HER2/neu as a Signaling and Therapeutic Marker in Uterine Serous Carcinoma
Abstract
1. Introduction
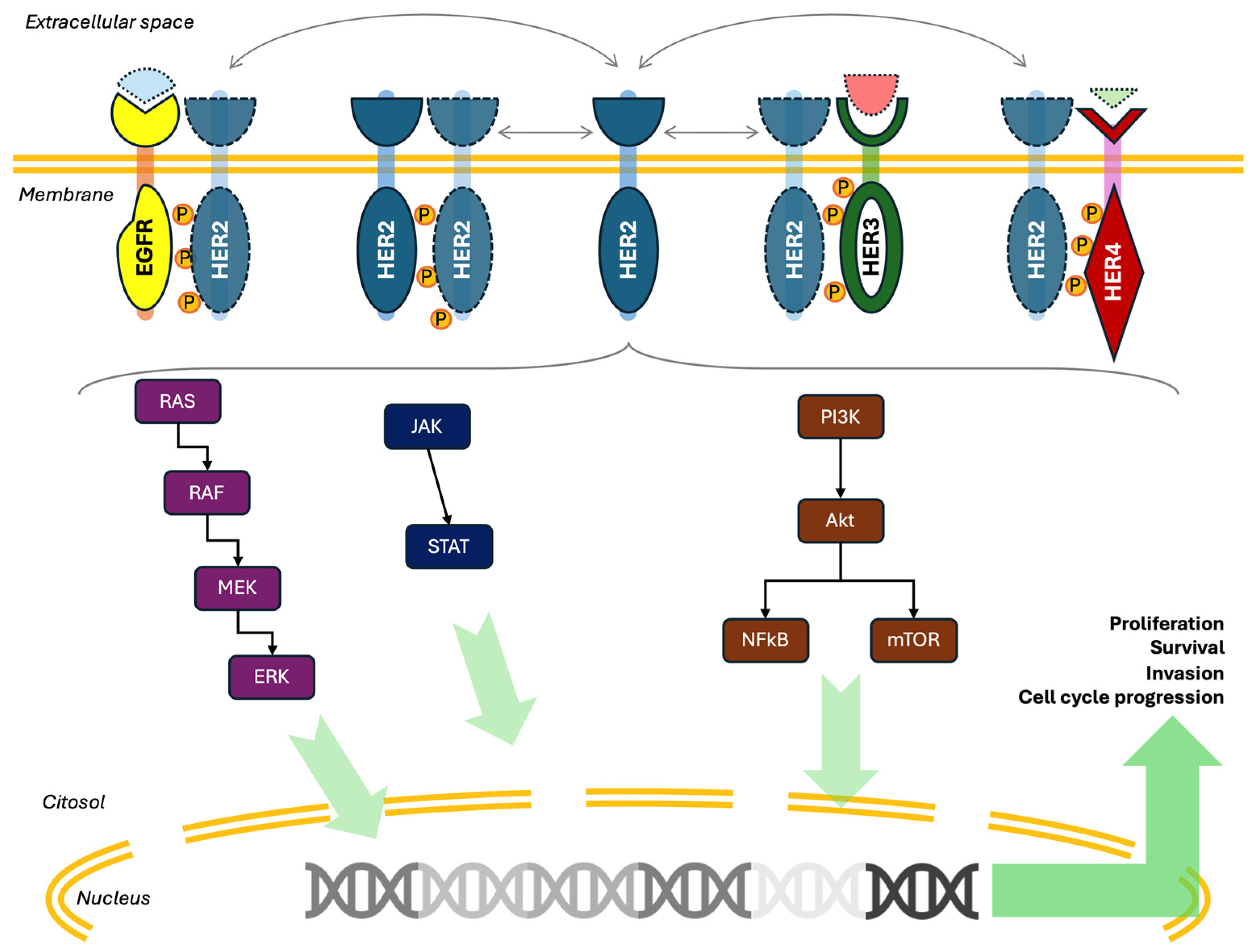
2. HER2 Mechanism of Action and Expression
2.1. The HER2 Pathway
2.2. HER2 Expression in Cancer
2.3. HER2 Expression in USC and Its Role in Carcinogenesis
3. HER2/neu-Directed Therapies
3.1. Trastuzumab and Pertuzumab
3.2. Trastuzumab Emtansine
3.3. Trastuzumab Deruxtecan
4. Mechanisms of Resistance
5. Discussion and Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| USC | Uterine Serous Carcinoma |
| ADC | Antibody–Drug Conjugate |
| TCGA | The Cancer Genome Atlas Network |
| T-DM1 | Trastuzumab Emastine |
| T-DXd | Trastuzumab Deruxtecan |
| FDA | US Food and Drug Administration |
| IHC | Immunohistochemistry |
| EGFR | Epidermal Growth Factor Receptor |
| EGF | Epidermal Growth Factor |
| FISH | Fluorescence In Situ Hybridization |
| CDK | Cyclin-Dependent Kinase |
| NK | Natural Killer |
| PFS | Progression-Free Survival |
| OS | Overall Survival |
| CS | Carcinosarcoma |
| ADCC | Antibody-Dependent Cell-Mediated Cytotoxicity |
| ILD | Interstitial Lung Disease |
| IGF-1R | Insulin-like Growth Factor 1 Receptor |
References
- National Cancer Institute. SEER Cancer Stat Facts: Uterine Cancer. 2025. Available online: https://seer.cancer.gov/statfacts/html/corp.html (accessed on 1 May 2025).
- Bogani, G.; Ray-Coquard, I.; Concin, N.; Ngoi, N.Y.L.; Morice, P.; Enomoto, T.; Takehara, K.; Denys, H.; Nout, R.A.; Lorusso, D.; et al. Uterine serous carcinoma. Gynecol. Oncol. 2021, 162, 226–234. [Google Scholar] [CrossRef]
- Ferriss, J.S.; Erickson, B.K.; Shih, I.M.; Fader, A.N. Uterine serous carcinoma: Key advances and novel treatment approaches. Int. J. Gynecol. Cancer. 2021, 31, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.; Yashar, C.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Chu, C.; et al. Uterine Neoplasms, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.A.; The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Zhao, S.; Choi, M.; Overton, J.D.; Bellone, S.; Roque, D.M.; Cocco, E.; Guzzo, F.; English, D.P.; Varughese, J.; Gasparrini, S.; et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc. Natl. Acad. Sci. USA 2013, 110, 2916–2921. [Google Scholar] [CrossRef]
- FDA. FDA Grants Accelerated Approval to Fam-Trastuzumab Deruxtecan-Nxki for Unresectable or Metastatic HER2-Positive Solid Tumors. 2024. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-enhertu-fam-trastuzumab-deruxtecan-nxki-unresectable-or (accessed on 1 May 2025).
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Gutierrez, C.; Schiff, R. HER2: Biology, detection, and clinical implications. Arch. Pathol. Lab. Med. 2011, 135, 55–62. [Google Scholar] [CrossRef]
- Arndt-Jovin, D.J.; Botelho, M.G.; Jovin, T.M. Structure-function relationships of ErbB RTKs in the plasma membrane of living cells. Cold Spring Harb. Perspect. Biol. 2014, 6, a008961. [Google Scholar] [CrossRef] [PubMed]
- Olayioye, M.A.; Neve, R.M.; Lane, H.A.; Hynes, N.E. The ErbB signaling network: Receptor heterodimerization in development and cancer. EMBO J. 2000, 19, 3159–3167. [Google Scholar] [CrossRef]
- Cheng, X. A Comprehensive Review of HER2 in Cancer Biology and Therapeutics. Genes 2024, 15, 903. [Google Scholar] [CrossRef]
- Klapper, L.N.; Glathe, S.; Vaisman, N.; Hynes, N.E.; Andrews, G.C.; Sela, M.; Yarden, Y. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc. Natl. Acad. Sci. USA 1999, 96, 4995–5000. [Google Scholar] [CrossRef]
- Song, H.; Huang, L.; Zhang, M.; Wang, X.; Song, S.; Yang, L. Transphosphorylation of EGFR at Y845 plays an important role in its autophosphorylation and kinase activity. Oncol. Rep. 2014, 31, 2393–2398. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A.; Schlessinger, J.; Ferguson, K.M. The EGFR family: Not so prototypical receptor tyrosine kinases. Cold Spring Harb. Perspect. Biol. 2014, 6, a020768. [Google Scholar] [CrossRef]
- Tzahar, E.; Waterman, H.; Chen, X.; Levkowitz, G.; Karunagaran, D.; Lavi, S.; Ratzkin, B.J.; Yarden, Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol. Cell. Biol. 1996, 16, 5276–5287. [Google Scholar] [CrossRef] [PubMed]
- Graus-Porta, D.; Beerli, R.R.; Daly, J.M.; Hynes, N.E. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997, 16, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Klapper, L.N.; Kirschbaum, M.H.; Sela, M.; Yarden, Y. Biochemical and clinical implications of the ErbB/HER signaling network of growth factor receptors. Adv. Cancer Res. 2000, 77, 25–79. [Google Scholar]
- Moasser, M.M. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef]
- Alimandi, M.; Romano, A.; Curia, M.C.; Muraro, R.; Fedi, P.; Aaronson, S.A.; Di Fiore, P.P.; Kraus, M.H. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene 1995, 10, 1813–1821. [Google Scholar]
- Pinkas-Kramarski, R.; Soussan, L.; Waterman, H.; Levkowitz, G.; Alroy, I.; Klapper, L.; Lavi, S.; Seger, R.; Ratzkin, B.J.; Sela, M.; et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996, 15, 2452–2467. [Google Scholar] [CrossRef]
- Arteaga, C.L.; Engelman, J.A. ERBB receptors: From oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 2014, 25, 282–303. [Google Scholar] [CrossRef]
- Pegram, M.; Jackisch, C.; Johnston, S.R.D. Estrogen/HER2 receptor crosstalk in breast cancer: Combination therapies to improve outcomes for patients with hormone receptor-positive/HER2-positive breast cancer. NPJ Breast Cancer 2023, 9, 1–19. [Google Scholar] [CrossRef]
- Yarden, Y.; Pines, G. The ERBB network: At last, cancer therapy meets systems biology. Nat. Rev. Cancer 2012, 12, 553–563. [Google Scholar] [CrossRef]
- Brandt, R.; Wong, A.M.; Hynes, N.E. Mammary glands reconstituted with Neu/ErbB2 transformed HC11 cells provide a novel orthotopic tumor model for testing anti-cancer agents. Oncogene 2001, 20, 5459–5465. [Google Scholar] [CrossRef]
- Schechter, A.L.; Stern, D.F.; Vaidyanathan, L.; Decker, S.J.; Drebin, J.A.; Greene, M.I.; Weinberg, R.A. The neu oncogene: An erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature 1984, 312, 513–516. [Google Scholar] [CrossRef]
- Shih, C.; Padhy, L.C.; Murray, M.; Weinberg, R.A. Transforming genes of carcinomas and neuroblastomas introduced into mouse fibroblasts. Nature 1981, 290, 261–264. [Google Scholar] [CrossRef]
- Muller, W.J.; Sinn, E.; Pattengale, P.K.; Wallace, R.; Leder, P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 1988, 54, 105–115. [Google Scholar] [CrossRef]
- Bouchard, L.; Lamarre, L.; Tremblay, P.J.; Jolicoeur, P. Stochastic appearance of mammary tumors in transgenic mice carrying the MMTV/c-neu oncogene. Cell 1989, 57, 931–936. [Google Scholar] [CrossRef]
- Guy, C.T.; Webster, M.A.; Schaller, M.; Parsons, T.J.; Cardiff, R.D.; Muller, W.J. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc. Natl. Acad. Sci. USA 1992, 89, 10578–10582. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Szabolcs, M.; Terwilliger, J.D.; Efstratiadis, A. Prostatic intraepithelial neoplasia and adenocarcinoma in mice expressing a probasin-Neu oncogenic transgene. Carcinogenesis 2006, 27, 1054–1067. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Chow, L.T.; Paterson, A.J.; Chin, E.; Kudlow, J.E. Conditional expression of the ErbB2 oncogene elicits reversible hyperplasia in stratified epithelia and up-regulation of TGFalpha expression in transgenic mice. Oncogene 1999, 18, 3593–3607. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, P.P.; Pierce, J.H.; Kraus, M.H.; Segatto, O.; King, C.R.; Aaronson, S.A. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science 1987, 237, 178–182. [Google Scholar] [CrossRef]
- Hudziak, R.M.; Schlessinger, J.; Ullrich, A. Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc. Natl. Acad. Sci. USA 1987, 84, 7159–7163. [Google Scholar] [CrossRef] [PubMed]
- Benz, C.C.; Scott, G.K.; Sarup, J.C.; Johnson, R.M.; Tripathy, D.; Coronado, E.; Shepard, H.M.; Osborne, C.K. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res. Treat. 1992, 24, 85–95. [Google Scholar] [CrossRef]
- Segatto, O.; King, C.R.; Pierce, J.H.; Di Fiore, P.P.; Aaronson, S.A. Different structural alterations upregulate in vitro tyrosine kinase activity and transforming potency of the erbB-2 gene. Mol. Cell. Biol. 1988, 8, 5570–5574. [Google Scholar]
- Finkle, D.; Quan, Z.R.; Asghari, V.; Kloss, J.; Ghaboosi, N.; Mai, E.; Wong, W.L.; Hollingshead, P.; Schwall, R.; Koeppen, H.; et al. HER2-targeted therapy reduces incidence and progression of midlife mammary tumors in female murine mammary tumor virus huHER2-transgenic mice. Clin. Cancer Res. 2004, 10, 2499–2511. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Bang, Y.J.; Feng-Yi, F.; Xu, J.M.; Lee, K.W.; Jiao, S.C.; Chong, J.L.; Lopez-Sanchez, R.I.; Price, T.; Gladkov, O.; et al. HER2 screening data from ToGA: Targeting HER2 in gastric and gastroesophageal junction cancer. Gastric. Cancer 2015, 18, 476–484. [Google Scholar] [CrossRef]
- Yan, M.; Schwaederle, M.; Arguello, D.; Millis, S.Z.; Gatalica, Z.; Kurzrock, R. HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev. 2015, 34, 157–164. [Google Scholar] [CrossRef]
- Buza, N.; Roque, D.M.; Santin, A.D. HER2/neu in Endometrial Cancer: A Promising Therapeutic Target With Diagnostic Challenges. Arch. Pathol. Lab. Med. 2014, 138, 343–350. [Google Scholar] [CrossRef]
- Fleischmann, A.; Rotzer, D.; Seiler, R.; Studer, U.E.; Thalmann, G.N. Her2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur. Urol. 2011, 60, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Lae, M.; Couturier, J.; Oudard, S.; Radvanyi, F.; Beuzeboc, P.; Vieillefond, A. Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: Results in 1005 patients. Ann. Oncol. 2010, 21, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Caner, V.; Turk, N.S.; Duzcan, F.; Tufan, N.L.; Kelten, E.C.; Zencir, S.; Dodurga, Y.; Bagci, H.; Duzcan, S.E. No strong association between HER-2/neu protein overexpression and gene amplification in high-grade invasive urothelial carcinomas. Pathol. Oncol. Res. 2008, 14, 261–266. [Google Scholar] [CrossRef]
- Gandour-Edwards, R.; Lara, P.N.; Folkins, A.K., Jr.; LaSalle, J.M.; Beckett, L.; Li, Y.; Meyers, F.J.; DeVere-White, R. Does HER2/neu expression provide prognostic information in patients with advanced urothelial carcinoma? Cancer 2002, 95, 1009–1015. [Google Scholar] [CrossRef]
- Varga, Z.; Noske, A.; Ramach, C.; Padberg, B.; Moch, H. Assessment of HER2 status in breast cancer: Overall positivity rate and accuracy by fluorescence in situ hybridization and immunohistochemistry in a single institution over 12 years: A quality control study. BMC Cancer 2013, 13, 615. [Google Scholar] [CrossRef]
- Schuell, B.; Gruenberger, T.; Scheithauer, W.; Zielinski, C.; Wrba, F. HER 2/neu protein expression in colorectal cancer. BMC Cancer 2006, 6, 123. [Google Scholar] [CrossRef] [PubMed]
- Heppner, B.I.; Behrens, H.M.; Balschun, K.; Haag, J.; Kruger, S.; Becker, T.; Rocken, C. HER2/neu testing in primary colorectal carcinoma. Br. J. Cancer 2014, 111, 1977–1984. [Google Scholar] [CrossRef]
- Pellegrini, C.; Falleni, M.; Marchetti, A.; Cassani, B.; Miozzo, M.; Buttitta, F.; Roncalli, M.; Coggi, G.; Bosari, S. HER-2/Neu alterations in non-small cell lung cancer: A comprehensive evaluation by real time reverse transcription-PCR, fluorescence in situ hybridization, and immunohistochemistry. Clin. Cancer Res. 2003, 9, 3645–3652. [Google Scholar]
- Rouquette, I.; Lauwers-Cances, V.; Allera, C.; Brouchet, L.; Milia, J.; Nicaise, Y.; Laurent, J.; Delisle, M.B.; Favre, G.; Didier, A.; et al. Characteristics of lung cancer in women: Importance of hormonal and growth factors. Lung Cancer 2012, 76, 280–285. [Google Scholar] [CrossRef]
- Langer, C.J.; Stephenson, P.; Thor, A.; Vangel, M.; Johnson, D.H. Trastuzumab in the treatment of advanced non-small-cell lung cancer: Is there a role? Focus on Eastern Cooperative Oncology Group study 2598. J. Clin. Oncol. 2004, 22, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Lara, P.N.; Laptalo, L., Jr.; Longmate, J.; Lau, D.H.; Gandour-Edwards, R.; Gumerlock, P.H.; Doroshow, J.H.; Gandara, D.R.; California Cancer, C. Trastuzumab plus docetaxel in HER2/neu-positive non-small-cell lung cancer: A California Cancer Consortium screening and phase II trial. Clin. Lung Cancer 2004, 5, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Heinmoller, P.; Gross, C.; Beyser, K.; Schmidtgen, C.; Maass, G.; Pedrocchi, M.; Ruschoff, J. HER2 status in non-small cell lung cancer: Results from patient screening for enrollment to a phase II study of herceptin. Clin. Cancer Res. 2003, 9, 5238–5243. [Google Scholar] [CrossRef]
- Santin, A.D.; Bellone, S.; Gokden, M.; Palmieri, M.; Dunn, D.; Agha, J.; Roman, J.J.; Hutchins, L.; Pecorelli, S.; O’Brien, T.; et al. Overexpression of HER-2/neu in uterine serous papillary cancer. Clin. Cancer Res. 2002, 8, 1271–1279. [Google Scholar] [PubMed]
- Villella, J.A.; Cohen, S.; Smith, D.H.; Hibshoosh, H.; Hershman, D. HER-2/neu overexpression in uterine papillary serous cancers and its possible therapeutic implications. Int. J. Gynecol. Cancer 2006, 16, 1897–1902. [Google Scholar] [CrossRef]
- Odicino, F.E.; Bignotti, E.; Rossi, E.; Pasinetti, B.; Tassi, R.A.; Donzelli, C.; Falchetti, M.; Fontana, P.; Grigolato, P.G.; Pecorelli, S. HER-2/neu overexpression and amplification in uterine serous papillary carcinoma: Comparative analysis of immunohistochemistry, real-time reverse transcription-polymerase chain reaction, and fluorescence in situ hybridization. Int. J. Gynecol. Cancer 2008, 18, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Nofech-Mozes, S.; Khalifa, M.A.; Ismiil, N.; Saad, R.S.; Hanna, W.M.; Covens, A.; Ghorab, Z. Immunophenotyping of serous carcinoma of the female genital tract. Mod. Pathol. 2008, 21, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Smith, C.L.; Cheetham, G.; Dodd, T.J.; Davy, M.L. Serous carcinoma of the uterus-determination of HER-2/neu status using immunohistochemistry, chromogenic in situ hybridization, and quantitative polymerase chain reaction techniques: Its significance and clinical correlation. Int. J. Gynecol. Cancer 2008, 18, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Slomovitz, B.M.; Broaddus, R.R.; Burke, T.W.; Sneige, N.; Soliman, P.T.; Wu, W.; Sun, C.C.; Munsell, M.F.; Gershenson, D.M.; Lu, K.H. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. J. Clin. Oncol. 2004, 22, 3126–3132. [Google Scholar] [CrossRef]
- Grushko, T.A.; Filiaci, V.L.; Mundt, A.J.; Ridderstrale, K.; Olopade, O.I.; Fleming, G.F.; Gynecologic Oncology, G. An exploratory analysis of HER-2 amplification and overexpression in advanced endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2008, 108, 3–9. [Google Scholar] [CrossRef]
- Santin, A.D.; Bellone, S.; Siegel, E.R.; Palmieri, M.; Thomas, M.; Cannon, M.J.; Kay, H.H.; Roman, J.J.; Burnett, A.; Pecorelli, S. Racial differences in the overexpression of epidermal growth factor type II receptor (HER2/neu): A major prognostic indicator in uterine serous papillary cancer. Am. J. Obs. Gynecol. 2005, 192, 813–818. [Google Scholar] [CrossRef]
- Santin, A.D.; Bellone, S.; Van Stedum, S.; Bushen, W.; De Las Casas, L.E.; Korourian, S.; Tian, E.; Roman, J.J.; Burnett, A.; Pecorelli, S. Determination of HER2/neu status in uterine serous papillary carcinoma: Comparative analysis of immunohistochemistry and fluorescence in situ hybridization. Gynecol. Oncol. 2005, 98, 24–30. [Google Scholar] [CrossRef]
- Chung, Y.W.; Kim, S.; Hong, J.H.; Lee, J.K.; Lee, N.W.; Lee, Y.S.; Song, J.Y. Overexpression of HER2/HER3 and clinical feature of ovarian cancer. J. Gynecol. Oncol. 2019, 30, e75. [Google Scholar] [CrossRef] [PubMed]
- Lassus, H.; Leminen, A.; Vayrynen, A.; Cheng, G.; Gustafsson, J.A.; Isola, J.; Butzow, R. ERBB2 amplification is superior to protein expression status in predicting patient outcome in serous ovarian carcinoma. Gynecol. Oncol. 2004, 92, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Mano, M.S.; Awada, A.; Di Leo, A.; Durbecq, V.; Paesmans, M.; Cardoso, F.; Larsimont, D.; Piccart, M. Rates of topoisomerase II-alpha and HER-2 gene amplification and expression in epithelial ovarian carcinoma. Gynecol. Oncol. 2004, 92, 887–895. [Google Scholar] [CrossRef]
- Camilleri-Broet, S.; Hardy-Bessard, A.C.; Le Tourneau, A.; Paraiso, D.; Levrel, O.; Leduc, B.; Bain, S.; Orfeuvre, H.; Audouin, J.; Pujade-Lauraine, E. HER-2 overexpression is an independent marker of poor prognosis of advanced primary ovarian carcinoma: A multicenter study of the GINECO group. Ann. Oncol. 2004, 15, 104–112. [Google Scholar] [CrossRef]
- Edwards, J.; Mukherjee, R.; Munro, A.F.; Wells, A.C.; Almushatat, A.; Bartlett, J.M. HER2 and COX2 expression in human prostate cancer. Eur. J. Cancer 2004, 40, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, A.; Sarkar, D.; Kumar Pal, D. Expression of HER2/NEU in newly diagnosed metastatic carcinoma prostate on TRUS biopsy specimen and its clinical correlation and progression. Urologia 2023, 90, 244–247. [Google Scholar] [CrossRef]
- Estephan, F.; Lap, C.J.; Banagan, J.; Antonio, M.; Liu, S.; Diao, G.; Rozalen, A.Z.; Rajendran, R.; Krasnow, S.; Subrahmanyam, R.; et al. The prevalence and clinical significance of HER2 expression in prostate adenocarcinoma. Ann. Diagn. Pathol. 2023, 67, 152219. [Google Scholar] [CrossRef]
- Reese, D.M.; Small, E.J.; Magrane, G.; Waldman, F.M.; Chew, K.; Sudilovsky, D. HER2 protein expression and gene amplification in androgen-independent prostate cancer. Am. J. Clin. Pathol. 2001, 116, 234–239. [Google Scholar] [CrossRef]
- Baek, K.H.; Hong, M.E.; Jung, Y.Y.; Lee, C.H.; Lee, T.J.; Park, E.S.; Kim, M.K.; Yoo, J.H.; Lee, S.W. Correlation of AR, EGFR, and HER2 Expression Levels in Prostate Cancer: Immunohistochemical Analysis and Chromogenic In Situ Hybridization. Cancer Res. Treat. 2012, 44, 50–56. [Google Scholar] [CrossRef]
- Tambo, M.; Higashihara, E.; Terado, Y.; Nutahara, K.; Okegawa, T. Comparison of serum HER2/neu with immunohistochemical HER2/neu expression for the prediction of biochemical progression in metastatic prostate cancer. Int. J. Urol. 2009, 16, 369–374. [Google Scholar] [CrossRef]
- Santin, A.D. HER2/neu overexpression: Has the Achilles’ heel of uterine serous papillary carcinoma been exposed? Gynecol. Oncol. 2003, 88, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Toboni, M.; Kurnit, K.; Erickson, B.; Powell, M.; Secord, A.A.; Fader, A.N. Updates and controversies in the management of uterine serous carcinoma and uterine carcinosarcoma. Int. J. Gynecol. Cancer 2025, 35, 101672. [Google Scholar] [CrossRef]
- Santin, A.D.; Bellone, S.; Van Stedum, S.; Bushen, W.; Palmieri, M.; Siegel, E.R.; De Las Casas, L.E.; Roman, J.J.; Burnett, A.; Pecorelli, S. Amplification of c-erbB2 oncogene: A major prognostic indicator in uterine serous papillary carcinoma. Cancer 2005, 104, 1391–1397. [Google Scholar] [CrossRef]
- Buza, N.; Hui, P. Marked heterogeneity of HER2/NEU gene amplification in endometrial serous carcinoma. Genes Chromosom. Cancer 2013, 52, 1178–1186. [Google Scholar] [CrossRef]
- Buza, N.; English, D.P.; Santin, A.D.; Hui, P. Toward standard HER2 testing of endometrial serous carcinoma: 4-year experience at a large academic center and recommendations for clinical practice. Mod. Pathol. 2013, 26, 1605–1612. [Google Scholar] [CrossRef]
- Hashem, S.; Zare, S.Y.; Fadare, O. HER2 Status Assessment in Endometrial Serous Carcinoma: Comparative Analysis of Two Proposed Testing and Interpretation Algorithms. Int. J. Gynecol. Pathol. 2024, 43, 4–14. [Google Scholar] [CrossRef]
- Rottmann, D.; Assem, H.; Matsumoto, N.; Wong, S.; Hui, P.; Buza, N. Does Specimen Type Have an Impact on HER2 Status in Endometrial Serous Carcinoma? Discordant HER2 Status of Paired Endometrial Biopsy and Hysterectomy Specimens in the Presence of Frequent Intratumoral Heterogeneity. Int. J. Gynecol. Pathol. 2021, 40, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Ma, W.; Brown, D.; Da Cruz Paula, A.; Zhou, Q.; Iaosonos, A.; Tessier-Cloutier, B.; Ross, D.S.; Troso-Sandoval, T.; Reis-Filho, J.S.; et al. HER2 Genetic Intratumor Heterogeneity Is Associated With Resistance to Trastuzumab and Trastuzumab Emtansine Therapy in Recurrent High-Grade Endometrial Cancer. Mod. Pathol. 2023, 36, 100299. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Hausdorf, D.E.; Schalper, K.A.; Bai, Y.; Black, J.; Santin, A.D.; Rimm, D.L. Objective, domain-specific HER2 measurement in uterine and ovarian serous carcinomas and its clinical significance. Gynecol. Oncol. 2017, 145, 154–158. [Google Scholar] [CrossRef]
- Template for Reporting Results of Biomarker Testing of Specimens from Patients with Carcinoma of Gynecologic Origin. Available online: https://documents.cap.org/protocols/Gynecologic.Bmk_1.0.0.0.REL_CAPCP.pdf (accessed on 1 May 2025).
- Fader, A.N.; Roque, D.M.; Siegel, E.; Buza, N.; Hui, P.; Abdelghany, O.; Chambers, S.K.; Secord, A.A.; Havrilesky, L.; O’Malley, D.M.; et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Versus Carboplatin-Paclitaxel-Trastuzumab in Uterine Serous Carcinomas That Overexpress Human Epidermal Growth Factor Receptor 2/neu. J. Clin. Oncol. 2018, 36, 2044–2051. [Google Scholar] [CrossRef]
- Buza, N. HER2 Testing in Endometrial Serous Carcinoma: Time for Standardized Pathology Practice to Meet the Clinical Demand. Arch. Pathol. Lab. Med. 2021, 145, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Klc, T.; Wu, S.; Wilhite, A.M.; Jones, N.L.; Powell, M.A.; Olawaiye, A.; Simpkins, F.; Girda, E.; Brown, J.; Puechl, A.; et al. HER2 in uterine serous carcinoma: Testing platforms and implications for targeted therapy. J. Clin. Oncol. 2021, 39, 5580. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 2018, 142, 1364–1382. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, R.; Shi, H.; Ye, L.; Wang, H.; Lu, B. Human epidermal growth factor 2 (HER2) amplification in uterine serous carcinoma: An analysis of prognosis and immune microenvironment. Virchows Arch. 2025, 486, 707–719. [Google Scholar] [CrossRef]
- Erickson, B.K.; Najjar, O.; Damast, S.; Blakaj, A.; Tymon-Rosario, J.; Shahi, M.; Santin, A.; Klein, M.; Dolan, M.; Cimino-Mathews, A.; et al. Human epidermal growth factor 2 (HER2) in early stage uterine serous carcinoma: A multi-institutional cohort study. Gynecol. Oncol. 2020, 159, 17–22. [Google Scholar] [CrossRef]
- Diaz-Montes, T.P.; Ji, H.; Smith Sehdev, A.E.; Zahurak, M.L.; Kurman, R.J.; Armstrong, D.K.; Bristow, R.E. Clinical significance of Her-2/neu overexpression in uterine serous carcinoma. Gynecol. Oncol. 2006, 100, 139–144. [Google Scholar] [CrossRef]
- Togami, S.; Sasajima, Y.; Oi, T.; Ishikawa, M.; Onda, T.; Ikeda, S.; Kato, T.; Tsuda, H.; Kasamatsu, T. Clinicopathological and prognostic impact of human epidermal growth factor receptor type 2 (HER2) and hormone receptor expression in uterine papillary serous carcinoma. Cancer Sci. 2012, 103, 926–932. [Google Scholar] [CrossRef]
- Kim, W.Y.; Yang, E.J.; Jang, E.B.; Lee, A.J.; So, K.A.; Shim, S.H.; Kim, T.J.; Lee, S.J. The Expression and Amplification of HER2 Has a Significant Impact on the Prognosis of Endometrial Carcinoma in Korean Patients. J. Clin. Med. 2024, 13, 2158. [Google Scholar] [CrossRef]
- English, D.P.; Bellone, S.; Cocco, E.; Bortolomai, I.; Pecorelli, S.; Lopez, S.; Silasi, D.A.; Schwartz, P.E.; Rutherford, T.; Santin, A.D. Oncogenic PIK3CA gene mutations and HER2/neu gene amplifications determine the sensitivity of uterine serous carcinoma cell lines to GDC-0980, a selective inhibitor of Class I PI3 kinase and mTOR kinase (TORC1/2). Am. J. Obstet. Gynecol. 2013, 209, 465.e1–465.e9. [Google Scholar] [CrossRef] [PubMed]
- English, D.P.; Roque, D.M.; Carrara, L.; Lopez, S.; Bellone, S.; Cocco, E.; Bortolomai, I.; Schwartz, P.E.; Rutherford, T.; Santin, A.D. HER2/neu gene amplification determines the sensitivity of uterine serous carcinoma cell lines to AZD8055, a novel dual mTORC1/2 inhibitor. Gynecol. Oncol. 2013, 131, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.; Schwab, C.L.; Cocco, E.; Bellone, S.; Bonazzoli, E.; English, D.P.; Schwartz, P.E.; Rutherford, T.; Angioli, R.; Santin, A.D. Taselisib, a selective inhibitor of PIK3CA, is highly effective on PIK3CA-mutated and HER2/neu amplified uterine serous carcinoma in vitro and in vivo. Gynecol. Oncol. 2014, 135, 312–317. [Google Scholar] [CrossRef]
- PubChem Compound Summary for Trastuzumab. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Trastuzumab (accessed on 1 May 2025).
- Albanell, J.; Codony, J.; Rovira, A.; Mellado, B.; Gascon, P. Mechanism of action of anti-HER2 monoclonal antibodies: Scientific update on trastuzumab and 2C4. Adv. Exp. Med. Biol. 2003, 532, 253–268. [Google Scholar] [PubMed]
- Delord, J.P.; Allal, C.; Canal, M.; Mery, E.; Rochaix, P.; Hennebelle, I.; Pradines, A.; Chatelut, E.; Bugat, R.; Guichard, S.; et al. Selective inhibition of HER2 inhibits AKT signal transduction and prolongs disease-free survival in a micrometastasis model of ovarian carcinoma. Ann. Oncol. 2005, 16, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Lane, H.A.; Motoyama, A.B.; Beuvink, I.; Hynes, N.E. Modulation of p27/Cdk2 complex formation through 4D5-mediated inhibition of HER2 receptor signaling. Ann. Oncol. 2001, 12, S21–S22. [Google Scholar] [CrossRef]
- Yakes, F.M.; Chinratanalab, W.; Ritter, C.A.; King, W.; Seelig, S.; Arteaga, C.L. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002, 62, 4132–4141. [Google Scholar] [PubMed]
- Wander, S.A.; Zhao, D.; Slingerland, J.M. p27: A barometer of signaling deregulation and potential predictor of response to targeted therapies. Clin. Cancer Res. 2011, 17, 12–18. [Google Scholar] [CrossRef]
- Maadi, H.; Soheilifar, M.H.; Choi, W.S.; Moshtaghian, A.; Wang, Z. Trastuzumab Mechanism of Action; 20 Years of Research to Unravel a Dilemma. Cancers 2021, 13, 3540. [Google Scholar] [CrossRef]
- Valabrega, G.; Montemurro, F.; Aglietta, M. Trastuzumab: Mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann. Oncol. 2007, 18, 977–984. [Google Scholar] [CrossRef]
- Cooley, S.; Burns, L.J.; Repka, T.; Miller, J.S. Natural killer cell cytotoxicity of breast cancer targets is enhanced by two distinct mechanisms of antibody-dependent cellular cytotoxicity against LFA-3 and HER2/neu. Exp. Hematol. 1999, 27, 1533–1541. [Google Scholar] [CrossRef]
- Lewis, G.D.; Figari, I.; Fendly, B.; Wong, W.L.; Carter, P.; Gorman, C.; Shepard, H.M. Differential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodies. Cancer Immunol. Immunother. 1993, 37, 255–263. [Google Scholar] [CrossRef]
- Clynes, R.A.; Towers, T.L.; Presta, L.G.; Ravetch, J.V. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 2000, 6, 443–446. [Google Scholar] [CrossRef]
- Gennari, R.; Menard, S.; Fagnoni, F.; Ponchio, L.; Scelsi, M.; Tagliabue, E.; Castiglioni, F.; Villani, L.; Magalotti, C.; Gibelli, N.; et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin. Cancer Res. 2004, 10, 5650–5655. [Google Scholar] [CrossRef]
- Rainone, V.; Martelli, C.; Ottobrini, L.; Biasin, M.; Texido, G.; Degrassi, A.; Borelli, M.; Lucignani, G.; Trabattoni, D.; Clerici, M. Immunological Characterization of Whole Tumour Lysate-Loaded Dendritic Cells for Cancer Immunotherapy. PLoS ONE 2016, 11, e0146622. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.M.; O’Donovan, N.; McGowan, P.M.; O’Sullivan, F.; Duffy, M.J.; Crown, J. Trastuzumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) in HER-2-non-amplified breast cancer cell lines. Ann. Oncol. 2012, 23, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Petricevic, B.; Laengle, J.; Singer, J.; Sachet, M.; Fazekas, J.; Steger, G.; Bartsch, R.; Jensen-Jarolim, E.; Bergmann, M. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J. Transl. Med. 2013, 11, 307. [Google Scholar] [CrossRef]
- Ault, A. Drugs in brief. Lancet 1998, 352, 1205. [Google Scholar] [CrossRef]
- Dawood, S.; Broglio, K.; Buzdar, A.U.; Hortobagyi, G.N.; Giordano, S.H. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: An institutional-based review. J. Clin. Oncol. 2010, 28, 92–98. [Google Scholar] [CrossRef]
- Scheck, M.K.; Hofheinz, R.D.; Lorenzen, S. HER2-Positive Gastric Cancer and Antibody Treatment: State of the Art and Future Developments. Cancers 2024, 16, 1336. [Google Scholar] [CrossRef]
- Fleming, G.F.; Sill, M.W.; Darcy, K.M.; McMeekin, D.S.; Thigpen, J.T.; Adler, L.M.; Berek, J.S.; Chapman, J.A.; DiSilvestro, P.A.; Horowitz, I.R.; et al. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2010, 116, 15–20. [Google Scholar] [CrossRef]
- Santin, A.D. Letter to the Editor referring to the manuscript entitled: “Phase II trial of trastuzumab in women with advanced or recurrent HER-positive endometrial carcinoma: A Gynecologic Oncology Group study” recently reported by Fleming et al., (Gynecol Oncol., 116;15–20;2010). Gynecol. Oncol. 2010, 118, 95–96. [Google Scholar] [CrossRef]
- Herceptin (Trastuzumab) Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf (accessed on 1 May 2025).
- Fader, A.N.; Roque, D.M.; Siegel, E.; Buza, N.; Hui, P.; Abdelghany, O.; Chambers, S.; Secord, A.A.; Havrilesky, L.; O’Malley, D.M.; et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Compared with Carboplatin-Paclitaxel-Trastuzumab in Advanced (Stage III-IV) or Recurrent Uterine Serous Carcinomas that Overexpress Her2/Neu (NCT01367002): Updated Overall Survival Analysis. Clin. Cancer Res. 2020, 26, 3928–3935. [Google Scholar] [CrossRef]
- Lu, T.F.; Sun, L.; Shih, Y.H.; Chen, Y.F.; Fan, C.T.; Wang, S.J.; Hsu, S.T.; Liu, C.K.; Hwang, S.F.; Lu, C.H. A retrospective study evaluating the effect of trastuzumab addition to carboplatin/paclitaxel on overall survival in patients with advanced-stage HER2/neu-overexpressing uterine serous carcinoma or carcinosarcoma. BMC Med. 2025, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zabaleta, M.E.; Forbes-Hernandez, T.Y.; Simal-Gandara, J.; Quiles, J.L.; Cianciosi, D.; Bullon, B.; Giampieri, F.; Battino, M. Effect of polyphenols on HER2-positive breast cancer and related miRNAs: Epigenomic regulation. Food Res. Int. 2020, 137, 109623. [Google Scholar] [CrossRef]
- Arnould, L.; Gelly, M.; Penault-Llorca, F.; Benoit, L.; Bonnetain, F.; Migeon, C.; Cabaret, V.; Fermeaux, V.; Bertheau, P.; Garnier, J.; et al. Trastuzumab-based treatment of HER2-positive breast cancer: An antibody-dependent cellular cytotoxicity mechanism? Br. J. Cancer 2006, 94, 259–267. [Google Scholar] [CrossRef]
- Agus, D.B.; Akita, R.W.; Fox, W.D.; Lewis, G.D.; Higgins, B.; Pisacane, P.I.; Lofgren, J.A.; Tindell, C.; Evans, D.P.; Maiese, K.; et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2002, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Mason, K.; Ramyar, K.X.; Stanley, A.M.; Gabelli, S.B.; Denney, D.W.; Leahy, D.J., Jr. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003, 421, 756–760. [Google Scholar] [CrossRef]
- Nahta, R.; Hung, M.C.; Esteva, F.J. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004, 64, 2343–2346. [Google Scholar] [CrossRef]
- Perjeta (Pertuzumab) Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125409lbl.pdf (accessed on 1 May 2025).
- von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef]
- El-Sahwi, K.; Bellone, S.; Cocco, E.; Cargnelutti, M.; Casagrande, F.; Bellone, M.; Abu-Khalaf, M.; Buza, N.; Tavassoli, F.A.; Hui, P.; et al. In vitro activity of pertuzumab in combination with trastuzumab in uterine serous papillary adenocarcinoma. Br. J. Cancer 2010, 102, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.R.; Rothe, M.; Mangat, P.K.; Garrett-Mayer, E.; Ali-Ahmad, H.M.; Chan, J.; Maitland, M.L.; Patel, S.R.; Reese, Z.; Balmanoukian, A.S.; et al. Pertuzumab Plus Trastuzumab in Patients With Endometrial Cancer With ERBB2/3 Amplification, Overexpression, or Mutation: Results From the TAPUR Study. JCO Precis. Oncol. 2023, 7, e2200609. [Google Scholar] [CrossRef]
- Erickson, B.; Enserro, D.; Lankes, H.A.; Dockery, L.E.; Ghamande, S.A.; Oliver, M.T.; Gressel, G.M.; Castellano, T.; Ratner, E.; Deery, A.; et al. Phase II/III study of paclitaxel/carboplatin alone or combined with either trastuzumab and hyaluronidase-oysk or pertuzumab, trastuzumab, and hyaluronidase-zzxf in HER2 positive, stage I-IV endometrial serous carcinoma or carcinosarcoma (NRG-GY026). J. Clin. Oncol. 2024, 42, TPS5641. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blattler, W.A.; Lambert, J.M.; Chari, R.V.; Lutz, R.J.; et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Barok, M.; Joensuu, H.; Isola, J. Trastuzumab emtansine: Mechanisms of action and drug resistance. Breast Cancer Res. 2014, 16, 209. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Dieras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- English, D.P.; Bellone, S.; Schwab, C.L.; Bortolomai, I.; Bonazzoli, E.; Cocco, E.; Buza, N.; Hui, P.; Lopez, S.; Ratner, E.; et al. T-DM1, a novel antibody-drug conjugate, is highly effective against primary HER2 overexpressing uterine serous carcinoma in vitro and in vivo. Cancer Med. 2014, 3, 1256–1265. [Google Scholar] [CrossRef]
- Santin, A.D.; Bellone, S.; Buza, N.; Schwartz, P.E. Regression of metastatic, radiation/chemotherapy-resistant uterine serous carcinoma overexpressing HER2/neu with trastuzumab emtansine (TDM-1). Gynecol. Oncol. Rep. 2017, 19, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Lopez, S.; Bellone, S.; Cocco, E.; Schwab, C.L.; Black, J.D.; Centritto, F.; Zhu, L.; Bonazzoli, E.; Buza, N.; et al. T-DM1, a novel antibody-drug conjugate, is highly effective against uterine and ovarian carcinosarcomas overexpressing HER2. Clin. Exp. Metastasis 2015, 32, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Thavaneswaran, S.; Lin, F.; Grady, J.P.; Espinoza, D.; Huang, M.L.; Chinchen, S.; Sebastian, L.; Kansara, M.; Mersiades, T.; Lee, C.K.; et al. A signal-seeking phase 2 study of Trastuzumab emtansine in tumours harbouring HER2 amplification or mutation. NPJ Precis. Oncol. 2024, 8, 195. [Google Scholar] [CrossRef]
- FDA. Kadcyla (Ado-Trastuzumab Emtansine) Label. 2025. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125427lbl.pdf (accessed on 1 May 2025).
- Karpel, H.C.; Powell, S.S.; Pothuri, B. Antibody-Drug Conjugates in Gynecologic Cancer. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e390772. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- McNamara, B.; Bellone, S.; Demirkiran, C.; Hartwich, T.M.P.; Santin, A.D. Trastuzumab deruxtecan in recurrent uterine serous carcinoma resistant to trastuzmab based-chemotherapy. Gynecol. Oncol. Rep. 2023, 48, 101219. [Google Scholar] [CrossRef]
- Mauricio, D.; Bellone, S.; Mutlu, L.; McNamara, B.; Manavella, D.D.; Demirkiran, C.; Verzosa, M.S.Z.; Buza, N.; Hui, P.; Hartwich, T.M.P.; et al. Trastuzumab deruxtecan (DS-8201a), a HER2-targeting antibody-drug conjugate with topoisomerase I inhibitor payload, shows antitumor activity in uterine and ovarian carcinosarcoma with HER2/neu expression. Gynecol. Oncol. 2023, 170, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, L.; Manavella, D.D.; Bellone, S.; McNamara, B.; Harold, J.A.; Mauricio, D.; Siegel, E.R.; Buza, N.; Hui, P.; Hartwich, T.M.P.; et al. In Vivo and In Vitro Efficacy of Trastuzumab Deruxtecan in Uterine Serous Carcinoma. Mol. Cancer Ther. 2023, 22, 1404–1412. [Google Scholar] [CrossRef]
- Cortes, J.; Kim, S.B.; Chung, W.P.; Im, S.A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.M.; Petry, V.; Chung, C.F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.; Modi, S.; Krop, I.; Park, Y.H.; Kim, S.B.; Tamura, K.; Iwata, H.; Tsurutani, J.; Sohn, J.; Mathias, E.; et al. Trastuzumab deruxtecan in previously treated patients with HER2-positive metastatic breast cancer: Updated survival results from a phase II trial (DESTINY-Breast01). Ann. Oncol. 2024, 35, 302–307. [Google Scholar] [CrossRef]
- Nishikawa, T.; Hasegawa, K.; Matsumoto, K.; Mori, M.; Hirashima, Y.; Takehara, K.; Ariyoshi, K.; Kato, T.; Yagishita, S.; Hamada, A.; et al. Trastuzumab Deruxtecan for Human Epidermal Growth Factor Receptor 2-Expressing Advanced or Recurrent Uterine Carcinosarcoma (NCCH1615): The STATICE Trial. J. Clin. Oncol. 2023, 41, 2789–2799. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.Y.; Banerjee, S.; Gonzalez-Martin, A.; Jung, K.H.; Lugowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef]
- FDA. Enhertu (Fam-Trastuzumab Deruxtecan-Nxki) Label. 2025. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761139s028lbl.pdf (accessed on 1 May 2025).
- Swain, S.M.; Nishino, M.; Lancaster, L.H.; Li, B.T.; Nicholson, A.G.; Bartholmai, B.J.; Naidoo, J.; Schumacher-Wulf, E.; Shitara, K.; Tsurutani, J.; et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis-Focus on proactive monitoring, diagnosis, and management. Cancer Treat. Rev. 2022, 106, 102378. [Google Scholar] [CrossRef]
- Guide to Enhertu Dosing and Administration. Available online: https://www.elaherehcp.com/dosing?utm_source=goog&utm_medium=cpc&utm_campaign=22024949250&utm_content=173829350564&utm_source=goog&utm_medium=cpc&utm_campaign=22024949250&utm_content=173829350564&gclsrc=aw.ds&&gclid=EAIaIQobChMIj_WI7MTJiwMVRENHAR03cAAOEAAYAiAAEgILdPD_BwE&gad_source=1 (accessed on 1 May 2025).
- Menderes, G.; Lopez, S.; Han, C.; Altwerger, G.; Gysler, S.; Varughese, J.; Schwartz, P.E.; Santin, A.D. Mechanisms of resistance to HER2-targeted therapies in HER2-amplified uterine serous carcinoma, and strategies to overcome it. Discov. Med. 2018, 26, 39–50. [Google Scholar]
- Black, J.D.; Lopez, S.; Cocco, E.; Bellone, S.; Altwerger, G.; Schwab, C.L.; English, D.P.; Bonazzoli, E.; Predolini, F.; Ferrari, F.; et al. PIK3CA oncogenic mutations represent a major mechanism of resistance to trastuzumab in HER2/neu overexpressing uterine serous carcinomas. Br. J. Cancer 2015, 113, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.L.; Xiu, J.; Reddy, S.K.; Burke, W.M.; Tergas, A.I.; Wright, J.D.; Hou, J.Y. Identification of potential therapeutic targets by molecular profiling of 628 cases of uterine serous carcinoma. Gynecol. Oncol. 2015, 138, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, H.; Xiu, J.; Reddy, S.K.; DeBernardo, R. Alteration in PI3K/mTOR, MAPK pathways and Her2 expression/amplification is more frequent in uterine serous carcinoma than ovarian serous carcinoma. J. Surg. Oncol. 2015, 112, 188–194. [Google Scholar] [CrossRef]
- Nagata, Y.; Lan, K.H.; Zhou, X.; Tan, M.; Esteva, F.J.; Sahin, A.A.; Klos, K.S.; Li, P.; Monia, B.P.; Nguyen, N.T.; et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 2004, 6, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Berns, K.; Horlings, H.M.; Hennessy, B.T.; Madiredjo, M.; Hijmans, E.M.; Beelen, K.; Linn, S.C.; Gonzalez-Angulo, A.M.; Stemke-Hale, K.; Hauptmann, M.; et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 2007, 12, 395–402. [Google Scholar] [CrossRef]
- Serra, V.; Markman, B.; Scaltriti, M.; Eichhorn, P.J.; Valero, V.; Guzman, M.; Botero, M.L.; Llonch, E.; Atzori, F.; Di Cosimo, S.; et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008, 68, 8022–8030. [Google Scholar] [CrossRef]
- Scaltriti, M.; Rojo, F.; Ocana, A.; Anido, J.; Guzman, M.; Cortes, J.; Di Cosimo, S.; Matias-Guiu, X.; Cajal, S.R.Y.; Arribas, J.; et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J. Natl. Cancer Inst. 2007, 99, 628–638. [Google Scholar] [CrossRef]
- Scott, G.K.; Robles, R.; Park, J.W.; Montgomery, P.A.; Daniel, J.; Holmes, W.E.; Lee, J.; Keller, G.A.; Li, W.L.; Fendly, B.M.; et al. A truncated intracellular HER2/neu receptor produced by alternative RNA processing affects growth of human carcinoma cells. Mol. Cell. Biol. 1993, 13, 2247–2257. [Google Scholar]
- Lu, Y.; Zi, X.; Zhao, Y.; Mascarenhas, D.; Pollak, M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J. Natl. Cancer Inst. 2001, 93, 1852–1857. [Google Scholar] [CrossRef]
- Lopez, S.; Cocco, E.; Black, J.; Bellone, S.; Bonazzoli, E.; Predolini, F.; Ferrari, F.; Schwab, C.L.; English, D.P.; Ratner, E.; et al. Dual HER2/PIK3CA Targeting Overcomes Single-Agent Acquired Resistance in HER2-Amplified Uterine Serous Carcinoma Cell Lines In Vitro and In Vivo. Mol. Cancer Ther. 2015, 14, 2519–2526. [Google Scholar] [CrossRef]
- Hernandez, S.F.; Chisholm, S.; Borger, D.; Foster, R.; Rueda, B.R.; Growdon, W.B. Ridaforolimus improves the anti-tumor activity of dual HER2 blockade in uterine serous carcinoma in vivo models with HER2 gene amplification and PIK3CA mutation. Gynecol. Oncol. 2016, 141, 570–579. [Google Scholar] [CrossRef]
- Todeschini, P.; Cocco, E.; Bellone, S.; Varughese, J.; Lin, K.; Carrara, L.; Guzzo, F.; Buza, N.; Hui, P.; Silasi, D.A.; et al. Her2/neu extracellular domain shedding in uterine serous carcinoma: Implications for immunotherapy with trastuzumab. Br. J. Cancer 2011, 105, 1176–1182. [Google Scholar] [CrossRef]
- Growdon, W.B.; Groeneweg, J.; Byron, V.; DiGloria, C.; Borger, D.R.; Tambouret, R.; Foster, R.; Chenna, A.; Sperinde, J.; Winslow, J.; et al. HER2 over-expressing high grade endometrial cancer expresses high levels of p95HER2 variant. Gynecol. Oncol. 2015, 137, 160–166. [Google Scholar] [CrossRef]
- Hunter, F.W.; Barker, H.R.; Lipert, B.; Rothe, F.; Gebhart, G.; Piccart-Gebhart, M.J.; Sotiriou, C.; Jamieson, S.M.F. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br. J. Cancer 2020, 122, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Walens, A.; Lin, J.; Damrauer, J.S.; McKinney, B.; Lupo, R.; Newcomb, R.; Fox, D.B.; Mabe, N.W.; Gresham, J.; Sheng, Z.; et al. Adaptation and selection shape clonal evolution of tumors during residual disease and recurrence. Nat. Commun. 2020, 11, 5017. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, Y.; Nowak, M.A.; Michor, F. Evolution of resistance during clonal expansion. Genetics 2006, 172, 2557–2566. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef]
- Tymon-Rosario, J.; Siegel, E.R.; Bellone, S.; Harold, J.; Adjei, N.; Zeybek, B.; Mauricio, D.; Altwerger, G.; Menderes, G.; Ratner, E.; et al. Trastuzumab tolerability in the treatment of advanced (stage III-IV) or recurrent uterine serous carcinomas that overexpress HER2/neu. Gynecol. Oncol. 2021, 163, 93–99. [Google Scholar] [CrossRef]
- A Phase II Evaluation of Afatinib in Patients with Persistent or Recurrent HER2-Positive Uterine Serous Carcinoma (Afatinib). Available online: https://clinicaltrials.gov/study/NCT02491099 (accessed on 1 May 2025).
- Ettorre, V.; Greenman, M.; Palmieri, L.; Demirkiran, C.; Santin, A.D. Successful sequential use of the antibody-drug-conjugate trastuzumab deruxtecan after progression on sacituzumab govitecan in a heavily pretreated, platinum-resistant, high grade serous ovarian cancer patient. SSRN Electron. J. 2025. [Google Scholar] [CrossRef]
- Buza, N.; Hui, P. Characteristics of HER2 Gene Amplification by Fluorescence In Situ Hybridization in Endometrial Serous Carcinoma. Arch. Pathol. Lab. Med. 2022, 147, 331–337. [Google Scholar] [CrossRef]
| Cancer Origin | Prevalence of HER2 Overexpression |
|---|---|
| Bladder | 8–70% [40,43,44,45,46] |
| Breast | 11–25% [47] |
| Stomach | 7–34% [40] |
| Colorectal | 1–5% [48,49] |
| Non-Small-Cell Lung Cancer | 6–20% [50,51,52,53,54] |
| USC | 14–80% [55,56,57,58,59,60,61,62,63] |
| Ovary | 4–16% [64,65,66,67] |
| Prostate | 1–24% [68,69,70,71,72,73] |
| IHC | Definition | Dual-Probe FISH | Results |
|---|---|---|---|
| 3+ | “Intense complete or basolateral/lateral membrane staining in >30% of tumor cells” | N/A | Positive |
| 2+ | “Intense complete or basolateral/lateral membrane staining in ≤30%, or weak to moderate in ≥10% of tumor cells” | “HER2/CEP17 ratio ≥ 2” | Positive |
| “HER2/CEP17 ratio < 2.0” | Negative | ||
| 1+ | “Faint/barely perceptible, incomplete membrane staining in any proportion, or weak complete in <10% of tumor cells” | N/A | Negative |
| 0 | “No staining in tumor cells” | N/A | Negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ettorre, V.M.; Palmieri, L.; Clemente, V.; Santin, A.D. HER2/neu as a Signaling and Therapeutic Marker in Uterine Serous Carcinoma. Cells 2025, 14, 1282. https://doi.org/10.3390/cells14161282
Ettorre VM, Palmieri L, Clemente V, Santin AD. HER2/neu as a Signaling and Therapeutic Marker in Uterine Serous Carcinoma. Cells. 2025; 14(16):1282. https://doi.org/10.3390/cells14161282
Chicago/Turabian StyleEttorre, Victoria M., Luca Palmieri, Valentino Clemente, and Alessandro D. Santin. 2025. "HER2/neu as a Signaling and Therapeutic Marker in Uterine Serous Carcinoma" Cells 14, no. 16: 1282. https://doi.org/10.3390/cells14161282
APA StyleEttorre, V. M., Palmieri, L., Clemente, V., & Santin, A. D. (2025). HER2/neu as a Signaling and Therapeutic Marker in Uterine Serous Carcinoma. Cells, 14(16), 1282. https://doi.org/10.3390/cells14161282





