Mitochondrial Reactive Oxygen Species Production in Vascular Dementia Following Experimental Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of T1D
2.2. Voluntary Urination Assay
2.3. Measurement of Visceral Fats
2.4. Behavior Tests
2.4.1. Acclimatization and Open Field
2.4.2. Object Position Tests
2.4.3. Novel Object Recognition Test (NORT)
2.4.4. Nest Building Test
2.5. Cerebral Blood Flow
2.6. Preparation of Cerebral Artery Smooth Muscle Cells (CASMCs)
2.7. Isolation of Mitochondria
2.8. ROS Generation Assays
2.9. DNA Damage
2.10. Tau Protein Phosphorylation
2.11. Statistical Analysis
3. Results
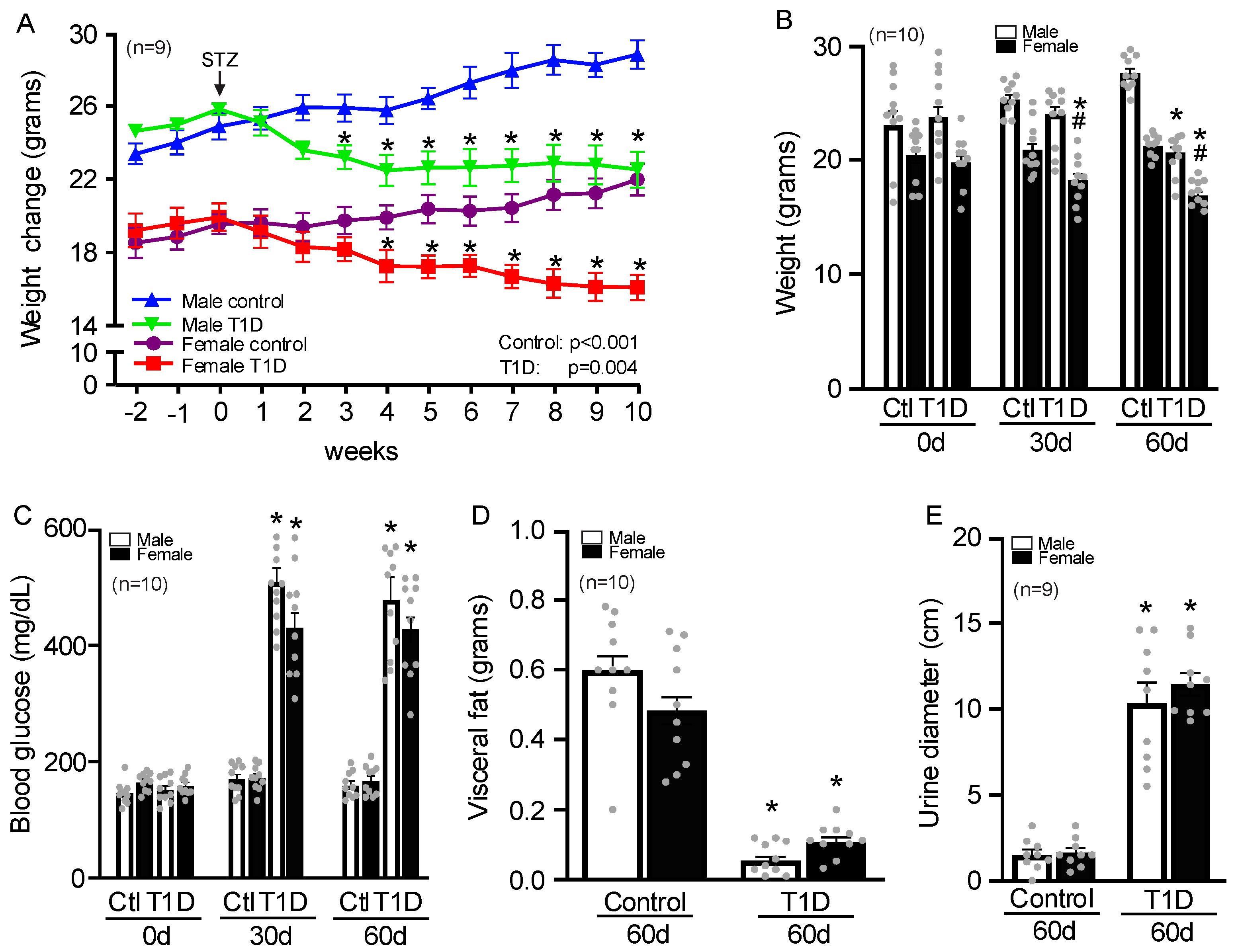
3.1. Sex-Specific Changes in Body Weight, Glucose, Visceral Fat, and Urination in T1D Mice
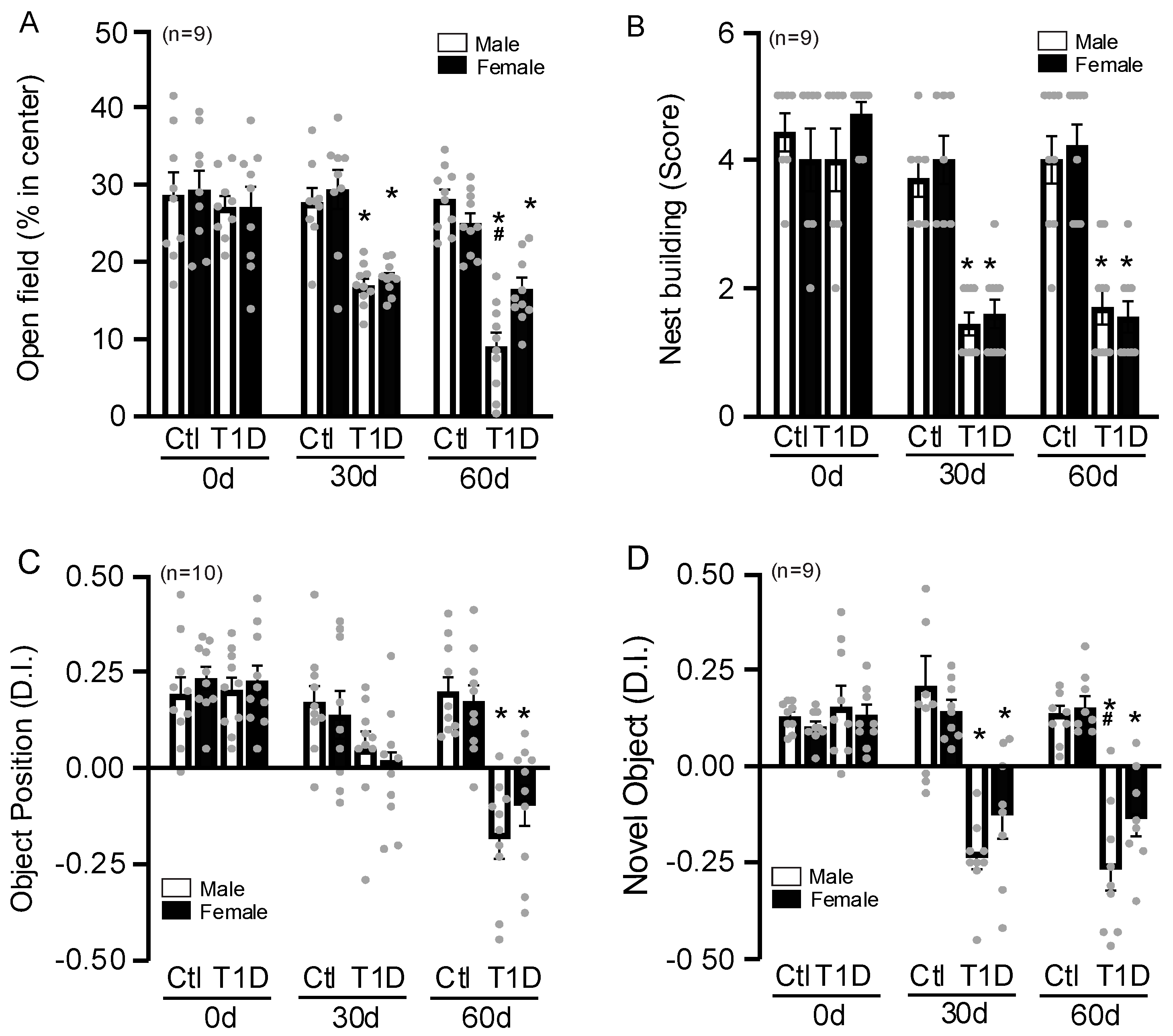
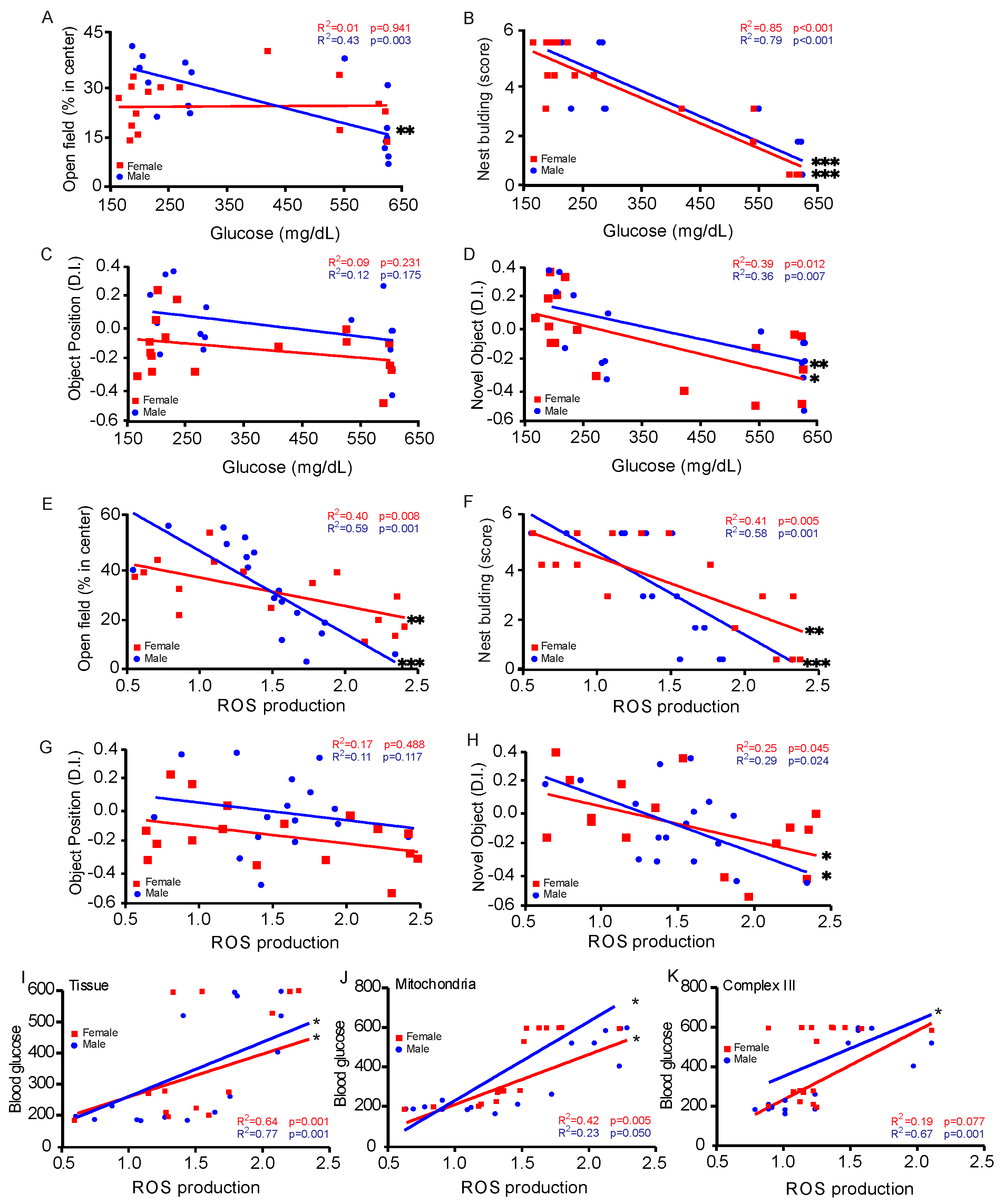
3.2. Sex-Specific Changes in Behavior Tests
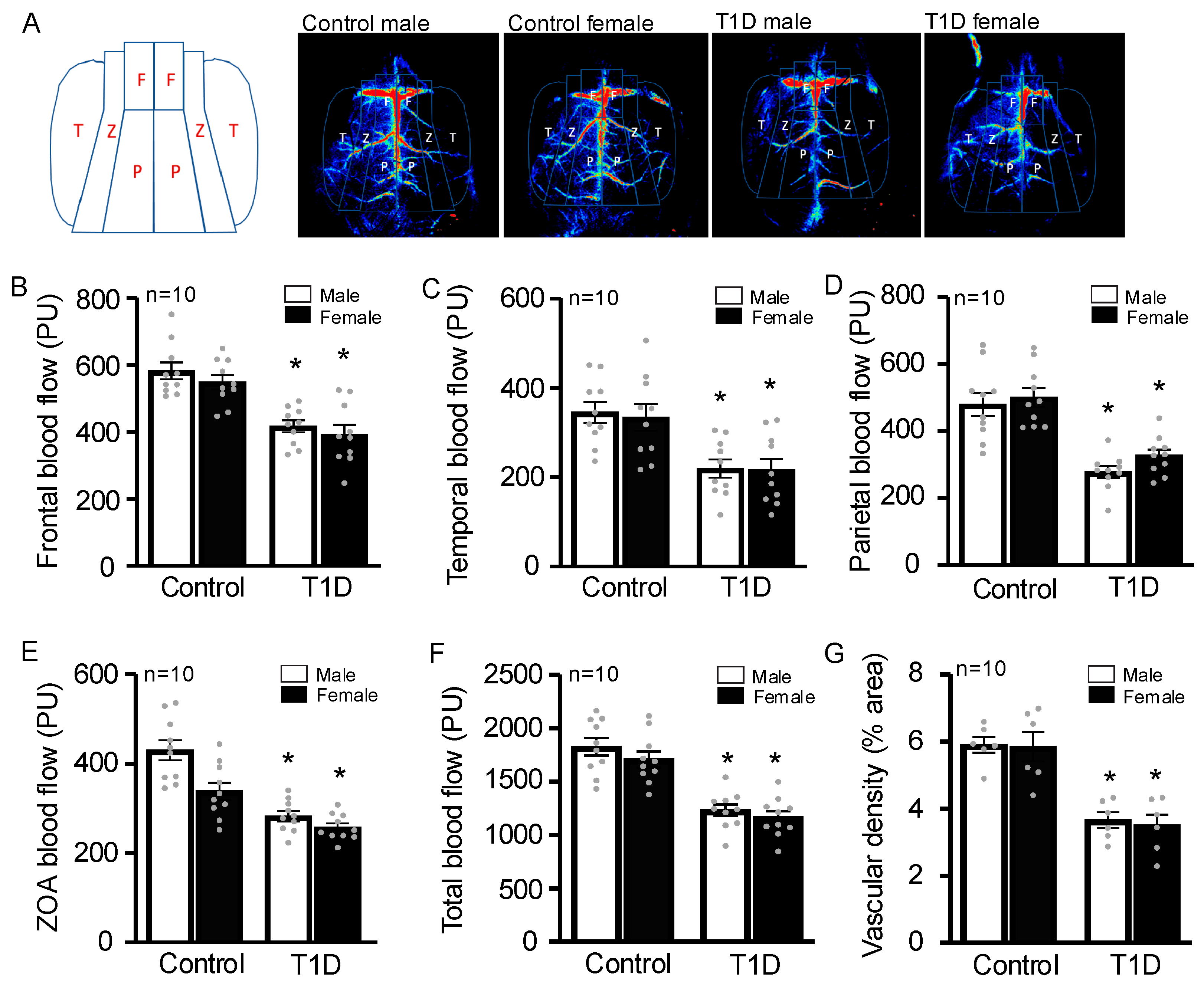
3.3. STZ-Injected Animals Have Decreased Cerebral Blood Flow
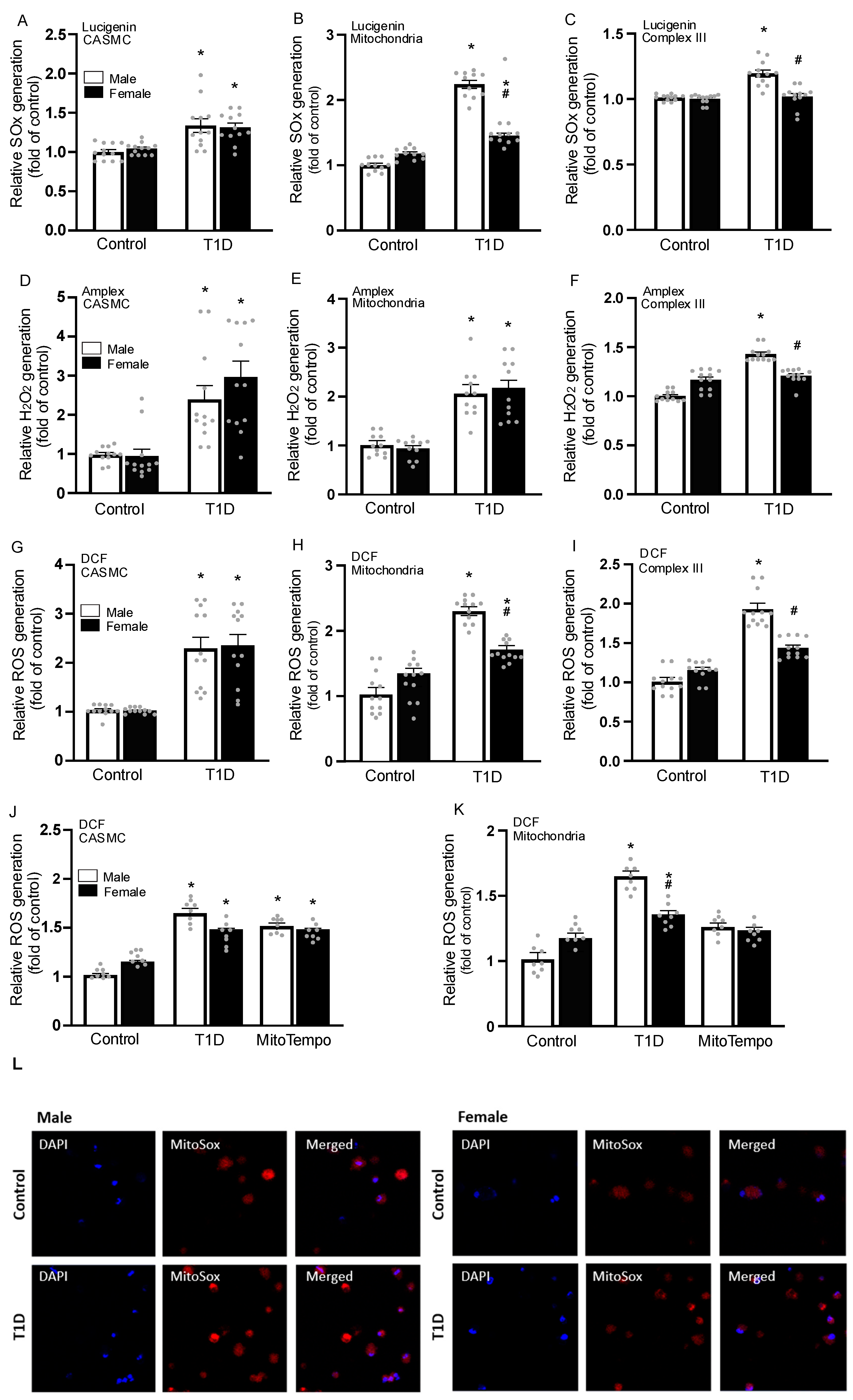
3.4. Sex-Specific Changes in ROS Generation
3.5. Correlation Between Blood Glucose Levels and Behavioral Assessments
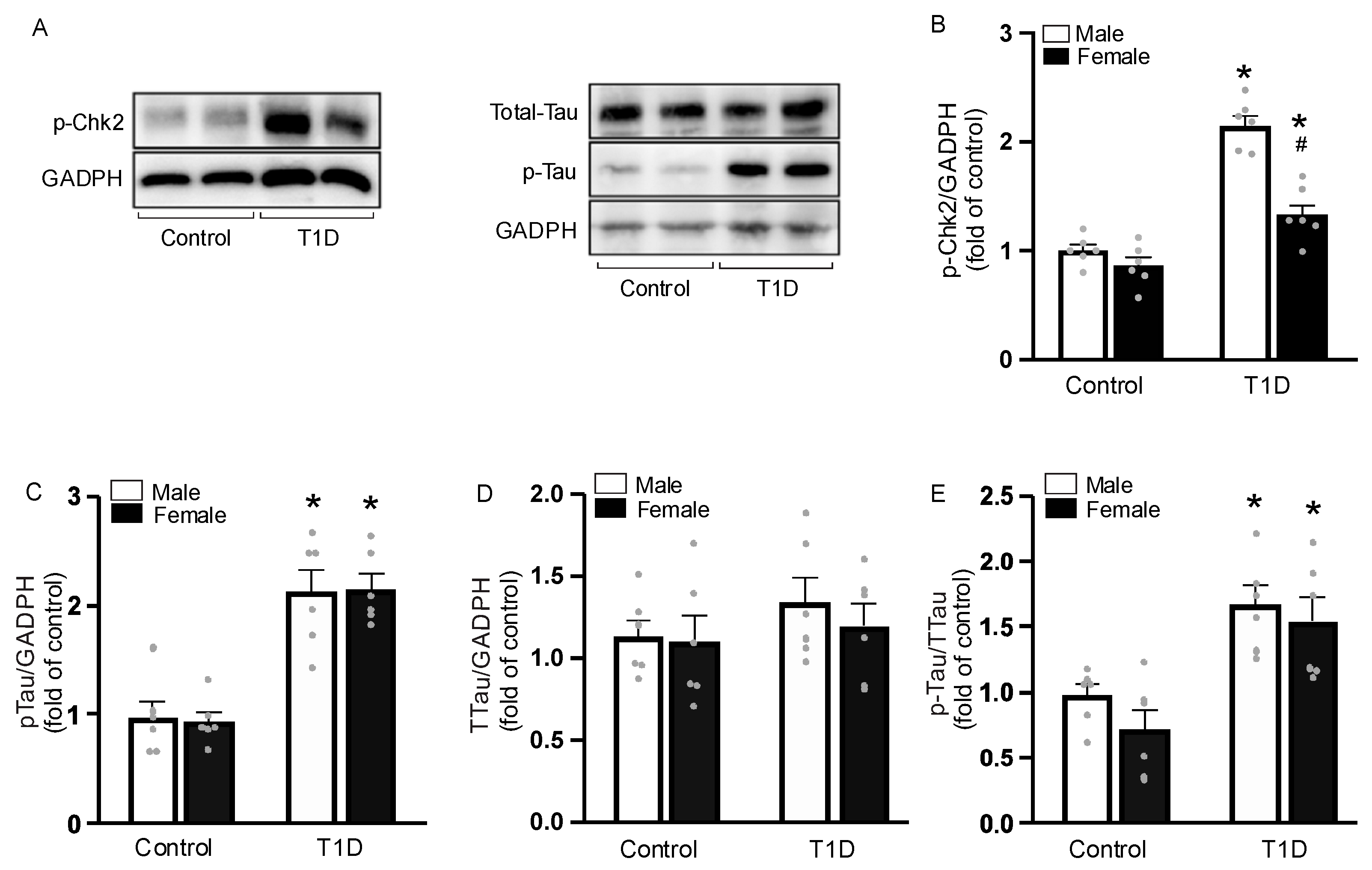
3.6. DNA Damage in CASMC
3.7. Sex-Specific Tau Phosphorylation
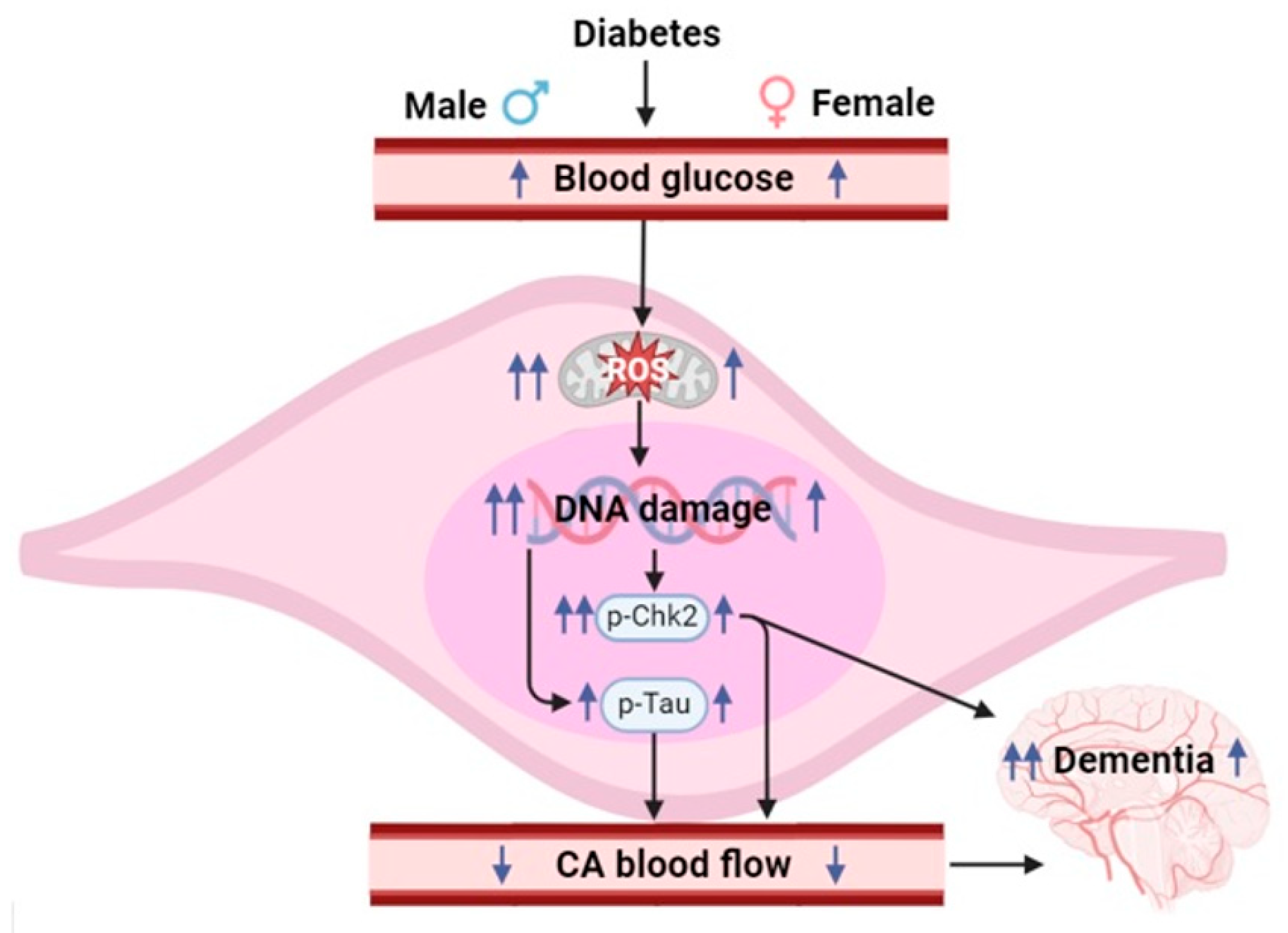
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas. Available online: https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/ (accessed on 8 June 2025).
- Parker, E.D.; Lin, J.; Mahoney, T.; Ume, N.; Yang, G.; Gabbay, R.A.; ElSayed, N.A.; Bannuru, R.R. Economic Costs of Diabetes in the U.S. in 2022. Diabetes Care 2024, 47, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.Z. Type 1 Diabetes Mellitus. Ann. Intern. Med. 2022, 175, ITC33–ITC48. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D. Diabetic complications of the central nervous system. Endocr. Rev. 1988, 9, 346–356. [Google Scholar] [CrossRef]
- Santos, E.W.; Khatoon, S.; Di Mise, A.; Zheng, Y.M.; Wang, Y.X. Mitochondrial Dynamics in Pulmonary Hypertension. Biomedicines 2023, 12, 53. [Google Scholar] [CrossRef]
- Truong, L.N.; Santos, E.W.; Zheng, Y.M.; Wang, Y.X. Rieske Iron-Sulfur Protein Mediates Pulmonary Hypertension Following Nicotine/Hypoxia Co-Exposure. Am. J. Respir. Cell Mol. Biol. 2023, 70, 193–202. [Google Scholar] [CrossRef]
- Busija, D.W.; Rutkai, I.; Dutta, S.; Katakam, P.V. Role of Mitochondria in Cerebral Vascular Function: Energy Production, Cellular Protection, and Regulation of Vascular Tone. Compr. Physiol. 2016, 6, 1529–1548. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef]
- Jiang, Q.; Xu, H.; Yan, J.; Xu, Q.; Zheng, Y.; Li, C.; Zhao, L.; Gao, H.; Zheng, H. Sex-specific metabolic alterations in the type 1 diabetic brain of mice revealed by an integrated method of metabolomics and mixed-model. Comput. Struct. Biotechnol. J. 2020, 18, 2063–2074. [Google Scholar] [CrossRef]
- Stenberg, L.; Dahlin, L.B. Gender differences in nerve regeneration after sciatic nerve injury and repair in healthy and in type 2 diabetic Goto-Kakizaki rats. BMC Neurosci. 2014, 15, 107. [Google Scholar] [CrossRef]
- Zeinivand, M.; Nahavandi, A.; Zare, M. Deferoxamine regulates neuroinflammation and oxidative stress in rats with diabetes-induced cognitive dysfunction. Inflammopharmacology 2020, 28, 575–583. [Google Scholar] [CrossRef]
- Dong, L.; Zheng, Y.M.; Van Riper, D.; Rathore, R.; Liu, Q.H.; Singer, H.A.; Wang, Y.X. Functional and molecular evidence for impairment of calcium-activated potassium channels in type-1 diabetic cerebral artery smooth muscle cells. J. Cereb. Blood. Flow. Metab. 2008, 28, 377–386. [Google Scholar] [CrossRef]
- Salinero, A.E.; Robison, L.S.; Gannon, O.J.; Riccio, D.; Mansour, F.; Abi-Ghanem, C.; Zuloaga, K.L. Sex-specific effects of high-fat diet on cognitive impairment in a mouse model of VCID. FASEB J. 2020, 34, 15108–15122. [Google Scholar] [CrossRef]
- Denninger, J.K.; Smith, B.M.; Kirby, E.D. Novel Object Recognition and Object Location Behavioral Testing in Mice on a Budget. J. Vis. Exp. 2018, 141, e58593. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. 2017, 126, 55718. [Google Scholar] [CrossRef]
- Deacon, R.M. Assessing nest building in mice. Nat. Protoc. 2006, 1, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Clayton, D.A.; Shadel, G.S. Isolation of mitochondria from tissue culture cells. Cold Spring Harb. Protoc. 2014, 2014, pdb.prot080002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Zheng, Y.M.; Dong, L.; Ho, Y.S.; Guo, Z.; Wang, Y.X. Role of mitochondrial reactive oxygen species in hypoxia-dependent increase in intracellular calcium in pulmonary artery myocytes. Free Radic. Biol. Med. 2007, 42, 642–653. [Google Scholar] [CrossRef]
- Yang, Z.; Song, T.; Truong, L.; Reyes-García, J.; Wang, L.; Zheng, Y.M.; Wang, Y.X. Important Role of Sarcoplasmic Reticulum Ca. Antioxid. Redox Signal. 2020, 32, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.M.; Wang, Q.S.; Liu, Q.H.; Rathore, R.; Yadav, V.; Wang, Y.X. Heterogeneous gene expression and functional activity of ryanodine receptors in resistance and conduit pulmonary as well as mesenteric artery smooth muscle cells. J. Vasc. Res. 2008, 45, 469–479. [Google Scholar] [CrossRef]
- Korde, A.S.; Yadav, V.R.; Zheng, Y.M.; Wang, Y.X. Primary role of mitochondrial Rieske iron-sulfur protein in hypoxic ROS production in pulmonary artery myocytes. Free Radic. Biol. Med. 2011, 50, 945–952. [Google Scholar] [CrossRef]
- Panov, A.; Dikalov, S.; Shalbuyeva, N.; Taylor, G.; Sherer, T.; Greenamyre, J.T. Rotenone model of Parkinson disease: Multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J. Biol. Chem. 2005, 280, 42026–42035. [Google Scholar] [CrossRef]
- van Jaarsveld, M.T.M.; Deng, D.; Ordoñez-Rueda, D.; Paulsen, M.; Wiemer, E.A.C.; Zi, Z. Cell-type-specific role of CHK2 in mediating DNA damage-induced G2 cell cycle arrest. Oncogenesis 2020, 9, 35. [Google Scholar] [CrossRef]
- Clodfelder-Miller, B.J.; Zmijewska, A.A.; Johnson, G.V.; Jope, R.S. Tau is hyperphosphorylated at multiple sites in mouse brain in vivo after streptozotocin-induced insulin deficiency. Diabetes 2006, 55, 3320–3325. [Google Scholar] [CrossRef] [PubMed]
- Zuloaga, K.L.; Johnson, L.A.; Roese, N.E.; Marzulla, T.; Zhang, W.; Nie, X.; Alkayed, F.N.; Hong, C.; Grafe, M.R.; Pike, M.M.; et al. High fat diet-induced diabetes in mice exacerbates cognitive deficit due to chronic hypoperfusion. J. Cereb. Blood Flow Metab. 2016, 36, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, Y.Y.; Nguyen, P.T.T.; Nam, H.; Suh, J.G. Sex differences in glucose metabolism of streptozotocin-induced diabetes inbred mice (C57BL/6J). Appl. Biol. Chem. 2020, 63, 59. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Le May, C.; Chu, K.; Hu, M.; Ortega, C.S.; Simpson, E.R.; Korach, K.S.; Tsai, M.J.; Mauvais-Jarvis, F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 9232–9237. [Google Scholar] [CrossRef]
- Crane, P.K.; Walker, R.; Hubbard, R.A.; Li, G.; Nathan, D.M.; Zheng, H.; Haneuse, S.; Craft, S.; Montine, T.J.; Kahn, S.E.; et al. Glucose levels and risk of dementia. N. Engl. J. Med. 2013, 369, 540–548. [Google Scholar] [CrossRef]
- Akhter, F.; Persaud, A.; Zaokari, Y.; Zhao, Z.; Zhu, D. Vascular Dementia and Underlying Sex Differences. Front. Aging. Neurosci. 2021, 13, 720715. [Google Scholar] [CrossRef]
- Kim, H.G. Cognitive dysfunctions in individuals with diabetes mellitus. Yeungnam Univ. J. Med. 2019, 36, 183–191. [Google Scholar] [CrossRef]
- Keleher, M.R.; Zaidi, R.; Patel, K.; Ahmed, A.; Bettler, C.; Pavlatos, C.; Shah, S.; Cheverud, J.M. The effect of dietary fat on behavior in mice. J. Diabetes Metab. Disord. 2018, 17, 297–307. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Nest Building Test in Mice for Assessment of General Well-Being. Methods Mol. Biol. 2019, 1916, 87–91. [Google Scholar] [CrossRef]
- Kamada, H.; Yu, F.; Nito, C.; Chan, P.H. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: Relation to blood-brain barrier dysfunction. Stroke 2007, 38, 1044–1049. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Zou, M.H. Activation of AMP-activated protein kinase alleviates high-glucose-induced dysfunction of brain microvascular endothelial cell tight-junction dynamics. Free Radic. Biol. Med. 2012, 53, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Gannon, O.J.; Robison, L.S.; Custozzo, A.J.; Zuloaga, K.L. Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochem. Int. 2019, 127, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Azarova, I.; Polonikov, A.; Klyosova, E. Molecular Genetics of Abnormal Redox Homeostasis in Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 4738. [Google Scholar] [CrossRef] [PubMed]
- Pickering, R.J.; Rosado, C.J.; Sharma, A.; Buksh, S.; Tate, M.; de Haan, J.B. Recent novel approaches to limit oxidative stress and inflammation in diabetic complications. Clin. Transl. Immunol. 2018, 7, e1016. [Google Scholar] [CrossRef]
- Forkink, M.; Basit, F.; Teixeira, J.; Swarts, H.G.; Koopman, W.J.H.; Willems, P.H.G.M. Complex I and complex III inhibition specifically increase cytosolic hydrogen peroxide levels without inducing oxidative stress in HEK293 cells. Redox Biol. 2015, 6, 607–616. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Berchtold, L.A.; Prause, M.; Størling, J.; Mandrup-Poulsen, T. Cytokines and Pancreatic β-Cell Apoptosis. Adv. Clin. Chem. 2016, 75, 99–158. [Google Scholar] [CrossRef]
- Wolff, S.P. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br. Med. Bull. 1993, 49, 642–652. [Google Scholar] [CrossRef]
- Ahmad, W.; Ijaz, B.; Shabbiri, K.; Ahmed, F.; Rehman, S. Oxidative toxicity in diabetes and Alzheimer’s disease: Mechanisms behind ROS/RNS generation. J. Biomed. Sci. 2017, 24, 76. [Google Scholar] [CrossRef]
- Rettberg, J.R.; Yao, J.; Brinton, R.D. Estrogen: A master regulator of bioenergetic systems in the brain and body. Front. Neuroendocrinol. 2014, 35, 8–30. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, C.; Piaggi, S.; Federico, G.; Scarpato, R. High levels of γ-H2AX foci and cell membrane oxidation in adolescents with type 1 diabetes. Mutat. Res. 2014, 770, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhanan, J.; Suresh, G.; Devanathan, V. Neurodegeneration in type 2 diabetes: Alzheimer’s as a case study. Brain. Behav. 2020, 10, e01577. [Google Scholar] [CrossRef]
- Guo, L.; Tian, J.; Du, H. Mitochondrial Dysfunction and Synaptic Transmission Failure in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1071–1086. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lin, Y.H.; Hsieh, H.H.; Lin, J.J.; Peng, S.L. Sex Differences in the Effect of Diabetes on Cerebral Glucose Metabolism. Biomedicines 2021, 9, 1661. [Google Scholar] [CrossRef]
- Tiano, J.P.; Delghingaro-Augusto, V.; Le May, C.; Liu, S.; Kaw, M.K.; Khuder, S.S.; Latour, M.G.; Bhatt, S.A.; Korach, K.S.; Najjar, S.M.; et al. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents β cell failure in rodent models of type 2 diabetes. J. Clin. Investig. 2011, 121, 3331–3342. [Google Scholar] [CrossRef]
- Crespo-Castrillo, A.; Yanguas-Casás, N.; Arevalo, M.A.; Azcoitia, I.; Barreto, G.E.; Garcia-Segura, L.M. The Synthetic Steroid Tibolone Decreases Reactive Gliosis and Neuronal Death in the Cerebral Cortex of Female Mice After a Stab Wound Injury. Mol. Neurobiol. 2018, 55, 8651–8667. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, E.W.; Khatoon, S.; Zheng, Y.-M.; Wang, Y.-X. Mitochondrial Reactive Oxygen Species Production in Vascular Dementia Following Experimental Diabetes. Cells 2025, 14, 1260. https://doi.org/10.3390/cells14161260
Santos EW, Khatoon S, Zheng Y-M, Wang Y-X. Mitochondrial Reactive Oxygen Species Production in Vascular Dementia Following Experimental Diabetes. Cells. 2025; 14(16):1260. https://doi.org/10.3390/cells14161260
Chicago/Turabian StyleSantos, Ed Wilson, Subika Khatoon, Yun-Min Zheng, and Yong-Xiao Wang. 2025. "Mitochondrial Reactive Oxygen Species Production in Vascular Dementia Following Experimental Diabetes" Cells 14, no. 16: 1260. https://doi.org/10.3390/cells14161260
APA StyleSantos, E. W., Khatoon, S., Zheng, Y.-M., & Wang, Y.-X. (2025). Mitochondrial Reactive Oxygen Species Production in Vascular Dementia Following Experimental Diabetes. Cells, 14(16), 1260. https://doi.org/10.3390/cells14161260






