1. Introduction
Osteosarcoma (OS) is a rare primary malignant bone tumor occurring primarily in children, with 3770 new cases estimated for 2025 [
1]. Historically, osteosarcoma was managed with surgery alone, and approximately 80% of patients developed metastatic disease. Although the introduction of adjuvant chemotherapy has improved 5-year survival from approximately 20% to 60%, metastasis continues to be the most important factor driving poor survival outcomes. Currently, OS treatment includes neoadjuvant chemotherapy followed by surgical resection and adjuvant therapy. While there is no consensus on a standard chemotherapy regimen, most regimens include cisplatin and doxorubicin with or without high-dose methotrexate [
2,
3].
Although primarily immune checkpoint inhibitors (ICIs) have revolutionized the treatment of many cancers, ICIs have been relatively ineffective for OS [
4,
5,
6]. The lack of activity of ICIs is associated with a “cold” tumor microenvironment, which is characterized by the presence of M2 macrophages and other cells that secrete immunosuppressive cytokines [
7]. In the tumor microenvironment, factors released by tumor cells can cause tumor-associated macrophages (TAMs) to develop an M2-like, anti-inflammatory phenotype. In general, M2 macrophages are associated with increased metastasis, angiogenesis, chemoresistance, and poor prognosis of many tumors. In primary OS, M2 makes up a large proportion of the TAMs and is associated with enhanced metastatic potential and worse survival outcomes [
8]. A therapy that reprograms M2 tumor-associated macrophages into the pro-inflammatory M1 subtype could transform immunologically “cold” tumors into “hot” tumors—making them more responsive to immune-based treatments. This approach represents a potentially paradigm-shifting advance in osteosarcoma therapy.
Extracellular vesicles (EVs) are naturally occurring nanoscale lipid membrane structures released by nearly all cell types that function in cell-to-cell communication. EVs have a lipid bilayer, lack a nucleus, and serve as carriers of proteins, nucleic acids, and other substances from their origin cells, which they deliver to recipient cells [
9]. EVs are under investigation as drug delivery vesicles and microenvironment modulators for a number of diseases; however, their low production rate in unaltered cell cultures and lack of batch reproducibility are current barriers to clinical development.
Manufactured cell-derived vesicles (MCDVs) are EV-like vesicles produced by cell disruption strategies. A novel method for generating via fragmentation of cell membranes was recently reported [
10]. These MCDVs retain properties of naturally occurring EVs, but importantly, can be produced at scale [
10,
11,
12]. Macrophage-manufactured vesicles (MVs) are a type of MCDV, made from human and mouse M1 macrophages. Previous studies demonstrate that cell-derived vesicles exhibit similar properties to exosomes in that they specifically target the cell from which they originate [
12]. As macrophages are the most abundant immune system cell within the tumor microenvironment, MVs formulated from macrophages have inherent targeting properties to most tumors. Previous animal data demonstrate precise localization of mouse MVs to cancer tumor xenografts in mice [
12,
13]. In addition, (MCDVs) can be engineered to carry chemotherapeutic agents, enabling targeted delivery directly to cancer cells [
12,
13]. This approach leverages the natural ability of MCDVs to interact with specific cell types through surface markers, thereby enhancing drug accumulation at the tumor site while minimizing off-target toxicity. By exploiting this cell-specific delivery mechanism, EV-based chemotherapy holds promise for improving therapeutic efficacy and reducing systemic side effects commonly associated with conventional cancer treatments.
The purpose of this study was to characterize MVs generated from both primary human monocytes and the RAW 264.7 mouse monocytic cell line, and to better understand cellular internalization and targeting. Anti-cancer efficacy and safety of empty (E-MVs) and cisplatin-loaded MVs (C-MVs) were assessed in vitro and in a mouse orthotopic xenograft model of OS (see Graphical Abstract).
2. Materials and Methods
2.1. Cell Lines and Culture Media
Human OS parental cells HOS (ATCC: CRL-1543), its derivative cell line 143B (CRL-8303), and mouse macrophage cell line RAW 264.7 (TIB 71) were obtained from ATCC, Manassas, VA, USA. HOS cells were maintained in Eagle’s Minimum Essential Medium (ATCC, Manassas, VA, USA #30-2003) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, Saint Louis, MO, USA #F0926). 143B cells were maintained in Minimum Essential Medium in Earle’s Balanced Salts (Sigma-Aldrich, Saint Louis, MO, USA #51412C) supplemented with 10% FBS, 1% penicillin-streptomycin (Gibco, Brooklyn, New York, NY, USA #15140-122), and 0.015 mg/mL 5-bromo-2′-deoxyuridine (BrdU) (Sigma-Aldrich, Saint Louis, MO, USA #B5002). RAW 264.7 cells were maintained in DMEM (Sigma-Aldrich, Saint Louis, MO, USA #D0822) media supplemented with 10% FBS and 1% penicillin-streptomycin.
2.2. Isolation of Human Peripheral Blood Mononuclear Cells (PBMCs) and Differentiation of M0 to M1 Macrophages
Human blood was obtained from the University of Kentucky Blood Center (IRB-58723 Approval for Exemption certification, 12 April 2020) and processed for the isolation of PBMCs through density gradient centrifugation using Ficoll-Paque (GE Healthcare, Tampa, FL, USA#17-5446-52). A total volume of 200–250 mL of blood product was isolated and pooled from 5 to 6 buffy coat bags for a single vesicle preparation. The Easy Sep human monocyte enrichment kit (Stemcell Technologies, Vancouver, BC, Canada, #19058) was used to isolate monocyte/M0 macrophages from human PBMCs using negative selection. Isolated M0 macrophages were cultured in RPMI 1640 media (Sigma-Aldrich, Saint Louis, MO, USA #R8758), supplemented with 10% heat-inactivated FBS, 1% penicillin-streptomycin, and recombinant human macrophage colony-stimulating factor (M-CSF) at 50 ng/mL (PeproTech, Oil City, PA, USA #300-03-5µG). Cells were maintained in a humidified incubator at 37 °C with 5% CO2 for 6 days, and the M-CSF-containing media was changed every 48 h. After 6 days, M-CSF-containing media were removed, and cells were stimulated to M1 macrophages by adding 20 ng/mL lipopolysaccharide (LPS) (Invitrogen, Carlsbad, CA, USA #tlrl-eblps) and 20 ng/mL recombinant human interferon-γ (IFN-γ) (PeproTech, Oil City, PA, USA #300-02-500UG) for 24 h.
2.3. RAW 264.7 Based M0 and M1 Macrophages Preparation
RAW 264.7 macrophage cells, at an approximate count of ~3 × 109, were used to prepare the murine (m) E-MVs and mC-MVs for in vivo experiments in mice. RAW 264.7 cells were stimulated with IFN-γ and LPS to polarize to the M1 phenotype as described previously for the human PBMC-derived macrophages.
2.4. Vesicles Generation
MVs were generated from M1 macrophages obtained from human monocytes and RAW 264.7 mouse cell lines through nitrogen (N2) cavitation as described previously [
8,
13]. Briefly, M1 macrophages were scraped from the cell culture flask using ice-cold phosphate-buffered saline (PBS) (Sigma-Aldrich, Saint Louis, MO, USA #P2272) with Pierce protease inhibitor (PI) mini-tablets (Thermo Scientific, Madison, WI, USA #A32953). Cells were centrifuged at 300×
g at room temperature (RT) to remove any remaining cytokines and washed with PBS containing PI. C-MVs or E-MVs were prepared as described in this section. Approximately 100 million human M1 macrophages were used to perform N
2 cavitation. To prepare C-MVs, an 8 mM solution of cisplatin (Tocris Bioscience, Bristol, UK #2251) in PBS was prepared and kept at 37 °C for 3–4 h to dissolve the cisplatin. Cells were scraped from the flask, pelleted down, and resuspended in 8 mL cisplatin solution. E-MVs were prepared by resuspending macrophage cell pellets in 10 mL of PBS. The cell suspensions prepared for either C-MVs or E-MVs were added to a pre-chilled pressurized chamber (Parr Instruments Company, Moline, IL, USA), which was then pressurized with 250 psi N
2 gas for 5 min at 4 °C to allow the cavitation to occur. The cell homogenate was collected from the chamber, and vesicles were purified from cellular debris by three centrifugation steps at increasing speeds. The first centrifugation was at 4000×
g for 20 min at 4 °C. The supernatant containing the vesicles was collected, and the cellular debris within the pellet was discarded. The supernatant was further centrifuged at 10,000×
g for 20 min at 4 °C to concentrate the vesicles. Again, the supernatant was collected and ultracentrifuged at 100,000×
g for 1 h at 4 °C was used to pellet the vesicles. The final vesicle pellet was resuspended in 1 mL of PBS.
2.5. Nanoparticle Tracking Analysis (NTA)
NTA was used to count the E-MVs and C-MVs using Nanosight NS300 (Malvern Panalytical, Malvern, UK). Samples were measured using constant camera and detection settings for all acquired images and videos. Each sample was recorded for 1 min, and a total of five repetitions with more than 200 tracks per video. The particle count analysis was performed using NTA version 3.4 software.
2.6. Characterization of Vesicles Through Scanning Electron Microscopy (SEM)
RAW 264.7 E-MVs were fixed in 2% formaldehyde (Thermo Fischer Scientific, Madison, WI, USA #J61899.AK) for 1 h and washed with PBS three times for 10 min at RT, as described previously [
13]. MVs were then further dehydrated using a series of ethanol (Sigma Aldrich, Saint Louis, MO, USA #1085430250) washes from 50%, 60%, 70%, 80%, 90%, 95%, and 100% for 10 min each. MVs were finally resuspended in 200-proof ethanol until imaged using SEM. MVs were briefly sonicated, and then a drop of the sample was transferred into a critical point dryer (EM CPD 300, Leica Microsystems). The sample was then metalized by sputter-coating 5 nm platinum (EM ACE 600, Leica Microsystems, Buffalo Grove, IL, USA) to enhance surface electrical conductivity. MVs were imaged using a field emission electron microscope, FE-SEM, Helios Nanolab 660 (Thermo Scientific, Madison, WI, USA).
2.7. Transmission Electron Microscopy
Human monocyte-derived E-MVs were tracked inside OS cells using immunogold silver enhancement staining (IGSS) and imaged using transmission electron microscopy (TEM). 143B cells were incubated with MVs for 24 h at 37 °C. After incubation, cells were washed with PBS and fixed with 4% paraformaldehyde for 40 min at RT. MVs were located using the CD86 antibody (Abcam, Cambridge, MA, USA #A243887), which acts as an M1 marker and primary antibody, and gold nanoparticles (Nanoprobes, Yaphank, NY, USA #2003) were used as a secondary stain. The immune gold silver enhancement staining (IGSS) (Nanoprobes, Yaphank, NY, USA #2012) method was performed, as described previously, to visualize the vesicles [
14].
2.8. LC-MS for Quantification of Cisplatin in C-MVs
Quantification of cisplatin was performed using the method described by Shaik et al. [
15]. A 10 mg/mL stock solution of cisplatin was prepared in dimethyl sulfoxide (DMSO) (Invitrogen, Carlsbad, CA, USA #D12345) and diluted in 50% methanol (Sigma-Aldrich, Saint Louis, MO, USA MX0486) in water to make a series of standard solutions (5, 10, 20, 50, 100, 200, 500 ng/mL) and quality controls (5, 25, 100, 500 ng/mL). To liberate cisplatin from C-MVs, vesicle samples were combined 1:1 with methanol, vortex mixed, and subjected to 5 successive freeze–thaw cycles using liquid nitrogen. 100 µL of each sample, standard, and quality control was combined in fresh polypropylene microcentrifuge tubes with 20 µL of 5 µg/mL transpalladin (trans-Diamminedichloropalladium (II)) (ThermoFisher Scientific, Madison, WI, USA #011037-02) and 20 µL of 1% diethyldithiocarbamic acid, sodium salt trihydrate (DTC) (Enzo Life Sciences, Farmingdale, NY, USA #ALX-400-003) in 0.1 N sodium hydroxide. The tubes were vortexed for 2 min, then incubated for 30 min at 4 °C to form a DDTC complex with platinum from cisplatin (Pt-DDTC) and palladium from transpalladin (Pd-DDTC). 300 µL of acetonitrile (Sigma-Aldrich, Saint Louis, MO, USA #AX0156) containing 50 ng/mL 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) (Sigma-Aldrich, Saint Louis, MO, USA #C101) was added, then vortexed for 2 min, and centrifuged at 15,000×
g for 5 min. Supernatant (250 µL) was transferred to 96-well polypropylene plates and dried under a stream of N
2 gas. The dried sample was resuspended in 100 μL of a 4:1 mixture of 0.1% formic acid (Agilent Technologies, Ankeny, IA, USA #G2453-85060) in water/acetonitrile and placed into the autosampler.
Samples were analyzed using a 1260 Infinity II LC system (Agilent Technologies, Ankeny, IA, USA) interfaced with a Ultivo triple quadrupole mass spectrometer (Agilent Technologies, Ankeny, IA, USA). Liquid chromatography was performed using an Agilent Infinity Lab Poroshell HPH-C18 column (100 mm × 2.1 mm, 2.7 μm). The mobile phase consisted of 0.1% formic acid in water (A) and acetonitrile (B) delivered as a linear gradient as follows: 0–0.5 min, 5% B; 0.5–1 min, 75% B; 1–1.5 min, 90% B; 1.5–2.5 min, 95% B; 2.5–4 min, 95% B; 4–5 min, 5% B and a 10 min equilibration before injection of the next sample. The flow rate was 0.35 mL/min. Electrospray ionization was operated in positive mode (ESI+) with nitrogen as both curtain and collision gas. The Ultivo instrument parameters were as follows: drying gas temperature, 30 °C; drying gas flow, 10 L/minute; sheath gas temperature, 25 °C; sheath gas flow, 11.5 L/minute; and nebulizer pressure, 45 psi. MRM used to detect the transitions of Pt-DDTC at m/z 491 → 88 (fragmentor voltage 240 V; collision energy 41 V), Pd-DDTC at m/z 403 → 116 (fragmentor voltage 190 V; collision energy 21 V), and DPCPX at m/z 305.2 → F178 (fragmentor voltage 98 V; collision energy 37 V). The data were analyzed using MassHunter Workstation (Agilent Technologies, Ankeny, IA, USA, version 1.1) software.
2.9. Drug Response Assay on HOS and 143B
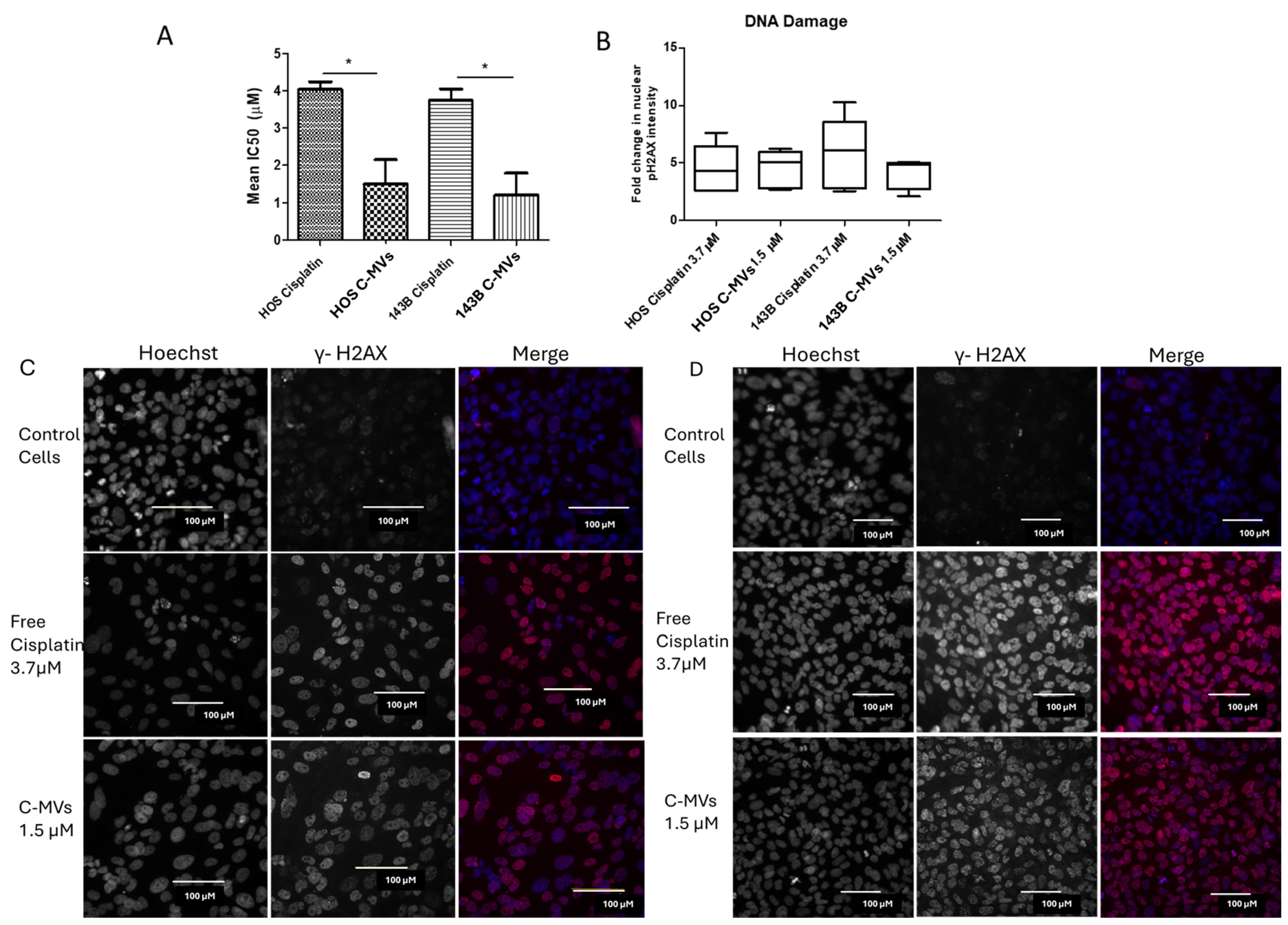
HOS and 143B cells were plated at 5000 cells/well onto a white-bottom 96-well cell culture plate and incubated for 24 h. Six different concentrations of serially, threefold diluted free cisplatin were used from 0.41 µM to 100 µM. Human (h) PBMC-derived hC-MVs were used at six different serial, threefold dilutions from 0.082% to 20%. The cisplatin concentration in the hC-MVs was determined by LC-MS to range from 0.3 ± 0.1 µM (0.083%) to 80 ± 33 µM (20%). Control cells were incubated in complete media without any added drugs. Cells were incubated for 96 h, and cell viability was assessed using Cell Titer-Glo 2.0 (Promega, Madison, WI, USA #G9243). Data were normalized to control cells and expressed as a percentage of cell viability. The assay was performed in 3 independent replicates, with each dose performed in duplicate (n = 3). Dose–response curves were generated using GraphPad Prism (version 5.1) and were used to calculate IC50 values.
2.10. DNA Damage Assay
HOS and 143B cells were seeded into a walled, clear-bottom 96-well cell culture plate (Thermo Scientific, Madison, WI, USA #165305) at 10,000 cells per well and allowed to adhere in a humidified CO2 incubator at 37 °C. After 24 h incubation, old media was replaced with fresh media having free cisplatin or hC-MVs at concentrations near the IC50 values for each cell line. Complete media, without any drug or vesicles, was used as a negative control. Cells were incubated for 24 h, followed by fixation with 4% paraformaldehyde (Thermo Scientific, Madison, WI, USA) in PBS at RT for 15 min. Cells were then permeabilized by incubating with 0.25% Triton X-100 (Alfa Aesar, Haverhill, MA, USA #A16046.AE) in PBS for 15 min and then blocked by incubation with 0.1% BSA (Proliant Biologicals, Ankeny, IA, USA #10842-662) in PBS for 1 h. Double-strand DNA breaks in cells were detected by a fluorescent antibody against phosphorylated histone H2AX (pH2AX) and nuclei were counter-stained using Hoechst 33342 (HCS DNA Damage Kit, Invitrogen, Carlsbad, CC, USA #H10292). Cells were imaged using the Cell Insight CX7 High Content Analysis Platform (Thermo Scientific, Madison, WI, USA), and quantification of nuclear pH2AX signal was performed using the HCS studio software (Thermo Scientific, Madison, WI, USA). Channel 1 defined the nucleus with Hoechst 33342 (using the segmentation tool) as an object with Hoechst/XF93 filters. Channel 3 determined the fluorescence intensity of the pH2AX signal using TRITC/XF93 filters in the nucleus outlined in channel 1. The fold change in pH2AX intensity was calculated by normalizingto matched control cells, and graphs were generated using GraphPad Prism (version 5.01).
2.11. In Vivo Mouse Experiment
Animal studies were approved by the University of Kentucky Institutional Animal Care and Use Committee (Approval #2017-2674). A total of 5 × 105 luciferase-labeled 143B cells were orthotopically injected into one tibia of 6–7-week-old female and male outbred homozygous nude mice (Jackson Laboratory, Bar Harbor, ME, USA #007850). A total of 32 mice were used in the study, with 8 mice in each group. The number of mice per group was determined a priori based on previously published xenograft growth experiments. Confounders of the order of treatments and the animal cage effect were not controlled for. Mice were treated with meloxicam analgesic at 5 mg/kg for 3 days after tumor cell injection. Tumor volume was assessed weekly using whole body bioluminescence imaging following intraperitoneal injection (IP) of 150 mg/kg of IVISbrite D-luciferin potassium salt (PerkinElmer, Waltham, MA, USA #760504) on Lago X animal imager (Spectral Instruments Imaging, Tucson, AZ, USA). The bioluminescence was not conducted by a blinded investigator; however, instrument settings were determined a priori and remained constant throughout the measurements. The bioluminescence values were converted to log values and baseline corrected. Animals were randomly assigned to four treatment groups when total body luminescence was between 2.0 and 7.5 × 107 photons/second: free cisplatin, mC-MVs, mE-MVs, and control (PBS). The concentration of cisplatin in mC-MVs was measured using LC-MS, and the amount of free cisplatin used for each treatment was matched to the amount of cisplatin in the mC-MVs treatment each week. The mice were treated IP once per week until they met criteria for euthanasia or for a total of 12 weeks. Mice not previously euthanized were monitored without treatment for 2 weeks post-end of treatment. Endpoint criteria included a 15% decrease in body weight from the start of treatment or a tumor burden that precluded movement. At the endpoint, mice were humanely euthanized, blood was collected using cardiac puncture, and the tumor, liver, spleen, kidney, and lungs were harvested.
2.12. MVs Labeling and Localization
Murine (m) RAW 264.7 cell-generated mMVs were resuspended in 2 mL of 250 mM sucrose buffer (VWR, Radnor, PA, USA #76177-754) in 10 mM HEPES (VWR, Radnor, PA, USA #97061-770), pH 7.5. Lipophilic DiR near-infrared fluorescent dye (Thermo Fisher Scientific, Madison, WI, USA #D12731) was diluted to a final concentration of 2 mM in sucrose buffer and incubated with mMVs for 30 min at 37 °C, after which, the vesicle solution was carefully layered with a 50% and 10% OptiPrep density gradient medium (Millipore Sigma, Madison, WI, USA). The sample was ultracentrifuged at 112,000× g for 1 h at 4 °C to separate labeled vesicles. Labeled vesicles were collected using a peristaltic pump in a 1.5 mL Eppendorf tube and were further purified using size exclusion PD Miditrap columns (Cytiva, Marlborough, MA, USA) to remove any free dye. One 6-week-old nude mouse bearing a human 143B tibia xenograft was used to investigate the localization of the mE-MVs. A total of 200 µL of dye-labeled E-MVs was injected intraperitoneally into the mouse and was imaged 48 h post-injection using Lago X animal imager (Spectral Instruments, Tucson, AZ, USA) at a fluorescence excitation of 710 nm and emission of 770 nm for 10 s to determine the localization of the mE-MVs in vivo. After imaging, a necropsy was performed, and the tumor, liver, lungs, and spleen were harvested and imaged in the same way as the whole mouse imaging. Fluorescent images were analyzed with Aura version 4.0.8 software.
2.13. Mouse Serum Profiles
Diagnostic chemistry profiling was performed on serum samples from all mice at the time they met criteria for euthanasia. Blood samples were collected via cardiac puncture under isoflurane anesthesia into Eppendorf tubes. The serum was separated from the blood within 30 min of collection by centrifugation at 2000× g for 15 min at RT. 100 µL of serum sample was used to perform serum chemistry for alkaline phosphatase (ALP), alanine aminotransferase (ALT), total albumin, creatinine, blood urea nitrogen (BUN), bilirubin, calcium, potassium, sodium, glucose, and phosphate using an automated analyzer, Abaxis VetScan version S2.
TNF-α was evaluated in mouse serum using a Quantikine ELISA kit (Bio-Techne, Minneapolis, MN, USA #MTA00B) according to the kit’s instructions. One mouse each in control and free cisplatin groups had TNF-α values below the level of quantification, and therefore, values were extrapolated using the equation of the standard curve [
16].
2.14. Histopathology of Mice Organs
Tissues, including the xenograft tumor on the tibia, liver, and kidney, were collected once the mice reached humane endpoints (described above) or after a total of 14 weeks. All tissues were fixed in 10% formalin (VWR, Radnor, PA, USA #100496-502) for 48 h and then transferred to 70% ethanol, followed by a series of dehydration before embedding in paraffin. Tissue sections were cut at 4–5 µm thickness and stained with hematoxylin and eosin (H&E) for histopathologic analysis by a blinded pathologist.
2.15. Immunohistochemistry for M1 and M2 Macrophages
Dewaxing of the parafilm-embedded sections was performed at 58 °C oven for 2 h. After dewaxing, the slides were cooled and passed through a series of 100% xylene (Sigma-Aldrich) and serially dehydrated from 100% to 70% ethanol. After dehydration, antigen retrieval was performed using a high-pH Tris base buffer (pH 9.0) (Abcam, Cambridge, MA, USA) for 15 minutes at 98 °C. The tumor sections were stained with anti-rabbit monoclonal antibody for CD86 (M1 macrophage, Abcam, Cambridge, MA, USA #A243887) and anti-rat monoclonal Ab for CD163 (M2 macrophage, Abcam, Cambridge, MA, USA #289979). The M1 and M2 macrophages were visualized by using anti-rabbit Alexa Fluor 488 (green fluorescence; Themo Fischer Scientific, Madison, WI, USA #A-11008) and anti-rat Alexa Fluor 594 fluorochromes (red fluorescence; Thermo Fischer Scientific, Madison, WI, USA #A11007) for M1 and M2 macrophages, respectively. The nuclei were visualized by DAPI (Thermo Fischer Scientific, Madison, WI, USA #62248) fluorescent staining. Slides were examined using a confocal microscope (Leica Biosystems, Buffalo Grove, IL, USA). Five similarly sized regions of interest (ROI) were randomly selected in the tumor slide, and total CD86 (M1) or CD163 (M2) positive cells were counted, and M1/M2 ratios were plotted for all the mice.
2.16. Statistical Analyses
All in vitro experiments included at least two technical replicates and were performed at least three times independently. Non-linear regression analysis was used to plot the dose–response curve and the log (inhibitor) vs. response curve. A variable slope equation was used to calculate the cell viability assays and IC50 calculations. The student’s unpaired t-test was used to analyze the difference between the IC50 values and the DNA damage assay between free cisplatin and C-MVs in cell lines. For mouse bioluminescence, two-way ANOVA was used. For analyzing the weight, serum chemistry, and IHC results, one-way ANOVA with post hoc test specified in the figure legend. For analyzing the Kaplan–Meier survival curves, a log-rank test was used.
4. Discussion
Endogenous EVs are nano-sized, cargo-baring, lipid bilayer vesicles naturally released by cells into the extracellular space. The unique cellular targeting ability of EVs has led to the exploration as therapeutic agents [
19]. Importantly, studies have shown that M1 macrophages actively secrete EVs that are able to repolarize M2 macrophages in the tumor microenvironment to the M1 or anti-cancer phenotype [
12]. Despite the promise of EVs as therapeutic agents, the field is limited by the inability to produce EVs on a large scale. More recently, advances have been made in scaling up the production of MVDVs using cell disruption strategies. We recently demonstrated that M1-MVs can be generated using nitrogen cavitation of LPS- and IFN-γ-stimulated macrophages, and that 100 million M1-macrophages generated approximately two trillion hM1-MVs. Importantly, manufactured hM1-MVs retained both the tumor-targeting and pro-inflammatory cytokine phenotypes of naturally occurring EVs released by M1 macrophages [
10].
In this work, we characterize human and mouse M1-MVs derived by nitrogen cavitation using scanning electron microscopy and show that they are similar in size and shape to naturally occurring extracellular vesicles, with rounded morphology and a size of approximately 100–200 nm in diameter [
20]. Interestingly, we also show via CD86-labeled, enhancement staining transmission electron microscopy, the internalization and location of hM1-MVs within an OS cancer cell line. We demonstrate the ability of hE-MVs to physically enter human OS cells and localize to intracellular compartments consistent with phagolysosomes, supporting a plausible delivery method for cargo.
EVs have garnered considerable interest as therapeutic delivery vehicles to transport cargo across cell membranes because they have been shown to precisely target cell types via distinct surface molecules [
21]. Previously, we demonstrated that MCDVs exhibit cell-specific targeting, preferentially homing to their cell of origin, and could easily be loaded to deliver cargo selectively to tumors in murine cancer models [
12]. We demonstrate that mE-MVs home to tumors and also localize to the mouse liver. Systemically administered MCDVs have been shown to preferentially accumulate in the liver, and hepatic clearance is the dominant elimination route [
22].
Here we also demonstrate that the incorporation of cisplatin into the MVs has superior anti-cancer efficacy against OS cell lines, as indicated by lower IC50 and compared with free cisplatin in vitro. C-MVs treatment resulted in DNA damage, indicating the cisplatin cargo, a DNA crosslinking agent, was effectively delivered inside the cell. In addition, the effectiveness of C-MVs was demonstrated in vivo using an orthotopic OS mouse model, with C-MV treatment resulting in a slower tumor growth rate and prolonged survival. Similarly, others have demonstrated the therapeutic effectiveness of chemotherapy-loaded endogenous M1 macrophage-derived exosomes in a breast cancer model [
23]. However, we may be the first to successfully use manufactured, chemotherapy-loaded vesicles as anti-cancer agents. We previously demonstrated that both E-MVs and C-MVs were effective in an ovarian cancer mouse xenograft model, with both exhibiting significantly longer survival than control-treated mice [
13]. In contrast, E-MV treatment was not superior to control in the current OS orthotopic model. This finding could be related to differences between the models, including tumor mutational burden, different mouse strains (nudes vs. BALBc/SCID), and the very rapid tumor growth rate of the OS model. Neither E-MVs nor C-MVs significantly reduced the formation of lung metastatic nodules, which may be attributed to the extended duration before euthanasia, allowing additional time for nodules to develop.
As cisplatin causes numerous off-target toxicities, often requiring patients to receive reduced doses or to discontinue therapy, toxicity was evaluated in mice treated with free cisplatin in comparison with equal doses of cisplatin incorporated into mM1-MVs. Mice treated with free cisplatin demonstrated significant weight loss, and although mC-MVs-treated mice did not increase in weight as fast as controls, none experienced weight loss. Cisplatin-treated mice also had significant elevations in serum BUN, ALP, and the albumin/globulin ratio and pathological changes indicative of both nephrotoxicity and hepatoxicity, which were not noted in mice treated with mE-MVs or mC-MVs treatment, suggesting mC-MVs are able to selectively deliver cisplatin to the tumor, reducing off-target toxicities.
In an orthotopic OS mouse model, we show that labeled M1-MVs selectively localize to the tumor xenograft and effectively increase the M1/M2 macrophage ratio within the tumor microenvironment, along with elevated plasma TNF-α levels. These findings suggest that M1-MVs can reprogram immunosuppressive M2 tumor-associated macrophages toward a pro-inflammatory M1 phenotype, potentially enhancing anti-tumor immune responses and improving the efficacy of PD-1/PD-L1 checkpoint inhibitors. The observed tumor selectivity, immunomodulatory activity, favorable safety profile, and capacity to reshape macrophage polarization support further investigation of M1-MVs as a promising therapeutic strategy for OS.