Immunoproteasomes in Skeletal Muscle Pathologies: Emerging Roles, Conflicting Evidence, and Future Directions
Abstract
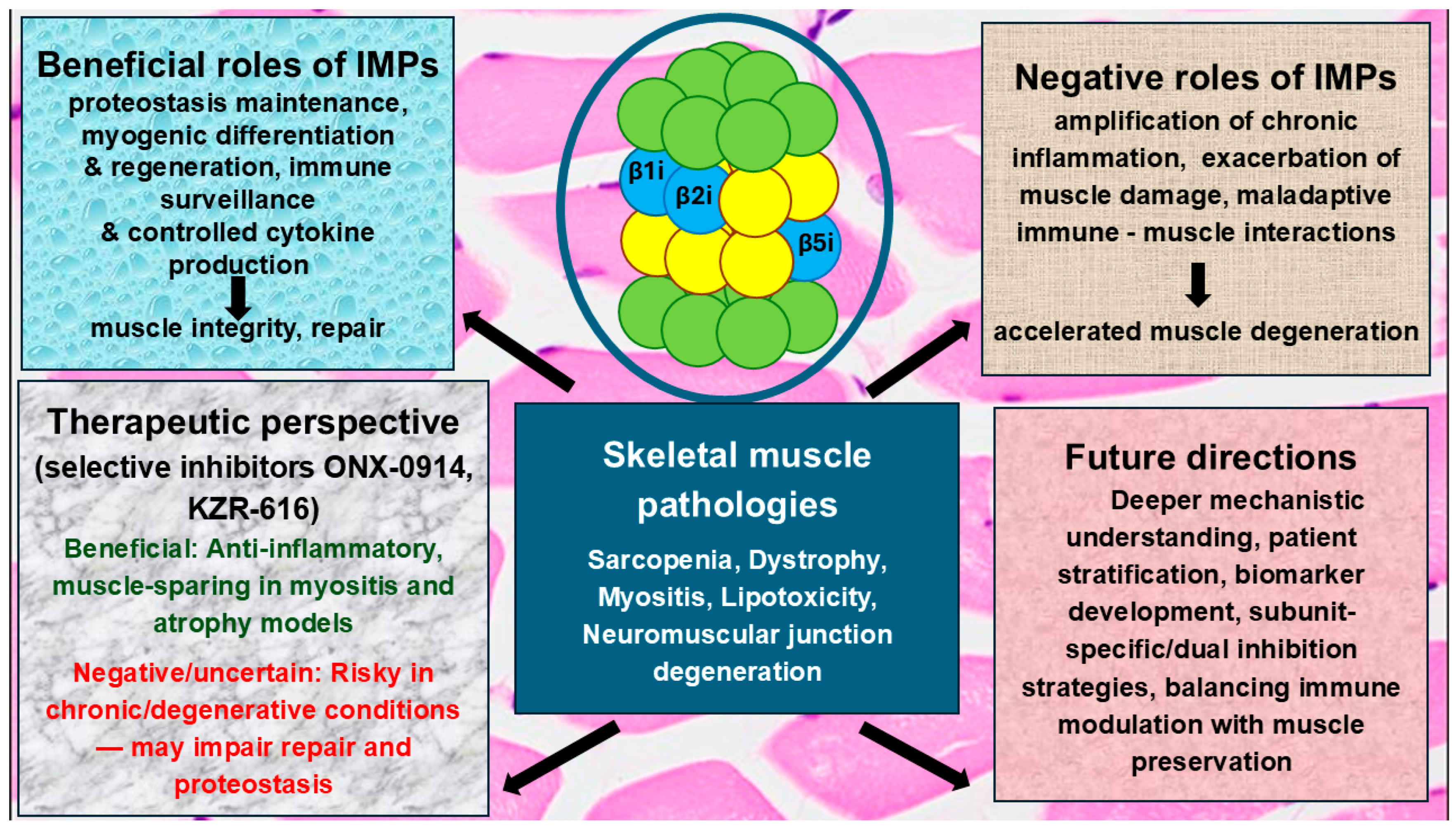
Highlights
- Immunoproteasomes (IMPs) act as a double-edged sword in muscle biology; they promote protein quality control and muscle differentiation under normal or early disease conditions, but they can drive muscle wasting when persistently activated.
- Excessive IMP activity fosters chronic inflammation and harmful crosstalk between immune and muscle cells, thereby worsening degeneration.
- Targeting IMPs with selective inhibitors holds therapeutic promise in skeletal muscle diseases and experimental models of muscle loss.
- However, in skeletal muscle disorders, inhibition may be a mixed blessing, potentially reducing damaging inflammation but at the cost of impairing muscle maintenance and repair.
Abstract
1. Introduction
2. Immunoproteasomes: A Brief Overview
2.1. Structure
2.2. Function
Inhibitors
3. Emerging Insights into the Role of Immunoproteasomes in Chronic Inflammation
4. Immunoproteasomes and Skeletal Muscle Pathologies: A Connection Gaining Recognition
5. Inflammasome Inhibitors in Skeletal Muscle Pathologies: A Double-Edged Sword
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef]
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing 2022, 51, afac220. [Google Scholar] [CrossRef]
- Coletta, G.; Phillips, S.M. An elusive consensus definition of sarcopenia impedes research and clinical treatment: A narrative review. Ageing Res. Rev. 2023, 86, 101883. [Google Scholar] [CrossRef]
- Bilski, J.; Pierzchalski, P.; Szczepanik, M.; Bonior, J.; Zoladz, J.A. Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines. Cells 2022, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Kalinkovich, A. Inflammaging as a common ground for the development and maintenance of sarcopenia, obesity, cardiomyopathy and dysbiosis. Ageing Res. Rev. 2019, 56, 100980. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, T.; Liu, H.; Li, Z.; Peng, L.; Wang, C.; Wang, T. Inflammaging: The ground for sarcopenia? Exp. Gerontol. 2022, 168, 111931. [Google Scholar] [CrossRef]
- Antuña, E.; Cachán-Vega, C.; Bermejo-Millo, J.C.; Potes, Y.; Caballero, B.; Vega-Naredo, I.; Coto-Montes, A.; Garcia-Gonzalez, C. Inflammaging: Implications in Sarcopenia. Int. J. Mol. Sci. 2022, 23, 15039. [Google Scholar] [CrossRef]
- Jimenez-Gutierrez, G.E.; Martínez-Gómez, L.E.; Martínez-Armenta, C.; Pineda, C.; Martínez-Nava, G.A.; Lopez-Reyes, A. Molecular Mechanisms of Inflammation in Sarcopenia: Diagnosis and Therapeutic Update. Cells 2022, 11, 2359. [Google Scholar] [CrossRef]
- Sun, C.; Shen, L.; Zhang, Z.; Xie, X. Therapeutic Strategies for Duchenne Muscular Dystrophy: An Update. Genes 2020, 11, 837. [Google Scholar] [CrossRef]
- Dourado, E.; Bottazzi, F.; Cardelli, C.; Conticini, E.; Schmidt, J.; Cavagna, L.; Barsotti, S. Idiopathic inflammatory myopathies: One year in review 2022. Clin. Exp. Rheumatol. 2023, 41, 199–213. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, R.; Choi, H.; Lee, S.J.; Bae, G.U. Understanding of sarcopenia: From definition to therapeutic strategies. Arch. Pharmacal Res. 2021, 44, 876–889. [Google Scholar] [CrossRef]
- Brook, M.S.; Wilkinson, D.J.; Atherton, P.J. An update on nutrient modulation in the management of disease-induced muscle wasting: Evidence from human studies. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 174–180. [Google Scholar] [CrossRef]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Yu, H.; Mim, C.; Matouschek, A. Regulated protein turnover: Snapshots of the proteasome in action. Nat. Rev. Mol. Cell Biol. 2014, 15, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Greene, E.R.; Dong, K.C.; Martin, A. Understanding the 26S proteasome molecular machine from a structural and conformational dynamics perspective. Curr. Opin. Struct. Biol. 2020, 61, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Sahu, I.; Glickman, M.H. Structural Insights into Substrate Recognition and Processing by the 20S Proteasome. Biomolecules 2021, 11, 148. [Google Scholar] [CrossRef]
- Kandel, R.; Jung, J.; Neal, S. Proteotoxic stress and the ubiquitin proteasome system. Semin. Cell Dev. Biol. 2024, 156, 107–120. [Google Scholar] [CrossRef]
- Aki, M.; Shimbara, N.; Takashina, M.; Akiyama, K.; Kagawa, S.; Tamura, T.; Tanahashi, N.; Yoshimura, T.; Tanaka, K.; Ichihara, A. Interferon-gamma induces different subunit organizations and functional diversity of proteasomes. J. Biochem. 1994, 115, 257–269. [Google Scholar] [CrossRef]
- Griffin, T.A.; Nandi, D.; Cruz, M.; Fehling, H.J.; Kaer, L.V.; Monaco, J.J.; Colbert, R.A. Immunoproteasome assembly: Cooperative incorporation of interferon gamma (IFN-gamma)-inducible subunits. J. Exp. Med. 1998, 187, 97–104. [Google Scholar] [CrossRef]
- Groettrup, M.; Kirk, C.J.; Basler, M. Proteasomes in immune cells: More than peptide producers? Nat. Rev. Immunol. 2010, 10, 73–78. [Google Scholar] [CrossRef]
- van den Eshof, B.L.; Medfai, L.; Nolfi, E.; Wawrzyniuk, M.; Sijts, A.J.A.M. The Function of Immunoproteasomes-An Immunologists’ Perspective. Cells 2021, 10, 3360. [Google Scholar] [CrossRef]
- Basler, M.; Groettrup, M. On the Role of the Immunoproteasome in Protein Homeostasis. Cells 2021, 10, 3216. [Google Scholar] [CrossRef]
- Husom, A.D.; Peters, E.A.; Kolling, E.A.; Fugere, N.A.; Thompson, L.V.; Ferrington, D.A. Altered proteasome function and subunit composition in aged muscle. Arch. Biochem. Biophys. 2004, 421, 67–76. [Google Scholar] [CrossRef]
- Cui, Z.; Hwang, S.M.; Gomes, A.V. Identification of the immunoproteasome as a novel regulator of skeletal muscle differentiation. Mol. Cell. Biol. 2014, 34, 96–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, S.; Zhang, F.; Yao, K.; Jin, S.; Gao, S.; Liu, Y.; Li, Y.; Zhang, C. Immunoproteasome subunit PSMB8 promotes skeletal muscle regeneration by regulating macrophage phenotyping switch in mice. Am. J. Physiol. Physiol. 2025, 328, C1716–C1729. [Google Scholar] [CrossRef]
- Farini, A.; Tripodi, L.; Villa, C.; Napolitano, F.; Strati, F.; Molinaro, D.; Facciotti, F.; Cassani, B.; Torrente, Y. Inhibition of the immunoproteasome modulates innate immunity to ameliorate muscle pathology of dysferlin-deficient BlAJ mice. Cell Death Dis. 2022, 13, 975. [Google Scholar] [CrossRef] [PubMed]
- Connolly, C.M.; Gupta, L.; Fujimoto, M.; Machado, P.M.; Paik, J.J. Idiopathic inflammatory myopathies: Current insights and future frontiers. Lancet Rheumatol. 2024, 6, e115–e127. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, K.; Martinez-Gamboa, L.; Spengler, L.; Krause, S.; Smiljanovic, B.; Bonin, M.; Bhattarai, S.; Grützkau, A.; Burmester, G.-R.; Häupl, T.; et al. Upregulation of immunoproteasome subunits in myositis indicates active inflammation with involvement of antigen presenting cells, CD8 T-cells and IFNΓ. PLoS ONE 2014, 9, e104048. [Google Scholar] [CrossRef] [PubMed]
- Del Rio Oliva, M.; Kirk, C.J.; Groettrup, M.; Basler, M. Effective therapy of polymyositis in mice via selective inhibition of the immunoproteasome. Eur. J. Immunol. 2022, 52, 1510–1522. [Google Scholar] [CrossRef]
- Dahlmann, B. Role of proteasomes in disease. BMC Biochem. 2007, 8 (Suppl. 1), S3. [Google Scholar] [CrossRef]
- Rousseau, A.; Bertolotti, A. Regulation of proteasome assembly and activity in health and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Thibaudeau, T.A.; Smith, D.M. A Practical Review of Proteasome Pharmacology. Pharmacol. Rev. 2019, 71, 170–197. [Google Scholar] [CrossRef]
- McCarthy, M.K.; Weinberg, J.B. The immunoproteasome and viral infection: A complex regulator of inflammation. Front. Microbiol. 2015, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Basler, M.; Mundt, S.; Bitzer, A.; Schmidt, C.; Groettrup, M. The immunoproteasome: A novel drug target for autoimmune diseases. Clin. Exp. Rheumatol. 2015, 33 (Suppl. 92), S74–S79. [Google Scholar]
- Arimochi, H.; Sasaki, Y.; Kitamura, A.; Yasutomo, K. Dysfunctional immunoproteasomes in autoinflammatory diseases. Inflamm. Regen. 2016, 36, 13. [Google Scholar] [CrossRef] [PubMed]
- Ettari, R.; Previti, S.; Bitto, A.; Grasso, S.; Zappalà, M. Immunoproteasome-Selective Inhibitors: A Promising Strategy to Treat Hematologic Malignancies, Autoimmune and Inflammatory Diseases. Curr. Med. Chem. 2016, 23, 1217–1238. [Google Scholar] [CrossRef]
- Malek, N.; Gladysz, R.; Stelmach, N.; Drag, M. Targeting Microglial Immunoproteasome: A Novel Approach in Neuroinflammatory-Related Disorders. ACS Chem. Neurosci. 2024, 15, 2532–2544. [Google Scholar] [CrossRef]
- Coux, O.; Zieba, B.A.; Meiners, S. The Proteasome System in Health and Disease. Adv. Exp. Med. Biol. 2020, 1233, 55–100. [Google Scholar]
- Collins, G.A.; Goldberg, A.L. The Logic of the 26S Proteasome. Cell 2017, 169, 792–806. [Google Scholar] [CrossRef]
- Bard, J.A.M.; Goodall, E.A.; Greene, E.R.; Jonsson, E.; Dong, K.C.; Martin, A. Structure and Function of the 26S Proteasome. Annu Rev Biochem. 2018, 87, 697–724. [Google Scholar] [CrossRef]
- Macagno, A.; Gilliet, M.; Sallusto, F.; Lanzavecchia, A.; Nestle, F.O.; Groettrup, M. Dendritic cells up-regulate immunoproteasomes and the proteasome regulator PA28 during maturation. Eur. J. Immunol. 1999, 29, 4037–4042. [Google Scholar] [CrossRef]
- Shin, E.-C.; Seifert, U.; Kato, T.; Rice, C.M.; Feinstone, S.M.; Kloetzel, P.M.; Rehermann, B. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J. Clin. Investig. 2006, 116, 3006–3014. [Google Scholar] [CrossRef]
- Reis, J.; Guan, X.Q.; Kisselev, A.F.; Papasian, C.J.; Qureshi, A.A.; Morrison, D.C.; Van Way, C.W., 3rd; Vogel, S.N.; Qureshi, N. LPS-induced formation of immunoproteasomes: TNF-α and nitric oxide production are regulated by altered composition of proteasome-active sites. Cell Biochem Biophys. 2011, 60, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Pickering, A.M.; Davies, K.J. Degradation of damaged proteins: The main function of the 20S proteasome. Prog. Mol. Biol. Transl. Sci. 2012, 109, 227–248. [Google Scholar]
- Fehling, H.J.; Swat, W.; Laplace, C.; Kühn, R.; Rajewsky, K.; Müller, U.; von Boehmer, H. MHC class I expression in mice lacking the proteasome subunit LMP-7. Science 1994, 265, 1234–1237. [Google Scholar] [CrossRef]
- Basler, M.; Moebius, J.; Elenich, L.; Groettrup, M.; Monaco, J.J. An altered T cell repertoire in MECL-1-deficient mice. J. Immunol. 2006, 176, 6665–6672. [Google Scholar] [CrossRef]
- Van Kaer, L.; Ashton-Rickardt, P.G.; Eichelberger, M.; Gaczynska, M.; Nagashima, K.; Rock, K.L.; Goldberg, A.L.; Doherty, P.C.; Tonegawa, S. Altered peptidase and viral-specific T cell response in LMP2 mutant mice. Immunity 1994, 1, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Krüger, E.; Kloetzel, P.M. Immunoproteasomes at the interface of innate and adaptive immune responses: Two faces of one enzyme. Curr. Opin. Immunol. 2012, 24, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Heink, S.; Ludwig, D.; Kloetzel, P.M.; Krüger, E. IFN-gamma-induced immune adaptation of the proteasome system is an accelerated and transient response. Proc. Natl. Acad. Sci. USA 2005, 102, 9241–9246. [Google Scholar] [CrossRef]
- Hirano, Y.; Kaneko, T.; Okamoto, K.; Bai, M.; Yashiroda, H.; Furuyama, K.; Kato, K.; Tanaka, K.; Murata, S. Dissecting beta-ring assembly pathway of the mammalian 20S proteasome. EMBO J. 2008, 27, 2204–2213. [Google Scholar] [CrossRef]
- De, M.; Jayarapu, K.; Elenich, L.; Monaco, J.J.; Colbert, R.A.; Griffin, T.A. Beta 2 subunit propeptides influence cooperative proteasome assembly. J. Biol. Chem. 2003, 278, 6153–6159. [Google Scholar] [CrossRef]
- Kingsbury, D.J.; Griffin, T.A.; Colbert, R.A. Novel propeptide function in 20 S proteasome assembly influences beta subunit composition. J. Biol. Chem. 2000, 275, 24156–24162. [Google Scholar] [CrossRef]
- Habib, J.A.; Lesenfants, J.; Vigneron, N.; Van den Eynde, B.J. Functional Differences between Proteasome Subtypes. Cells 2022, 11, 421. [Google Scholar] [CrossRef]
- Seifert, U.; Bialy, L.P.; Ebstein, F.; Bech-Otschir, D.; Voigt, A.; Schröter, F.; Prozorovski, T.; Lange, N.; Steffen, J.; Rieger, M.; et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell 2010, 142, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Nathan, J.A.; Spinnenhirn, V.; Schmidtke, G.; Basler, M.; Groettrup, M.; Goldberg, A.L. Immuno- and constitutive proteasomes do not differ in their abilities to degrade ubiquitinated proteins. Cell 2013, 152, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Inholz, K.; Anderl, J.L.; Klawitter, M.; Goebel, H.; Maurits, E.; Kirk, C.J.; Fan, R.A.; Basler, M. Proteasome composition in immune cells implies special immune-cell-specific immunoproteasome function. Eur. J. Immunol. 2024, 54, e2350613. [Google Scholar] [CrossRef]
- Moebius, J.; Broek, M.; Groettrup, M.; Basler, M. Immunoproteasomes are essential for survival and expansion of T cells in virus-infected mice. Eur. J. Immunol. 2010, 40, 3439–3449. [Google Scholar] [CrossRef] [PubMed]
- Caudill, C.M.; Jayarapu, K.; Elenich, L.; Monaco, J.J.; Colbert, R.A.; Griffin, T.A. T cells lacking immunoproteasome subunits MECL-1 and LMP7 hyperproliferate in response to polyclonal mitogens. J. Immunol. 2006, 176, 4075–4082. [Google Scholar] [CrossRef]
- Kalim, K.W.; Basler, M.; Kirk, C.J.; Groettrup, M. Immunoproteasome subunit LMP7 deficiency and inhibition suppresses Th1 and Th17 but enhances regulatory T cell differentiation. J. Immunol. 2012, 189, 4182–4193. [Google Scholar] [CrossRef]
- Vachharajani, N.; Joeris, T.; Luu, M.; Hartmann, S.; Pautz, S.; Jenike, E.; Pantazis, G.; Prinz, I.; Hofer, M.J.; Steinhoff, U.; et al. Prevention of colitis-associated cancer by selective targeting of immunoproteasome subunit LMP7. Oncotarget 2017, 8, 50447–50459. [Google Scholar] [CrossRef]
- Oliveri, F.; Mink, D.; Muchamuel, T.; Basler, M. Immunoproteasome Inhibition Impairs Differentiation but Not Survival of T Helper 17 Cells. Cells 2025, 14, 689. [Google Scholar] [CrossRef]
- Chen, S.; Kammerl, I.E.; Vosyka, O.; Baumann, T.; Yu, Y.; Wu, Y.; Irmler, M.; Overkleeft, H.S.; Beckers, J.; Eickelberg, O.; et al. Immunoproteasome dysfunction augments alternative polarization of alveolar macrophages. Cell Death Differ. 2016, 23, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xie, Y.; Lin, Q.; Yang, X.; An, X.; Xia, Y.; Du, J.; Wang, F.; Li, H.H. Immunoproteasome subunit β5i regulates diet-induced atherosclerosis through altering MERTK-mediated efferocytosis in Apoe knockout mice. J. Pathol. 2020, 250, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Kremer, M.; Henn, A.; Kolb, C.; Basler, M.; Moebius, J.; Guillaume, B.; Leist, M.; Van den Eynde, B.J.; Groettrup, M. Reduced immunoproteasome formation and accumulation of immunoproteasomal precursors in the brains of lymphocytic choriomeningitis virus-infected mice. J. Immunol. 2010, 185, 5549–5560. [Google Scholar] [CrossRef]
- Mundt, S.; Engelhardt, B.; Kirk, C.J.; Groettrup, M.; Basler, M. Inhibition and deficiency of the immunoproteasome subunit LMP7 attenuates LCMV-induced meningitis. Eur. J. Immunol. 2016, 46, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Basler, M.; Li, J.; Groettrup, M. On the role of the immunoproteasome in transplant rejection. Immunogenetics 2019, 71, 263–271. [Google Scholar] [CrossRef]
- Keller, I.E.; Vosyka, O.; Takenaka, S.; Kloß, A.; Dahlmann, B.; Willems, L.I.; Verdoes, M.; Overkleeft, H.S.; Marcos, E.; Adnot, S.; et al. Regulation of immunoproteasome function in the lung. Sci. Rep. 2015, 5, 10230. [Google Scholar] [CrossRef]
- Kammerl, I.E.; Dann, A.; Mossina, A.; Brech, D.; Lukas, C.; Vosyka, O.; Nathan, P.; Conlon, T.M.; Wagner, D.E.; Overkleeft, H.S.; et al. Impairment of Immunoproteasome Function by Cigarette Smoke and in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2016, 193, 1230–1241. [Google Scholar] [CrossRef]
- Desterke, C.; Turhan, A.G.; Bennaceur-Griscelli, A.; Griscelli, F. HLA-dependent heterogeneity and macrophage immunoproteasome activation during lung COVID-19 disease. J. Transl. Med. 2021, 19, 290. [Google Scholar] [CrossRef]
- Davidson, K.; Pickering, A.M. The proteasome: A key modulator of nervous system function, brain aging, and neurodegenerative disease. Front. Cell Dev. Biol. 2023, 11, 1124907. [Google Scholar] [CrossRef]
- Church, T.R.; Margolis, S.S. Mechanisms of ubiquitin-independent proteasomal degradation and their roles in age-related neurodegenerative disease. Front. Cell Dev. Biol. 2025, 12, 1531797. [Google Scholar] [CrossRef]
- Nie, Y.; Ma, Z.; Zhang, B.; Sun, M.; Zhang, D.; Li, H.H.; Song, X. The role of the immunoproteasome in cardiovascular disease. Pharmacol. Res. 2024, 204, 107215. [Google Scholar] [CrossRef]
- Ott, C. Mapping the interplay of immunoproteasome and autophagy in different heart failure phenotypes. Free. Radic. Biol. Med. 2024, 218, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Zhuang, R.; Kong, L.; He, R.; Zhu, H.; Zhang, J. Immunoproteasome-selective inhibitors: An overview of recent developments as potential drugs for hematologic malignancies and autoimmune diseases. Eur. J. Med. Chem. 2019, 182, 111646. [Google Scholar] [CrossRef] [PubMed]
- Abramson, H.N. Immunotherapy of Multiple Myeloma: Current Status as Prologue to the Future. Int. J. Mol. Sci. 2023, 24, 15674. [Google Scholar] [CrossRef]
- Arastu-Kapur, S.; Anderl, J.L.; Kraus, M.; Parlati, F.; Shenk, K.D.; Lee, S.J.; Muchamuel, T.; Bennett, M.K.; Driessen, C.; Ball, A.J.; et al. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: A link to clinical adverse events. Clin. Cancer Res. 2011, 17, 2734–2743. [Google Scholar] [CrossRef]
- Muchamuel, T.; Basler, M.; Aujay, M.A.; Suzuki, E.; Kalim, K.W.; Lauer, C.; Sylvain, C.; Ring, E.R.; Shields, J.; Jiang, J.; et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat. Med. 2009, 15, 781–787. [Google Scholar] [CrossRef]
- Huber, E.M.; Basler, M.; Schwab, R.; Heinemeyer, W.; Kirk, C.J.; Groettrup, M.; Groll, M. Immuno- and constitutive proteasome crystal structures reveal differences in substrate and inhibitor specificity. Cell 2012, 148, 727–738. [Google Scholar] [CrossRef]
- Kisselev, A.F. Site-Specific Proteasome Inhibitors. Biomolecules 2021, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Muchamuel, T.; Fan, R.A.; Anderl, J.L.; Bomba, D.J.; Johnson, H.W.B.; Lowe, E.; Tuch, B.B.; McMinn, D.L.; Millare, B.; Kirk, C.J. Zetomipzomib (KZR-616) attenuates lupus in mice via modulation of innate and adaptive immune responses. Front. Immunol. 2023, 14, 1043680. [Google Scholar] [CrossRef]
- Klein, M.; Busch, M.; Friese-Hamim, M.; Crosignani, S.; Fuchss, T.; Musil, D.; Rohdich, F.; Sanderson, M.P.; Seenisamy, J.; Walter-Bausch, G.; et al. Structure-Based Optimization and Discovery of M3258, a Specific Inhibitor of the Immunoproteasome Subunit LMP7 (β5i). J. Med. Chem. 2021, 64, 10230–10245. [Google Scholar] [CrossRef] [PubMed]
- Karreci, E.S.; Fan, H.; Uehara, M.; Mihali, A.B.; Singh, P.K.; Kurdi, A.T.; Solhjou, Z.; Riella, L.V.; Ghobrial, I.; Laragione, T.; et al. Brief treatment with a highly selective immunoproteasome inhibitor promotes long-term cardiac allograft acceptance in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E8425–E8432. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.J.; Hunsucker, S.A.; Chen, Q.; Voorhees, P.M.; Orlowski, M.; Orlowski, R.Z. Targeted inhibition of the immunoproteasome is a potent strategy against models of multiple myeloma that overcomes resistance to conventional drugs and nonspecific proteasome inhibitors. Blood 2009, 113, 4667–4676. [Google Scholar] [CrossRef]
- Xin, B.-T.; Huber, E.M.; de Bruin, G.; Heinemeyer, W.; Maurits, E.; Espinal, C.; Du, Y.; Janssens, M.; Weyburne, E.S.; Kisselev, A.F.; et al. Structure-Based Design of Inhibitors Selective for Human Proteasome β2c or β2i Subunits. J. Med. Chem. 2019, 62, 1626–1642. [Google Scholar] [CrossRef]
- Zhang, H.M.; Fu, J.; Hamilton, R.; Diaz, V.; Zhang, Y. The mammalian target of rapamycin modulates the immunoproteasome system in the heart. J. Mol. Cell. Cardiol. 2015, 86, 158–167. [Google Scholar] [CrossRef]
- Chen, C.; Zou, L.X.; Lin, Q.Y.; Yan, X.; Bi, H.L.; Xie, X.; Wang, S.; Wang, Q.S.; Zhang, Y.L.; Li, H.H. Resveratrol as a new inhibitor of immunoproteasome prevents PTEN degradation and attenuates cardiac hypertrophy after pressure overload. Redox Biol. 2019, 20, 390–401. [Google Scholar] [CrossRef]
- Tripathi, S.C.; Vedpathak, D.; Ostrin, E.J. The Functional and Mechanistic Roles of Immunoproteasome Subunits in Cancer. Cells 2021, 10, 3587. [Google Scholar] [CrossRef]
- Huber, E.M.; Groll, M. A Nut for Every Bolt: Subunit-Selective Inhibitors of the Immunoproteasome and Their Therapeutic Potential. Cells 2021, 10, 1929. [Google Scholar] [CrossRef]
- Mancuso, F.; Di Chio, C.; Di Matteo, F.; Smaldone, G.; Iraci, N.; Giofrè, S.V. Recent Advances in the Development of Immunoproteasome Inhibitors as Anti-Cancer Agents: The Past 5 Years. Molecules 2025, 30, 755. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.Y.R.; David, R. Alzheimer’s Disease, β-Amyloid Peptides, and Ubiquitin-Proteasome System: Nutritherapeutic Insights. Neurodegener. Dis. 2025, 25, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Kloetzel, P.M.; Ossendorp, F. Proteasome and peptidase function in MHC-class-I-mediated antigen presentation. Curr. Opin. Immunol. 2004, 16, 76–81. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Yao, N.; Lin, Z. Immunoproteasome modulates NLRP3 inflammasome-mediated neuroinflammation under cerebral ischaemia and reperfusion conditions. J. Cell. Mol. Med. 2022, 26, 462–474. [Google Scholar] [CrossRef]
- Lee, Y.; Yoon, B.; Son, S.; Cho, E.; Kim, K.B.; Choi, E.Y.; Kim, D.E. Inhibition of Immunoproteasome Attenuates NLRP3 Inflammasome Response by Regulating E3 Ubiquitin Ligase TRIM31. Cells 2024, 13, 675. [Google Scholar] [CrossRef]
- Kanarek, N.; Ben-Neriah, Y. Regulation of NF-κB by ubiquitination and degradation of the IκBs. Immunol. Rev. 2012, 246, 77–94. [Google Scholar] [CrossRef]
- Bitzer, A.; Basler, M.; Krappmann, D.; Groettrup, M. Immunoproteasome subunit deficiency has no influence on the canonical pathway of NF-κB activation. Mol. Immunol. 2017, 83, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Jeong, J.; Yoo, C.G. Long-term incubation with proteasome inhibitors (PIs) induces IκBα degradation via the lysosomal pathway in an IκB kinase (IKK)-dependent and IKK-independent manner. J. Biol. Chem. 2013, 288, 32777–32786. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Santoro, A.; Capri, M. The complex relationship between Immunosenescence and Inflammaging: Special issue on the New Biomedical Perspectives. Semin. Immunopathol. 2020, 42, 517–520. [Google Scholar] [CrossRef]
- Nathan, C. Nonresolving inflammation redux. Immunity 2022, 55, 592–605. [Google Scholar] [CrossRef]
- Johnston-Carey, H.K.; Pomatto, L.C.D.; Davies, K.J.A. The Immunoproteasome in oxidative stress, aging, and disease. Crit. Rev. Biochem. Mol. Biol. 2015, 51, 268–281. [Google Scholar] [CrossRef]
- Ichikawa, H.T.; Conley, T.; Muchamuel, T.; Jiang, J.; Lee, S.; Owen, T.; Barnard, J.; Nevarez, S.; Goldman, B.I.; Kirk, C.J.; et al. Beneficial effect of novel proteasome inhibitors in murine lupus via dual inhibition of type I interferon and autoantibody-secreting cells. Arthritis Rheum. 2012, 64, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.d.R.; Mellett, M.; Basler, M. Immunoproteasome inhibition attenuates experimental psoriasis. Front. Immunol. 2022, 13, 1075615. [Google Scholar] [CrossRef]
- Schaftenaar, F.H.; van Dam, A.D.; de Bruin, G.; Depuydt, M.A.; de Mol, J.; Amersfoort, J.; Douna, H.; Meijer, M.; Kröner, M.J.; van Santbrink, P.J.; et al. Immunoproteasomal Inhibition With ONX-0914 Attenuates Atherosclerosis and Reduces White Adipose Tissue Mass and Metabolic Syndrome in Mice. Arter. Thromb. Vasc. Biol. 2024, 44, 1346–1364. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Q.; Li, W.; Liu, Q.; Zhang, L.; Tian, X.; Ye, L.; Wang, G.; Peng, Q. Immunoproteasome subunit β5i promotes perifascicular muscle atrophy in dermatomyositis by upregulating RIG-I. RMD Open 2023, 9, e002818. [Google Scholar] [CrossRef]
- Du, S.H.; Xiang, Y.J.; Liu, L.; Nie, M.; Hou, Y.; Wang, L.; Li, B.B.; Xu, M.; Teng, Q.L.; Peng, J.; et al. Co-Inhibition of the Immunoproteasome Subunits LMP2 and LMP7 Ameliorates Immune Thrombocytopenia. Front. Immunol. 2021, 11, 603278. [Google Scholar] [CrossRef]
- Chang, H.H.; Lin, Y.H.; Chen, T.M.; Tsai, Y.L.; Lai, C.R.; Tsai, W.C.; Cheng, Y.C.; Chen, Y. ONX-0914 Induces Apoptosis and Autophagy with p53 Regulation in Human Glioblastoma Cells. Cancers 2022, 14, 5712. [Google Scholar] [CrossRef]
- Li, J.; Liu, N.; Zhou, H.; Xian, P.; Song, Y.; Tang, X.; Li, Y.; Basler, M. Immunoproteasome inhibition prevents progression of castration-resistant prostate cancer. Br. J. Cancer 2023, 128, 1377–1390. [Google Scholar] [CrossRef]
- Downey-Kopyscinski, S.; Daily, E.W.; Gautier, M.; Bhatt, A.; Florea, B.I.; Mitsiades, C.S.; Richardson, P.G.; Driessen, C.; Overkleeft, H.S.; Kisselev, A.F. An inhibitor of proteasome β2 sites sensitizes myeloma cells to immunoproteasome inhibitors. Blood Adv. 2018, 2, 2443–2451. [Google Scholar] [CrossRef]
- Bhattarai, D.; Lee, M.J.; Baek, A.; Yeo, I.J.; Miller, Z.; Baek, Y.M.; Lee, S.; Kim, D.E.; Hong, J.T.; Kim, K.B. LMP2 Inhibitors as a Potential Treatment for Alzheimer’s Disease. J. Med. Chem. 2020, 63, 3763–3783. [Google Scholar] [CrossRef] [PubMed]
- Kioon, M.D.A.; Pierides, M.; Pannellini, T.; Lin, G.; Nathan, C.F.; Barrat, F.J. Noncytotoxic Inhibition of the Immunoproteasome Regulates Human Immune Cells In Vitro and Suppresses Cutaneous Inflammation in the Mouse. J. Immunol. 2021, 206, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lee, J.; Manyam, G.C.; Pearson, T.; Walter-Bausch, G.; Friese-Hamim, M.; Zhao, S.; Jabs, J.; Manginelli, A.A.; Piske, N.; et al. LMP7-Specific Inhibitor M3258 Modulates the Tumor Microenvironment of Triple-Negative Breast Cancer and Inflammatory Breast Cancer. Cancers 2025, 17, 1887. [Google Scholar] [CrossRef]
- Moallemian, R.; Rehman, A.U.; Zhao, N.; Wang, H.; Chen, H.; Lin, G.; Ma, X.; Yu, J. Immunoproteasome inhibitor DPLG3 attenuates experimental colitis by restraining NF-κB activation. Biochem. Pharmacol. 2020, 177, 113964. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Pais, A.; Ferreira, R.; Oliveira, P.A.; Duarte, J.A. A neuromuscular perspective of sarcopenia pathogenesis: Deciphering the signaling pathways involved. Geroscience 2022, 44, 1199–1213. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Coelho-Júnior, H.J.; Landi, F.; Picca, A. Mitochondrial Quantity and Quality in Age-Related Sarcopenia. Int. J. Mol. Sci. 2024, 25, 2052. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Zhu, C.-F. Causal relationship between insulin resistance and sarcopenia. Diabetol. Metab. Syndr. 2023, 15, 46. [Google Scholar] [CrossRef]
- Wang, L.; Valencak, T.G.; Shan, T. Fat infiltration in skeletal muscle: Influential triggers and regulatory mechanism. iScience 2024, 27, 109221. [Google Scholar] [CrossRef]
- Gomarasca, M.; Banfi, G.; Lombardi, G. Myokines: The endocrine coupling of skeletal muscle and bone. Adv. Clin. Chem. 2020, 94, 155–218. [Google Scholar]
- Xie, G.; Jin, H.; Mikhail, H.; Pavel, V.; Yang, G.; Ji, B.; Lu, B.; Li, Y. Autophagy in sarcopenia: Possible mechanisms and novel therapies. Biomed. Pharmacother. 2023, 165, 115147. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xie, W.; Li, H.; Jin, H.; Zhang, Y.; Li, Y. Cellular Senescence in Sarcopenia: Possible Mechanisms and Therapeutic Potential. Front. Cell Dev. Biol. 2022, 9, 793088. [Google Scholar] [CrossRef] [PubMed]
- Kalinkovich, A.; Becker, M.; Livshits, G. New Horizons in the Treatment of Age-Associated Obesity, Sarcopenia and Osteoporosis. Drugs Aging 2022, 39, 673–683. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Izzo, A.; Massimino, E.; Riccardi, G.; Della Pepa, G. A Narrative Review on Sarcopenia in Type 2 Diabetes Mellitus: Prevalence and Associated Factors. Nutrients 2021, 13, 183. [Google Scholar] [CrossRef]
- Losasso, M.R.; Parussolo, M.L.C.; Silva, A.O.; Direito, R.; Quesada, K.; Detregiachi, C.R.P.; Bechara, M.D.; Méndez-Sánchez, N.; Abenavoli, L.; Araújo, A.C.; et al. Unraveling the Metabolic Pathways Between Metabolic-Associated Fatty Liver Disease (MAFLD) and Sarcopenia. Int. J. Mol. Sci. 2025, 26, 4673. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenia-The search for emerging biomarkers. Ageing Res. Rev. 2015, 22, 58–71. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Nishikawa, H.; Fukunishi, S.; Asai, A.; Yokohama, K.; Nishiguchi, S.; Higuchi, K. Pathophysiology and mechanisms of primary sarcopenia (Review). Int. J. Mol. Med. 2021, 48, 156. [Google Scholar] [CrossRef]
- Wu, J.; Ding, P.; Wu, H.; Yang, P.; Guo, H.; Tian, Y.; Meng, L.; Zhao, Q. Sarcopenia: Molecular regulatory network for loss of muscle mass and function. Front. Nutr. 2023, 10, 1037200. [Google Scholar] [CrossRef]
- Liu, D.; Wang, S.; Liu, S.; Wang, Q.; Che, X.; Wu, G. Frontiers in sarcopenia: Advancements in diagnostics, molecular mechanisms, and therapeutic strategies. Mol. Asp. Med. 2024, 97, 101270. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.R.; Lee, S.; Song, S.K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef]
- Rolland, Y.; Dray, C.; Vellas, B.; Barreto, P.D.S. Current and investigational medications for the treatment of sarcopenia. Metabolism 2023, 149, 155597. [Google Scholar] [CrossRef] [PubMed]
- Najm, A.; Niculescu, A.-G.; Grumezescu, A.M.; Beuran, M. Emerging Therapeutic Strategies in Sarcopenia: An Updated Review on Pathogenesis and Treatment Advances. Int. J. Mol. Sci. 2024, 25, 4300. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Nichetti, M.; Peroni, G.; Faliva, M.A.; Naso, M.; Gasparri, C.; Perna, S.; Oberto, L.; Di Paolo, E.; Riva, A.; et al. Where to Find Leucine in Food and How to Feed Elderly With Sarcopenia in Order to Counteract Loss of Muscle Mass: Practical Advice. Front. Nutr. 2021, 7, 622391. [Google Scholar] [CrossRef]
- Shen, Y.; Shi, Q.; Nong, K.; Li, S.; Yue, J.; Huang, J.; Dong, B.; Beauchamp, M.; Hao, Q. Exercise for sarcopenia in older people: A systematic review and network meta-analysis. J. Cachex. Sarcopenia Muscle 2023, 14, 1199–1211. [Google Scholar] [CrossRef]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef]
- Devries, M.C.; McGlory, C.; Bolster, D.R.; Kamil, A.; Rahn, M.; Harkness, L.; Baker, S.K.; Phillips, S.M. Protein leucine content is a determinant of shorter- and longer-term muscle protein synthetic responses at rest and following resistance exercise in healthy older women: A randomized, controlled trial. Am. J. Clin. Nutr. 2018, 107, 217–226. [Google Scholar] [CrossRef]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Buigues, C.; Castillo, Y.; Molina, P.; Hoogland, A.J.; van Doesburg, F.; Pruimboom, L.; Fernández-Garrido, J.; Cauli, O. Effects of Leucine Administration in Sarcopenia: A Randomized and Placebo-controlled Clinical Trial. Nutrients 2020, 12, 932. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Aoi, W.; Yamaguchi, A. Molecular mechanism of sarcopenia and cachexia: Recent research advances. Pflug. Arch. 2017, 469, 573–591. [Google Scholar] [CrossRef]
- Haberecht-Müller, S.; Krüger, E.; Fielitz, J. Out of Control: The Role of the Ubiquitin Proteasome System in Skeletal Muscle during Inflammation. Biomolecules 2021, 11, 1327. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, P.; Chen, X.; Liu, W. Ubiquitin-proteasome pathway in skeletal muscle atrophy. Front. Physiol. 2023, 14, 1289537. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Kinoshita, A.; Mishima, H.; Kanazawa, N.; Kaneko, T.; Mizushima, T.; Ichinose, K.; Nakamura, H.; Tsujino, A.; Kawakami, A.; et al. Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proc. Natl. Acad. Sci. USA 2011, 108, 14914–14919. [Google Scholar] [CrossRef]
- Ayaki, T.; Murata, K.; Kanazawa, N.; Uruha, A.; Ohmura, K.; Sugie, K.; Kasagi, S.; Li, F.; Mori, M.; Nakajima, R.; et al. Myositis with sarcoplasmic inclusions in Nakajo–Nishimura syndrome: A genetic inflammatory myopathy. Neuropathol. Appl. Neurobiol. 2020, 46, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Ferrington, D.A.; Baumann, C.W.; Thompson, L.V. Denervation-Induced Activation of the Standard Proteasome and Immunoproteasome. PLoS ONE 2016, 11, e0166831. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.W.; Kwak, D.; Ferrington, D.A.; Thompson, L.V. Downhill exercise alters immunoproteasome content in mouse skeletal muscle. Cell Stress Chaperon. 2018, 23, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yue, X.; Sun, Z.; Hambright, W.S.; Feng, Q.; Cui, Y.; Huard, J.; Robbins, P.D.; Wang, Z.; Mu, X. Senolytic elimination of senescent macrophages restores muscle stem cell function in severely dystrophic muscle. Aging 2022, 14, 7650–7661. [Google Scholar] [CrossRef]
- Farini, A.; Sitzia, C.; Cassani, B.; Cassinelli, L.; Rigoni, R.; Colleoni, F.; Fusco, N.; Gatti, S.; Bella, P.; Villa, C.; et al. Therapeutic Potential of Immunoproteasome Inhibition in Duchenne Muscular Dystrophy. Mol. Ther. 2016, 24, 1898–1912. [Google Scholar] [CrossRef]
- Tripodi, L.; Molinaro, D.; Fortunato, F.; Mella, C.; Cassani, B.; Torrente, Y.; Farini, A. Immunoproteasome Inhibition Ameliorates Aged Dystrophic Mouse Muscle Environment. Int. J. Mol. Sci. 2022, 23, 14657. [Google Scholar] [CrossRef]
- Buch, A.; Carmeli, E.; Boker, L.K.; Marcus, Y.; Shefer, G.; Kis, O.; Berner, Y.; Stern, N. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age—An overview. Exp. Gerontol. 2016, 76, 25–32. [Google Scholar] [CrossRef]
- Nishi, H.; Higashihara, T.; Inagi, R. Lipotoxicity in Kidney, Heart, and Skeletal Muscle Dysfunction. Nutrients 2019, 11, 1664. [Google Scholar] [CrossRef]
- Prado, C.M.; Batsis, J.A.; Donini, L.M.; Gonzalez, M.C.; Siervo, M. Sarcopenic obesity in older adults: A clinical overview. Nat. Rev. Endocrinol. 2024, 20, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachex. Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef]
- Cleasby, M.E.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J. Endocrinol. 2016, 229, R67–R81. [Google Scholar] [CrossRef]
- Fletcher, E.; Gordon, P.M. Obesity-induced alterations to the immunoproteasome: A potential link to intramuscular lipotoxicity. Appl. Physiol. Nutr. Metab. 2021, 46, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.; Wiggs, M.; Greathouse, K.L.; Morgan, G.B.; Gordon, P.M. Impaired proteostasis in obese skeletal muscle relates to altered immunoproteasome activity. Appl. Physiol. Nutr. Metab. 2022, 47, 555–564. [Google Scholar] [CrossRef]
- Kimura, H.; Usui, F.; Karasawa, T.; Kawashima, A.; Shirasuna, K.; Inoue, Y.; Komada, T.; Kobayashi, M.; Mizushina, Y.; Kasahara, T.; et al. Immunoproteasome subunit LMP7 Deficiency Improves Obesity and Metabolic Disorders. Sci. Rep. 2015, 5, 15883. [Google Scholar] [CrossRef]
- Lelegren, M.; Liu, Y.; Ross, C.; Tardif, S.; Salmon, A.B. Pharmaceutical inhibition of mTOR in the common marmoset: Effect of rapamycin on regulators of proteostasis in a non-human primate. Pathobiol. Aging Age-Relat. Dis. 2016, 6, 31793. [Google Scholar] [CrossRef]
- Bhattarai, S.; Ghannam, K.; Krause, S.; Benveniste, O.; Marg, A.; de Bruin, G.; Xin, B.T.; Overkleeft, H.S.; Spuler, S.; Stenzel, W.; et al. The immunoproteasomes are key to regulate myokines and MHC class I expression in idiopathic inflammatory myopathies. J. Autoimmun. 2016, 75, 118–129. [Google Scholar] [CrossRef]
- Holzer, M.T.; Uruha, A.; Roos, A.; Hentschel, A.; Schänzer, A.; Weis, J.; Claeys, K.G.; Schoser, B.; Montagnese, F.; Goebel, H.-H.; et al. Anti-Ku + myositis: An acquired inflammatory protein-aggregate myopathy. Acta Neuropathol. 2024, 148, 6. [Google Scholar] [CrossRef]
- Dong, H.; Lyu, Y.; Huang, C.Y.; Tsai, S.Y. Limiting cap-dependent translation increases 20S proteasomal degradation and protects the proteomic integrity in autophagy-deficient skeletal muscle. Autophagy 2025, 21, 1212–1227. [Google Scholar] [CrossRef]
- Miller, Z.; Ao, L.; Kim, K.B.; Lee, W. Inhibitors of the immunoproteasome: Current status and future directions. Curr. Pharm. Des. 2013, 19, 4140–4151. [Google Scholar] [CrossRef] [PubMed]
- Kodroń, A.; Kowalski, K.; Mussulini, B.H.M.; Hazir, C.; Borrero-Landazabal, M.A.; Ngati, S.; Wasilewski, M.; Chacinska, A. Immunoproteasome-specific Subunit Alterations as a Potential Therapeutic Target for Mitochondriopathies. J. Mol. Biol. 2025, 437, 169229. [Google Scholar] [CrossRef]
- Tzemach, R.; Gur, C.; Phan, T.S.; David, E.; Zada, M.; Shmueli, M.D.; Mazuz, K.; Sheban, F.; Kurilovich, A.; Ben Yehuda, M.; et al. Dissection of the immune landscape in psoriatic arthritis defines immunoproteasome up-regulation in treatment resistance. Sci. Immunol. 2025, 10, eadu0284. [Google Scholar] [CrossRef] [PubMed]
- Basler, M.; Groettrup, M. Recent insights how combined inhibition of immuno/proteasome subunits enables therapeutic efficacy. Genes Immun. 2020, 21, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Basler, M.; Lindstrom, M.M.; LaStant, J.J.; Bradshaw, J.M.; Owens, T.D.; Schmidt, C.; Maurits, E.; Tsu, C.; Overkleeft, H.S.; Kirk, C.J.; et al. Co-inhibition of immunoproteasome subunits LMP2 and LMP7 is required to block autoimmunity. Embo Rep. 2018, 19, e46512. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.W.B.; Lowe, E.; Anderl, J.L.; Fan, A.; Muchamuel, T.; Bowers, S.; Moebius, D.C.; Kirk, C.; McMinn, D.L. Required Immunoproteasome Subunit Inhibition Profile for Anti-Inflammatory Efficacy and Clinical Candidate KZR-616 ((2S,3R)-N-((S)-3-(Cyclopent-1-en-1-yl)-1-((R)-2-methyloxiran-2-yl)-1-oxopropan-2-yl)-3-hydroxy-3-(4-methoxyphenyl)-2-((S)-2-(2-morpholinoacetamido)propanamido)propenamide). J. Med. Chem. 2018, 61, 11127–11143. [Google Scholar] [CrossRef] [PubMed]
- Besse, A.; Besse, L.; Kraus, M.; Mendez-Lopez, M.; Bader, J.; Xin, B.T.; de Bruin, G.; Maurits, E.; Overkleeft, H.S.; Driessen, C. Proteasome Inhibition in Multiple Myeloma: Head-to-Head Comparison of Currently Available Proteasome Inhibitors. Cell Chem. Biol. 2019, 26, 340–351.e3. [Google Scholar] [CrossRef]

| Name and Target | Selectivity | Model | Effects and Mechanisms | Refs. |
|---|---|---|---|---|
| ONX-0914, LMP2, and LMP7 | LMP7 subunit (20- to 40-fold more selective than β5c or LMP2) | Murine PBMC culture; murine collagen-induced arthritis (CIA) | Reduces the production of IL-23 by activated monocytes and IFNγ and IL-2 by T cells. Attenuates the severity of arthritis, reduces cellular infiltration, IL-1β, IL-6, and TNFα production, and autoantibody levels. | [78] |
| ONX-0914 | Murine lupus model | Prevents nephritis progression, decreases serum autoantibody levels, and reduces IFNα production of TLR-activated plasmacytoid dendritic cells. | [101] | |
| ONX-0914 | Murine models of psoriasis, spontaneously developing and imiquimod-induced | Reduces skin thickness, inflammation scores, and pathological lesions, normalizes the expression of several pro-inflammatory genes in the ear and significantly reduced the inflammatory infiltrate, accompanied by a significant alteration in the αβ+ and γδ+ T cell subsets. | [102] | |
| ONX-0914 | Murine models of atherosclerosis | Reduces atherosclerosis, dendritic cell, and macrophage levels and their activation and the levels of antigen-experienced T cells and Th1 cells. Reduces accumulation of neutrophils and macrophages in white adipose tissue, intestinal triglyceride uptake and gastric emptying, and improves markers of metabolic syndrome. | [103] | |
| ONX-0914 | Human skeletal muscle myoblasts | Recovers reduced cell viability after LMP7 overexpression. | [104] | |
| ONX-0914 | PBMCs of immune thrombocytopenia (ITP) patients; ITP murine model | Increases the number of platelets, decreases the expression of FcγRI in ITP mice and decreases that of FcγRIII in ITP patients, inhibits the activation of CD4+ T cells, and affects the differentiation of Th1 cells in patients with ITP. | [105] | |
| ONX-0914 | LU-102, inhibitor of β2 (MECL-1) | Human LN229, GBM8401, and U87MG glioblastoma cells; orthotopic mouse glioblastoma model | Induces cell cycle arrest, apoptosis, and autophagy; reduced BCL-2 expression of ONX-0914 also induced in glioblastoma cells. In vivo, TMZ plus ONX-0914 reduced tumor progression better than the control or TMZ alone. | [106] |
| ONX-0914 | Castration-resistant prostate cancer (CRPC) tumor graft model | Suppresses the “tumor-elicited” Th17-type inflammatory response, angiogenesis, and epithelial–mesenchymal transition via inactivation of COX-2/VEGF-A signaling and β-catenin/Snail signaling. | [107] | |
| ONX-0914 and LU-102 | Human multiple myeloma (MM) cell lines | ONX-914 induces MM cell cytotoxicity, which is enhanced by IFNγ. LU-102, and dramatically sensitizes MM cells ONX-0914. ONX-0914 synergizes with all FDA-approved proteasome inhibitors in MM in vitro and in vivo. | [108] | |
| YU102 and LMP7 | Murine model of LPS-induced neuroinflammation | Attenuates disease progression, reduces the number of reactive astrocytes and microglia, and suppresses the secretion of IL-1α, IL-1β, and CCL12 from microglial cells. | [109] | |
| KZR-616, LMP2, and LMP7 | LMP7 and LMP2 subunits (18- and 81-fold more selective than β5c and β1c) | Murine lupus model, healthy volunteers | Completely resolves proteinuria mediated by alterations in T and B cell activation, including reduced numbers of short- and long-lived plasma cells in mice. Selectively inhibits immunoproteasomes and blocks cytokine production following ex vivo stimulation. | [81] |
| KZR-616 | Murine model of C-protein-induced myositis | Similarly to ONX 0914, it prevents loss of grip strength, reduces leukocyte infiltration of the muscle, and prevents increased serum creatine kinase levels. | [30] | |
| PKS3053 and LMP7 | LMP7 subunit (5600- and 13,600-fold more selective than β5c) | Human PBMCs, murine model of cutaneous lupus erythematosus | Reduces TLR-dependent activation of plasmacytoid dendritic cells and decreases their maturation, IFNα response, and T cell proliferation. Decreases inflammation, cellular infiltration, and skin damage. | [110] |
| M3258 and LMP7 | LMP7 subunit (>500-fold more selective than β5c) | Human breast cancer (BC) samples and cell lines, murine BC model | Reduces viability and induced cell apoptosis in vitro, reduces tumor growth and the tumor abundance of M2 macrophages, activates tumor-infiltrating CD8+ T cells, and suppresses the expression of specific inflammatory pathway gene signatures in immune cells. | [111] |
| DPLG3 and LMP7 | LMP7 subunit (7200-fold more selective than β5c) | Murine dextran sulphate (DSS)-induced colitis | Attenuates disease progression and decreases the production of IL-6, IL-1β, IFNγ, and TNFα and the influx of effector T cells and macrophages in colon tissues while increasing the number of Tregs; reduces the expression of NF-κB p50 and p65. | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinkovich, A.; Livshits, G. Immunoproteasomes in Skeletal Muscle Pathologies: Emerging Roles, Conflicting Evidence, and Future Directions. Cells 2025, 14, 1586. https://doi.org/10.3390/cells14201586
Kalinkovich A, Livshits G. Immunoproteasomes in Skeletal Muscle Pathologies: Emerging Roles, Conflicting Evidence, and Future Directions. Cells. 2025; 14(20):1586. https://doi.org/10.3390/cells14201586
Chicago/Turabian StyleKalinkovich, Alexander, and Gregory Livshits. 2025. "Immunoproteasomes in Skeletal Muscle Pathologies: Emerging Roles, Conflicting Evidence, and Future Directions" Cells 14, no. 20: 1586. https://doi.org/10.3390/cells14201586
APA StyleKalinkovich, A., & Livshits, G. (2025). Immunoproteasomes in Skeletal Muscle Pathologies: Emerging Roles, Conflicting Evidence, and Future Directions. Cells, 14(20), 1586. https://doi.org/10.3390/cells14201586






