Therapeutic Strategies Targeting Aerobic Glycolysis in Cancer and Dynamic Monitoring of Associated Metabolites
Abstract
1. Introduction
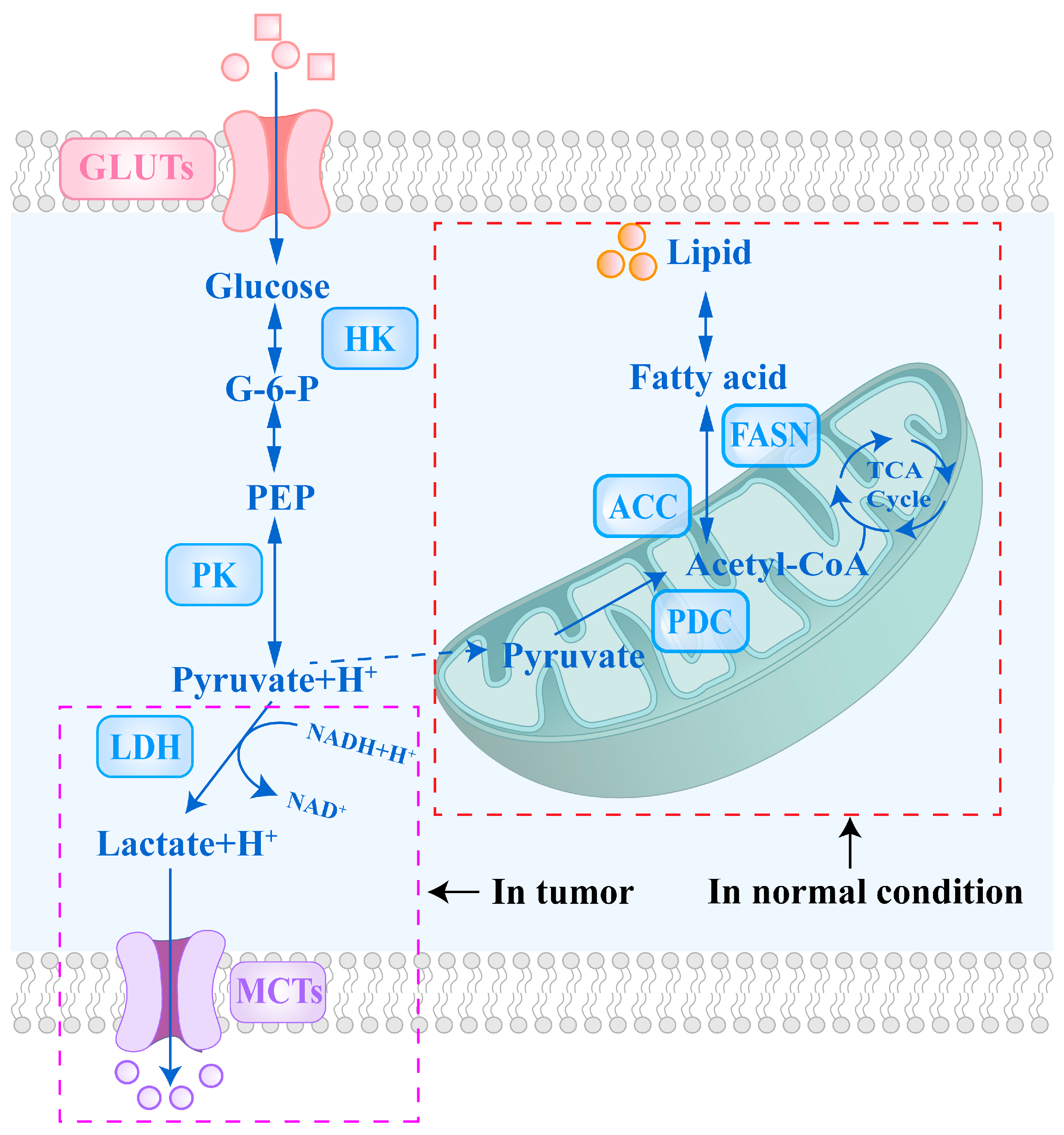
2. The Mechanisms of Key Glycolytic Enzymes and Transport Proteins in Tumor Cells
3. Targeted Therapeutic Strategies for Tumor Glucose Metabolic Pathways
3.1. GLUTs-Targeted Therapeutic Agents
3.2. HK-Targeted Therapeutic Agents
4. Targeted Therapeutic Strategies for Tumor Pyruvate Metabolic Pathways
4.1. PKM2-Targeted Therapeutic Agents
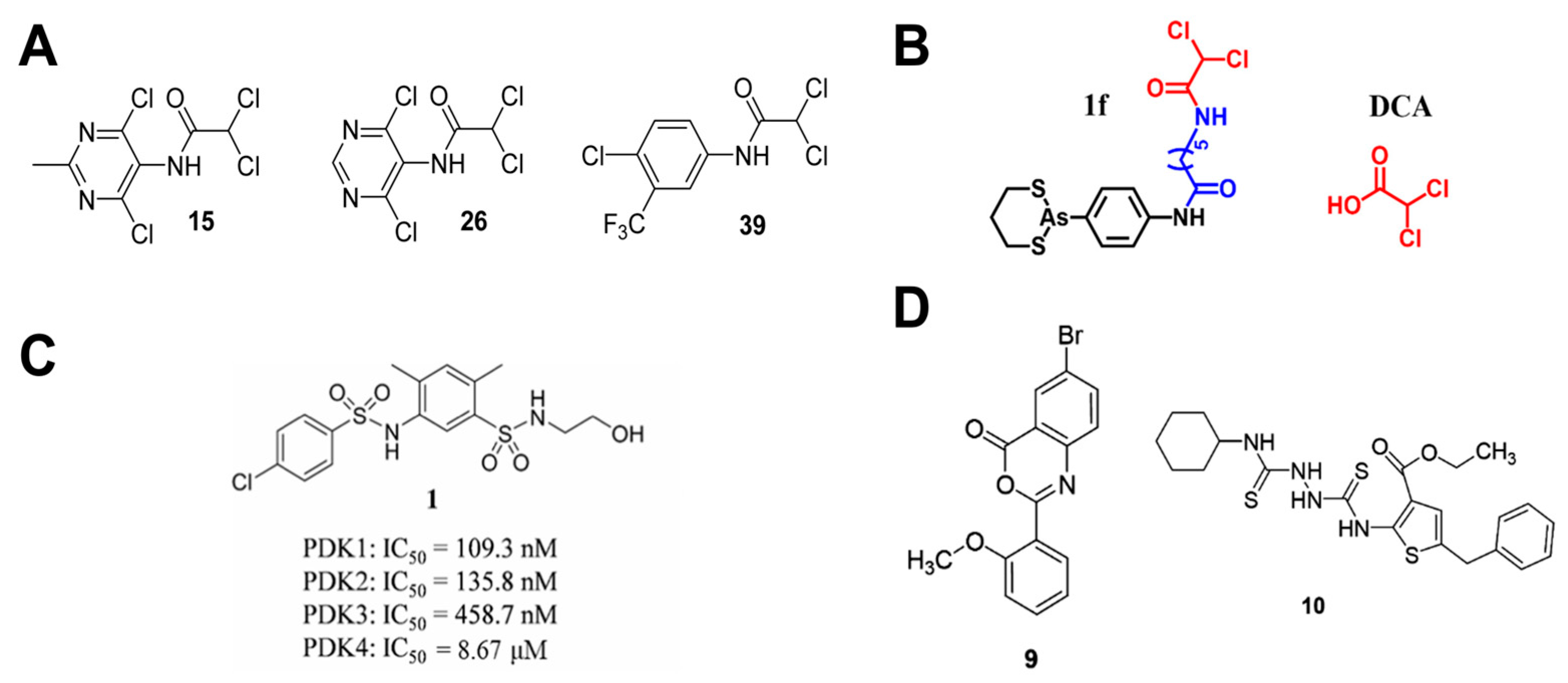
4.2. PDK-Targeted Therapeutic Agents
5. Targeted Therapeutic Strategies for Tumor Lactate Metabolic Pathways
5.1. LDH-Targeted Therapeutic Agents
5.2. MCTs-Targeted Therapeutic Agents
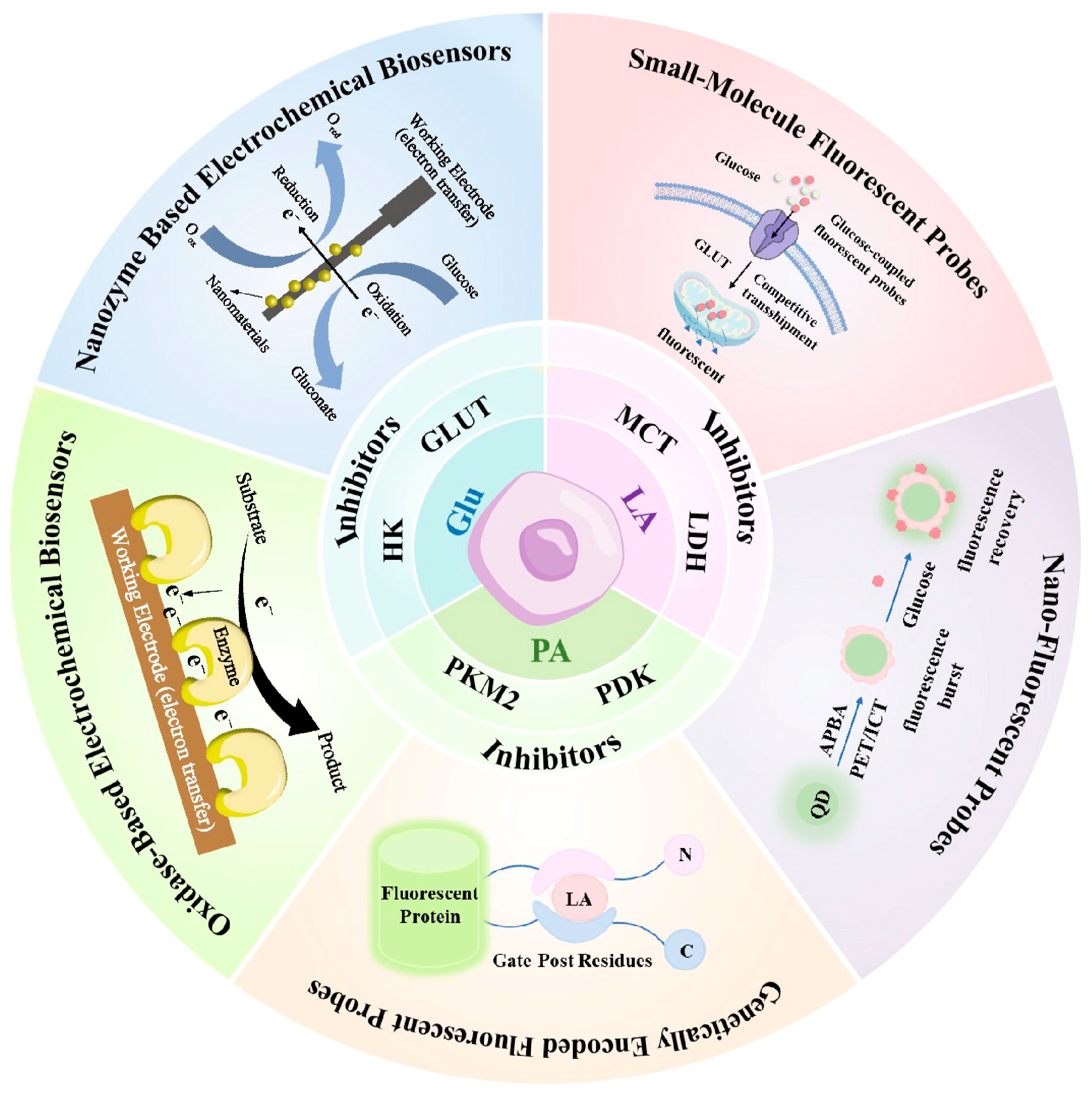
6. Dynamic Monitoring of Metabolic Biomarkers
6.1. Electrochemical Methods
6.1.1. Oxidase-Based Electrochemical Biosensors

| Analyte | Electrode Composition | Linear Range | LOD | Practical Analysis Sample | Monitoring Characteristics | Ref. |
|---|---|---|---|---|---|---|
| Glu | PHF-MWNT-PB-GOx | 0–32 mM | / | PC9 cells | 3-day continuous monitoring | [126] |
| GOx MPs | 0–30 mM | / | H1299 cells | 2-day continuous monitoring | [129] | |
| SWNT-GOx-GA/Pd NPs-Pt | 2–1000 μM | 0.5 μM | MGC803 cells | intermittent monitoring | [132] | |
| GC/GO-Ph-AuNP/GOx | 0.3–20 mM | 0.3 mM | HeLa cells | intermittent monitoring | [136] | |
| P(VI-AA)-Os/GOx/CNE | 0–4 mM | / | NG108-15 cells | intermittent monitoring | [137] | |
| PLL-GA-GOx/QNP | 0.1–8 mM | / | MDA-MB-231; MCF7 cells | intermittent monitoring | [138] | |
| GCE/CNT-GR-GOx | 0–21 mM | 2.99 µM | MiaPaCa2 cells | intermittent monitoring | [139] | |
| LA | LOx/ZnO NWs/Ti | 0–27 mM | 1.3 mM | 4T1-luc cells | intermittent monitoring | [130] |
| LOx/PCP | 0.02–1 mM | 0.75 μM | C6 glioma cells | continuous monitoring | [133] | |
| CS/CNTs/LOx/PB/Pt | 0–1 mM | 4.5 μM | HepG2 cells | intermittent monitoring | [134] | |
| JACM/CeO2 NPs/ LOx/CS | 3–300 μM | 300 nM | HepG2; A549; Hela cells | intermittent monitoring | [135] | |
| LOx/MWCNT-PEDOT: PSS/Nafion | 1–10 mM | 4.0 ± 5 μM | MCF-7 cells | intermittent monitoring | [140] | |
| LOx/Au/CNT/RGO/Pt | 0.05–100 mM | 2.3 μM | A549 cells | intermittent monitoring | [141] | |
| PS/LOx/PEDOT-HFM/Pt NPs-PANI-HFM | 0.1–6 mM | 10 µM | PC9 cells | intermittent monitoring | [142] | |
| PA | POx NPs/PGE | 0.001–6000 μM | 0.58 µM | serum | intermittent monitoring | [143] |
| POx NPs/AuE | 0.01–5000 μM | 0.67 µM | serum | intermittent monitoring | [144] | |
| POx NPs/PtE | 10–5000 μM | 5 µM | serum | intermittent monitoring | [145] |
6.1.2. Nanozyme Based Electrochemical Biosensors
6.2. Fluorescence-Based Methods
6.2.1. Small-Molecule Fluorescent Probes
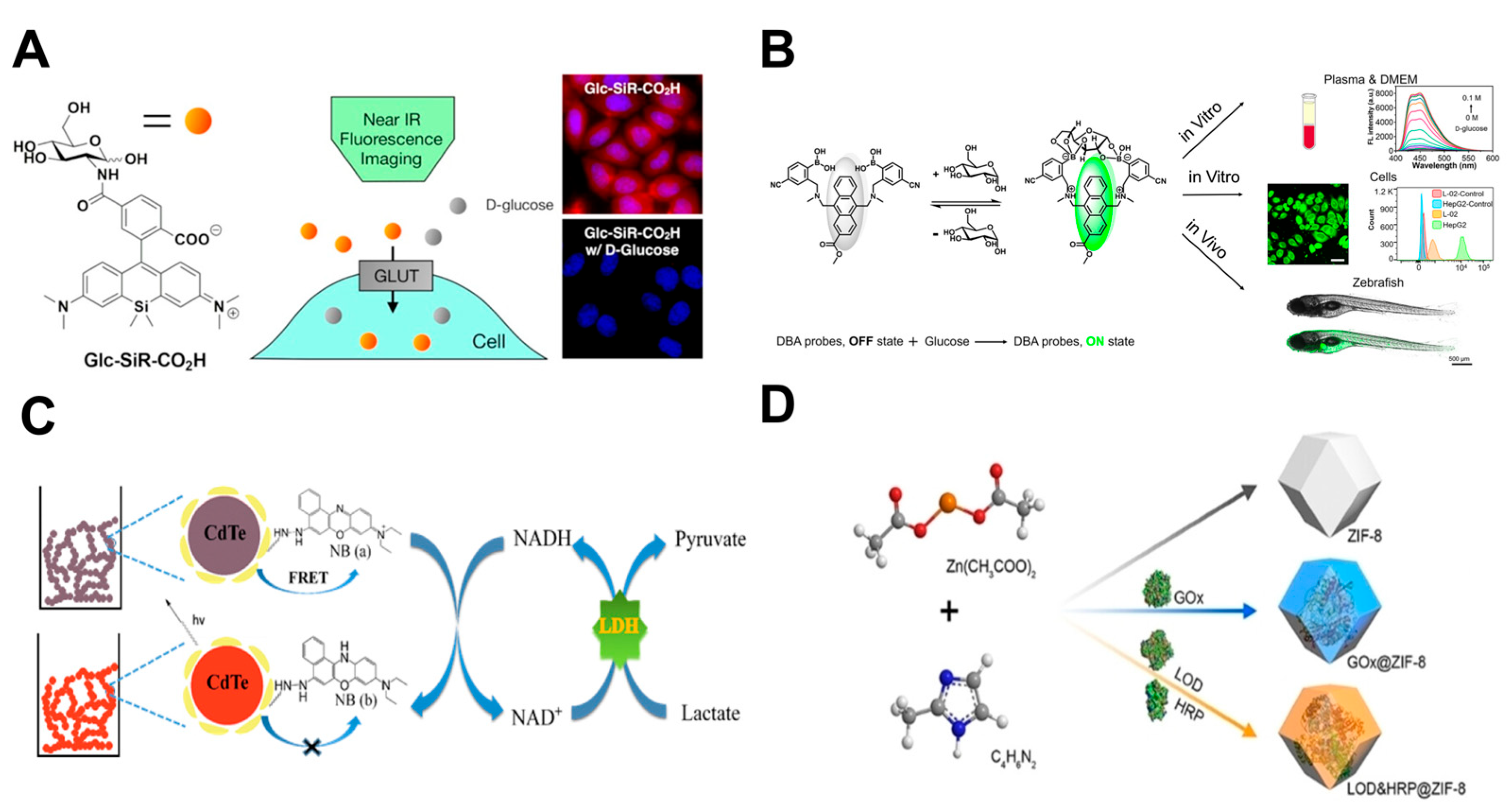
6.2.2. Nano-Fluorescent Probes
6.2.3. Genetically Encoded Fluorescent Probes
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Siyu, Z.; Zhang, X.; Du, Y.; Ni, T.; Hao, S. Crosstalk between metabolic and epigenetic modifications during cell carcinogenesis. iScience 2024, 27, 111359. [Google Scholar] [CrossRef]
- Kalyanaraman, B. Teaching the basics of cancer metabolism: Developing antitumor strategies by exploiting the differences between normal and cancer cell metabolism. Redox Biol. 2017, 12, 833–842. [Google Scholar] [CrossRef]
- Wallrabe, H.; Svindrych, Z.; Alam, S.R.; Siller, K.H.; Wang, T.; Kashatus, D.; Hu, S.; Periasamy, A. Segmented cell analyses to measure redox states of autofluorescent NAD(P)H, FAD & Trp in cancer cells by FLIM. Sci. Rep. 2018, 8, 79. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, S.; Hong, B.-J.; Lee, C.-J.; Kim, Y.-E.; Bok, S.; Oh, J.-M.; Gwak, S.-H.; Yoo, M.Y.; Lee, M.S.; et al. Tumor-Associated Macrophages Enhance Tumor Hypoxia and Aerobic Glycolysis. Cancer Res. 2019, 79, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.Y.; Stanton, R.C. The Warburg Effect, Lactate, and Nearly a Century of Trying to Cure Cancer. Semin. Nephrol. 2019, 39, 380–393. [Google Scholar] [CrossRef]
- Elia, I.; Haigis, M.C. Metabolites and the tumour microenvironment: From cellular mechanisms to systemic metabolism. Nat. Metab. 2021, 3, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhuo, L.; Wang, X. Metabolic reprogramming of carcinoma-associated fibroblasts and its impact on metabolic heterogeneity of tumors. Semin. Cell Dev. Biol. 2017, 64, 125–131. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Song, H.; Li, Y.; Liu, Z.; Ye, Z.; Zhao, J.; Wu, Y.; Tang, J.; Yao, M. Metabolic reprogramming in tumor immune microenvironment: Impact on immune cell function and therapeutic implications. Cancer Lett. 2024, 597, 217076. [Google Scholar] [CrossRef]
- Su, C.K.; Tseng, P.J.; Chiu, H.T.; Del Vall, A.; Huang, Y.F.; Sun, Y.C. Sequential enzymatic derivatization coupled with online microdialysis sampling for simultaneous profiling of mouse tumor extracellular hydrogen peroxide, lactate, and glucose. Anal. Chim. Acta 2017, 956, 24–31. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell. Mol. Life Sci. 2015, 73, 377–392. [Google Scholar] [CrossRef]
- Yu, L.; Chen, X.; Sun, X.; Wang, L.; Chen, S. The Glycolytic Switch in Tumors: How Many Players Are Involved? J. Cancer 2017, 8, 3430–3440. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.F.; Mahmoud, W.; Al-Harizy, R.M. Targeting glucose metabolism to suppress cancer progression: Prospective of anti-glycolytic cancer therapy. Pharmacol. Res. 2019, 150, 104511. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Hao, Q.; Cai, M.; Wang, X.; An, W. Harnessing glucose metabolism with nanomedicine for cancer treatment. Theranostics 2024, 14, 6831–6882. [Google Scholar] [CrossRef]
- Ghanavat, M.; Shahrouzian, M.; Deris Zayeri, Z.; Banihashemi, S.; Kazemi, S.M.; Saki, N. Digging deeper through glucose metabolism and its regulators in cancer and metastasis. Life Sci. 2021, 264, 118603. [Google Scholar] [CrossRef]
- Gill, K.S.; Fernandes, P.; O’Donovan, T.R.; McKenna, S.L.; Doddakula, K.K.; Power, D.G.; Soden, D.M.; Forde, P.F. Glycolysis inhibition as a cancer treatment and its role in an anti-tumour immune response. Biochim. Biophys. Acta Rev. Cancer 2016, 1866, 87–105. [Google Scholar] [CrossRef]
- Cordani, M.; Michetti, F.; Zarrabi, A.; Zarepour, A.; Rumio, C.; Strippoli, R.; Marcucci, F. The role of glycolysis in tumorigenesis: From biological aspects to therapeutic opportunities. Neoplasia 2024, 58, 101076. [Google Scholar] [CrossRef]
- Agrawal, S.; Singh, G.K.; Tiwari, S. Focused starvation of tumor cells using glucose oxidase: A comprehensive review. Int. J. Biol. Macromol. 2024, 281, 136444. [Google Scholar] [CrossRef]
- Fan, S.; Guo, J.; Nie, H.; Xiong, H.; Xia, Y. Aberrant Energy Metabolism in Tumors and Potential Therapeutic Targets. Genes Chromosom. Cancer 2024, 63, e70008. [Google Scholar] [CrossRef] [PubMed]
- Hay, N. Reprogramming glucose metabolism in cancer: Can it be exploited for cancer therapy? Nat. Rev. Cancer 2016, 16, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Barron, C.C.; Bilan, P.J.; Tsakiridis, T.; Tsiani, E. Facilitative glucose transporters: Implications for cancer detection, prognosis and treatment. Metabolism 2016, 65, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Na, K.J.; Choi, H.; Oh, H.R.; Kim, Y.H.; Lee, S.B.; Jung, Y.J.; Koh, J.; Park, S.; Lee, H.J.; Jeon, Y.K.; et al. Reciprocal change in Glucose metabolism of Cancer and Immune Cells mediated by different Glucose Transporters predicts Immunotherapy response. Theranostics 2020, 10, 9579–9590. [Google Scholar] [CrossRef]
- Shriwas, P.; Chen, X.; Kinghorn, A.D.; Ren, Y. Plant-derived glucose transport inhibitors with potential antitumor activity. Phytother. Res. 2019, 34, 1027–1040. [Google Scholar] [CrossRef]
- Reckzeh, E.S.; Waldmann, H. Small-Molecule Inhibition of Glucose Transporters GLUT-1–4. ChemBioChem 2019, 21, 45–52. [Google Scholar] [CrossRef]
- Gunnink, L.K.; Alabi, O.D.; Kuiper, B.D.; Gunnink, S.M.; Schuiteman, S.J.; Strohbehn, L.E.; Hamilton, K.E.; Wrobel, K.E.; Louters, L.L. Curcumin directly inhibits the transport activity of GLUT1. Biochimie 2016, 125, 179–185. [Google Scholar] [CrossRef]
- Brito, A.F.; Ribeiro, M.; Abrantes, A.M.; Mamede, A.C.; Laranjo, M.; Casalta-Lopes, J.E.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Tralhão, J.G.; Botelho, M.F. New Approach for Treatment of Primary Liver Tumors: The Role of Quercetin. Nutr. Cancer 2016, 68, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Gwak, H.; Haegeman, G.; Tsang, B.K.; Song, Y.S. Cancer-specific interruption of glucose metabolism by resveratrol is mediated through inhibition of Akt/GLUT1 axis in ovarian cancer cells. Mol. Carcinog. 2014, 54, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mei, Z.; Jin, G.; Liu, H.; Lv, S.; Fu, R.; Li, M.; Yao, C. In situ sustained release hydrogel system delivering GLUT1 inhibitor and chemo-drug for cancer post-surgical treatment. Bioact. Mater. 2024, 36, 541–550. [Google Scholar] [CrossRef]
- De, A.; Wadhwani, A.; Sauraj; Roychowdhury, P.; Kang, J.H.; Ko, Y.T.; Kuppusamy, G. WZB117 Decorated Metformin-Carboxymethyl Chitosan Nanoparticles for Targeting Breast Cancer Metabolism. Polymers 2023, 15, 976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-S.; Li, Z.-K.; Liu, J.; Deng, Y.-T.; Jiang, Y. WZB117 enhanced the anti-tumor effect of apatinib against melanoma via blocking STAT3/PKM2 axis. Front. Pharmacol. 2022, 13, 976117. [Google Scholar] [CrossRef] [PubMed]
- Temre, M.K.; Kumar, A.; Singh, S.M. An appraisal of the current status of inhibition of glucose transporters as an emerging antineoplastic approach: Promising potential of new pan-GLUT inhibitors. Front. Pharmacol. 2022, 13, 1035510. [Google Scholar] [CrossRef]
- Miller, Z.A.; Muthuswami, S.; Mueller, A.; Ma, R.Z.; Sywanycz, S.M.; Naik, A.; Huang, L.; Brody, R.M.; Diab, A.; Carey, R.M.; et al. GLUT1 inhibitor BAY-876 induces apoptosis and enhances anti-cancer effects of bitter receptor agonists in head and neck squamous carcinoma cells. Cell Death Discov. 2024, 10, 1–13. [Google Scholar] [CrossRef]
- Ceballos, J.; Schwalfenberg, M.; Karageorgis, G.; Reckzeh, E.S.; Sievers, S.; Ostermann, C.; Pahl, A.; Sellstedt, M.; Nowacki, J.; Carnero Corrales, M.A.; et al. Synthesis of Indomorphan Pseudo-Natural Product Inhibitors of Glucose Transporters GLUT-1 and -3. Angew. Chem. Int. Ed. Engl. 2019, 58, 17016–17025. [Google Scholar] [CrossRef]
- Karageorgis, G.; Reckzeh, E.S.; Ceballos, J.; Schwalfenberg, M.; Sievers, S.; Ostermann, C.; Pahl, A.; Ziegler, S.; Waldmann, H. Chromopynones are pseudo natural product glucose uptake inhibitors targeting glucose transporters GLUT-1 and -3. Nat. Chem. 2018, 10, 1103–1111. [Google Scholar] [CrossRef]
- Shan, W.; Zhou, Y.; Tam, K.Y. The development of small-molecule inhibitors targeting hexokinase 2. Drug Discov. Today 2022, 27, 2574–2585. [Google Scholar] [CrossRef]
- Bao, F.; Yang, K.; Wu, C.; Gao, S.; Wang, P.; Chen, L.; Li, H. New natural inhibitors of hexokinase 2 (HK2): Steroids from Ganoderma sinense. Fitoterapia 2018, 125, 123–129. [Google Scholar] [CrossRef]
- Pei, W.-J.; Zhou, H.; Zeng, Y.; Ji, Q.-L.; Zhang, J.-J.; Wang, X. 3-Bromopyruvate modified with cholesterol enhances anti-hepatoma activity by inducing ferroptosis and apoptosis. J. Drug Deliv. Sci. Technol. 2025, 103, 106426. [Google Scholar] [CrossRef]
- Juszczak, K.; Szczepankiewicz, W.; Walczak, K. Synthesis and Primary Activity Assay of Novel Benitrobenrazide and Benserazide Derivatives. Molecules 2024, 29, 629. [Google Scholar] [CrossRef]
- Juszczak, K.; Kubicka, A.; Kitel, R.; Dzido, G.; Łabieniec-Watała, M.; Zawadzki, S.; Marczak, A.; Walczak, K.; Matczak, K.; Tomczyk, M.D. Hexokinase 2 Inhibition and Biological Effects of BNBZ and Its Derivatives: The Influence of the Number and Arrangement of Hydroxyl Groups. Int. J. Mol. Sci. 2022, 23, 2616. [Google Scholar] [CrossRef]
- Zheng, M.; Wu, C.; Yang, K.; Yang, Y.; Liu, Y.; Gao, S.; Wang, Q.; Li, C.; Chen, L.; Li, H. Novel selective hexokinase 2 inhibitor Benitrobenrazide blocks cancer cells growth by targeting glycolysis. Pharmacol. Res. 2021, 164, 105367. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, M.; Wu, S.; Gao, S.; Yang, M.; Li, Z.; Min, Q.; Sun, W.; Chen, L.; Xiang, G.; et al. Benserazide, a dopadecarboxylase inhibitor, suppresses tumor growth by targeting hexokinase 2. J. Exp. Clin. Cancer Res. 2017, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, X.; Zhou, L.; Li, J.; Li, M.; Li, W.; Gao, F. Sinomenine Inhibits Non-Small Cell Lung Cancer via Downregulation of Hexokinases II-Mediated Aerobic Glycolysis. Onco Targets Ther. 2020, 13, 3209–3221. [Google Scholar] [CrossRef]
- Liu, W.; Li, W.; Liu, H.; Yu, X. Xanthohumol inhibits colorectal cancer cells via downregulation of Hexokinases II-mediated glycolysis. Int. J. Biol. Sci. 2019, 15, 2497–2508. [Google Scholar] [CrossRef]
- Yuan, J.; Peng, G.; Xiao, G.; Yang, Z.; Huang, J.; Liu, Q.; Yang, Z.; Liu, D. Xanthohumol suppresses glioblastoma via modulation of Hexokinase 2 -mediated glycolysis. J. Cancer 2020, 11, 4047–4058. [Google Scholar] [CrossRef]
- Xu, D.; Jin, J.; Yu, H.; Zhao, Z.; Ma, D.; Zhang, C.; Jiang, H. Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J. Exp. Clin. Cancer Res. 2017, 36, 1–11. [Google Scholar] [CrossRef]
- Lin, G.; Wu, Y.; Cai, F.; Li, Z.; Su, S.; Wang, J.; Cao, J.; Ma, L. Matrine Promotes Human Myeloid Leukemia Cells Apoptosis Through Warburg Effect Mediated by Hexokinase 2. Front. Pharmacol. 2019, 10, 1069. [Google Scholar] [CrossRef]
- Tan, S.M.; Luo, L.; He, Y.F.; Li, W.; Wan, X.X. Daurisoline inhibits glycolysis of lung cancer by targeting the AKT-HK2 axis. Cancer Biol. Ther. 2025, 26, 2442556. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Pan, Y.; Yang, G.; Liu, Y.; Zou, J.; Zhao, H.; Yin, G.; Wu, Y.; Li, X.; Wei, Z.; et al. Kaempferol impairs aerobic glycolysis against melanoma metastasis via inhibiting the mitochondrial binding of HK2 and VDAC1. Eur. J. Pharmacol. 2022, 931, 175226. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Peiris-Pages, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer metabolism: A therapeutic perspective. Nat. Rev. Clin. Oncol. 2017, 14, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.A.; Schell, J.C.; Rutter, J. Pyruvate and Metabolic Flexibility: Illuminating a Path Toward Selective Cancer Therapies. Trends Biochem. Sci. 2016, 41, 219–230. [Google Scholar] [CrossRef]
- Alquraishi, M.; Puckett, D.L.; Alani, D.S.; Humidat, A.S.; Frankel, V.D.; Donohoe, D.R.; Whelan, J.; Bettaieb, A. Pyruvate kinase M2: A simple molecule with complex functions. Free Radic. Biol. Med. 2019, 143, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, S.; Yu, D. Protein kinase function of pyruvate kinase M2 and cancer. Cancer Cell Int. 2020, 20, 523. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, Y.; Zhang, X.; Liu, H.; Yin, M.; Chen, X.; Peng, C. Pyruvate kinase M2 (PKM2) in cancer and cancer therapeutics. Cancer Lett. 2021, 503, 240–248. [Google Scholar] [CrossRef]
- El-Far, A.H.; Al Jaouni, S.K.; Li, X.; Fu, J. Cancer metabolism control by natural products: Pyruvate kinase M2 targeting therapeutics. Phytother. Res. 2022, 36, 3181–3201. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Luo, S.; Tan, Q.; Deng, J.; Zhou, S.; Peng, M.; Tao, T.; Yang, X. The role of pyruvate kinase M2 in anticancer therapeutic treatments. Oncol. Lett. 2019, 18, 5663–5672. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, S.; Wu, L.; Yu, Q.; Li, J.; Feng, J.; Zhang, J.; Chen, J.; Zhou, Y.; Ji, J.; et al. Experimental Study of Hepatocellular Carcinoma Treatment by Shikonin Through Regulating PKM2. J. Hepatocell. Carcinoma 2020, 7, 19–31. [Google Scholar] [CrossRef]
- James, A.D.; Richardson, D.A.; Oh, I.W.; Sritangos, P.; Attard, T.; Barrett, L.; Bruce, J.I.E. Cutting off the fuel supply to calcium pumps in pancreatic cancer cells: Role of pyruvate kinase-M2 (PKM2). Br. J. Cancer 2020, 122, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, Z.; Su, J.; Li, J.; Zhao, S.; Wu, L.; Zhang, J.; He, Y.; Zhang, G.; Tao, J.; et al. Benserazide is a novel inhibitor targeting PKM2 for melanoma treatment. Int. J. Cancer 2020, 147, 139–151. [Google Scholar] [CrossRef]
- Dimitrijevs, P.; Makrecka-Kuka, M.; Bogucka, A.; Hyvonen, M.; Pantelejevs, T.; Arsenyan, P. Development of isoselenazolium chlorides as selective pyruvate kinase isoform M2 inhibitors. Eur. J. Med. Chem. 2023, 257, 115504. [Google Scholar] [CrossRef]
- Wang, X.; Shen, X.; Yan, Y.; Li, H. Pyruvate dehydrogenase kinases (PDKs): An overview toward clinical applications. Biosci. Rep. 2021, 41, BSR20204402. [Google Scholar] [CrossRef]
- Anwar, S.; Shamsi, A.; Mohammad, T.; Islam, A.; Hassan, M.I. Targeting pyruvate dehydrogenase kinase signaling in the development of effective cancer therapy. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188568. [Google Scholar] [CrossRef]
- Bessho, Y.; Akaki, T.; Hara, Y.; Yamakawa, M.; Obika, S.; Mori, G.; Ubukata, M.; Yasue, K.; Nakane, Y.; Terasako, Y.; et al. Structure-based drug design of novel and highly potent pyruvate dehydrogenase kinase inhibitors. Bioorganic Med. Chem. 2021, 52, 116514. [Google Scholar] [CrossRef] [PubMed]
- Woolbright, B.L.; Rajendran, G.; Harris, R.A.; Taylor, J.A., 3rd. Metabolic Flexibility in Cancer: Targeting the Pyruvate Dehydrogenase Kinase:Pyruvate Dehydrogenase Axis. Mol. Cancer Ther. 2019, 18, 1673–1681. [Google Scholar] [CrossRef]
- Masini, T.; Birkaya, B.; van Dijk, S.; Mondal, M.; Hekelaar, J.; Jäger, M.; Terwisscha van Scheltinga, A.C.; Patel, M.S.; Hirsch, A.K.H.; Moman, E. Furoates and thenoates inhibit pyruvate dehydrogenase kinase 2 allosterically by binding to its pyruvate regulatory site. J. Enzym. Inhib. Med. Chem. 2016, 31, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-L.; Hu, X.; Zhang, W.; Tam, K.Y. Unexpected Discovery of Dichloroacetate Derived Adenosine Triphosphate Competitors Targeting Pyruvate Dehydrogenase Kinase To Inhibit Cancer Proliferation. J. Med. Chem. 2016, 59, 3562–3568. [Google Scholar] [CrossRef] [PubMed]
- She, W.; Liu, T.; Li, H.; Wang, Z.; Guo, Z.; Liu, Y.; Liu, Y. Reprogramming Energy Metabolism with Synthesized PDK Inhibitors Based on Dichloroacetate Derivatives and Targeted Delivery Systems for Enhanced Cancer Therapy. J. Med. Chem. 2023, 66, 14683–14699. [Google Scholar] [CrossRef]
- Gan, L.; Yang, Y.; Liang, Z.; Zhang, M.; He, Y.; Zhang, S.-L. Targeting the pyruvate dehydrogenase complex/pyruvate dehydrogenase kinase (PDC/PDK) axis to discover potent PDK inhibitors through structure-based virtual screening and pharmacological evaluation. Eur. J. Med. Chem. 2024, 264, 116008. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, X.; Chakravarty, H.; Yang, Z.; Tam, K.Y. Identification of Novel Pyruvate Dehydrogenase Kinase 1 (PDK1) Inhibitors by Kinase Activity-Based High-Throughput Screening for Anticancer Therapeutics. ACS Comb. Sci. 2018, 20, 660–671. [Google Scholar] [CrossRef]
- Chan, C.-Y.; Hong, S.-C.; Chang, C.-M.; Chen, Y.-H.; Liao, P.-C.; Huang, C.-Y. Oral Squamous Cell Carcinoma Cells with Acquired Resistance to Erlotinib Are Sensitive to Anti-Cancer Effect of Quercetin via Pyruvate Kinase M2 (PKM2). Cells 2023, 12, 179. [Google Scholar] [CrossRef]
- Dahiya, R.; Mohammad, T.; Roy, S.; Anwar, S.; Gupta, P.; Haque, A.; Khan, P.; Kazim, S.N.; Islam, A.; Ahmad, F.; et al. Investigation of inhibitory potential of quercetin to the pyruvate dehydrogenase kinase 3: Towards implications in anticancer therapy. Int. J. Biol. Macromol. 2019, 136, 1076–1085. [Google Scholar] [CrossRef]
- Jairajpuri, D.S.; Khan, S.; Anwar, S.; Hussain, A.; Alajmi, M.F.; Hassan, I. Investigating the role of thymol as a promising inhibitor of pyruvate dehydrogenase kinase 3 for targeted cancer therapy. Int. J. Biol. Macromol. 2024, 259, 129314. [Google Scholar] [CrossRef]
- Alotaibi, B.S.; Hakami, M.A.; Anwar, S.; Mawkili, W.; Albaqami, A.; Hassan, M.I. Structure-based investigation of pyruvate dehydrogenase kinase-3 inhibitory potential of thymoquinone, targeting lung cancer therapy. Int. J. Biol. Macromol. 2024, 265, 131064. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.-K. Tumor lactic acid: A potential target for cancer therapy. Arch. Pharm. Res. 2023, 46, 90–110. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Peng, W.-B.; Zhang, P.; Yang, X.-P.; Zhou, Q. Lactate in the tumour microenvironment: From immune modulation to therapy. eBioMedicine 2021, 73, 103627. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wu, J.; Zhao, Q.; Fu, S.; Jin, J. Emerging roles of aerobic glycolysis in breast cancer. Clin. Transl. Oncol. 2020, 22, 631–646. [Google Scholar] [CrossRef]
- Jiang, B. Aerobic glycolysis and high level of lactate in cancer metabolism and microenvironment. Genes Dis. 2017, 4, 25–27. [Google Scholar] [CrossRef]
- Varghese, E.; Samuel, S.M.; Líšková, A.; Samec, M.; Kubatka, P.; Büsselberg, D. Targeting Glucose Metabolism to Overcome Resistance to Anticancer Chemotherapy in Breast Cancer. Cancers 2020, 12, 2252. [Google Scholar] [CrossRef] [PubMed]
- Morrot, A.; da Fonseca, L.M.; Salustiano, E.J.; Gentile, L.B.; Conde, L.; Filardy, A.A.; Franklim, T.N.; da Costa, K.M.; Freire-de-Lima, C.G.; Freire-de-Lima, L. Metabolic Symbiosis and Immunomodulation: How Tumor Cell-Derived Lactate May Disturb Innate and Adaptive Immune Responses. Front. Oncol. 2018, 8, 81. [Google Scholar] [CrossRef]
- Lin, J.; Liu, G.; Chen, L.; Kwok, H.F.; Lin, Y. Targeting lactate-related cell cycle activities for cancer therapy. Semin. Cancer Biol. 2022, 86, 1231–1243. [Google Scholar] [CrossRef]
- Mortazavi Farsani, S.S.; Verma, V. Lactate mediated metabolic crosstalk between cancer and immune cells and its therapeutic implications. Front. Oncol. 2023, 13, 1175532. [Google Scholar] [CrossRef]
- Jafary, F.; Ganjalikhany, M.R.; Moradi, A.; Hemati, M.; Jafari, S. Novel Peptide Inhibitors for Lactate Dehydrogenase A (LDHA): A Survey to Inhibit LDHA Activity via Disruption of Protein-Protein Interaction. Sci. Rep. 2019, 9, 4686. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, M.; Rani, R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics. Semin. Cancer Biol. 2022, 87, 184–195. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhang, Y.; He, J.; Zang, Z.; Zhou, Z.; Pei, X.; Zheng, X.; Zhang, W.; Yang, H.; Li, S. Lactate dehydrogenase A promotes the invasion and proliferation of pituitary adenoma. Sci. Rep. 2017, 7, 4734. [Google Scholar] [CrossRef] [PubMed]
- Valvona, C.J.; Fillmore, H.L. Oxamate, but Not Selective Targeting of LDH-A, Inhibits Medulloblastoma Cell Glycolysis, Growth and Motility. Brain Sci. 2018, 8, 56. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Ozpinar, A. Oxamate targeting aggressive cancers with special emphasis to brain tumors. Biomed. Pharmacother. 2022, 147, 112686. [Google Scholar] [CrossRef]
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018, 7, 6124–6136. [Google Scholar] [CrossRef]
- Kim, E.Y.; Chung, T.W.; Han, C.W.; Park, S.Y.; Park, K.H.; Jang, S.B.; Ha, K.T. A Novel Lactate Dehydrogenase Inhibitor, 1-(Phenylseleno)-4-(Trifluoromethyl) Benzene, Suppresses Tumor Growth through Apoptotic Cell Death. Sci. Rep. 2019, 9, 3969. [Google Scholar] [CrossRef]
- Li, Z.; Cui, J. Targeting the lactic acid metabolic pathway for antitumor therapy. Mol. Ther. Oncolytics 2023, 31, 100740. [Google Scholar] [CrossRef]
- Rellinger, E.J.; Craig, B.T.; Alvarez, A.L.; Dusek, H.L.; Kim, K.W.; Qiao, J.; Chung, D.H. FX11 inhibits aerobic glycolysis and growth of neuroblastoma cells. Surgery 2017, 161, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, C.; Zhang, T.; Chang, C.Y.; Wang, J.; Bazile, L.; Zhang, L.; Haffty, B.G.; Hu, W.; Feng, Z. Metabolic enzyme LDHA activates Rac1 GTPase as a noncanonical mechanism to promote cancer. Nat. Metab. 2022, 4, 1830–1846. [Google Scholar] [CrossRef]
- Hou, X.; Shi, X.; Zhang, W.; Li, D.; Hu, L.; Yang, J.; Zhao, J.; Wei, S.; Wei, X.; Ruan, X.; et al. LDHA induces EMT gene transcription and regulates autophagy to promote the metastasis and tumorigenesis of papillary thyroid carcinoma. Cell Death Dis. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Oshima, N.; Ishida, R.; Kishimoto, S.; Beebe, K.; Brender, J.R.; Yamamoto, K.; Urban, D.; Rai, G.; Johnson, M.S.; Benavides, G.; et al. Dynamic Imaging of LDH Inhibition in Tumors Reveals Rapid In Vivo Metabolic Rewiring and Vulnerability to Combination Therapy. Cell Rep. 2020, 30, 1798–1810.E4. [Google Scholar] [CrossRef]
- Woodford, M.R.; Chen, V.Z.; Backe, S.J.; Gennady, B.; Mollapour, M. Structural and Functional Regulation of Lactate dehydrogenase-A in Cancer. Future Med. Chem. 2020, 12, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Li, X.; Zhou, Y.; Li, M.; Zhu, M. Lactate metabolic pathway regulates tumor cell metastasis and its use as a new therapeutic target. Explor. Med. 2023, 4, 541–559. [Google Scholar] [CrossRef]
- Angulo-Elizari, E.; Gaviria-Soteras, L.; Zubiri, I.; Ramos-Inza, S.; Sanmartin, C.; Plano, D. Unmasking the Warburg Effect: Unleashing the Power of Enzyme Inhibitors for Cancer Therapy. Drugs Drug Candidates 2023, 2, 728–769. [Google Scholar] [CrossRef]
- D’Andrea, F.; Vagelli, G.; Granchi, C.; Guazzelli, L.; Tuccinardi, T.; Poli, G.; Iacopini, D.; Minutolo, F.; Di Bussolo, V. Synthesis and Biological Evaluation of New Glycoconjugated LDH Inhibitors as Anticancer Agents. Molecules 2019, 24, 3520. [Google Scholar] [CrossRef]
- Jafary, F.; Moradi, A.; Ganjalikhany, M.R.; Hassanpour Dehnavi, A.; Seifati, S.M.; Khodarahmi, A.; Hemati, M. Investigation of the inhibitory peptide effect as novel strategy in cancer treatment: Targeting the tetramerization of lactate dehydrogenase A. Int. J. Biol. Macromol. 2025, 306, 140878. [Google Scholar] [CrossRef]
- Tang, Y.; Gu, S.; Zhu, L.; Wu, Y.; Zhang, W.; Zhao, C. LDHA: The Obstacle to T cell responses against tumor. Front. Oncol. 2022, 12, 1036477. [Google Scholar] [CrossRef]
- Wang, N.; Jiang, X.; Zhang, S.; Zhu, A.; Yuan, Y.; Xu, H.; Lei, J.; Yan, C. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell 2021, 184, 370–383.e313. [Google Scholar] [CrossRef] [PubMed]
- Payen, V.L.; Mina, E.; Van Hée, V.F.; Porporato, P.E.; Sonveaux, P. Monocarboxylate transporters in cancer. Mol. Metab. 2020, 33, 48–66. [Google Scholar] [CrossRef]
- Babl, N.; Decking, S.M.; Voll, F.; Althammer, M.; Sala-Hojman, A.; Ferretti, R.; Korf, C.; Schmidl, C.; Schmidleithner, L.; Nerb, B.; et al. MCT4 blockade increases the efficacy of immune checkpoint blockade. J. Immunother. Cancer 2023, 11, e007349. [Google Scholar] [CrossRef]
- Wu, P.; Zhou, Y.; Guo, Y.; Zhang, S.-L.; Tam, K.Y. Recent developments of human monocarboxylate transporter (hMCT) inhibitors as anticancer agents. Drug Discov. Today 2021, 26, 836–844. [Google Scholar] [CrossRef]
- Nancolas, B.; Sessions, R.B.; Halestrap, A.P. Identification of key binding site residues of MCT1 for AR-C155858 reveals the molecular basis of its isoform selectivity. Biochem. J. 2015, 466, 177–188. [Google Scholar] [CrossRef]
- Guan, X.; Bryniarski, M.A.; Morris, M.E. In Vitro and In Vivo Efficacy of the Monocarboxylate Transporter 1 Inhibitor AR-C155858 in the Murine 4T1 Breast Cancer Tumor Model. AAPS J. 2018, 21, 3. [Google Scholar] [CrossRef]
- Beloueche-Babari, M.; Casals Galobart, T.; Delgado-Goni, T.; Wantuch, S.; Parkes, H.G.; Tandy, D.; Harker, J.A.; Leach, M.O. Monocarboxylate transporter 1 blockade with AZD3965 inhibits lipid biosynthesis and increases tumour immune cell infiltration. Br. J. Cancer 2020, 122, 895–903. [Google Scholar] [CrossRef]
- Puri, S.; Juvale, K. Monocarboxylate transporter 1 and 4 inhibitors as potential therapeutics for treating solid tumours: A review with structure-activity relationship insights. Eur. J. Med. Chem. 2020, 199, 112393. [Google Scholar] [CrossRef]
- Barbato, A.; Giallongo, C.; Giallongo, S.; Romano, A.; Scandura, G.; Concetta, S.; Zuppelli, T.; Lolicato, M.; Lazzarino, G.; Parrinello, N.; et al. Lactate trafficking inhibition restores sensitivity to proteasome inhibitors and orchestrates immuno-microenvironment in multiple myeloma. Cell Prolif. 2023, 56, e13388. [Google Scholar] [CrossRef]
- Guan, X.; Rodriguez-Cruz, V.; Morris, M.E. Cellular Uptake of MCT1 Inhibitors AR-C155858 and AZD3965 and Their Effects on MCT-Mediated Transport of L-Lactate in Murine 4T1 Breast Tumor Cancer Cells. AAPS J. 2019, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, F.W.; Kettle, J.G.; Lamont, G.M.; Buttar, D.; Ting, A.K.T.; McGuire, T.M.; Cook, C.R.; Beattie, D.; Morentin Gutierrez, P.; Kavanagh, S.L.; et al. Discovery of Clinical Candidate AZD0095, a Selective Inhibitor of Monocarboxylate Transporter 4 (MCT4) for Oncology. J. Med. Chem. 2022, 66, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Goreczny, G.; Noonepalle, S.K.; Palmer, E.; Garcia-Hernandez, M.; Banerjee, D.; Escobedo, J.; Villagra, A.; Sandanayaka, V. Abstract 1268: A novel treatment approach for melanoma by dually targeting MCT1 and MCT4 lactate transporters. Cancer Res. 2021, 81, 1268. [Google Scholar] [CrossRef]
- Goreczny, G.J.; Escobedo, J.; Sandanayaka, V. Abstract 1335: Dual MCT1/4 inhibition promotes anti-tumor immunity in triple-negative breast cancer. Cancer Res. 2021, 81, 1335. [Google Scholar] [CrossRef]
- Singh, M.; Afonso, J.; Sharma, D.; Gupta, R.; Kumar, V.; Rani, R.; Baltazar, F.; Kumar, V. Targeting monocarboxylate transporters (MCTs) in cancer: How close are we to the clinics? Semin. Cancer Biol. 2023, 90, 1–14. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, S.; Wang, Y.; Lu, D.; Sun, Y.; Wu, Y. Proton-coupled monocarboxylate transporters in cancer: From metabolic crosstalk, immunosuppression and anti-apoptosis to clinical applications. Front. Cell Dev. Biol. 2022, 10, 1069555. [Google Scholar] [CrossRef]
- Yang, J.; Davis, T.; Kazerouni, A.S.; Chen, Y.I.; Bloom, M.J.; Yeh, H.C.; Yankeelov, T.E.; Virostko, J. Longitudinal FRET Imaging of Glucose and Lactate Dynamics and Response to Therapy in Breast Cancer Cells. Mol. Imaging Biol. 2022, 24, 144–155. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Wang, J.; Guo, X.; Song, Y.; Fu, K.; Gao, Z.; Liu, D.; He, W.; Yang, L.-L. Energy metabolism in health and diseases. Signal Transduct. Target. Ther. 2025, 10, 69. [Google Scholar] [CrossRef]
- Alhusban, A.A.; Hamadneh, L.A.; Albustanji, S.; Shallan, A.I. Lactate and pyruvate levels correlation with lactate dehydrogenase gene expression and glucose consumption in Tamoxifen-resistant MCF-7 cells using capillary electrophoresis with contactless conductivity detection (CE-C4D). Electrophoresis 2022, 43, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Prabhakaran, V.; Laskin, J. Quantitative Extraction and Mass Spectrometry Analysis at a Single-Cell Level. Anal. Chem. 2018, 90, 7937–7945. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Recent advancements in optical biosensors for cancer detection. Biosens. Bioelectron. 2022, 197, 113805. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Yang, Y.; Huang, Y.; Ma, G.; Luo, Y.; Huang, P.; Lin, J. Glucose Oxidase-Instructed Fluorescence Amplification Strategy for Intracellular Glucose Detection. ACS Appl. Mater. Interfaces 2019, 11, 10554–10558. [Google Scholar] [CrossRef]
- Koo, K.-M.; Kim, C.-D.; Kim, T.-H. Recent Advances in Electrochemical Detection of Cell Energy Metabolism. Biosensors 2024, 14, 46. [Google Scholar] [CrossRef]
- Noreen, S.; Ishaq, I.; Saleem, M.H.; Ali, B.; Muhammad Ali, S.; Iqbal, J. Electrochemical biosensing in oncology: A review advancements and prospects for cancer diagnosis. Cancer Biol. Ther. 2025, 26, 2475581. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Zhou, Z.; Zhou, H.S. Review—Measurement and Analysis of Cancer Biomarkers Based on Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037525. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Sumitha, M.S.; Xavier, T.S. Recent advances in electrochemical biosensors—A brief review. Hybrid. Adv. 2023, 2, 100023. [Google Scholar] [CrossRef]
- Zahirinejad, S.; Hemmati, R.; Homaei, A.; Dinari, A.; Hosseinkhani, S.; Mohammadi, S.; Vianello, F. Nano-organic supports for enzyme immobilization: Scopes and perspectives. Colloids Surf. B Biointerfaces 2021, 204, 111774. [Google Scholar] [CrossRef]
- De Zio, S.; Becconi, M.; Solda, A.; Malferrari, M.; Lesch, A.; Rapino, S. Glucose micro-biosensor for scanning electrochemical microscopy characterization of cellular metabolism in hypoxic microenvironments. Bioelectrochemistry 2023, 150, 108343. [Google Scholar] [CrossRef]
- Ma, Z.; Luo, Y.; Zhu, Q.; Jiang, M.; Pan, M.; Xie, T.; Huang, X.; Chen, D. In-situ monitoring of glucose metabolism in cancer cell microenvironments based on hollow fiber structure. Biosens. Bioelectron. 2020, 162, 112261. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-C.; Gurudatt, N.G.; Park, D.-S.; Kim, K.B.; Choi, C.S.; Shim, Y.-B. Microneedle array sensor for monitoring glucose in single cell using glucose oxidase-bonded polyterthiophene coated on AuZn oxide layer. Sens. Actuators B Chem. 2020, 320, 128416. [Google Scholar] [CrossRef]
- Ding, L.; Zhao, M.; Fan, S.; Ma, Y.; Liang, J.; Wang, X.; Song, Y.; Chen, S. Preparing Co3O4 urchin-like hollow microspheres self-supporting architecture for improved glucose biosensing performance. Sens. Actuators B Chem. 2016, 235, 162–169. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Liu, Y.; Li, J.; Zhu, L.; Lu, Y.; Guo, X.; Chen, D. An enzyme-particle hybrid ink for one step screen-printing and long-term metabolism monitoring. Anal. Chim. Acta 2022, 1221, 340168. [Google Scholar] [CrossRef]
- Lin, J.; Yuan, P.; Lin, R.; Xue, X.; Chen, M.; Xing, L. A Self-Powered Lactate Sensor Based on the Piezoelectric Effect for Assessing Tumor Development. Sensors 2024, 24, 2161. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-M.; Dong, C. Recent advances in nano-carrier immobilized enzymes and their applications. Process Biochem. 2020, 92, 464–475. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Zhao, M.; Zhou, M.; Xiao, Y.; Sun, Z.; Tong, L. Determination of glucose in human stomach cancer cell extracts and single cells by capillary electrophoresis with a micro-biosensor. J. Chromatogr. A 2016, 1469, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.-Y.; Qin, Y.; Fan, W.-T.; Yan, L.-P.; Chen, M.; Liu, Y.-L.; Huang, W.-H. A three-dimensional electrochemical biosensor integrated with hydrogel for cells culture and lactate release monitoring. J. Electroanal. Chem. 2022, 915, 116338. [Google Scholar] [CrossRef]
- Mi, S.; Xia, J.; Xu, Y.; Du, Z.; Sun, W. An integrated microchannel biosensor platform to analyse low density lactate metabolism in HepG2 cells in vitro. RSC Adv. 2019, 9, 9006–9013. [Google Scholar] [CrossRef]
- Bi, Y.; Ye, L.; Mao, Y.; Wang, L.; Qu, H.; Liu, J.; Zheng, L. Porous carbon supported nanoceria derived from one step in situ pyrolysis of Jerusalem artichoke stalk for functionalization of solution-gated graphene transistors for real-time detection of lactic acid from cancer cell metabolism. Biosens. Bioelectron. 2019, 140, 111271. [Google Scholar] [CrossRef]
- Qi, M.; Zhang, Y.; Cao, C.; Lu, Y.; Liu, G. Increased sensitivity of extracellular glucose monitoring based on AuNP decorated GO nanocomposites. RSC Adv. 2016, 6, 39180–39187. [Google Scholar] [CrossRef]
- Marquitan, M.; Ruff, A.; Bramini, M.; Herlitze, S.; Mark, M.D.; Schuhmann, W. Polymer/enzyme-modified HF-etched carbon nanoelectrodes for single-cell analysis. Bioelectrochemistry 2020, 133, 107487. [Google Scholar] [CrossRef]
- Nascimento, R.A.; Ozel, R.E.; Mak, W.H.; Mulato, M.; Singaram, B.; Pourmand, N. Single Cell “Glucose Nanosensor” Verifies Elevated Glucose Levels in Individual Cancer Cells. Nano Lett. 2016, 16, 1194–1200. [Google Scholar] [CrossRef]
- Madhurantakam, S.; Jayanth Babu, K.; Balaguru Rayappan, J.B.; Krishnan, U.M. Fabrication of mediator-free hybrid nano-interfaced electrochemical biosensor for monitoring cancer cell proliferation. Biosens. Bioelectron. 2017, 87, 832–841. [Google Scholar] [CrossRef]
- Ali, M.; Khalid, M.A.U.; Kim, Y.S.; Soomro, A.M.; Hussain, S.; Doh, Y.H.; Choi, K.H. MWCNTs/PEDOT: PSS Composite as Guiding Layer on Screen-Printed Carbon Electrode for Linear Range Lactate Detection. J. Electrochem. Soc. 2021, 168, 037507. [Google Scholar] [CrossRef]
- Hashemzadeh, S.; Omidi, Y.; Rafii-Tabar, H. Amperometric lactate nanobiosensor based on reduced graphene oxide, carbon nanotube and gold nanoparticle nanocomposite. Mikrochim. Acta 2019, 186, 680. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Wei, C.; Wang, C.; Jiang, M.; Hong, X.; Xu, Z.; Chen, D.; Huang, X. Modular assembly of enzyme loaded nanoparticles in 3D hollow fiber electrode for electrochemical sensing. Chem. Eng. J. 2021, 421, 129721. [Google Scholar] [CrossRef]
- Malik, M.; Chaudhary, R.; Pundir, C.S. An amperometric pyruvate biosensor based on pyruvate oxidase nanoparticles immobilized onto pencil graphite electrode. Process Biochem. 2020, 93, 12–20. [Google Scholar] [CrossRef]
- Malik, M.; Chaudhary, R.; Pundir, C.S. An improved enzyme nanoparticles based amperometric pyruvate biosensor for detection of pyruvate in serum. Enzym. Microb. Technol. 2019, 123, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Kucherenko, I.S.; Soldatkin, O.O.; Topolnikova, Y.V.; Dzyadevych, S.V.; Soldatkin, A.P. Novel Multiplexed Biosensor System for the Determination of Lactate and Pyruvate in Blood Serum. Electroanalysis 2019, 31, 1608–1614. [Google Scholar] [CrossRef]
- Bilal, M.; Khaliq, N.; Ashraf, M.; Hussain, N.; Baqar, Z.; Zdarta, J.; Jesionowski, T.; Iqbal, H.M.N. Enzyme mimic nanomaterials as nanozymes with catalytic attributes. Colloids Surf. B Biointerfaces 2023, 221, 112950. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.-W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef]
- Wei, M.; Qiao, Y.; Zhao, H.; Liang, J.; Li, T.; Luo, Y.; Lu, S.; Shi, X.; Lu, W.; Sun, X. Electrochemical non-enzymatic glucose sensors: Recent progress and perspectives. Chem. Commun. 2020, 56, 14553–14569. [Google Scholar] [CrossRef]
- Saputra, H.A. Electrochemical sensors: Basic principles, engineering, and state of the art. Monatsh. Chem. 2023, 154, 1083–1100. [Google Scholar] [CrossRef]
- Hassan, M.H.; Vyas, C.; Grieve, B.; Bartolo, P. Recent Advances in Enzymatic and Non-Enzymatic Electrochemical Glucose Sensing. Sensors 2021, 21, 4672. [Google Scholar] [CrossRef]
- Kader, M.A.; Suhaity Azmi, N.; Kafi, A.K.M. Recent advances in gold nanoparticles modified electrodes in electrochemical nonenzymatic sensing of chemical and biological compounds. Inorg. Chem. Commun. 2023, 153, 110767. [Google Scholar] [CrossRef]
- Utagawa, Y.; Ino, K.; Shinoda, Y.; Yamazaki, M.; Abe, H.; Shiku, H. Enzyme-Free In-Situ Electrochemical Measurement Using a Porous Membrane Electrode for Glucose Transport into Cell Spheroids. ACS Sens. 2024, 9, 4248–4255. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Fan, H.; Gong, Y.; Xu, Y.; Lv, Q.; Xu, Y.; Xiao, F.; Wang, S.; Wang, Z.; et al. In vitro and in vivo detection of lactate with nanohybrid-functionalized Pt microelectrode facilitating assessment of tumor development. Biosens. Bioelectron. 2021, 191, 113474. [Google Scholar] [CrossRef]
- Wang, W.; Mao, Z.; Lan, X.; Tian, D.; Peng, J.; Chen, Y. Non-enzymatic monitoring of organoid culture media using a microfluidic device with screen-printed electrodes. Mater. Res. Bull. 2025, 185, 113320. [Google Scholar] [CrossRef]
- Grasso, G.; Colella, F.; Forciniti, S.; Onesto, V.; Iuele, H.; Siciliano, A.C.; Carnevali, F.; Chandra, A.; Gigli, G.; Del Mercato, L.L. Fluorescent nano- and microparticles for sensing cellular microenvironment: Past, present and future applications. Nanoscale Adv. 2023, 5, 4311–4336. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sun, R.; Xia, K.; Xia, Q.; Liu, Y.; Zhang, X. Design and Application of Fluorescent Probes to Detect Cellular Physical Microenvironments. Chem. Rev. 2024, 124, 1738–1861. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Wu, F.G. Aggregation-induced emission-based fluorescent probes for cellular microenvironment detection. Biosens. Bioelectron. 2025, 274, 117130. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Yin, C.; Huo, F. Small-Molecule Fluorescent Probes for Detecting Several Abnormally Expressed Substances in Tumors. Micromachines 2022, 13, 1328. [Google Scholar] [CrossRef]
- Jun, J.V.; Chenoweth, D.M.; Petersson, E.J. Rational design of small molecule fluorescent probes for biological applications. Org. Biomol. Chem. 2020, 18, 5747–5763. [Google Scholar] [CrossRef]
- Wu, L.; Liu, J.; Li, P.; Tang, B.; James, T.D. Two-photon small-molecule fluorescence-based agents for sensing, imaging, and therapy within biological systems. Chem. Soc. Rev. 2021, 50, 702–734. [Google Scholar] [CrossRef]
- Liang, Z.; Pang, H.; Zeng, G.; Chen, T. Bioorthogonal Light-Up Fluorescent Probe Enables Wash-Free Real-Time Dynamic Monitoring of Cellular Glucose Uptake. Anal. Chem. 2022, 94, 8293–8301. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Shabir, G.; Li, X.; Fang, L.; Xu, L.; Zhang, H.; Li, E. Development of a deep-red fluorescent glucose-conjugated bioprobe for in vivo tumor targeting. Chem. Commun. 2020, 56, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.; Sung, J.; Lee, S.; Nam, H.; Lee, H.W.; Park, J.; Kim, H.M.; Kim, E.; Park, S.B. Near-IR Fluorescent Tracer for Glucose-Uptake Monitoring in Live Cells. Bioconjug. Chem. 2018, 29, 3394–3401. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, R.; Zhao, X.; Ma, Y.; Ren, L.; Ren, Y.; Chen, G.; Ye, D.; Wu, J.; Hu, X.; et al. Reversible Recognition-Based Boronic Acid Probes for Glucose Detection in Live Cells and Zebrafish. J. Am. Chem. Soc. 2023, 145, 8408–8416. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Chen, L.; Li, X.; Liu, C. An “off-on” fluorescent probe for imaging pyruvic acid in living systems. Talanta 2025, 284, 127225. [Google Scholar] [CrossRef]
- Rajalakshmi, K.; Muthusamy, S.; Nam, Y.S.; Li, Y.; Lee, K.B.; Xu, Y. A new recognition moiety diphenylborinate in the detection of pyruvate via Lewis acid/base sensing pathway and its bioimaging applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 267, 120457. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, S.; Cao, S.; Zhu, A.; Shi, G. Functional surface engineering of quantum dot hydrogels for selective fluorescence imaging of extracellular lactate release. Biosens. Bioelectron. 2016, 80, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Y.; He, B.; Pan, Y.; Zhou, D.; Xiong, M.; Song, Y. An Enzyme-Loaded Metal-Organic Framework-Assisted Microfluidic Platform Enables Single-Cell Metabolite Analysis. Angew. Chem. Int. Ed. Engl. 2023, 62, e202302000. [Google Scholar] [CrossRef]
- Sargazi, S.; Fatima, I.; Hassan Kiani, M.; Mohammadzadeh, V.; Arshad, R.; Bilal, M.; Rahdar, A.; Diez-Pascual, A.M.; Behzadmehr, R. Fluorescent-based nanosensors for selective detection of a wide range of biological macromolecules: A comprehensive review. Int. J. Biol. Macromol. 2022, 206, 115–147. [Google Scholar] [CrossRef]
- Mohammadi, R.; Naderi-Manesh, H.; Farzin, L.; Vaezi, Z.; Ayarri, N.; Samandari, L.; Shamsipur, M. Fluorescence sensing and imaging with carbon-based quantum dots for early diagnosis of cancer: A review. J. Pharm. Biomed. Anal. 2022, 212, 114628. [Google Scholar] [CrossRef]
- Henrique, R.B.L.; Santos, A.L.F.; Pereira, M.I.A.; Oliveira, W.F.; Santos, B.S.; Pereira, G.; Fontes, A.; Cabral Filho, P.E. A fluorescent glyconanoprobe based on quantum dots and thiolated glucose: Applications in monolayers and spheroids of cancer cells. Biochim. Biophys. Acta Gen. Subj. 2023, 1867, 130474. [Google Scholar] [CrossRef]
- Ripoll, C.; Orte, A.; Paniza, L.; Ruedas-Rama, M.J. A Quantum Dot-Based FLIM Glucose Nanosensor. Sensors 2019, 19, 4992. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shu, T.; Ma, J.; Dai, Q.; Peng, P.; Zhou, Z.; Zhou, X.; Su, L.; Zhang, X. Rational Design of ZIF-8 for Constructing Luminescent Biosensors with Glucose Oxidase and AIE-Type Gold Nanoclusters. Anal. Chem. 2022, 94, 3408–3417. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Ge, J. Multienzyme System in Amorphous Metal-Organic Frameworks for Intracellular Lactate Detection. Nano Lett. 2022, 22, 5029–5036. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, A.; Shi, M.; Chen, X.; Liu, R.; Li, T.; Zhang, C.; Zhang, Z.; Zhu, L.; Ju, Z.; et al. Analysis of redox landscapes and dynamics in living cells and in vivo using genetically encoded fluorescent sensors. Nat. Protoc. 2018, 13, 2362–2386. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, X.; Zhao, Y.; Yang, Y. Lighting Up Live-Cell and In Vivo Central Carbon Metabolism with Genetically Encoded Fluorescent Sensors. Annu. Rev. Anal. Chem. 2020, 13, 293–314. [Google Scholar] [CrossRef] [PubMed]
- Hario, S.; Le, G.N.T.; Sugimoto, H.; Takahashi-Yamashiro, K.; Nishinami, S.; Toda, H.; Li, S.; Marvin, J.S.; Kuroda, S.; Drobizhev, M.; et al. High-Performance Genetically Encoded Green Fluorescent Biosensors for Intracellular l-Lactate. ACS Cent. Sci. 2024, 10, 402–416. [Google Scholar] [CrossRef]
- Nasu, Y.; Murphy-Royal, C.; Wen, Y.; Haidey, J.N.; Molina, R.S.; Aggarwal, A.; Zhang, S.; Kamijo, Y.; Paquet, M.E.; Podgorski, K.; et al. A genetically encoded fluorescent biosensor for extracellular L-lactate. Nat. Commun. 2021, 12, 7058. [Google Scholar] [CrossRef] [PubMed]
- Nasu, Y.; Aggarwal, A.; Le, G.N.T.; Vo, C.T.; Kambe, Y.; Wang, X.; Beinlich, F.R.M.; Lee, A.B.; Ram, T.R.; Wang, F.; et al. Lactate biosensors for spectrally and spatially multiplexed fluorescence imaging. Nat. Commun. 2023, 14, 6598. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Xu, L.; Wang, A.; Zou, Y.; Li, T.; Huang, L.; Chen, W.; Liu, S.; Jiang, K.; et al. Ultrasensitive sensors reveal the spatiotemporal landscape of lactate metabolism in physiology and disease. Cell Metab. 2023, 35, 200–211.e9. [Google Scholar] [CrossRef]
- Ruiz-Rodado, V.; Lita, A.; Larion, M. Advances in measuring cancer cell metabolism with subcellular resolution. Nat. Methods 2022, 19, 1048–1063. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.; Zheng, K.; Zhang, L.; Kan, Y.; Zhao, J.; Chen, D. Therapeutic Strategies Targeting Aerobic Glycolysis in Cancer and Dynamic Monitoring of Associated Metabolites. Cells 2025, 14, 1288. https://doi.org/10.3390/cells14161288
Hu M, Zheng K, Zhang L, Kan Y, Zhao J, Chen D. Therapeutic Strategies Targeting Aerobic Glycolysis in Cancer and Dynamic Monitoring of Associated Metabolites. Cells. 2025; 14(16):1288. https://doi.org/10.3390/cells14161288
Chicago/Turabian StyleHu, Mengjie, Kaijie Zheng, Lijiao Zhang, Yue Kan, Jiaqian Zhao, and Dajing Chen. 2025. "Therapeutic Strategies Targeting Aerobic Glycolysis in Cancer and Dynamic Monitoring of Associated Metabolites" Cells 14, no. 16: 1288. https://doi.org/10.3390/cells14161288
APA StyleHu, M., Zheng, K., Zhang, L., Kan, Y., Zhao, J., & Chen, D. (2025). Therapeutic Strategies Targeting Aerobic Glycolysis in Cancer and Dynamic Monitoring of Associated Metabolites. Cells, 14(16), 1288. https://doi.org/10.3390/cells14161288






