Alternative Lengthening of Telomeres: A Prognostic Paradox in Cancer
Abstract
Highlights
- ALT functions as a context-dependent double-edged sword: it drives aggressiveness in rapidly proliferating cancers but imposes replicative and metabolic stress in slow-growing, immune-cold tumors such as glioblastoma and chondrosarcoma.
- Loss of ATRX/DAXX and ALT activation creates unique DNA repair vulnerabilities, including hypersensitivity to ATR and PARP inhibition, opening avenues for targeted therapy.
- Refines prognostic interpretation of ALT positivity—transforming it from a uniformly negative marker into a tumor-type-specific indicator of clinical outcome.
- Positions ALT as a precision oncology target, where integrated biomarkers (C-circle as say, APB detection, ATRX/DAXX mutation profiling) enable patient stratification and therapeutic decision-making.
Abstract
1. Introduction
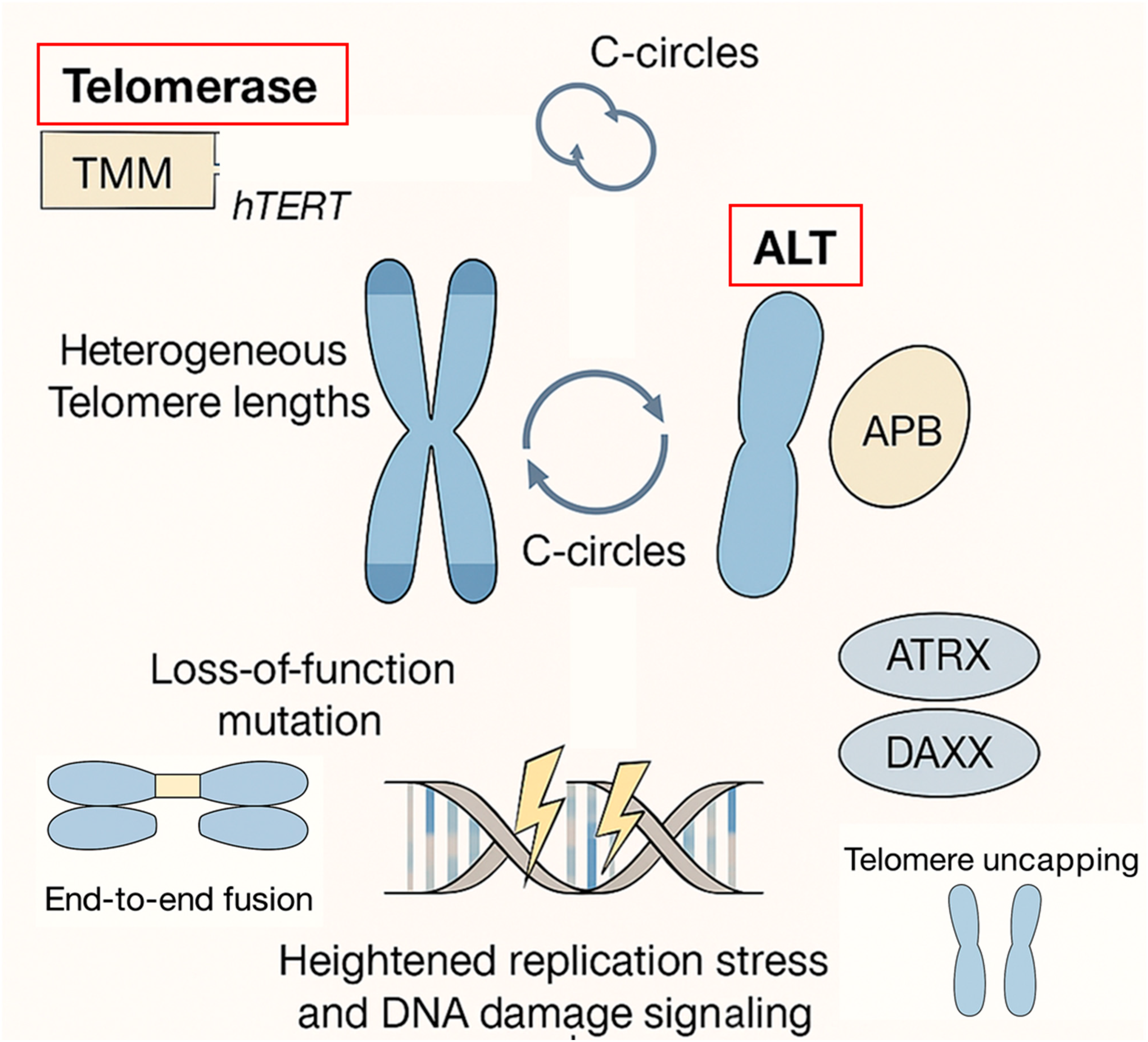
2. ALT and Poor Prognosis in Most Cancers
2.1. Genomic Instability as a Double-Edged Sword
2.2. ALT and Tumor Progression
2.3. Clinical Observations Across Cancer Types
2.4. Summary
3. Favorable Prognosis of ALT in GBM and Chondrosarcoma
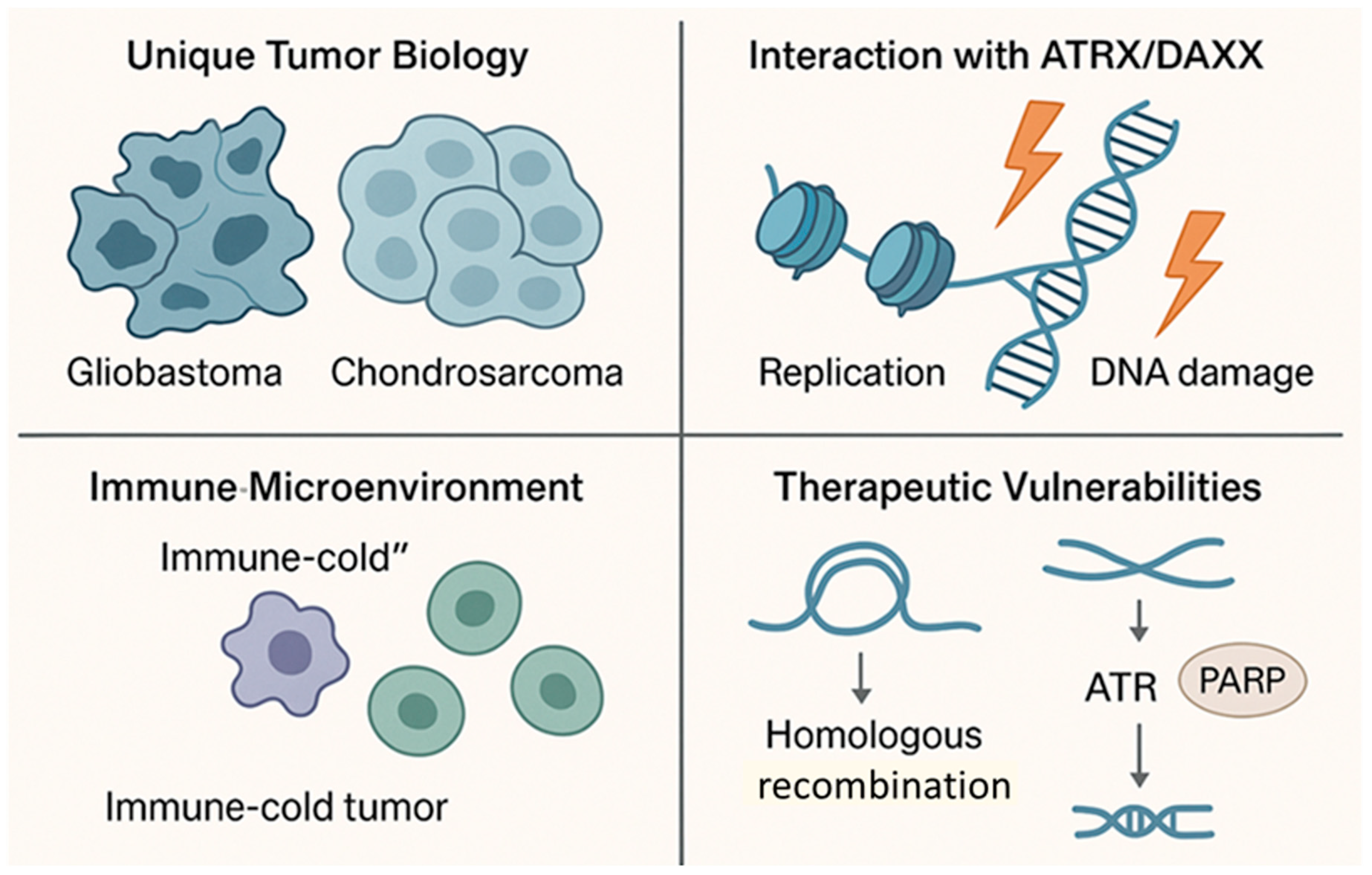
3.1. Unique Tumor Biology
3.2. Interaction with ATRX/DAXX Mutations
3.3. Immune Microenvironment
3.4. Therapeutic Vulnerabilities
3.5. Summary
4. A Context-Dependent Model
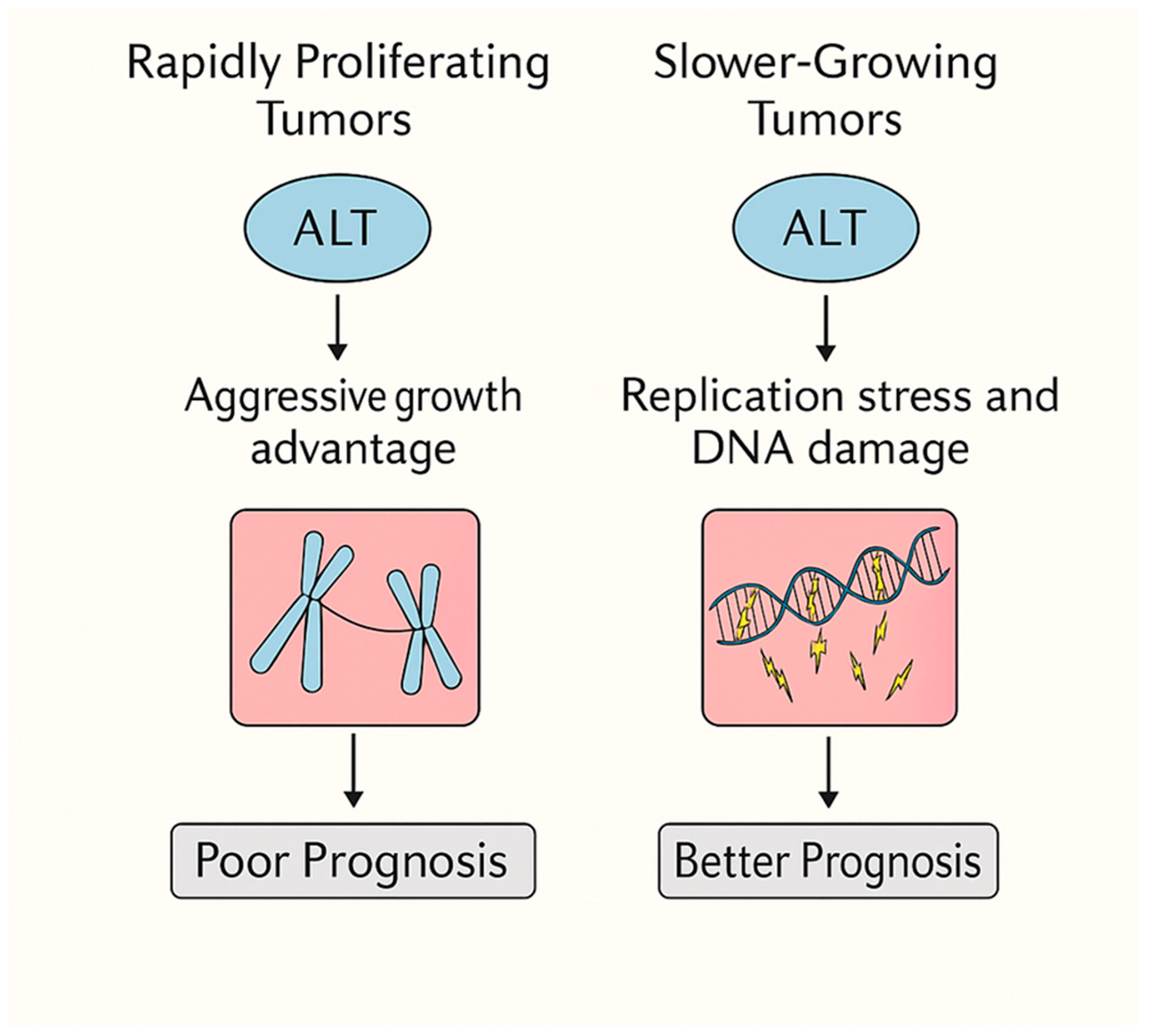
4.1. ALT as a Driver of Aggressiveness in Rapidly Proliferating Tumors
4.2. ALT as a Fitness Burden in Slow-Growing, Immune-Cold Tumors
4.3. ALT as a Double-Edged Sword
4.4. Broader Implications
4.5. ALT, p53, and Tumor Suppressor Networks
4.6. Oncogenic and Tumor-Suppressive Alterations in ALT-Driven Cancers
4.7. Oxidative Stress as a Modulator of ALT Dynamics
4.8. Epigenetic Regulation, Metabolism, and the Tumor Microenvironment
5. Clinical Implications
5.1. Prognostic Stratification
5.2. Therapeutic Targeting
5.3. Precision Oncology
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.C.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef]
- Cesare, A.J.; Reddel, R.R. Alternative lengthening of telomeres: Models, mechanisms and implications. Nat. Rev. Genet. 2010, 11, 319–330. [Google Scholar] [CrossRef]
- Heaphy, C.M.; Subhawong, A.P.; Hong, S.-M.; Goggins, M.G.; Montgomery, E.A.; Gabrielson, E.; Netto, G.J.; Epstein, J.I.; Lotan, T.L.; Westra, W.H.; et al. Prevalence of the Alternative Lengthening of Telomeres Telomere Maintenance Mechanism in Human Cancer Subtypes. Am. J. Pathol. 2011, 179, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Yu, Y.; Li, D.; Zhang, Y.; Zhang, K.; Tong, J.; Yang, K.; Jia, S. Alternative Lengthening of Telomeres and Mediated Telomere Synthesis. Cancers 2022, 14, 2194. [Google Scholar] [CrossRef] [PubMed]
- Rosso, I.; Jones-Weinert, C.; Rossiello, F.; Cabrini, M.; Brambillasca, S.; Munoz-Sagredo, L.; Lavagnino, Z.; Martini, E.; Tedone, E.; Garre’, M.; et al. Alternative lengthening of telomeres (ALT) cells viability is dependent on C-rich telomeric RNAs. Nat. Commun. 2023, 14, 7086. [Google Scholar] [CrossRef]
- Heaphy, C.M.; de Wilde, R.F.; Jiao, Y.; Klein, A.P.; Edil, B.H.; Shi, C.; Bettegowda, C.; Rodriguez, F.J.; Eberhart, C.G.; Hebbar, S.; et al. Altered Telomeres in Tumors with ATRX and DAXX Mutations. Science 2011, 333, 425. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, C.A.; Li, W.; Reisenweber, S.; Thongthip, S.; Bruno, J.; de Lange, T.; De, S.; Petrini, J.H.; Sung, P.A.; Jasin, M.; et al. Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway. PLoS Genet. 2012, 8, e1002772. [Google Scholar] [CrossRef]
- Garbarino, J.; Eckroate, J.; Sundaram, R.K.; Jensen, R.B.; Bindra, R.S. Loss of ATRX confers DNA repair defects and PARP inhibitor sensitivity. Transl. Oncol. 2021, 14, 101147. [Google Scholar] [CrossRef]
- Sung, J.-Y.; Lim, H.-W.; Joung, J.-G.; Park, W.-Y. Pan-Cancer Analysis of Alternative Lengthening of Telomere Activity. Cancers 2020, 12, 2207. [Google Scholar] [CrossRef] [PubMed]
- Desmaze, C.; Soria, J.-C.; Freulet-Marrière, M.-A.; Mathieu, N.; Sabatier, L. Telomere-driven genomic instability in cancer cells. Cancer Lett. 2003, 194, 173–182. [Google Scholar] [CrossRef]
- Sobinoff, A.P.; Pickett, H.A. Alternative Lengthening of Telomeres: DNA Repair Pathways Converge. Trends Genet. 2017, 33, 921–932. [Google Scholar] [CrossRef]
- Naderlinger, E.; Holzmann, K. Epigenetic Regulation of Telomere Maintenance for Therapeutic Interventions in Gliomas. Genes 2017, 8, 145. [Google Scholar] [CrossRef]
- Sung, J.-Y.; Cheong, J.-H. Pan-Cancer Analysis of Clinical Relevance via Telomere Maintenance Mechanism. Int. J. Mol. Sci. 2021, 22, 11101. [Google Scholar] [CrossRef]
- Sung, J.-Y.; Kim, J.-H.; Kim, Y.-J. Alternative lengthening of telomeres confers favorable prognosis in chondrosarcomas. J. Transl. Med. 2025, 23, 536. [Google Scholar] [CrossRef]
- Sohn, E.J.; Goralsky, J.A.; Shay, J.W.; Min, J. The Molecular Mechanisms and Therapeutic Prospects of Alternative Lengthening of Telomeres (ALT). Cancers 2023, 15, 1945. [Google Scholar] [CrossRef]
- Lawlor, R.T.; Veronese, N.; Pea, A.; Nottegar, A.; Smith, L.; Pilati, C.; Demurtas, J.; Fassan, M.; Cheng, L.; Luchini, C. Alternative lengthening of telomeres (ALT) influences survival in soft tissue sarcomas: A systematic review with meta-analysis. BMC Cancer 2019, 19, 232. [Google Scholar] [CrossRef]
- Sung, J.-Y.; Cheong, J.-H. Pan-Cancer Analysis Reveals Distinct Metabolic Reprogramming in Different Epithelial–Mesenchymal Transition Activity States. Cancers 2021, 13, 1778. [Google Scholar] [CrossRef]
- Hakin-Smith, V.; Jellinek, D.A.; Levy, D.; Carroll, T.; Teo, M.; Timperley, W.R.; McKay, M.J.; Reddel, R.R.; Royds, J.A. Alternative lengthening of telomeres and survival in patients with glioblastoma multiforme. Lancet 2003, 361, 836–838. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.L.; McDonnell, J.; Muntoni, A.; Henson, J.D.; Hegi, M.E.; von Deimling, A.; Wheeler, H.R.; Cook, R.J.; Biggs, M.T.; Little, N.S.; et al. Presence of Alternative Lengthening of Telomeres Mechanism in Patients With Glioblastoma Identifies a Less Aggressive Tumor Type With Longer Survival. J. Neuropathol. Exp. Neurol. 2010, 69, 729–736. [Google Scholar] [CrossRef] [PubMed]
- La Torre, D.; Aguennouz, M.; Conti, A.; Giusa, M.; Raffa, G.; Abbritti, R.V.; Germano’, A.; Angileri, F.F. Potential Clinical Role of Telomere Length in Human Glioblastoma. Transl. Med. @ UniSa 2011, 1, 243–270. [Google Scholar] [PubMed]
- Dilley, R.L.; Greenberg, R.A. ALTernative Telomere Maintenance and Cancer. Trends Cancer 2015, 1, 145–156. [Google Scholar] [CrossRef]
- Udugama, M.; Hii, L.; Garvie, A.; Cervini, M.; Vinod, B.; Chan, F.-L.; Das, P.P.; Mann, J.R.; Collas, P.; Voon, H.P.J.; et al. Mutations inhibiting KDM4B drive ALT activation in ATRX-mutated glioblastomas. Nat. Commun. 2021, 12, 2584. [Google Scholar] [CrossRef]
- Episkopou, H.; Draskovic, I.; Van Beneden, A.; Tilman, G.; Mattiussi, M.; Gobin, M.; Arnoult, N.; Londoño-Vallejo, A.; Decottignies, A. Alternative Lengthening of Telomeres is characterized by reduced compaction of telomeric chromatin. Nucleic Acids Res. 2014, 42, 4391–4405. [Google Scholar] [CrossRef]
- Yu, E.Y.; Pérez-Martín, J.; Holloman, W.K.; Lue, N.F. Mre11 and Blm-Dependent Formation of ALT-Like Telomeres in Ku-Deficient Ustilago maydis. PLoS Genet. 2015, 11, e1005570. [Google Scholar] [CrossRef]
- Pompili, L.; Leonetti, C.; Biroccio, A.; Salvati, E. Diagnosis and treatment of ALT tumors: Is Trabectedin a new therapeutic option? J. Exp. Clin. Cancer Res. 2017, 36, 189. [Google Scholar] [CrossRef]
- Singh, M.; MacKenzie, D.; Desai, S.; Batista, N.; Zhang, D. Diagnostic Biomarkers and Therapeutic Targets of ALT-Positive Cancers. Insid. Precis. Med. 2022, 9, 48–50. [Google Scholar] [CrossRef]
- Hoang, S.M.; O’sullivan, R.J. Alternative Lengthening of Telomeres: Building Bridges To Connect Chromosome Ends. Trends Cancer 2020, 6, 247–260. [Google Scholar] [CrossRef]
- Zhang, J.-M.; Zou, L. Alternative lengthening of telomeres: From molecular mechanisms to therapeutic outlooks. Cell Biosci. 2020, 10, 30. [Google Scholar] [CrossRef]
- Mori, J.O.; Keegan, J.; Flynn, R.L.; Heaphy, C.M. Alternative lengthening of telomeres: Mechanism and the pathogenesis of cancer. J. Clin. Pathol. 2023, 77, 82–86. [Google Scholar] [CrossRef]
- Sung, J.-Y.; Lee, J.W. Telomere maintenance mechanism subtype reveals different immune activity in vestibular schwannoma. J. Neuro-Oncology 2023, 165, 113–126. [Google Scholar] [CrossRef]
- Singhi, A.D.; Liu, T.-C.; Roncaioli, J.L.; Cao, D.; Zeh, H.J.; Zureikat, A.H.; Tsung, A.; Marsh, J.W.; Lee, K.K.; Hogg, M.E.; et al. Alternative Lengthening of Telomeres and Loss of DAXX/ATRX Expression Predicts Metastatic Disease and Poor Survival in Patients with Pancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2017, 23, 600–609. [Google Scholar] [CrossRef]
- MacKenzie, D.; Watters, A.K.; To, J.T.; Young, M.W.; Muratori, J.; Wilkoff, M.H.; Abraham, R.G.; Plummer, M.M.; Zhang, D. ALT Positivity in Human Cancers: Prevalence and Clinical Insights. Cancers 2021, 13, 2384. [Google Scholar] [CrossRef]
- Sun, H.; Chen, G.; Guo, B.; Lv, S.; Yuan, G. Potential clinical treatment prospects behind the molecular mechanism of alternative lengthening of telomeres (ALT). J. Cancer 2023, 14, 417–433. [Google Scholar] [CrossRef]
- Sung, J.; Cheong, J. Alternative lengthening of telomeres is mechanistically linked to potential therapeutic vulnerability in the stem-like subtype of gastric cancer. Clin. Transl. Med. 2021, 11, e561. [Google Scholar] [CrossRef]
- Royds, J.A.; Al Nadaf, S.; Wiles, A.K.; Chen, Y.J.; Ahn, A.; Shaw, A.; Bowie, S.; Lam, F.; Baguley, B.C.; Braithwaite, A.W.; et al. The CDKN2A G500 Allele Is More Frequent in GBM Patients with No Defined Telomere Maintenance Mechanism Tumors and Is Associated with Poorer Survival. PLoS ONE 2011, 6, e26737. [Google Scholar] [CrossRef]
- Henson, J.D.; Hannay, J.A.; McCarthy, S.W.; Royds, J.A.; Yeager, T.R.; Robinson, R.A.; Wharton, S.B.; Jellinek, D.A.; Arbuckle, S.M.; Yoo, J.; et al. A Robust Assay for Alternative Lengthening of Telomeres in Tumors Shows the Significance of Alternative Lengthening of Telomeres in Sarcomas and Astrocytomas. Clin. Cancer Res. 2005, 11, 217–225. [Google Scholar] [CrossRef]
- Aguilera, P.; López-Contreras, A.J. ATRX, a guardian of chromatin. Trends Genet. 2023, 39, 505–519. [Google Scholar] [CrossRef]
- Koschmann, C.; Lowenstein, P.R.; Castro, M.G. ATRX mutations and glioblastoma: Impaired DNA damage repair, alternative lengthening of telomeres, and genetic instability. Mol. Cell. Oncol. 2016, 3, e1167158. [Google Scholar] [CrossRef]
- Dyer, M.A.; Qadeer, Z.A.; Valle-Garcia, D.; Bernstein, E. ATRX and DAXX: Mechanisms and Mutations. Cold Spring Harb. Perspect. Med. 2017, 7, a026567. [Google Scholar] [CrossRef]
- Carson, L.M.; Flynn, R.L. Highlighting vulnerabilities in the alternative lengthening of telomeres pathway. Curr. Opin. Pharmacol. 2023, 70, 102380. [Google Scholar] [CrossRef]
- George, S.L.; Lorenzi, F.; King, D.; Hartlieb, S.; Campbell, J.; Pemberton, H.; Toprak, U.H.; Barker, K.; Tall, J.; da Costa, B.M.; et al. Therapeutic vulnerabilities in the DNA damage response for the treatment of ATRX mutant neuroblastoma. EBioMedicine 2020, 59, 102971. [Google Scholar] [CrossRef]
- Stundon, J.L.; Ijaz, H.; Gaonkar, K.S.; Kaufman, R.S.; Jin, R.; Karras, A.; Vaksman, Z.; Kim, J.; Corbett, R.J.; Lueder, M.R.; et al. Alternative lengthening of telomeres (ALT) in pediatric high-grade gliomas can occur without ATRX mutation and is enriched in patients with pathogenic germline mismatch repair (MMR) variants. Neuro-Oncology 2022, 25, 1331–1342. [Google Scholar] [CrossRef]
- Li, F.; Deng, Z.; Zhang, L.; Wu, C.; Jin, Y.; Hwang, I.; Vladimirova, O.; Xu, L.; Yang, L.; Lu, B.; et al. ATRX loss induces telomere dysfunction and necessitates induction of alternative lengthening of telomeres during human cell immortalization. EMBO J. 2019, 38, e96659. [Google Scholar] [CrossRef]
- Lu, R.; Pickett, H.A. Telomeric replication stress: The beginning and the end for alternative lengthening of telomeres cancers. Open Biol. 2022, 12, 220011. [Google Scholar] [CrossRef]
- Ebata, H.; Loo, T.M.; Takahashi, A. Telomere Maintenance and the cGAS-STING Pathway in Cancer. Cells 2022, 11, 1958. [Google Scholar] [CrossRef]
- Zheng, J.; Mo, J.; Zhu, T.; Zhuo, W.; Yi, Y.; Hu, S.; Yin, J.; Zhang, W.; Zhou, H.; Liu, Z. Comprehensive elaboration of the cGAS-STING signaling axis in cancer development and immunotherapy. Mol. Cancer 2020, 19, 133. [Google Scholar] [CrossRef]
- Khoo, L.T.; Chen, L. Role of the cGAS–STING pathway in cancer development and oncotherapeutic approaches. Embo Rep. 2018, 19, e46935. [Google Scholar] [CrossRef]
- Flynn, R.L.; Cox, K.E.; Jeitany, M.; Wakimoto, H.; Bryll, A.R.; Ganem, N.J.; Bersani, F.; Pineda, J.R.; Suvà, M.L.; Benes, C.H.; et al. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science 2015, 347, 273–277. [Google Scholar] [CrossRef]
- Yuan, M.; Eberhart, C.G.; Pratilas, C.A.; Blakeley, J.O.; Davis, C.; Stojanova, M.; Reilly, K.; Meeker, A.K.; Heaphy, C.M.; Rodriguez, F.J. Therapeutic Vulnerability to ATR Inhibition in Concurrent NF1 and ATRX-Deficient/ALT-Positive High-Grade Solid Tumors. Cancers 2022, 14, 3015. [Google Scholar] [CrossRef]
- Bisht, P.; Kumar, V.U.; Pandey, R.; Velayutham, R.; Kumar, N. Role of PARP Inhibitors in Glioblastoma and Perceiving Challenges as Well as Strategies for Successful Clinical Development. Front. Pharmacol. 2022, 13, 939570. [Google Scholar] [CrossRef]
- Bhargava, R.; Lynskey, M.L.; O’sUllivan, R.J. New twists to the ALTernative endings at telomeres. DNA Repair 2022, 115, 103342. [Google Scholar] [CrossRef] [PubMed]
- Gulve, N.; Su, C.; Deng, Z.; Soldan, S.S.; Vladimirova, O.; Wickramasinghe, J.; Zheng, H.; Kossenkov, A.V.; Lieberman, P.M. DAXX-ATRX regulation of p53 chromatin binding and DNA damage response. Nat. Commun. 2022, 13, 5033. [Google Scholar] [CrossRef]
- Koneru, B.; Farooqi, A.; Nguyen, T.H.; Chen, W.H.; Hindle, A.; Eslinger, C.; Makena, M.R.; Burrow, T.A.; Wilson, J.; Smith, A.; et al. ALT neuroblastoma chemoresistance due to telomere dysfunction–induced ATM activation is reversible with ATM inhibitor AZD0156. Sci. Transl. Med. 2021, 13, eabd5750. [Google Scholar] [CrossRef]
- Macha, S.J.; Koneru, B.; Burrow, T.A.; Zhu, C.; Savitski, D.; Rahman, R.L.; Ronaghan, C.A.; Nance, J.; McCoy, K.; Eslinger, C.; et al. Alternative Lengthening of Telomeres in Cancer Confers a Vulnerability to Reactivation of p53 Function. Cancer Res. 2022, 82, 3345–3358. [Google Scholar] [CrossRef]
- Goncalves, T.; Cunniffe, S.; Ma, T.S.; Mattis, N.; Rose, A.W.; Kent, T.; Mole, D.R.; Geiller, H.E.B.; van Bijsterveldt, L.; Humphrey, T.C.; et al. Elevated reactive oxygen species can drive the alternative lengthening of telomeres pathway in ATRX-null cancers. Nucleic Acids Res. 2025, 53, gkaf061. [Google Scholar] [CrossRef] [PubMed]
- Doksani, Y. The Response to DNA Damage at Telomeric Repeats and Its Consequences for Telomere Function. Genes 2019, 10, 318. [Google Scholar] [CrossRef]
- Roumelioti, F.-M.; Sotiriou, S.K.; Katsini, V.; Chiourea, M.; Halazonetis, T.D.; Gagos, S. Alternative lengthening of human telomeres is a conservative DNA replication process with features of break-induced replication. EMBO Rep. 2016, 17, 1731–1737. [Google Scholar] [CrossRef]
- Opresko, P.L.; Fan, J.; Danzy, S.; Wilson, D.M., 3rd; Bohr, V.A. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005, 33, 1230–1239. [Google Scholar] [CrossRef]
- Coluzzi, E.; Leone, S.; Sgura, A. Oxidative Stress Induces Telomere Dysfunction and Senescence by Replication Fork Arrest. Cells 2019, 8, 19. [Google Scholar] [CrossRef]
- Porreca, R.M.; Herrera-Moyano, E.; Skourti, E.; Law, P.P.; Franco, R.G.; Montoya, A.; Faull, P.; Kramer, H.; Vannier, J.-B. TRF1 averts chromatin remodelling, recombination and replication dependent-break induced replication at mouse telomeres. eLife 2020, 9, e49817. [Google Scholar] [CrossRef] [PubMed]
- Billard, P.; Poncet, D.A. Replication Stress at Telomeric and Mitochondrial DNA: Common Origins and Consequences on Ageing. Int. J. Mol. Sci. 2019, 20, 4959. [Google Scholar] [CrossRef]
- Armendáriz-Castillo, I.; García-Cárdenas, J.; Espinosa, P.; Hidalgo-Fernández, K.; Peña-Zúñiga, L.; Martínez, R.; Moromenacho, J.; Herrera-Yela, A.; Cruz-Varela, J.; Saucedo-Sariñana, A.; et al. Metabolic pathways of Alternative Lengthening of Telomeres in pan-carcinoma. PLoS ONE 2025, 20, e0314012. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Rose, M.; Burgess, J.T.; O’bYrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef]
- Kanev, P.-B.; Atemin, A.; Stoynov, S.; Aleksandrov, R. PARP1 roles in DNA repair and DNA replication: The basi(c)s of PARP inhibitor efficacy and resistance. Semin. Oncol. 2023, 51, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Parsels, L.A.; Karnak, D.; Parsels, J.D.; Zhang, Q.; Vélez-Padilla, J.; Reichert, Z.R.; Wahl, D.R.; Maybaum, J.; O’Connor, M.J.; Lawrence, T.S.; et al. PARP1 Trapping and DNA Replication Stress Enhance Radiosensitization with Combined WEE1 and PARP Inhibitors. Mol. Cancer Res. 2018, 16, 222–232. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, Y.; Chen, S.; Weng, X.; Rao, Y.; Fang, H. Mechanism and current progress of Poly ADP-ribose polymerase (PARP) inhibitors in the treatment of ovarian cancer. Biomed. Pharmacother. 2020, 123, 109661. [Google Scholar] [CrossRef] [PubMed]
- Vaitsiankova, A.; Burdova, K.; Sobol, M.; Gautam, A.; Benada, O.; Hanzlikova, H.; Caldecott, K.W. PARP inhibition impedes the maturation of nascent DNA strands during DNA replication. Nat. Struct. Mol. Biol. 2022, 29, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.; Murai, J.; Lee, J.-M. Restored replication fork stabilization, a mechanism of PARP inhibitor resistance, can be overcome by cell cycle checkpoint inhibition. Cancer Treat. Rev. 2018, 71, 1–7. [Google Scholar] [CrossRef]
- Slade, D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020, 34, 360–394. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Dagg, R.; Zhang, Y.; Lee, J.H.Y.; Lu, R.; La Rotta, N.M.; Sampl, S.; Korkut-Demirbaş, M.; Holzmann, K.; Lau, L.M.S.; et al. The C-Circle Biomarker Is Secreted by Alternative-Lengthening-of-Telomeres Positive Cancer Cells inside Exosomes and Provides a Blood-Based Diagnostic for ALT Activity. Cancers 2021, 13, 5369. [Google Scholar] [CrossRef]
- Henson, J.D.; Lau, L.M.; Koch, S.; La Rotta, N.M.; Dagg, R.A.; Reddel, R.R. The C-Circle Assay for alternative-lengthening-of-telomeres activity. Methods 2017, 114, 74–84. [Google Scholar] [CrossRef]
- van T Veld, B.R.; Hackeng, W.M.; Luchini, C.; Brosens, L.A.A.; Dreijerink, K.M.A. Clinical Relevance of ATRX/DAXX Gene Mutations and ALT in Functioning Pancreatic Neuroendocrine Tumors. Endocr. Pathol. 2025, 36, 3. [Google Scholar] [CrossRef] [PubMed]
- Udroiu, I.; Sgura, A. Alternative Lengthening of Telomeres: The Need for ATRX Mutations Is Lineage-Dependent. Int. J. Mol. Sci. 2025, 26, 6765. [Google Scholar] [CrossRef] [PubMed]
- Gaela, V.M.; Hsia, H.-Y.; Joseph, N.A.; Tzeng, W.-Y.; Ting, P.-C.; Shen, Y.-L.; Tsai, C.-T.; Boudier, T.; Chen, L.-Y. Orphan nuclear receptors-induced ALT-associated PML bodies are targets for ALT inhibition. Nucleic Acids Res. 2024, 52, 6472–6489. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Functional Role in ALT Activation | Effect on Tumor Phenotype | Interaction with Other Factors |
|---|---|---|---|
| Level of DNA Damage | Persistent replication stress and telomeric DNA breaks initiate homologous recombination-based telomere elongation. | High levels favor ALT activation but can impair proliferative fitness when excessive. | Synergizes with ATRX/DAXX loss and impaired checkpoint signaling. |
| ATRX/DAXX Mutations | Disrupt chromatin remodeling at telomeric regions, facilitating recombination-mediated telomere synthesis. | Promote genomic instability; context-dependent effect (protective vs. deleterious). | Strongly linked to replication stress and p53 status. |
| Checkpoint Mutations (ATR/ATM Pathways) | Impair DNA damage sensing and repair coordination. | Can enhance ALT activation under chronic stress; may also promote cell death if damage exceeds tolerance. | Modulate replication stress response in combination with ATRX/DAXX loss. |
| Proliferation Rate | Determines cellular tolerance to replication stress and repair efficiency. | Low proliferation favors stable ALT activation (e.g., GBM, chondrosarcoma), while high proliferation supports telomerase-based maintenance. | Influenced by metabolic capacity and cell cycle control. |
| p53 Status (Secondary factor) | Controls cell-cycle arrest and apoptosis in response to DNA damage. | p53 loss allows accumulation of DNA lesions; intact p53 suppresses uncontrolled ALT proliferation. | Interacts with ATRX/DAXX and checkpoint pathways. |
| Epigenetic Regulation (Secondary factor) | Modifies chromatin accessibility at telomeric/subtelomeric loci. | Aberrant histone modification or DNA methylation can trigger or stabilize ALT activation. | Affects ATRX/DAXX recruitment and replication timing. |
| Metabolic State (Secondary factor) | Alters nucleotide pool balance and redox state, influencing DNA replication fidelity. | Metabolic stress enhances ALT vulnerability; metabolic adaptation supports ALT persistence. | Links cellular energy metabolism to replication stress resilience. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, J.-Y. Alternative Lengthening of Telomeres: A Prognostic Paradox in Cancer. Cells 2025, 14, 1613. https://doi.org/10.3390/cells14201613
Sung J-Y. Alternative Lengthening of Telomeres: A Prognostic Paradox in Cancer. Cells. 2025; 14(20):1613. https://doi.org/10.3390/cells14201613
Chicago/Turabian StyleSung, Ji-Yong. 2025. "Alternative Lengthening of Telomeres: A Prognostic Paradox in Cancer" Cells 14, no. 20: 1613. https://doi.org/10.3390/cells14201613
APA StyleSung, J.-Y. (2025). Alternative Lengthening of Telomeres: A Prognostic Paradox in Cancer. Cells, 14(20), 1613. https://doi.org/10.3390/cells14201613






