.; Fu, Q.; Tian, S.; Zhao, L.; Li, S.; Shi, L.; Li, R.; Chen, B. Hypovirus infection induces proliferation and perturbs functions of mitochondria in the chestnut blight fungus. Front. Microbiol. 2023, 14, 1206603. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Sperm Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Motility | Progressive Motility | Fast Motility | Circle Motility | Slow Motility | Local Motility | Immotile | |
| Day 0 | 87.37 ± 0.50 | 78.78 ± 1.57 | 24.04 ± 3.66 | 0.34 ± 0.11 | 54.38 ± 3.83 | 8.61 ± 1.14 | 12.63 ± 0.50 |
| Day 3 | |||||||
| VC | 76.65 ± 0.13 c | 64.36 ± 3.81 b | 21.95 ± 6.15 b | 0.37 ± 0.12 a | 42.06 ± 3.19 a | 12.29 ± 3.86 a | 23.36 ± 0.12 a |
| MLT | 86.77 ± 1.06 a | 78.11 ± 1.75 a | 40.00 ± 4.92 a | 0.18 ± 0.07 a | 37.92 ± 4.07 a | 8.64 ± 2.47 a | 13.26 ± 1.06 c |
| 3-TYP | 75.97 ± 1.27 c | 62.30 ± 3.77 b | 17.03 ± 3.80 b | 0.32 ± 0.12 a | 44.98 ± 2.74 a | 13.63 ± 3.70 a | 24.04 ± 1.27 a |
| MLT + 3-TYP | 81.83 ± 0.33 b | 69.66 ± 3.13 ab | 22.27 ± 4.75 b | 0.20 ± 0.08 a | 47.20 ± 3.29 a | 12.14 ± 3.26 a | 18.18 ± 0.33 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, N.; Lei, H.; Jiang, X.; Jia, P.; Li, B.; Xia, D. SIRT3 Mediates Coordination Between Energy Metabolism and SOD Activity in Melatonin-Enhanced Boar Sperm Motility. Cells 2025, 14, 1633. https://doi.org/10.3390/cells14201633
Lu N, Lei H, Jiang X, Jia P, Li B, Xia D. SIRT3 Mediates Coordination Between Energy Metabolism and SOD Activity in Melatonin-Enhanced Boar Sperm Motility. Cells. 2025; 14(20):1633. https://doi.org/10.3390/cells14201633
Chicago/Turabian StyleLu, Naisheng, Hulong Lei, Xueyuan Jiang, Peng Jia, Bushe Li, and Dong Xia. 2025. "SIRT3 Mediates Coordination Between Energy Metabolism and SOD Activity in Melatonin-Enhanced Boar Sperm Motility" Cells 14, no. 20: 1633. https://doi.org/10.3390/cells14201633
APA StyleLu, N., Lei, H., Jiang, X., Jia, P., Li, B., & Xia, D. (2025). SIRT3 Mediates Coordination Between Energy Metabolism and SOD Activity in Melatonin-Enhanced Boar Sperm Motility. Cells, 14(20), 1633. https://doi.org/10.3390/cells14201633