Lipoxins as Modulators of Diseases
Abstract
1. Introduction
2. Biosynthesis of Lipoxins
3. LX Receptors
4. Synthetic LX Modulators
5. LXs and Diseases
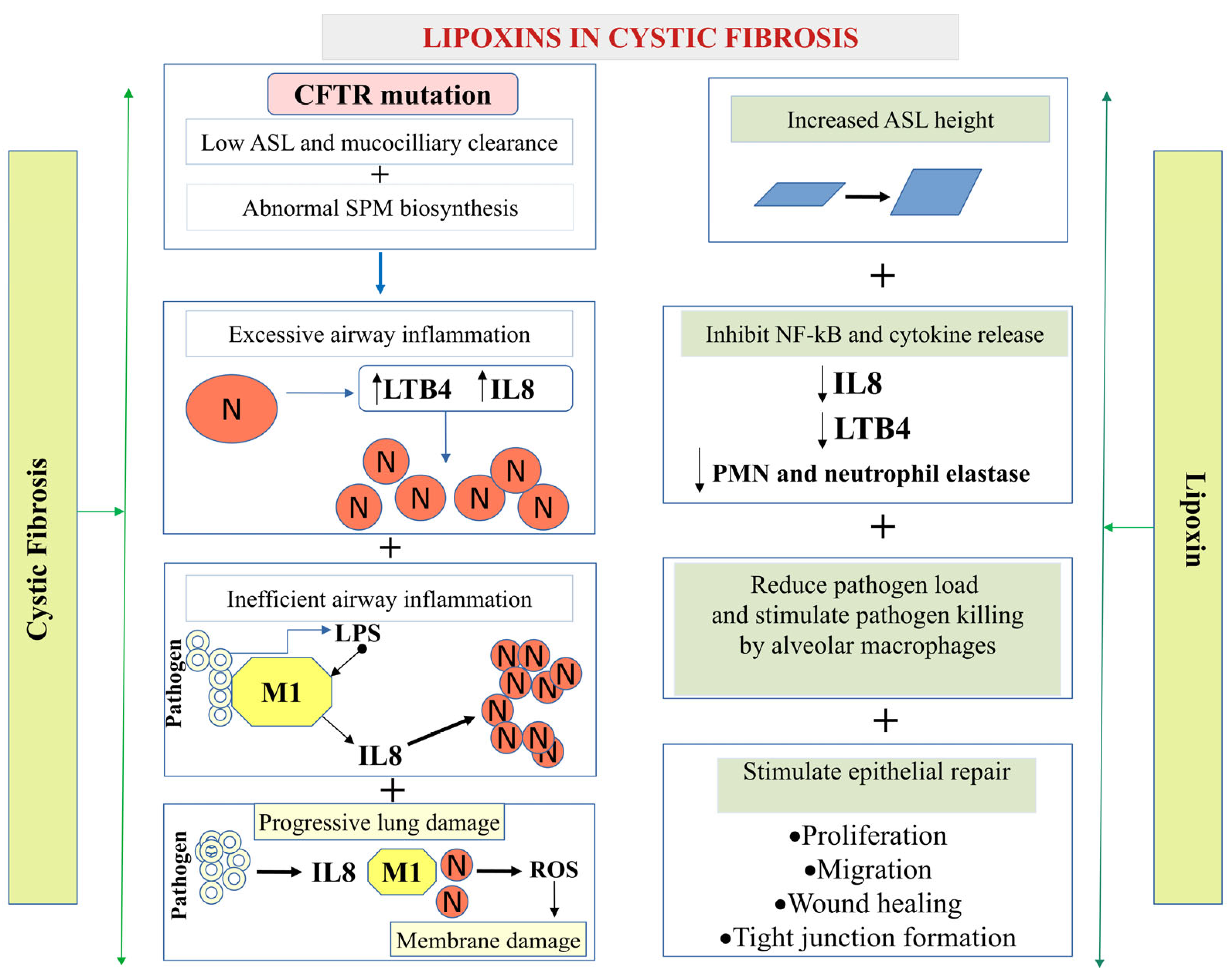
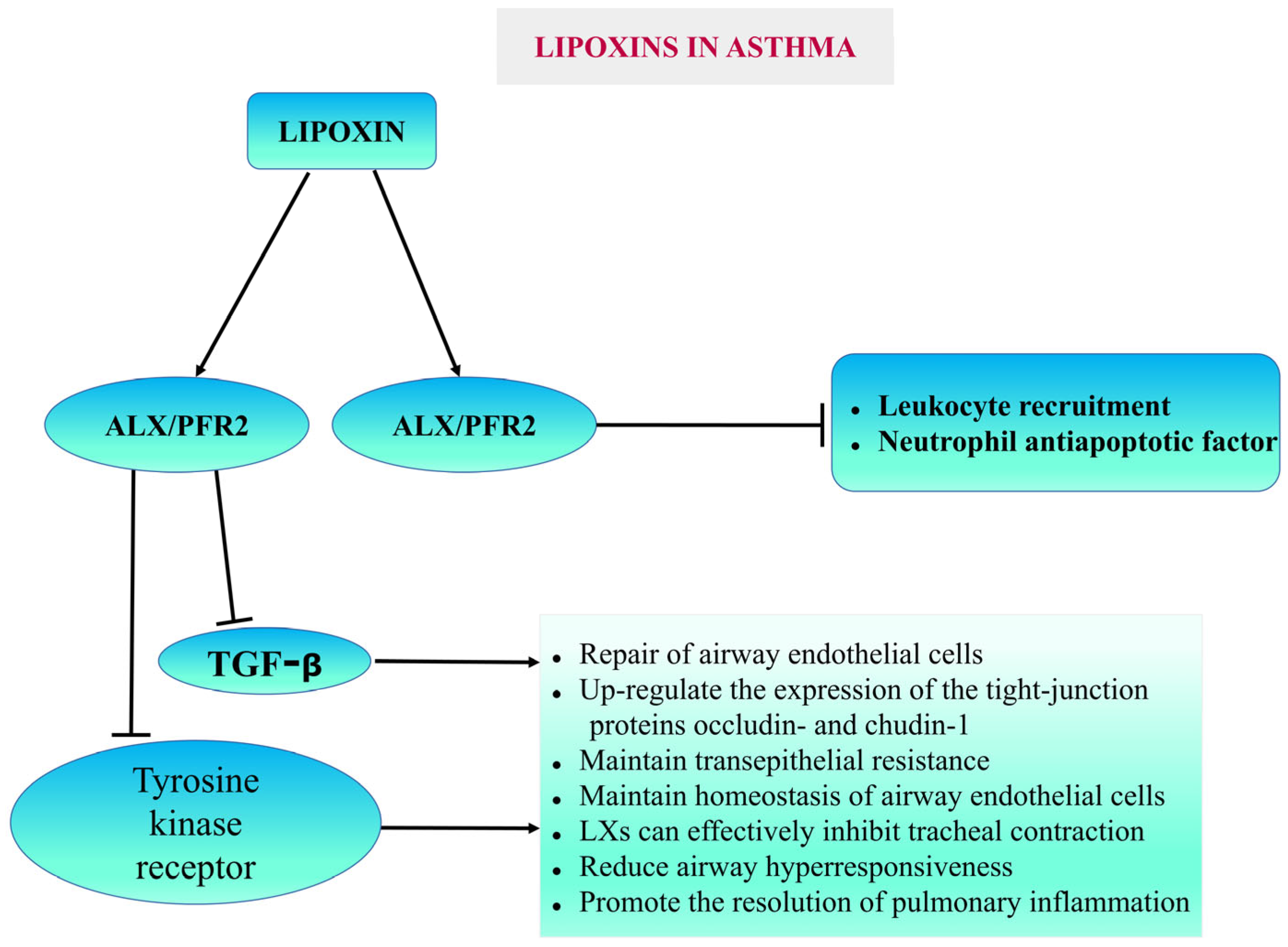
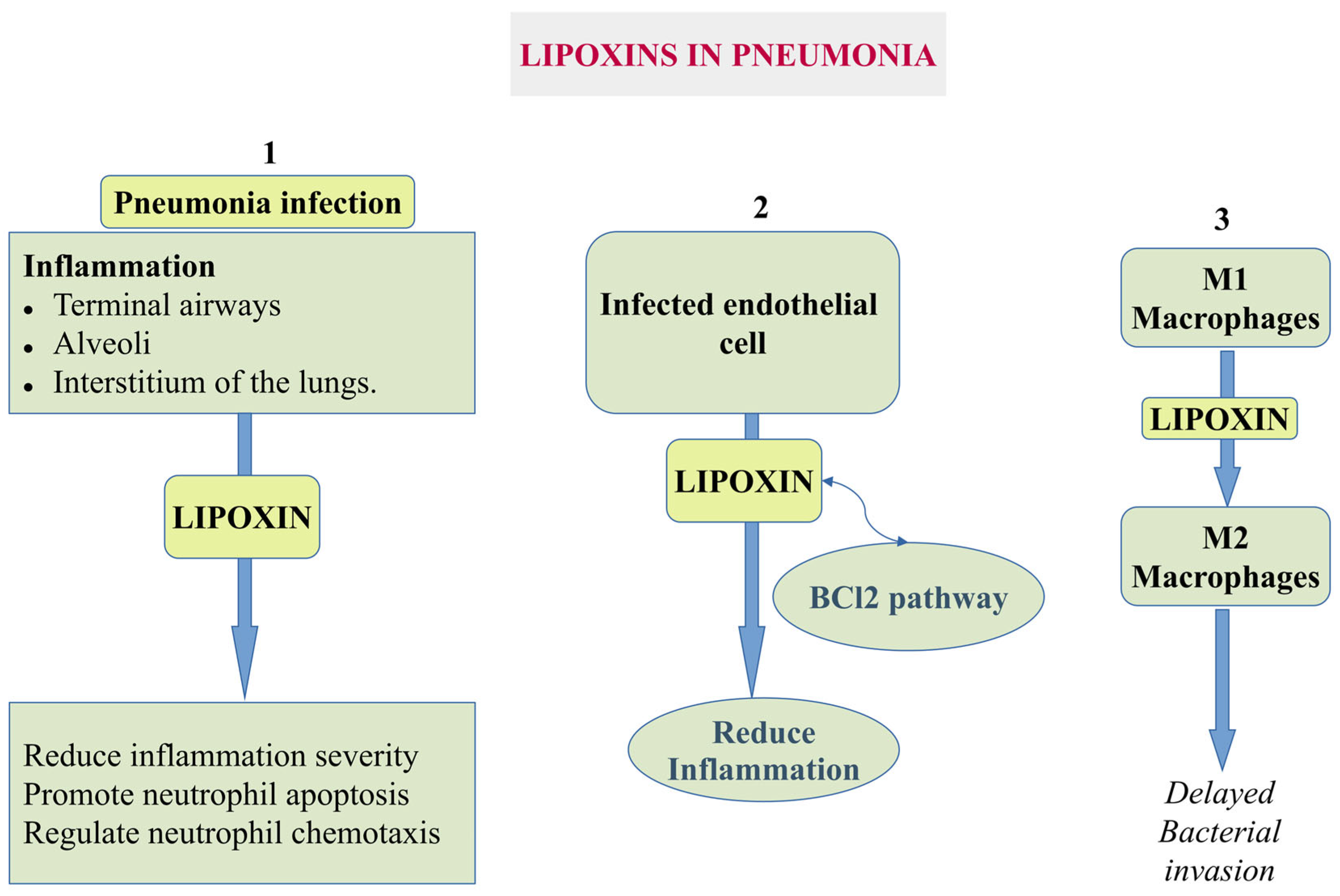
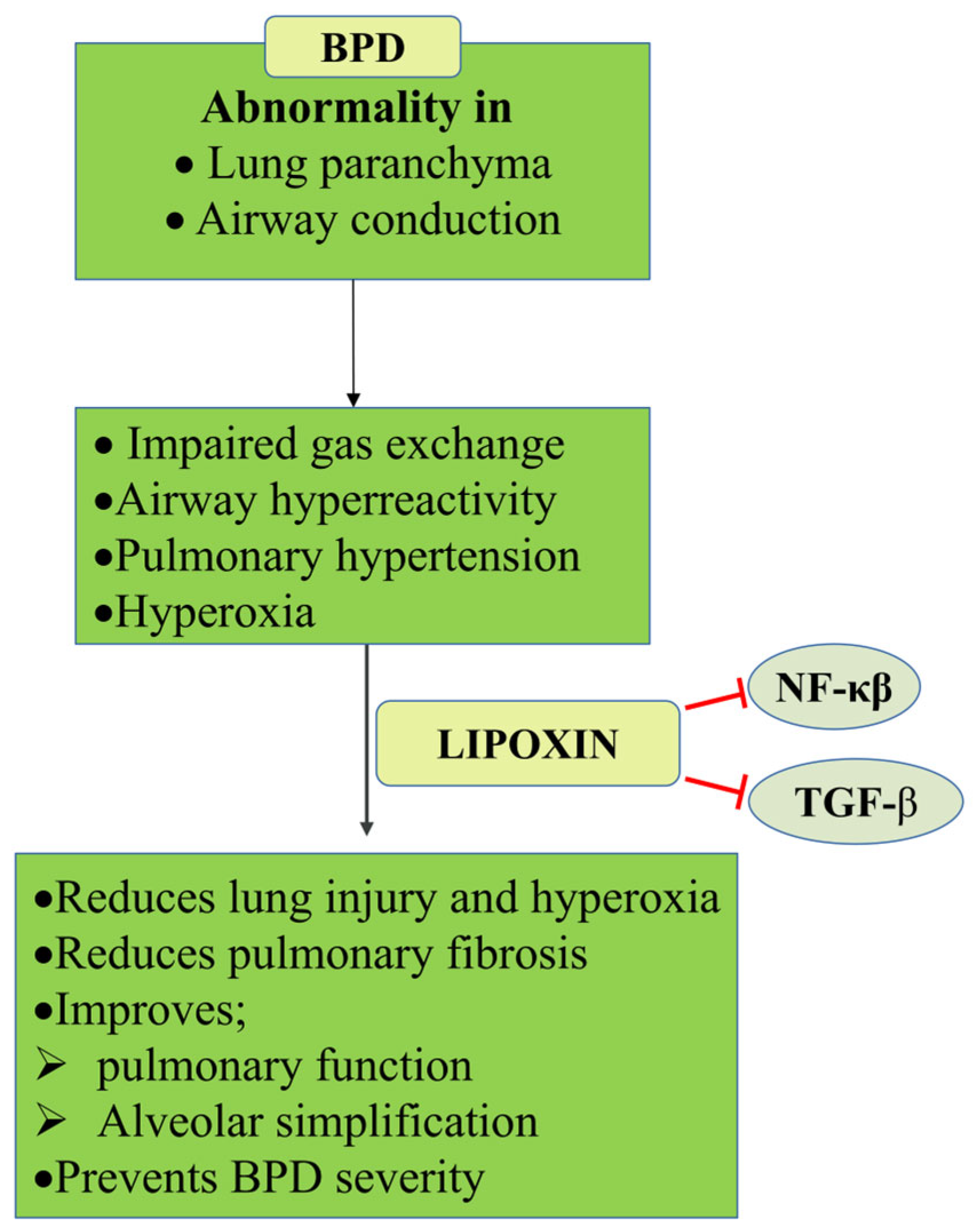
5.1. Lung Diseases
5.1.1. Cystic Fibrosis (CF)
5.1.2. Asthma
5.1.3. Pneumonia
5.1.4. Acute Lung Injury (ALI) and Malaria
5.1.5. Bronchopulmonary Dysplasia (BPD)
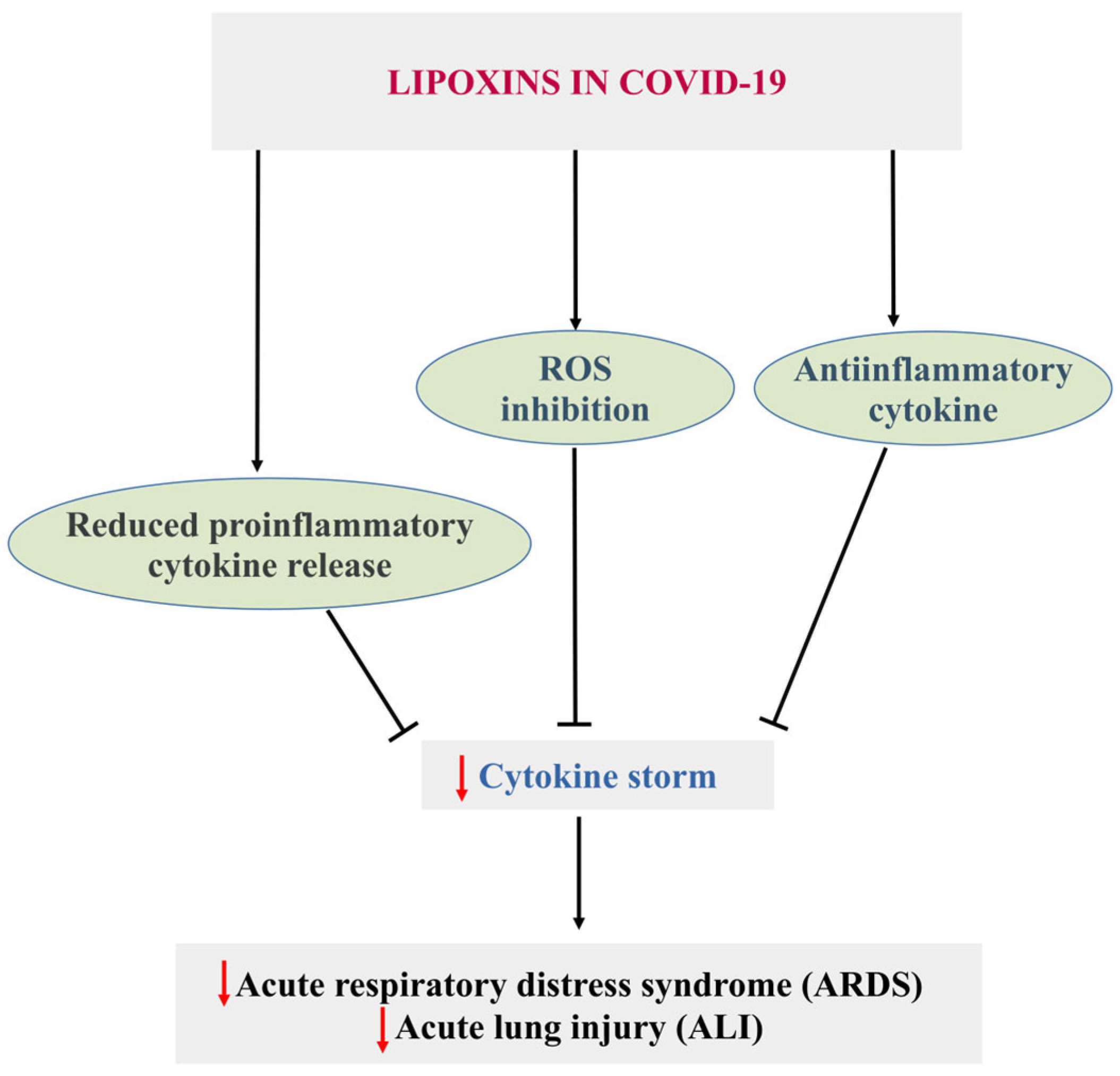
5.2. COVID-19
5.3. Cardiovascular Diseases
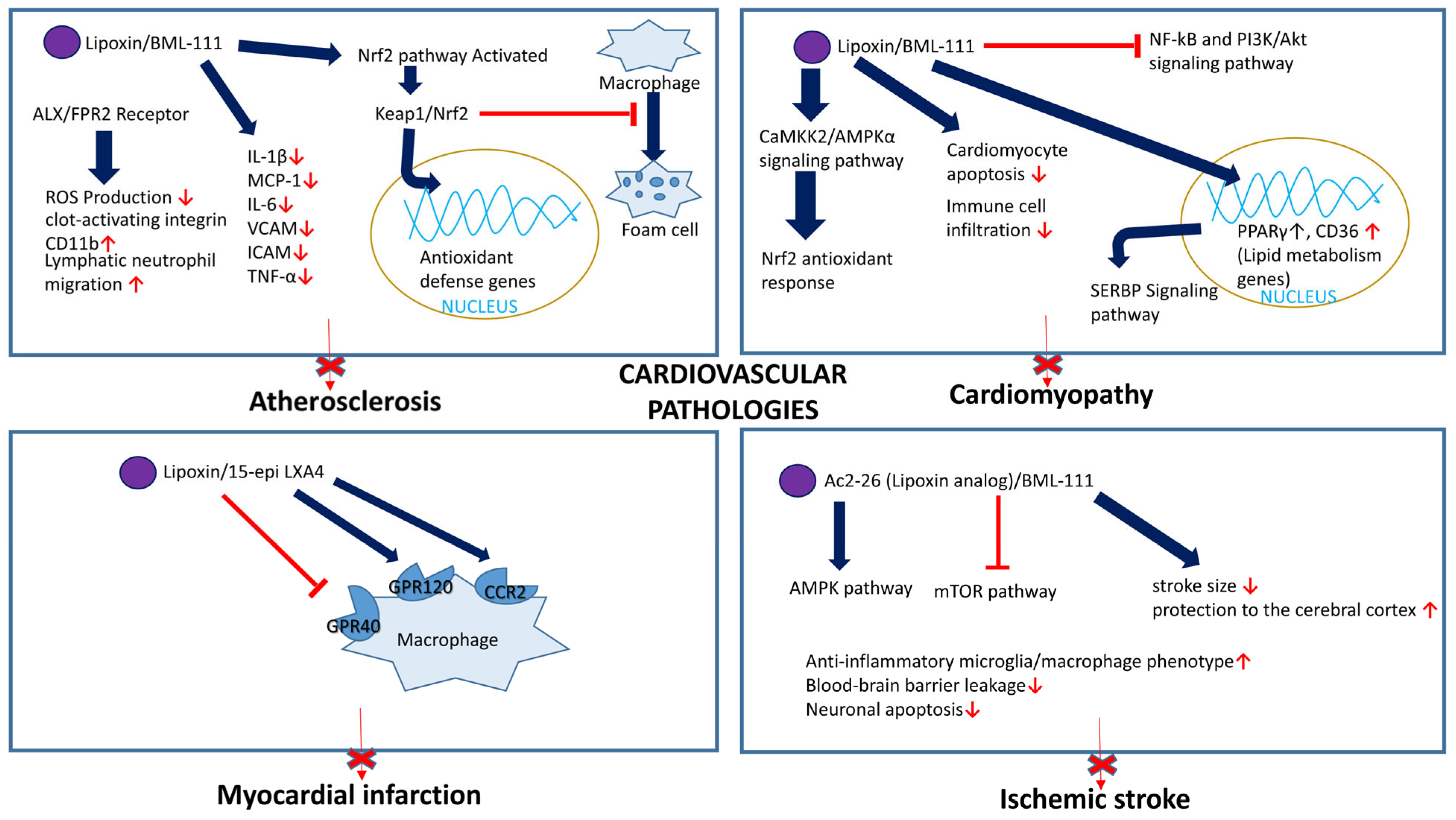
5.3.1. Atherosclerosis
5.3.2. Abdominal Aortic Aneurysm
5.3.3. Cardiomyopathy
5.3.4. Myocardial Infarction
5.3.5. Other Thrombotic Vascular Disorder
5.4. Microbial Infection
5.4.1. Staphylococcus aureus
5.4.2. Klebsiella pneumoniae
5.4.3. Borrelia burgdorferi
5.4.4. Pseudomonas aeruginosa
5.4.5. Cryptococcus neoformans
5.4.6. Toxoplasma gondii
5.4.7. Trypanosoma cruzi
5.4.8. Porphyromonas gingivalis
5.5. LXs in Sepsis
5.6. Stem Cells
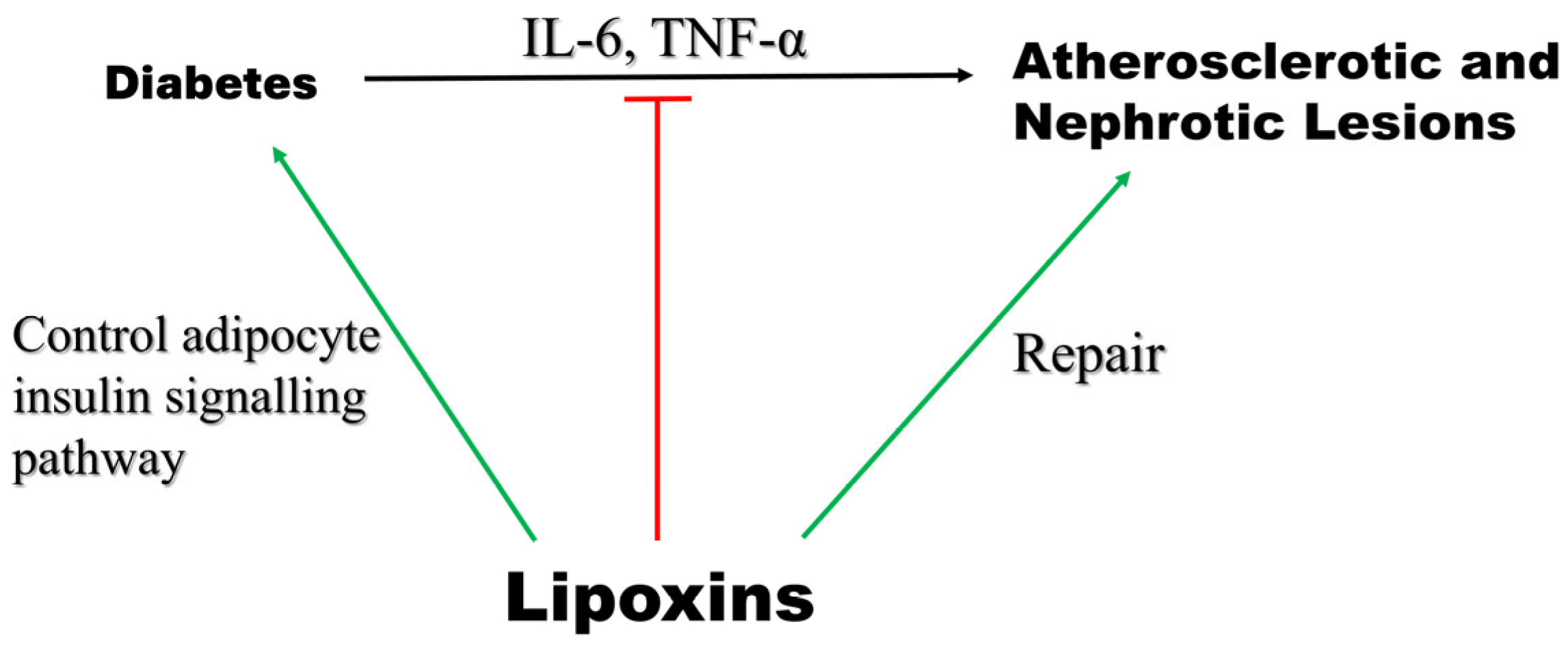
5.7. Diabetes
5.8. Neurological Disorders
5.8.1. Alzheimer’s Disease
5.8.2. Multiple Sclerosis (MS)
5.8.3. Ischemic Stroke
5.8.4. Hemorrhagic Stroke
5.8.5. Spinal Cord Injury (SCI)
5.8.6. Neonatal Hypoxia–Ischemia Encephalopathy
5.8.7. Traumatic Brain Injury
5.8.8. Neuropathic Pain
5.9. Cancer
5.9.1. Pancreatic Cancer
5.9.2. Kaposi’s Sarcoma (KS)
5.9.3. Colorectal Cancer
5.9.4. Prostate Cancer
5.9.5. Breast Cancer
5.9.6. Aspirin as a Potential Cancer Therapy
5.10. Endometriosis
5.11. Clinical Trails Related to LXs
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| Akt | Protein kinase B |
| ALXR | Lipoxin A4 receptor |
| ANX-A1 | Annexin A1 |
| ATL | Aspirin triggered lipoxin |
| BCL2 | The B-cell lymphoma-2 |
| BBB | Brain-blood barrier |
| BPD | Bronchopulmonary dysplasia |
| CF | Cystic fibrosis |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| COVID-19 | Coronavirus disease 2019 |
| FPR | Formyl peptide receptor |
| GC | Glucocorticoids |
| IL-1β | Interleukin 1b |
| IL-6 | Interleukin 6 |
| KS | Kaposi’s sarcoma |
| LOX | Lipoxygenase |
| LTs | leukotrienes (LTs) |
| LTA4 | Leukotriene A4 |
| LTB4 | Leukotriene B4 |
| LX | Lipoxin |
| LXA4 | Lipoxin A4 |
| LXB4 | Lipoxin B4 |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3-kinase |
| PKC | Protein kinase C |
| PGDH | Prostaglandin dehydrogenase |
| SARS | Severe acute respiratory syndrome |
| SPMs | Specialized pro-resolving mediators |
| TGF-β | Transforming growth factor beta |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor alpha |
| VEGFR-2 | Vascular endothelial growth factor receptor 2 |
References
- Serhan, C.N.; Hamberg, M.; Samuelsson, B. Trihydroxytetraenes: A novel series of compounds formed from arachidonic acid in human leukocytes. Biochem. Biophys. Res. Commun. 1984, 118, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Hamberg, M.; Samuelsson, B. Lipoxins: Novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc. Natl. Acad. Sci. USA 1984, 81, 5335–5339. [Google Scholar] [CrossRef]
- Romano, M.; Cianci, E.; Simiele, F.; Recchiuti, A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur. J. Pharmacol. 2015, 760, 49–63. [Google Scholar] [CrossRef]
- Sharma, N.P.; Dong, L.; Yuan, C.; Noon, K.R.; Smith, W.L. Asymmetric acetylation of the cyclooxygenase-2 homodimer by aspirin and its effects on the oxygenation of arachidonic, eicosapentaenoic, and docosahexaenoic acids. Mol. Pharmacol. 2010, 77, 979–986. [Google Scholar] [CrossRef]
- Mulugeta, S.; Suzuki, T.; Hernandez, N.T.; Griesser, M.; Boeglin, W.E.; Schneider, C. Identification and absolute configuration of dihydroxy-arachidonic acids formed by oxygenation of 5S-HETE by native and aspirin-acetylated COX-2. J. Lipid Res. 2010, 51, 575–585. [Google Scholar] [CrossRef]
- Chandrasekharan, J.A.; Sharma-Walia, N. Lipoxins: Nature’s way to resolve inflammation. J. Inflamm. Res. 2015, 8, 181–192. [Google Scholar] [CrossRef]
- Prieto, P.; Cuenca, J.; Traves, P.G.; Fernandez-Velasco, M.; Martin-Sanz, P.; Bosca, L. Lipoxin A4 impairment of apoptotic signaling in macrophages: Implication of the PI3K/Akt and the ERK/Nrf-2 defense pathways. Cell Death Differ. 2010, 17, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Godson, C.; Mitchell, S.; Harvey, K.; Petasis, N.A.; Hogg, N.; Brady, H.R. Cutting edge: Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 2000, 164, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, A.; Tolar, M.; Peters, O.A. Lipoxin A(4) Attenuates the Inflammatory Response in Stem Cells of the Apical Papilla via ALX/FPR2. Sci. Rep. 2018, 8, 8921. [Google Scholar] [CrossRef]
- Ramon, S.; Bancos, S.; Serhan, C.N.; Phipps, R.P. Lipoxin A(4) modulates adaptive immunity by decreasing memory B-cell responses via an ALX/FPR2-dependent mechanism. Eur. J. Immunol. 2014, 44, 357–369. [Google Scholar] [CrossRef]
- Fu, T.; Mohan, M.; Brennan, E.P.; Woodman, O.L.; Godson, C.; Kantharidis, P.; Ritchie, R.H.; Qin, C.X. Therapeutic Potential of Lipoxin A(4) in Chronic Inflammation: Focus on Cardiometabolic Disease. ACS Pharmacol. Transl. Sci. 2020, 3, 43–55. [Google Scholar] [CrossRef]
- Eltay, E.G.; Van Dyke, T. Resolution of inflammation in oral diseases. Pharmacol. Ther. 2023, 247, 108453. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Falcato, F.; Bandarra, N.; Rauter, A.P. Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation. Molecules 2022, 27, 1677. [Google Scholar] [CrossRef] [PubMed]
- Fiore, S.; Serhan, C.N. Formation of lipoxins and leukotrienes during receptor-mediated interactions of human platelets and recombinant human granulocyte/macrophage colony-stimulating factor-primed neutrophils. J. Exp. Med. 1990, 172, 1451–1457. [Google Scholar] [CrossRef]
- Serhan, C.N. Lipoxins and novel aspirin-triggered 15-epi-lipoxins (ATL): A jungle of cell-cell interactions or a therapeutic opportunity? Prostaglandins 1997, 53, 107–137. [Google Scholar] [CrossRef]
- Claria, J.; Serhan, C.N. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc. Natl. Acad. Sci. USA 1995, 92, 9475–9479. [Google Scholar] [CrossRef]
- Folco, G.; Murphy, R.C. Eicosanoid transcellular biosynthesis: From cell-cell interactions to in vivo tissue responses. Pharmacol. Rev. 2006, 58, 375–388. [Google Scholar] [CrossRef]
- Serhan, C.N.; Maddox, J.F.; Petasis, N.A.; Akritopoulou-Zanze, I.; Papayianni, A.; Brady, H.R.; Colgan, S.P.; Madara, J.L. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry 1995, 34, 14609–14615. [Google Scholar] [CrossRef]
- Samuelsson, B.; Dahlen, S.E.; Lindgren, J.A.; Rouzer, C.A.; Serhan, C.N. Leukotrienes and lipoxins: Structures, biosynthesis, and biological effects. Science 1987, 237, 1171–1176. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D.; Clish, C.B.; Gronert, K.; Chiang, N. Lipoxins, aspirin-triggered 15-epi-lipoxin stable analogs and their receptors in anti-inflammation: A window for therapeutic opportunity. In Advances in Eicosanoid Research; Ernst Schering Research Foundation Workshop; Springer: Berlin/Heidelberg, Germany, 2000; pp. 143–185. [Google Scholar] [CrossRef]
- Edenius, C.; Heidvall, K.; Lindgren, J.A. Novel transcellular interaction: Conversion of granulocyte-derived leukotriene A4 to cysteinyl-containing leukotrienes by human platelets. Eur. J. Biochem. 1988, 178, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.X.; Norling, L.V.; Vecchio, E.A.; Brennan, E.P.; May, L.T.; Wootten, D.; Godson, C.; Perretti, M.; Ritchie, R.H. Formylpeptide receptor 2: Nomenclature, structure, signalling and translational perspectives: IUPHAR review 35. Br. J. Pharmacol. 2022, 179, 4617–4639. [Google Scholar] [CrossRef] [PubMed]
- Cooray, S.N.; Gobbetti, T.; Montero-Melendez, T.; McArthur, S.; Thompson, D.; Clark, A.J.; Flower, R.J.; Perretti, M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc. Natl. Acad. Sci. USA 2013, 110, 18232–18237. [Google Scholar] [CrossRef] [PubMed]
- Tylek, K.; Trojan, E.; Regulska, M.; Lacivita, E.; Leopoldo, M.; Basta-Kaim, A. Formyl peptide receptor 2, as an important target for ligands triggering the inflammatory response regulation: A link to brain pathology. Pharmacol. Rep. 2021, 73, 1004–1019. [Google Scholar] [CrossRef]
- Schaldach, C.M.; Riby, J.; Bjeldanes, L.F. Lipoxin A4: A new class of ligand for the Ah receptor. Biochemistry 1999, 38, 7594–7600. [Google Scholar] [CrossRef]
- Sanchez-Garcia, S.; Jaen, R.I.; Fernandez-Velasco, M.; Delgado, C.; Bosca, L.; Prieto, P. Lipoxin-mediated signaling: ALX/FPR2 interaction and beyond. Pharmacol. Res. 2023, 197, 106982. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Fan, M.; Jin, W. Lipoxins in the Nervous System: Brighter Prospects for Neuroprotection. Front. Pharmacol. 2022, 13, 781889. [Google Scholar] [CrossRef]
- Wu, J.; Ding, D.H.; Li, Q.Q.; Wang, X.Y.; Sun, Y.Y.; Li, L.J. Lipoxin A4 Regulates Lipopolysaccharide-Induced BV2 Microglial Activation and Differentiation via the Notch Signaling Pathway. Front. Cell. Neurosci. 2019, 13, 19. [Google Scholar] [CrossRef]
- Wang, Y.P.; Wu, Y.; Li, L.Y.; Zheng, J.; Liu, R.G.; Zhou, J.P.; Yuan, S.Y.; Shang, Y.; Yao, S.L. Aspirin-triggered lipoxin A4 attenuates LPS-induced pro-inflammatory responses by inhibiting activation of NF-kappaB and MAPKs in BV-2 microglial cells. J. Neuroinflamm. 2011, 8, 95. [Google Scholar] [CrossRef]
- Maderna, P.; Cottell, D.C.; Toivonen, T.; Dufton, N.; Dalli, J.; Perretti, M.; Godson, C. FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J. 2010, 24, 4240–4249. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Topete, D.; Cidlowski, J.A. One hormone, two actions: Anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation 2015, 22, 20–32. [Google Scholar] [CrossRef]
- Hashimoto, A.; Murakami, Y.; Kitasato, H.; Hayashi, I.; Endo, H. Glucocorticoids co-interact with lipoxin A4 via lipoxin A4 receptor (ALX) up-regulation. Biomed. Pharmacother. 2007, 61, 81–85. [Google Scholar] [CrossRef]
- Qin, C.; Yang, Y.H.; May, L.; Gao, X.; Stewart, A.G.; Tu, Y.; Woodman, O.L.; Ritchie, R.H. Cardioprotective potential of annexin-A1 mimetics in myocardial infarction. Pharmacol. Ther. 2015, 148, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Colby, J.K.; Gott, K.M.; Wilder, J.A.; Levy, B.D. Lipoxin Signaling in Murine Lung Host Responses to Cryptococcus neoformans Infection. Am. J. Respir. Cell Mol. Biol. 2016, 54, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.D.; Blomgran, R.; Bergstrom, I.; Sotak, M.; Clark, M.; Rani, A.; Rajan, M.R.; Dalli, J.; Nystrom, S.; Quiding-Jarbrink, M.; et al. Lipoxins modulate neutrophil oxidative burst, integrin expression and lymphatic transmigration differentially in human health and atherosclerosis. FASEB J. 2022, 36, e22173. [Google Scholar] [CrossRef]
- Petri, M.H.; Laguna-Fernandez, A.; Gonzalez-Diez, M.; Paulsson-Berne, G.; Hansson, G.K.; Back, M. The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovasc. Res. 2015, 105, 65–74. [Google Scholar] [CrossRef]
- Peng, C.; Vecchio, E.A.; Nguyen, A.T.N.; De Seram, M.; Tang, R.; Keov, P.; Woodman, O.L.; Chen, Y.C.; Baell, J.; May, L.T.; et al. Biased receptor signalling and intracellular trafficking profiles of structurally distinct formylpeptide receptor 2 agonists. Br. J. Pharmacol. 2024, 181, 4677–4692. [Google Scholar] [CrossRef]
- Dufton, N.; Hannon, R.; Brancaleone, V.; Dalli, J.; Patel, H.B.; Gray, M.; D’Acquisto, F.; Buckingham, J.C.; Perretti, M.; Flower, R.J. Anti-inflammatory role of the murine formyl-peptide receptor 2: Ligand-specific effects on leukocyte responses and experimental inflammation. J. Immunol. 2010, 184, 2611–2619. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol. Asp. Med. 2017, 58, 114–129. [Google Scholar] [CrossRef]
- Sordi, R.; Menezes-de-Lima, O., Jr.; Horewicz, V.; Scheschowitsch, K.; Santos, L.F.; Assreuy, J. Dual role of lipoxin A4 in pneumosepsis pathogenesis. Int. Immunopharmacol. 2013, 17, 283–292. [Google Scholar] [CrossRef]
- Gilroy, D.W. The role of aspirin-triggered lipoxins in the mechanism of action of aspirin. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 203–210. [Google Scholar] [CrossRef]
- Koudelka, A.; Buchan, G.J.; Cechova, V.; O’Brien, J.P.; Stevenson, E.R.; Uvalle, C.E.; Liu, H.; Woodcock, S.R.; Mullett, S.J.; Zhang, C.; et al. Lipoxin A(4) yields an electrophilic 15-oxo metabolite that mediates FPR2 receptor-independent anti-inflammatory signaling. J. Lipid Res. 2025, 66, 100705. [Google Scholar] [CrossRef]
- Fierro, I.M.; Colgan, S.P.; Bernasconi, G.; Petasis, N.A.; Clish, C.B.; Arita, M.; Serhan, C.N. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit human neutrophil migration: Comparisons between synthetic 15 epimers in chemotaxis and transmigration with microvessel endothelial cells and epithelial cells. J. Immunol. 2003, 170, 2688–2694. [Google Scholar] [CrossRef]
- Andrews, D.; Godson, C. Lipoxins and synthetic lipoxin mimetics: Therapeutic potential in renal diseases. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158940. [Google Scholar] [CrossRef]
- de Gaetano, M.; Butler, E.; Gahan, K.; Zanetti, A.; Marai, M.; Chen, J.; Cacace, A.; Hams, E.; Maingot, C.; McLoughlin, A.; et al. Asymmetric synthesis and biological evaluation of imidazole- and oxazole-containing synthetic lipoxin A(4) mimetics (sLXms). Eur. J. Med. Chem. 2019, 162, 80–108. [Google Scholar] [CrossRef]
- Maddox, J.F.; Colgan, S.P.; Clish, C.B.; Petasis, N.A.; Fokin, V.V.; Serhan, C.N. Lipoxin B4 regulates human monocyte/neutrophil adherence and motility: Design of stable lipoxin B4 analogs with increased biologic activity. FASEB J. 1998, 12, 487–494. [Google Scholar] [CrossRef]
- Takano, T.; Fiore, S.; Maddox, J.F.; Brady, H.R.; Petasis, N.A.; Serhan, C.N. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: Evidence for anti-inflammatory receptors. J. Exp. Med. 1997, 185, 1693–1704. [Google Scholar] [CrossRef]
- Hachicha, M.; Pouliot, M.; Petasis, N.A.; Serhan, C.N. Lipoxin (LX)A4 and aspirin-triggered 15-epi-LXA4 inhibit tumor necrosis factor 1alpha-initiated neutrophil responses and trafficking: Regulators of a cytokine-chemokine axis. J. Exp. Med. 1999, 189, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Kieran, N.E.; Doran, P.P.; Connolly, S.B.; Greenan, M.C.; Higgins, D.F.; Leonard, M.; Godson, C.; Taylor, C.T.; Henger, A.; Kretzler, M.; et al. Modification of the transcriptomic response to renal ischemia/reperfusion injury by lipoxin analog. Kidney Int. 2003, 64, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Clish, C.B.; O’Brien, J.A.; Gronert, K.; Stahl, G.L.; Petasis, N.A.; Serhan, C.N. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc. Natl. Acad. Sci. USA 1999, 96, 8247–8252. [Google Scholar] [CrossRef]
- Bannenberg, G.; Moussignac, R.L.; Gronert, K.; Devchand, P.R.; Schmidt, B.A.; Guilford, W.J.; Bauman, J.G.; Subramanyam, B.; Perez, H.D.; Parkinson, J.F.; et al. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br. J. Pharmacol. 2004, 143, 43–52. [Google Scholar] [CrossRef]
- Sun, Y.P.; Tjonahen, E.; Keledjian, R.; Zhu, M.; Yang, R.; Recchiuti, A.; Pillai, P.S.; Petasis, N.A.; Serhan, C.N. Anti-inflammatory and pro-resolving properties of benzo-lipoxin A(4) analogs. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 357–366. [Google Scholar] [CrossRef]
- Brennan, E.P.; Mohan, M.; McClelland, A.; Tikellis, C.; Ziemann, M.; Kaspi, A.; Gray, S.P.; Pickering, R.; Tan, S.M.; Ali-Shah, S.T.; et al. Lipoxins Regulate the Early Growth Response-1 Network and Reverse Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 1437–1448. [Google Scholar] [CrossRef]
- Brennan, E.P.; Mohan, M.; McClelland, A.; de Gaetano, M.; Tikellis, C.; Marai, M.; Crean, D.; Dai, A.; Beuscart, O.; Derouiche, S.; et al. Lipoxins Protect Against Inflammation in Diabetes-Associated Atherosclerosis. Diabetes 2018, 67, 2657–2667. [Google Scholar] [CrossRef]
- Levy, B.D.; Bonnans, C.; Silverman, E.S.; Palmer, L.J.; Marigowda, G.; Israel, E.; Severe Asthma Research Program, N.H.L.; Blood, I. Diminished lipoxin biosynthesis in severe asthma. Am. J. Respir. Crit. Care Med. 2005, 172, 824–830. [Google Scholar] [CrossRef]
- Wang, S.; Qian, X.; Shen, C.; Sun, Q.; Jing, Y.; Liu, B.; Zhang, K.; Li, M.; Wang, J.; Zhou, H.; et al. The protective effects of lipoxin A4 on type 2 diabetes mellitus: A Chinese prospective cohort study. Front Endocrinol 2023, 14, 1109747. [Google Scholar] [CrossRef] [PubMed]
- Borgeson, E.; Johnson, A.M.; Lee, Y.S.; Till, A.; Syed, G.H.; Ali-Shah, S.T.; Guiry, P.J.; Dalli, J.; Colas, R.A.; Serhan, C.N.; et al. Lipoxin A4 Attenuates Obesity-Induced Adipose Inflammation and Associated Liver and Kidney Disease. Cell Metab. 2015, 22, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E. Pro-resolving mediators in the regulation of periodontal disease. Mol. Aspects Med. 2017, 58, 21–36. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, F.; Lin, F.; Xiang, H.; Wang, N.; Ye, D.; Huang, Y. Preeclampsia is associated with a deficiency of lipoxin A4, an endogenous anti-inflammatory mediator. Fertil. Steril. 2014, 102, 282–290.e284. [Google Scholar] [CrossRef]
- Lin, F.; Zeng, P.; Xu, Z.; Ye, D.; Yu, X.; Wang, N.; Tang, J.; Zhou, Y.; Huang, Y. Treatment of Lipoxin A(4) and its analogue on low-dose endotoxin induced preeclampsia in rat and possible mechanisms. Reprod. Toxicol. 2012, 34, 677–685. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Pham, T.L.; Kakazu, A.H.; Ponnath, A.; Do, K.V.; Bazan, H.E.P. Lipoxin A4 (LXA4) Reduces Alkali-Induced Corneal Inflammation and Neovascularization and Upregulates a Repair Transcriptome. Biomolecules 2023, 13, 831. [Google Scholar] [CrossRef] [PubMed]
- Conte, F.P.; Menezes-de-Lima, O., Jr.; Verri, W.A., Jr.; Cunha, F.Q.; Penido, C.; Henriques, M.G. Lipoxin A(4) attenuates zymosan-induced arthritis by modulating endothelin-1 and its effects. Br. J. Pharmacol. 2010, 161, 911–924. [Google Scholar] [CrossRef]
- Saraiva-Santos, T.; Zaninelli, T.H.; Manchope, M.F.; Andrade, K.C.; Ferraz, C.R.; Bertozzi, M.M.; Artero, N.A.; Franciosi, A.; Badaro-Garcia, S.; Staurengo-Ferrari, L.; et al. Therapeutic activity of lipoxin A(4) in TiO(2)-induced arthritis in mice: NF-kappaB and Nrf2 in synovial fluid leukocytes and neuronal TRPV1 mechanisms. Front. Immunol. 2023, 14, 949407. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, J.; Huang, W.; Xu, Q.; Ye, D.; Sun, R.; Zhang, D. Colorectal Cancer Is Associated with a Deficiency of Lipoxin A(4), an Endogenous Anti-inflammatory Mediator. J. Cancer 2019, 10, 4719–4730. [Google Scholar] [CrossRef]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.L.; et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef]
- Cantin, A.M.; Hartl, D.; Konstan, M.W.; Chmiel, J.F. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros. 2015, 14, 419–430. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Fanning, A.S.; Bridges, A.; Anderson, J.M. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell 2009, 20, 3930–3940. [Google Scholar] [CrossRef]
- Higgins, G.; Fustero Torre, C.; Tyrrell, J.; McNally, P.; Harvey, B.J.; Urbach, V. Lipoxin A4 prevents tight junction disruption and delays the colonization of cystic fibrosis bronchial epithelial cells by Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L1053–L1061. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, P.J.; McNally, P.; Harvey, B.J.; Urbach, V. Lipoxin A(4)-mediated KATP potassium channel activation results in cystic fibrosis airway epithelial repair. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L193–L201. [Google Scholar] [CrossRef]
- Higgins, G.; Buchanan, P.; Perriere, M.; Al-Alawi, M.; Costello, R.W.; Verriere, V.; McNally, P.; Harvey, B.J.; Urbach, V. Activation of P2RY11 and ATP release by lipoxin A4 restores the airway surface liquid layer and epithelial repair in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2014, 51, 178–190. [Google Scholar] [CrossRef] [PubMed]
- El Kebir, D.; Filep, J.G. Role of neutrophil apoptosis in the resolution of inflammation. Sci. World J. 2010, 10, 1731–1748. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Gras, D.; Chavis, C.; Mainprice, B.; Vachier, I.; Godard, P.; Chanez, P. Synthesis and anti-inflammatory effect of lipoxins in human airway epithelial cells. Biomed. Pharmacother. 2007, 61, 261–267. [Google Scholar] [CrossRef]
- Bonnans, C.; Mainprice, B.; Chanez, P.; Bousquet, J.; Urbach, V. Lipoxin A4 stimulates a cytosolic Ca2+ increase in human bronchial epithelium. J. Biol. Chem. 2003, 278, 10879–10884. [Google Scholar] [CrossRef] [PubMed]
- Verriere, V.; Higgins, G.; Al-Alawi, M.; Costello, R.W.; McNally, P.; Chiron, R.; Harvey, B.J.; Urbach, V. Lipoxin A4 stimulates calcium-activated chloride currents and increases airway surface liquid height in normal and cystic fibrosis airway epithelia. PLoS ONE 2012, 7, e37746. [Google Scholar] [CrossRef]
- Hodges, R.R.; Li, D.; Shatos, M.A.; Bair, J.A.; Lippestad, M.; Serhan, C.N.; Dartt, D.A. Lipoxin A(4) activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion. Mucosal Immunol. 2017, 10, 46–57. [Google Scholar] [CrossRef]
- Christie, P.E.; Spur, B.W.; Lee, T.H. The effects of lipoxin A4 on airway responses in asthmatic subjects. Am. Rev. Respir. Dis. 1992, 145, 1281–1284. [Google Scholar] [CrossRef]
- Tian, C.; Gao, F.; Li, X.; Li, Z. Icariside II attenuates eosinophils-induced airway inflammation and remodeling via inactivation of NF-kappaB and STAT3 in an asthma mouse model. Exp. Mol. Pathol. 2020, 113, 104373. [Google Scholar] [CrossRef]
- Kazani, S.; Planaguma, A.; Ono, E.; Bonini, M.; Zahid, M.; Marigowda, G.; Wechsler, M.E.; Levy, B.D.; Israel, E. Exhaled breath condensate eicosanoid levels associate with asthma and its severity. J. Allergy Clin. Immunol. 2013, 132, 547–553. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, L.; He, C.; Chen, R.; Meng, L. Lipoxin A4 inhibits ovalbumin-induced airway inflammation and airway remodeling in a mouse model of asthma. Chem. Biol. Interact. 2021, 349, 109660. [Google Scholar] [CrossRef]
- Kumar, V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef]
- Yang, A.; Yang, X.; Wang, J.; Wang, X.; Wu, H.; Fan, L.; Li, H.; Li, J. Effects of the Tight Junction Protein CLDN6 on Cell Migration and Invasion in High-Grade Meningioma. World Neurosurg. 2021, 151, e208–e216. [Google Scholar] [CrossRef]
- Padua, T.A.; Torres, N.D.; Candea, A.L.P.; Costa, M.F.S.; Silva, J.D.; Silva-Filho, J.L.; Costa, F.T.M.; Rocco, P.R.M.; Souza, M.C.; Henriques, M.G. Therapeutic effect of Lipoxin A(4) in malaria-induced acute lung injury. J. Leukoc. Biol. 2018, 103, 657–670. [Google Scholar] [CrossRef]
- O’Reilly, M.; Sozo, F.; Harding, R. Impact of preterm birth and bronchopulmonary dysplasia on the developing lung: Long-term consequences for respiratory health. Clin. Exp. Pharmacol. Physiol. 2013, 40, 765–773. [Google Scholar] [CrossRef]
- Ji, Y.D.; Luo, Z.L.; Chen, C.X.; Li, B.; Gong, J.; Wang, Y.X.; Chen, L.; Yao, S.L.; Shang, Y. BML-111 suppresses TGF-beta1-induced lung fi broblast activation in vitro and decreases experimental pulmonary fibrosis in vivo. Int. J. Mol. Med. 2018, 42, 3083–3092. [Google Scholar] [CrossRef]
- Kindermann, A.; Binder, L.; Baier, J.; Gundel, B.; Simm, A.; Haase, R.; Bartling, B. Severe but not moderate hyperoxia of newborn mice causes an emphysematous lung phenotype in adulthood without persisting oxidative stress and inflammation. BMC Pulm. Med. 2019, 19, 245. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chong, L.; Shao, Y.; Chen, S.; Li, C. Lipoxin A4 reduces hyperoxia-induced lung injury in neonatal rats through PINK1 signaling pathway. Int. Immunopharmacol. 2019, 73, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Adu-Agyeiwaah, Y.; Grant, M.B.; Obukhov, A.G. The Potential Role of Osteopontin and Furin in Worsening Disease Outcomes in COVID-19 Patients with Pre-Existing Diabetes. Cells 2020, 9, 2528. [Google Scholar] [CrossRef] [PubMed]
- Obukhov, A.G.; Stevens, B.R.; Prasad, R.; Li Calzi, S.; Boulton, M.E.; Raizada, M.K.; Oudit, G.Y.; Grant, M.B. SARS-CoV-2 Infections and ACE2: Clinical Outcomes Linked With Increased Morbidity and Mortality in Individuals With Diabetes. Diabetes 2020, 69, 1875–1886. [Google Scholar] [CrossRef]
- Attallah, N.G.M.; El-Kadem, A.H.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Elmongy, E.I.; Altwaijry, N.; Alanazi, A.S.; Al-Hamoud, G.A.; Ragab, A.E. Promising Antiviral Activity of Agrimonia pilosa Phytochemicals against Severe Acute Respiratory Syndrome Coronavirus 2 Supported with In Vivo Mice Study. Pharmaceuticals 2021, 14, 1313. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Bioactive Lipids in COVID-19-Further Evidence. Arch. Med. Res. 2021, 52, 107–120. [Google Scholar] [CrossRef]
- Pal, A.; Gowdy, K.M.; Oestreich, K.J.; Beck, M.; Shaikh, S.R. Obesity-Driven Deficiencies of Specialized Pro-resolving Mediators May Drive Adverse Outcomes During SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 1997. [Google Scholar] [CrossRef]
- Shah, V.K.; Firmal, P.; Alam, A.; Ganguly, D.; Chattopadhyay, S. Overview of Immune Response During SARS-CoV-2 Infection: Lessons From the Past. Front. Immunol. 2020, 11, 1949. [Google Scholar] [CrossRef]
- Rex, D.A.B.; Dagamajalu, S.; Kandasamy, R.K.; Raju, R.; Prasad, T.S.K. SARS-CoV-2 signaling pathway map: A functional landscape of molecular mechanisms in COVID-19. J. Cell Commun. Signal. 2021, 15, 601–608. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, X.; Yin, Z.; Yu, X.; Yang, Q.; Guo, Q.; Tian, D.; Xiong, X.; Xu, G.; Kuang, X. The anti-inflammatory effect of BML-111 on COPD may be mediated by regulating NLRP3 inflammasome activation and ROS production. Prostaglandins Other Lipid Mediat. 2018, 138, 23–30. [Google Scholar] [CrossRef]
- Lindstrom, M.; DeCleene, N.; Dorsey, H.; Fuster, V.; Johnson, C.O.; LeGrand, K.E.; Mensah, G.A.; Razo, C.; Stark, B.; Varieur Turco, J.; et al. Global Burden of Cardiovascular Diseases and Risks Collaboration, 1990–2021. J. Am. Coll. Cardiol. 2022, 80, 2372–2425. [Google Scholar] [CrossRef]
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Narula, J.; et al. The Heart of the World. Glob. Heart 2024, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Serna, M.F.; Mosquera Escudero, M.; Garcia-Perdomo, H.A. Lipoxins and their relationship with inflammation-associated diseases. A systematic review. Obes. Res. Clin. Pract. 2023, 17, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Solanki, K.; Bezsonov, E.; Orekhov, A.; Parihar, S.P.; Vaja, S.; White, F.A.; Obukhov, A.G.; Baig, M.S. Effect of reactive oxygen, nitrogen, and sulfur species on signaling pathways in atherosclerosis. Vasc. Pharmacol. 2024, 154, 107282. [Google Scholar] [CrossRef]
- Caso, V.M.; Manzo, V.; Pecchillo Cimmino, T.; Conti, V.; Caso, P.; Esposito, G.; Russo, V.; Filippelli, A.; Ammendola, R.; Cattaneo, F. Regulation of Inflammation and Oxidative Stress by Formyl Peptide Receptors in Cardiovascular Disease Progression. Life 2021, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Perretti, M.; Godson, C. Formyl peptide receptor type 2 agonists to kick-start resolution pharmacology. Br. J. Pharmacol. 2020, 177, 4595–4600. [Google Scholar] [CrossRef]
- Petri, M.H.; Laguna-Fernandez, A.; Arnardottir, H.; Wheelock, C.E.; Perretti, M.; Hansson, G.K.; Back, M. Aspirin-triggered lipoxin A4 inhibits atherosclerosis progression in apolipoprotein E(-/-) mice. Br. J. Pharmacol. 2017, 174, 4043–4054. [Google Scholar] [CrossRef]
- Hashem, S.; Dougha, A.; Tuffery, P. Ligand-Induced Biased Activation of GPCRs: Recent Advances and New Directions from In Silico Approaches. Molecules 2025, 30, 1047. [Google Scholar] [CrossRef]
- Mai, J.; Liu, W.; Fang, Y.; Zhang, S.; Qiu, Q.; Yang, Y.; Wang, X.; Huang, T.; Zhang, H.; Xie, Y.; et al. The atheroprotective role of lipoxin A(4) prevents oxLDL-induced apoptotic signaling in macrophages via JNK pathway. Atherosclerosis 2018, 278, 259–268. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, J.; Zhou, X.; Hao, H. Lipoxin A(4) and its analog attenuate high fat diet-induced atherosclerosis via Keap1/Nrf2 pathway. Exp. Cell Res. 2022, 412, 113025. [Google Scholar] [CrossRef]
- O’Brien, C.W.; Juraschek, S.P.; Wee, C.C. Prevalence of Aspirin Use for Primary Prevention of Cardiovascular Disease in the United States: Results From the 2017 National Health Interview Survey. Ann. Intern. Med. 2019, 171, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Lu, Y.; Wei, J.; Wu, J.; Yang, J.; Cai, Z. Abdominal Aortic Aneurysm: Roles of Inflammatory Cells. Front. Immunol. 2020, 11, 609161. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.H.; Thul, S.; Andonova, T.; Lindquist-Liljeqvist, M.; Jin, H.; Skenteris, N.T.; Arnardottir, H.; Maegdefessel, L.; Caidahl, K.; Perretti, M.; et al. Resolution of Inflammation Through the Lipoxin and ALX/FPR2 Receptor Pathway Protects Against Abdominal Aortic Aneurysms. JACC Basic. Transl. Sci. 2018, 3, 719–727. [Google Scholar] [CrossRef]
- Jaen, R.I.; Fernandez-Velasco, M.; Terron, V.; Sanchez-Garcia, S.; Zaragoza, C.; Canales-Bueno, N.; Val-Blasco, A.; Vallejo-Cremades, M.T.; Bosca, L.; Prieto, P. BML-111 treatment prevents cardiac apoptosis and oxidative stress in a mouse model of autoimmune myocarditis. FASEB J. 2020, 34, 10531–10546. [Google Scholar] [CrossRef] [PubMed]
- Reina-Couto, M.; Carvalho, J.; Valente, M.J.; Vale, L.; Afonso, J.; Carvalho, F.; Bettencourt, P.; Sousa, T.; Albino-Teixeira, A. Impaired resolution of inflammation in human chronic heart failure. Eur. J. Clin. Investig. 2014, 44, 527–538. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, H.; Zhang, H.Z.; Zhao, X.Y.; Jin, J.; Wang, H.Y. Lipoxin A4 mitigates experimental autoimmune myocarditis by regulating inflammatory response, NF-kappaB and PI3K/Akt signaling pathway in mice. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1850–1859. [Google Scholar]
- Thakur, M.; Tupe, R.S. Lipoxin and glycation in SREBP signaling: Insight into diabetic cardiomyopathy and associated lipotoxicity. Prostaglandins Other Lipid Mediat. 2023, 164, 106698. [Google Scholar] [CrossRef]
- Chen, R.; Li, J.; Zhou, J.; Wang, Y.; Zhao, X.; Li, N.; Liu, W.; Liu, C.; Zhou, P.; Chen, Y.; et al. Prognostic impacts of Lipoxin A4 in patients with acute myocardial infarction: A prospective cohort study. Pharmacol. Res. 2023, 187, 106618. [Google Scholar] [CrossRef]
- Kain, V.; Liu, F.; Kozlovskaya, V.; Ingle, K.A.; Bolisetty, S.; Agarwal, A.; Khedkar, S.; Prabhu, S.D.; Kharlampieva, E.; Halade, G.V. Resolution Agonist 15-epi-Lipoxin A(4) Programs Early Activation of Resolving Phase in Post-Myocardial Infarction Healing. Sci. Rep. 2017, 7, 9999. [Google Scholar] [CrossRef]
- Kang, G.J.; Kim, E.J.; Lee, C.H. Therapeutic Effects of Specialized Pro-Resolving Lipids Mediators on Cardiac Fibrosis via NRF2 Activation. Antioxidants 2020, 9, 1259. [Google Scholar] [CrossRef]
- Wallace, J.L.; Zamuner, S.R.; McKnight, W.; Dicay, M.; Mencarelli, A.; del Soldato, P.; Fiorucci, S. Aspirin, but not NO-releasing aspirin (NCX-4016), interacts with selective COX-2 inhibitors to aggravate gastric damage and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G76–G81. [Google Scholar] [CrossRef]
- Das, U.N. Can COX-2 inhibitor-induced increase in cardiovascular disease risk be modified by essential fatty acids? J. Assoc. Physicians India 2005, 53, 623–627. [Google Scholar]
- Weisman, S.M.; Graham, D.Y. Evaluation of the benefits and risks of low-dose aspirin in the secondary prevention of cardiovascular and cerebrovascular events. Arch. Intern. Med. 2002, 162, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- Tulowiecka, N.; Kotlega, D.; Bohatyrewicz, A.; Szczuko, M. Could Lipoxins Represent a New Standard in Ischemic Stroke Treatment? Int. J. Mol. Sci. 2021, 22, 4207. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gao, W.; Li, L.; Hao, J.; Yang, B.; Wang, T.; Li, L.; Bai, X.; Li, F.; Ren, H.; et al. Annexin A1 protects against cerebral ischemia-reperfusion injury by modulating microglia/macrophage polarization via FPR2/ALX-dependent AMPK-mTOR pathway. J. Neuroinflamm. 2021, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.; Chambers, S.T.; Nolan, I.; Gallagher, K.; Werno, A.; Browne, M.; Stamp, L.K. Native Joint Septic Arthritis: Epidemiology, Clinical Features, and Microbiological Causes in a New Zealand Population. J. Rheumatol. 2015, 42, 2392–2397. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.Y.; Abu-Khattab, M.; Baagar, K.; Mohamed, S.F.; Elgendy, I.; Anand, D.; Malallah, H.; Sanjay, D. Characteristics of patients with definite septic arthritis at Hamad General Hospital, Qatar: A hospital-based study from 2006 to 2011. Clin. Rheumatol. 2013, 32, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Boff, D.; Oliveira, V.L.S.; Queiroz Junior, C.M.; Galvao, I.; Batista, N.V.; Gouwy, M.; Menezes, G.B.; Cunha, T.M.; Verri Junior, W.A.; Proost, P.; et al. Lipoxin A(4) impairs effective bacterial control and potentiates joint inflammation and damage caused by Staphylococcus aureus infection. FASEB J. 2020, 34, 11498–11510. [Google Scholar] [CrossRef]
- Blaho, V.A.; Zhang, Y.; Hughes-Hanks, J.M.; Brown, C.R. 5-Lipoxygenase-deficient mice infected with Borrelia burgdorferi develop persistent arthritis. J. Immunol. 2011, 186, 3076–3084. [Google Scholar] [CrossRef]
- Zhang, Y.; Olson, R.M.; Brown, C.R. Macrophage LTB(4) drives efficient phagocytosis of Borrelia burgdorferi via BLT1 or BLT2. J. Lipid Res. 2017, 58, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Takai, D.; Nagase, T.; Shimizu, T. New therapeutic key for cystic fibrosis: A role for lipoxins. Nat. Immunol. 2004, 5, 357–358. [Google Scholar] [CrossRef]
- Machado, F.S.; Aliberti, J. Role of lipoxin in the modulation of immune response during infection. Int. Immunopharmacol. 2008, 8, 1316–1319. [Google Scholar] [CrossRef]
- Machado, F.S.; Johndrow, J.E.; Esper, L.; Dias, A.; Bafica, A.; Serhan, C.N.; Aliberti, J. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat. Med. 2006, 12, 330–334. [Google Scholar] [CrossRef]
- Aliberti, J.; Serhan, C.; Sher, A. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J. Exp. Med. 2002, 196, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Shirey, K.A.; Pletneva, L.M.; Puche, A.C.; Keegan, A.D.; Prince, G.A.; Blanco, J.C.; Vogel, S.N. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4R alpha-, TLR4-, and IFN-beta-dependent. Mucosal Immunol. 2010, 3, 291–300. [Google Scholar] [CrossRef]
- Genis, P.; Jett, M.; Bernton, E.W.; Boyle, T.; Gelbard, H.A.; Dzenko, K.; Keane, R.W.; Resnick, L.; Mizrachi, Y.; Volsky, D.J.; et al. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: Implications for the neuropathogenesis of HIV disease. J. Exp. Med. 1992, 176, 1703–1718. [Google Scholar] [CrossRef]
- Molina-Berrios, A.; Campos-Estrada, C.; Henriquez, N.; Faundez, M.; Torres, G.; Castillo, C.; Escanilla, S.; Kemmerling, U.; Morello, A.; Lopez-Munoz, R.A.; et al. Protective role of acetylsalicylic acid in experimental Trypanosoma cruzi infection: Evidence of a 15-epi-lipoxin A(4)-mediated effect. PLoS Negl. Trop. Dis. 2013, 7, e2173. [Google Scholar] [CrossRef] [PubMed]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Serhan, C.N.; Jain, A.; Marleau, S.; Clish, C.; Kantarci, A.; Behbehani, B.; Colgan, S.P.; Stahl, G.L.; Merched, A.; Petasis, N.A.; et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J. Immunol. 2003, 171, 6856–6865. [Google Scholar] [CrossRef]
- Parolini, C. Sepsis and high-density lipoproteins: Pathophysiology and potential new therapeutic targets. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167761. [Google Scholar] [CrossRef]
- Gounni, A.S.; Lamkhioued, B.; Ochiai, K.; Tanaka, Y.; Delaporte, E.; Capron, A.; Kinet, J.P.; Capron, M. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature 1994, 367, 183–186. [Google Scholar] [CrossRef]
- Ashour, D.S. Toll-like receptor signaling in parasitic infections. Expert. Rev. Clin. Immunol. 2015, 11, 771–780. [Google Scholar] [CrossRef]
- Wu, B.; Walker, J.; Spur, B.; Rodriguez, A.; Yin, K. Effects of Lipoxin A4 on antimicrobial actions of neutrophils in sepsis. Prostaglandins Leukot. Essent. Fat. Acids 2015, 94, 55–64. [Google Scholar] [CrossRef]
- Bafica, A.; Scanga, C.A.; Serhan, C.; Machado, F.; White, S.; Sher, A.; Aliberti, J. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J. Clin. Investig. 2005, 115, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, C.C.B.; Fisher, L.; Amaral, E.P.; Mittereder, L.; McCann, K.; Correa, A.A.S.; Namasivayam, S.; Swamydas, M.; Moayeri, M.; Weiss, J.M.; et al. Mycobacterium tuberculosis Induces Irg1 in Murine Macrophages by a Pathway Involving Both TLR-2 and STING/IFNAR Signaling and Requiring Bacterial Phagocytosis. Front. Cell. Infect. Microbiol. 2022, 12, 862582. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wang, M.; Shi, P.; Xie, X.; Duan, D.; Meng, S.; Yuan, Q.; Wu, Y.; Wang, J. Effect of lipoxin A4 on the osteogenic differentiation of periodontal ligament stem cells under lipopolysaccharide-induced inflammatory conditions. Eur. J. Oral. Sci. 2023, 131, e12932. [Google Scholar] [CrossRef]
- Cianci, E.; Recchiuti, A.; Trubiani, O.; Diomede, F.; Marchisio, M.; Miscia, S.; Colas, R.A.; Dalli, J.; Serhan, C.N.; Romano, M. Human Periodontal Stem Cells Release Specialized Proresolving Mediators and Carry Immunomodulatory and Prohealing Properties Regulated by Lipoxins. Stem Cells Transl. Med. 2016, 5, 20–32. [Google Scholar] [CrossRef]
- Stenke, L.; Reizenstein, P.; Lindgren, J.A. Leukotrienes and lipoxins--new potential performers in the regulation of human myelopoiesis. Leuk. Res. 1994, 18, 727–732. [Google Scholar] [CrossRef]
- Stenke, L.; Mansour, M.; Edenius, C.; Reizenstein, P.; Lindgren, J.A. Formation and proliferative effects of lipoxins in human bone marrow. Biochem. Biophys. Res. Commun. 1991, 180, 255–261. [Google Scholar] [CrossRef]
- Lindgren, J.A.; Stenke, L.; Mansour, M.; Edenius, C.; Lauren, L.; Nasman-Glaser, B.; Ericsson, I.; Reizenstein, P. Formation and effects of leukotrienes and lipoxins in human bone marrow. J. Lipid Mediat. 1993, 6, 313–320. [Google Scholar] [PubMed]
- Romano, M.; Patruno, S.; Pomilio, A.; Recchiuti, A. Proresolving Lipid Mediators and Receptors in Stem Cell Biology: Concise Review. Stem Cells Transl. Med. 2019, 8, 992–998. [Google Scholar] [CrossRef]
- Desplat, V.; Dupuis, F.; Trimoreau, F.; Dulery, C.; Praloran, V.; Denizot, Y. Effects of lipoxygenase metabolites of arachidonic acid on the growth of human mononuclear marrow cells and marrow stromal cell cultures. Mediat. Inflamm. 1998, 7, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N. Novel endogenous small molecules as the checkpoint controllers in inflammation and resolution: Entree for resoleomics. Rheum. Dis. Clin. N. Am. 2004, 30, 69–95. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liao, J.; Feng, F.; Chen, S.; Liao, E.; Li, D.; Dai, X.; Dong, J.; Shao, Y. Combined Application of Exosomes and FPR2 Agonist LXA4 in Controlling Fetal Membrane Inflammation and Promoting Fetal Membrane Tissue Repair. Reprod. Sci. 2023, 30, 1979–1993. [Google Scholar] [CrossRef]
- Tan, J.L.; Tan, Y.Z.; Muljadi, R.; Chan, S.T.; Lau, S.N.; Mockler, J.C.; Wallace, E.M.; Lim, R. Amnion Epithelial Cells Promote Lung Repair via Lipoxin A(4). Stem Cells Transl. Med. 2017, 6, 1085–1095. [Google Scholar] [CrossRef]
- Murphy, S.; Lim, R.; Dickinson, H.; Acharya, R.; Rosli, S.; Jenkin, G.; Wallace, E. Human amnion epithelial cells prevent bleomycin-induced lung injury and preserve lung function. Cell Transplant. 2011, 20, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, S.H.; Han, S.Y.; Lee, Y.S.; Cho, J.; Kim, J.M. LXA(4)-FPR2 signaling regulates radiation-induced pulmonary fibrosis via crosstalk with TGF-beta/Smad signaling. Cell Death Dis. 2020, 11, 653. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, J.; He, Z.; Yang, M.; Li, L.; Jiang, H. Mesenchymal Stem Cells Reverse Diabetic Nephropathy Disease via Lipoxin A4 by Targeting Transforming Growth Factor beta (TGF-beta)/smad Pathway and Pro-Inflammatory Cytokines. Med. Sci. Monit. 2019, 25, 3069–3076. [Google Scholar] [CrossRef]
- Wada, K.; Arita, M.; Nakajima, A.; Katayama, K.; Kudo, C.; Kamisaki, Y.; Serhan, C.N. Leukotriene B4 and lipoxin A4 are regulatory signals for neural stem cell proliferation and differentiation. FASEB J. 2006, 20, 1785–1792. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.; Zhou, T.; Liao, T.; Huang, X.; Xiang, H.; Zhang, Q.; Huang, Y.; Lin, F.; Ye, D.; et al. Lipoxin A4 interferes with embryo implantation via suppression of epithelial-mesenchymal transition. Am. J. Reprod. Immunol. 2019, 81, e13107. [Google Scholar] [CrossRef]
- Jaen, R.I.; Sanchez-Garcia, S.; Fernandez-Velasco, M.; Bosca, L.; Prieto, P. Resolution-Based Therapies: The Potential of Lipoxins to Treat Human Diseases. Front. Immunol. 2021, 12, 658840. [Google Scholar] [CrossRef] [PubMed]
- Elias, I.; Ferre, T.; Vila, L.; Munoz, S.; Casellas, A.; Garcia, M.; Molas, M.; Agudo, J.; Roca, C.; Ruberte, J.; et al. ALOX5AP Overexpression in Adipose Tissue Leads to LXA4 Production and Protection Against Diet-Induced Obesity and Insulin Resistance. Diabetes 2016, 65, 2139–2150. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Arachidonic acid and lipoxin A4 as possible endogenous anti-diabetic molecules. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 201–210. [Google Scholar] [CrossRef]
- Spite, M.; Claria, J.; Serhan, C.N. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. PUFAs, BDNF and lipoxin A4 inhibit chemical-induced cytotoxicity of RIN5F cells in vitro and streptozotocin-induced type 2 diabetes mellitus in vivo. Lipids Health Dis. 2019, 18, 214. [Google Scholar] [CrossRef]
- Schwab, J.M.; Serhan, C.N. Lipoxins and new lipid mediators in the resolution of inflammation. Curr. Opin. Pharmacol. 2006, 6, 414–420. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Dunn, H.C.; Ager, R.R.; Baglietto-Vargas, D.; Cheng, D.; Kitazawa, M.; Cribbs, D.H.; Medeiros, R. Restoration of lipoxin A4 signaling reduces Alzheimer’s disease-like pathology in the 3xTg-AD mouse model. J. Alzheimers Dis. 2015, 43, 893–903. [Google Scholar] [CrossRef]
- Pamplona, F.A.; Ferreira, J.; Menezes de Lima, O., Jr.; Duarte, F.S.; Bento, A.F.; Forner, S.; Villarinho, J.G.; Bellocchio, L.; Wotjak, C.T.; Lerner, R.; et al. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 21134–21139. [Google Scholar] [CrossRef] [PubMed]
- Trovato, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Fronte, V.; Koverech, G.; Luca, M.; Serra, A.; Toscano, M.A.; Petralia, A.; et al. Redox modulation of cellular stress response and lipoxin A4 expression by Coriolus versicolor in rat brain: Relevance to Alzheimer’s disease pathogenesis. Neurotoxicology 2016, 53, 350–358. [Google Scholar] [CrossRef]
- Lee, J.Y.; Han, S.H.; Park, M.H.; Baek, B.; Song, I.S.; Choi, M.K.; Takuwa, Y.; Ryu, H.; Kim, S.H.; He, X.; et al. Neuronal SphK1 acetylates COX2 and contributes to pathogenesis in a model of Alzheimer’s Disease. Nat. Commun. 2018, 9, 1479. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, M.; Hjorth, E.; Cortes-Toro, V.; Eyjolfsdottir, H.; Graff, C.; Nennesmo, I.; Palmblad, J.; Eriksdotter, M.; Sambamurti, K.; et al. Resolution of inflammation is altered in Alzheimer’s disease. Alzheimers Dement. 2015, 11, 40–50.e2. [Google Scholar] [CrossRef]
- Kantarci, A.; Aytan, N.; Palaska, I.; Stephens, D.; Crabtree, L.; Benincasa, C.; Jenkins, B.G.; Carreras, I.; Dedeoglu, A. Combined administration of resolvin E1 and lipoxin A4 resolves inflammation in a murine model of Alzheimer’s disease. Exp. Neurol. 2018, 300, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Loma, I.; Heyman, R. Multiple sclerosis: Pathogenesis and treatment. Curr. Neuropharmacol. 2011, 9, 409–416. [Google Scholar] [CrossRef]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Gadoth, N. Multiple sclerosis in children. Brain Dev. 2003, 25, 229–232. [Google Scholar] [CrossRef]
- Kooij, G.; Troletti, C.D.; Leuti, A.; Norris, P.C.; Riley, I.; Albanese, M.; Ruggieri, S.; Libreros, S.; van der Pol, S.M.A.; van Het Hof, B.; et al. Specialized pro-resolving lipid mediators are differentially altered in peripheral blood of patients with multiple sclerosis and attenuate monocyte and blood-brain barrier dysfunction. Haematologica 2020, 105, 2056–2070. [Google Scholar] [CrossRef]
- Derada Troletti, C.; Enzmann, G.; Chiurchiu, V.; Kamermans, A.; Tietz, S.M.; Norris, P.C.; Jahromi, N.H.; Leuti, A.; van der Pol, S.M.A.; Schouten, M.; et al. Pro-resolving lipid mediator lipoxin A(4) attenuates neuro-inflammation by modulating T cell responses and modifies the spinal cord lipidome. Cell Rep. 2021, 35, 109201. [Google Scholar] [CrossRef] [PubMed]
- Ntaios, G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 333–340. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Davis, P.H.; Leira, E.C.; Chang, K.C.; Bendixen, B.H.; Clarke, W.R.; Woolson, R.F.; Hansen, M.D. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999, 53, 126–131. [Google Scholar] [CrossRef]
- Marcheselli, V.L.; Hong, S.; Lukiw, W.J.; Tian, X.H.; Gronert, K.; Musto, A.; Hardy, M.; Gimenez, J.M.; Chiang, N.; Serhan, C.N.; et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol. Chem. 2003, 278, 43807–43817. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.S.; Kho, A.R.; Lee, S.H.; Choi, B.Y.; Kang, S.H.; Koh, J.Y.; Suh, S.W.; Song, D.K. Changes in plasma lipoxin A4, resolvins and CD59 levels after ischemic and traumatic brain injuries in rats. Korean J. Physiol. Pharmacol. 2020, 24, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Sobrado, M.; Pereira, M.P.; Ballesteros, I.; Hurtado, O.; Fernandez-Lopez, D.; Pradillo, J.M.; Caso, J.R.; Vivancos, J.; Nombela, F.; Serena, J.; et al. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J. Neurosci. 2009, 29, 3875–3884. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, X.H.; Guo, P.P.; Xu, S.P.; Wang, J.; Yuan, S.Y.; Yao, S.L.; Shang, Y. Neuroprotective effect of lipoxin A4 methyl ester in a rat model of permanent focal cerebral ischemia. J. Mol. Neurosci. 2010, 42, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.P.; Guo, P.; Ye, X.H.; Wang, J.; Yuan, S.Y.; Yao, S.L.; Shang, Y. A lipoxin A4 analog ameliorates blood-brain barrier dysfunction and reduces MMP-9 expression in a rat model of focal cerebral ischemia-reperfusion injury. J. Mol. Neurosci. 2012, 46, 483–491. [Google Scholar] [CrossRef]
- Li, Q.Q.; Ding, D.H.; Wang, X.Y.; Sun, Y.Y.; Wu, J. Lipoxin A4 regulates microglial M1/M2 polarization after cerebral ischemia-reperfusion injury via the Notch signaling pathway. Exp. Neurol. 2021, 339, 113645. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Miao, S.; Zou, L.B.; Wu, P.; Hao, H.; Tang, K.; Zeng, P.; Xiong, J.; Li, H.H.; Wu, Q.; et al. Lipoxin A4 inhibits 5-lipoxygenase translocation and leukotrienes biosynthesis to exert a neuroprotective effect in cerebral ischemia/reperfusion injury. J. Mol. Neurosci. 2012, 48, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.S.; Guo, P.P.; Jin, Z.; Li, X.Y.; Yang, X.; Ke, J.J.; Wang, Y.L.; Feng, X.B. Effects of Lipoxin A4 Pretreatment on Cognitive Function of Aged Rats after Global Cerebral Ischemia Reperfusion. Curr. Med. Sci. 2018, 38, 666–671. [Google Scholar] [CrossRef]
- Kotlega, D.; Zembron-Lacny, A.; Golab-Janowska, M.; Nowacki, P.; Szczuko, M. The Association of Free Fatty Acids and Eicosanoids with the Severity of Depressive Symptoms in Stroke Patients. Int. J. Mol. Sci. 2020, 21, 5220. [Google Scholar] [CrossRef]
- Unnithan, A.K.A.; Das, J.M.; Mehta, P. Hemorrhagic Stroke. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kitagawa, K. Blood pressure management for secondary stroke prevention. Hypertens. Res. 2022, 45, 936–943. [Google Scholar] [CrossRef]
- Castello, J.P.; Pasi, M.; Kubiszewski, P.; Abramson, J.R.; Charidimou, A.; Kourkoulis, C.; DiPucchio, Z.; Schwab, K.; Anderson, C.D.; Gurol, M.E.; et al. Cerebral Small Vessel Disease and Depression Among Intracerebral Hemorrhage Survivors. Stroke 2022, 53, 523–531. [Google Scholar] [CrossRef]
- Guo, Z.; Hu, Q.; Xu, L.; Guo, Z.N.; Ou, Y.; He, Y.; Yin, C.; Sun, X.; Tang, J.; Zhang, J.H. Lipoxin A4 Reduces Inflammation Through Formyl Peptide Receptor 2/p38 MAPK Signaling Pathway in Subarachnoid Hemorrhage Rats. Stroke 2016, 47, 490–497. [Google Scholar] [CrossRef]
- Song, Y.; Yang, Y.; Cui, Y.; Gao, J.; Wang, K.; Cui, J. Lipoxin A4 Methyl Ester Reduces Early Brain Injury by Inhibition of the Nuclear Factor Kappa B (NF-kappaB)-Dependent Matrix Metallopeptidase 9 (MMP-9) Pathway in a Rat Model of Intracerebral Hemorrhage. Med. Sci. Monit. 2019, 25, 1838–1847. [Google Scholar] [CrossRef]
- Eckert, M.J.; Martin, M.J. Trauma: Spinal Cord Injury. Surg. Clin. N. Am. 2017, 97, 1031–1045. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Martini, A.C.; Berta, T.; Forner, S.; Chen, G.; Bento, A.F.; Ji, R.R.; Rae, G.A. Lipoxin A4 inhibits microglial activation and reduces neuroinflammation and neuropathic pain after spinal cord hemisection. J. Neuroinflamm. 2016, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Miao, G.S.; Liu, Z.H.; Wei, S.X.; Luo, J.G.; Fu, Z.J.; Sun, T. Lipoxin A4 attenuates radicular pain possibly by inhibiting spinal ERK, JNK and NF-kappaB/p65 and cytokine signals, but not p38, in a rat model of non-compressive lumbar disc herniation. Neuroscience 2015, 300, 10–18. [Google Scholar] [CrossRef]
- Svensson, C.I.; Zattoni, M.; Serhan, C.N. Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J. Exp. Med. 2007, 204, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Wu, X.; Wei, N.; Liu, X.; Zhou, Y.; Shang, C.; Duan, Y.; Dong, Y. Lipoxin A4 protects against spinal cord injury via regulating Akt/nuclear factor (erythroid-derived 2)-like 2/heme oxygenase-1 signaling. Biomed. Pharmacother. 2018, 97, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef]
- Zhu, J.J.; Yu, B.Y.; Fu, C.C.; He, M.Z.; Zhu, J.H.; Chen, B.W.; Zheng, Y.H.; Chen, S.Q.; Fu, X.Q.; Li, P.J.; et al. LXA4 protects against hypoxic-ischemic damage in neonatal rats by reducing the inflammatory response via the IkappaB/NF-kappaB pathway. Int. Immunopharmacol. 2020, 89, 107095. [Google Scholar] [CrossRef]
- Nguyen, T.; Nguyen, N.; Cochran, A.G.; Smith, J.A.; Al-Juboori, M.; Brumett, A.; Saxena, S.; Talley, S.; Campbell, E.M.; Obukhov, A.G.; et al. Repeated closed-head mild traumatic brain injury-induced inflammation is associated with nociceptive sensitization. J. Neuroinflamm. 2023, 20, 196. [Google Scholar] [CrossRef]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.R. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell Transplant. 2017, 26, 1118–1130. [Google Scholar] [CrossRef]
- Moretti, L.; Cristofori, I.; Weaver, S.M.; Chau, A.; Portelli, J.N.; Grafman, J. Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurol. 2012, 11, 1103–1112. [Google Scholar] [CrossRef]
- Sandsmark, D.K.; Elliott, J.E.; Lim, M.M. Sleep-Wake Disturbances After Traumatic Brain Injury: Synthesis of Human and Animal Studies. Sleep 2017, 40, zsx044. [Google Scholar] [CrossRef]
- Luo, C.L.; Li, Q.Q.; Chen, X.P.; Zhang, X.M.; Li, L.L.; Li, B.X.; Zhao, Z.Q.; Tao, L.Y. Lipoxin A4 attenuates brain damage and downregulates the production of pro-inflammatory cytokines and phosphorylated mitogen-activated protein kinases in a mouse model of traumatic brain injury. Brain Res. 2013, 1502, 1–10. [Google Scholar] [CrossRef]
- Wang, Z.F.; Li, Q.; Liu, S.B.; Mi, W.L.; Hu, S.; Zhao, J.; Tian, Y.; Mao-Ying, Q.L.; Jiang, J.W.; Ma, H.J.; et al. Aspirin-triggered Lipoxin A4 attenuates mechanical allodynia in association with inhibiting spinal JAK2/STAT3 signaling in neuropathic pain in rats. Neuroscience 2014, 273, 65–78. [Google Scholar] [CrossRef]
- Terracciano, A.; Iacono, D.; O’Brien, R.J.; Troncoso, J.C.; An, Y.; Sutin, A.R.; Ferrucci, L.; Zonderman, A.B.; Resnick, S.M. Personality and resilience to Alzheimer’s disease neuropathology: A prospective autopsy study. Neurobiol. Aging 2013, 34, 1045–1050. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Hao, H.; Zhou, X.Y. The role of lipoxin in regulating tumor immune microenvironments. Prostaglandins Other Lipid Mediat. 2019, 144, 106341. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjose, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Kinlen, L.J.; Balkwill, A. Infective cause of childhood leukaemia and wartime population mixing in Orkney and Shetland, UK. Lancet 2001, 357, 858. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N. Endogenous pro-resolving and anti-inflammatory lipid mediators: A new pharmacologic genus. Br. J. Pharmacol. 2008, 153 (Suppl. 1), S200–S215. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Chen, K.; Jiang, Z.; Chen, X.; Sun, L.; Ma, J.; Zhou, C.; Xu, Q.; Duan, W.; Han, L.; et al. Lipoxin A4 reverses mesenchymal phenotypes to attenuate invasion and metastasis via the inhibition of autocrine TGF-beta1 signaling in pancreatic cancer. J. Exp. Clin. Cancer Res. 2017, 36, 181. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Li, J.; Chen, X.; Chen, K.; Li, W.; Li, X.; Zhang, L.; Duan, W.; Lei, J.; Xu, Q.; et al. Lipoxin A4 Attenuates Cell Invasion by Inhibiting ROS/ERK/MMP Pathway in Pancreatic Cancer. Oxid. Med. Cell Longev. 2016, 2016, 6815727. [Google Scholar] [CrossRef]
- Varani, J.; Ward, P.A. Mechanisms of endothelial cell injury in acute inflammation. Shock 1994, 2, 311–312. [Google Scholar] [CrossRef]
- Marginean, A.; Sharma-Walia, N. Lipoxins exert antiangiogenic and anti-inflammatory effects on Kaposi’s sarcoma cells. Transl. Res. 2015, 166, 111–133. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Baker, N.; O’Meara, S.J.; Scannell, M.; Maderna, P.; Godson, C. Lipoxin A4: Anti-inflammatory and anti-angiogenic impact on endothelial cells. J. Immunol. 2009, 182, 3819–3826. [Google Scholar] [CrossRef] [PubMed]
- Fujio, Y.; Walsh, K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J. Biol. Chem. 1999, 274, 16349–16354. [Google Scholar] [CrossRef]
- Tsopanoglou, N.E.; Pipili-Synetos, E.; Maragoudakis, M.E. Leukotrienes C4 and D4 promote angiogenesis via a receptor-mediated interaction. Eur. J. Pharmacol. 1994, 258, 151–154. [Google Scholar] [CrossRef]
- Cezar-de-Mello, P.F.; Vieira, A.M.; Nascimento-Silva, V.; Villela, C.G.; Barja-Fidalgo, C.; Fierro, I.M. ATL-1, an analogue of aspirin-triggered lipoxin A4, is a potent inhibitor of several steps in angiogenesis induced by vascular endothelial growth factor. Br. J. Pharmacol. 2008, 153, 956–965. [Google Scholar] [CrossRef]
- Gil-Villa, A.M.; Norling, L.V.; Serhan, C.N.; Cordero, D.; Rojas, M.; Cadavid, A. Aspirin triggered-lipoxin A4 reduces the adhesion of human polymorphonuclear neutrophils to endothelial cells initiated by preeclamptic plasma. Prostaglandins Leukot. Essent. Fat. Acids 2012, 87, 127–134. [Google Scholar] [CrossRef]
- Orme, J.J.; Pagliaro, L.C.; Quevedo, J.F.; Park, S.S.; Costello, B.A. Rational Second-Generation Antiandrogen Use in Prostate Cancer. Oncologist 2022, 27, 110–124. [Google Scholar] [CrossRef]
- Tong, M.; Tai, H.H. Synergistic induction of the nicotinamide adenine dinucleotide-linked 15-hydroxyprostaglandin dehydrogenase by an androgen and interleukin-6 or forskolin in human prostate cancer cells. Endocrinology 2004, 145, 2141–2147. [Google Scholar] [CrossRef] [PubMed]
- Shappell, S.B.; Boeglin, W.E.; Olson, S.J.; Kasper, S.; Brash, A.R. 15-lipoxygenase-2 (15-LOX-2) is expressed in benign prostatic epithelium and reduced in prostate adenocarcinoma. Am. J. Pathol. 1999, 155, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Jack, G.S.; Brash, A.R.; Olson, S.J.; Manning, S.; Coffey, C.S.; Smith, J.A., Jr.; Shappell, S.B. Reduced 15-lipoxygenase-2 immunostaining in prostate adenocarcinoma: Correlation with grade and expression in high-grade prostatic intraepithelial neoplasia. Hum. Pathol. 2000, 31, 1146–1154. [Google Scholar] [CrossRef]
- Jia, G.; Wang, X.; Wu, W.; Zhang, Y.; Chen, S.; Zhao, J.; Zhao, W.; Li, W.; Sun, X.; Han, B. LXA4 enhances prostate cancer progression by facilitating M2 macrophage polarization via inhibition of METTL3. Int. Immunopharmacol. 2022, 107, 108586. [Google Scholar] [CrossRef]
- Claria, J.; Lee, M.H.; Serhan, C.N. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Mol. Med. 1996, 2, 583–596. [Google Scholar] [CrossRef]
- Browne, E.; Chen, H.; Vencken, S.; Condron, C.M. Lipoxin A4 a novel therapeutic agent for the treatment of breast cancer metastases. Integr. Cancer Sci. Ther. 2020, 7, 1–7. [Google Scholar] [CrossRef]
- Hurst, D.R.; Edmonds, M.D.; Scott, G.K.; Benz, C.C.; Vaidya, K.S.; Welch, D.R. Breast cancer metastasis suppressor 1 up-regulates miR-146, which suppresses breast cancer metastasis. Cancer Res. 2009, 69, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhou, X.; Lin, L.; Xu, J.; Feng, Y.; He, Y.; Hao, H. BML-111, the agonist of lipoxin A4, suppresses epithelial-mesenchymal transition and migration of MCF-7 cells via regulating the lipoxygenase pathway. Int. J. Immunopathol. Pharmacol. 2023, 37, 3946320231223826. [Google Scholar] [CrossRef]
- Khau, T.; Langenbach, S.Y.; Schuliga, M.; Harris, T.; Johnstone, C.N.; Anderson, R.L.; Stewart, A.G. Annexin-1 signals mitogen-stimulated breast tumor cell proliferation by activation of the formyl peptide receptors (FPRs) 1 and 2. FASEB J. 2011, 25, 483–496. [Google Scholar] [CrossRef]
- Song, L.; Li, H.; Ma, R.R.; Liu, S.; Zhang, G.H.; Guo, X.Y.; Zhao, R.N.; Wu, X.J.; Zhang, K.; Gao, P. E2F1-initiated transcription of PRSS22 promotes breast cancer metastasis by cleaving ANXA1 and activating FPR2/ERK signaling pathway. Cell Death Dis. 2022, 13, 982. [Google Scholar] [CrossRef]
- Simiele, F.; Recchiuti, A.; Patruno, S.; Plebani, R.; Pierdomenico, A.M.; Codagnone, M.; Romano, M. Epigenetic regulation of the formyl peptide receptor 2 gene. Biochim. Biophys. Acta 2016, 1859, 1252–1258. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Wilson, M.; Elwin, C.E.; Norrving, B.; Algra, A.; Warlow, C.P.; Meade, T.W. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010, 376, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Burn, J.; Gerdes, A.M.; Macrae, F.; Mecklin, J.P.; Moeslein, G.; Olschwang, S.; Eccles, D.; Evans, D.G.; Maher, E.R.; Bertario, L.; et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: An analysis from the CAPP2 randomised controlled trial. Lancet 2011, 378, 2081–2087. [Google Scholar] [CrossRef]
- Burn, J.; Sheth, H.; Elliott, F.; Reed, L.; Macrae, F.; Mecklin, J.P.; Moslein, G.; McRonald, F.E.; Bertario, L.; Evans, D.G.; et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: A double-blind, randomised, placebo-controlled trial. Lancet 2020, 395, 1855–1863. [Google Scholar] [CrossRef]
- Simoni, O.; Scarpa, M.; Castagliuolo, I.; Stepanyan, A.; Angriman, I.; Kotsafti, A.; Nacci, C.; Scognamiglio, F.; Negro, S.; D’Angelo, A.; et al. IMMUNOREACT 7: Regular aspirin use is associated with immune surveillance activation in colorectal cancer. Cancer 2024, 130, 2272–2286. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xia, W.; Wu, B.; Sun, C.; Jiang, Y.; Liu, H.; Lowe, S.; Zhou, Z.; Xie, P.; Gao, J.; et al. Effect of aspirin on incidence, recurrence, and mortality in prostate cancer patients: Integrating evidence from randomized controlled trials and real-world studies. Eur. J. Clin. Pharmacol. 2023, 79, 1475–1503. [Google Scholar] [CrossRef]
- Koo, H.Y.; Jeong, S.M.; Cho, M.H.; Chun, S.; Shin, D.W.; Park, J. Population-wide impacts of aspirin, statins, and metformin use on prostate cancer incidence and mortality. Sci. Rep. 2021, 11, 16171. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Guo, C.; Sun, C.; Han, T.; Zhang, H.; Qu, G.; Jiang, Y.; Zhou, Q.; Sun, Y. Aspirin Use and Risk of Breast Cancer: A Meta-analysis of Observational Studies from 1989 to 2019. Clin. Breast Cancer 2021, 21, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Ballman, K.V.; Partridge, A.H.; Hahn, O.M.; Briccetti, F.M.; Irvin, W.J.; Symington, B.; Visvanathan, K.; Pohlmann, P.R.; Openshaw, T.H.; et al. Aspirin vs Placebo as Adjuvant Therapy for Breast Cancer: The Alliance A011502 Randomized Trial. JAMA 2024, 331, 1714–1721. [Google Scholar] [CrossRef]
- Benjamin, D.J.; Haslam, A.; Prasad, V. Cardiovascular/anti-inflammatory drugs repurposed for treating or preventing cancer: A systematic review and meta-analysis of randomized trials. Cancer Med. 2024, 13, e7049. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yamashita-Kanemaru, Y.; Morris, B.I.; Contursi, A.; Trajkovski, D.; Xu, J.; Patrascan, I.; Benson, J.; Evans, A.C.; Conti, A.G.; et al. Aspirin prevents metastasis by limiting platelet TXA(2) suppression of T cell immunity. Nature 2025, 640, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zhu, M.; Wu, R.; Lin, D.; Huang, Z.; Ren, L.; Huang, S.; Cheng, L.; Chen, Q. Lipoxin A(4) Suppresses IL-1beta-Induced Cyclooxygenase-2 Expression Through Inhibition of p38 MAPK Activation in Endometriosis. Reprod. Sci. 2019, 26, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Young, S.L.; Lessey, B.A. Progesterone function in human endometrium: Clinical perspectives. Semin. Reprod. Med. 2010, 28, 5–16. [Google Scholar] [CrossRef]
- Lai, Z.Z.; Yang, H.L.; Ha, S.Y.; Chang, K.K.; Mei, J.; Zhou, W.J.; Qiu, X.M.; Wang, X.Q.; Zhu, R.; Li, D.J.; et al. Cyclooxygenase-2 in Endometriosis. Int. J. Biol. Sci. 2019, 15, 2783–2797. [Google Scholar] [CrossRef]
- Takenaka, Y.; Taniguchi, F.; Miyakoda, H.; Takai, E.; Terakawa, N.; Harada, T. Lipopolysaccharide promoted proliferation and invasion of endometriotic stromal cells via induction of cyclooxygenase-2 expression. Fertil. Steril. 2010, 93, 325–327. [Google Scholar] [CrossRef]
- Binion, D.G.; Otterson, M.F.; Rafiee, P. Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut 2008, 57, 1509–1517. [Google Scholar] [CrossRef]
- Martinez-Limon, A.; Joaquin, M.; Caballero, M.; Posas, F.; de Nadal, E. The p38 Pathway: From Biology to Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 1913. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, W.; Pu, D.; Li, Z.; Huang, Q.; Chen, Q. The inhibitory effect of 15-R-LXA4 on experimental endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 145, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhou, W.; Chen, S.; Shi, Y.; Su, L.; Zhu, M.; Chen, Q.; Chen, Q. Lipoxin A4 suppresses the development of endometriosis in an ALX receptor-dependent manner via the p38 MAPK pathway. Br. J. Pharmacol. 2014, 171, 4927–4940. [Google Scholar] [CrossRef]
- Hasturk, H.; Schulte, F.; Martins, M.; Sherzai, H.; Floros, C.; Cugini, M.; Chiu, C.J.; Hardt, M.; Van Dyke, T. Safety and Preliminary Efficacy of a Novel Host-Modulatory Therapy for Reducing Gingival Inflammation. Front. Immunol. 2021, 12, 704163. [Google Scholar] [CrossRef] [PubMed]
- Bozinovski, S.; Uddin, M.; Vlahos, R.; Thompson, M.; McQualter, J.L.; Merritt, A.S.; Wark, P.A.; Hutchinson, A.; Irving, L.B.; Levy, B.D.; et al. Serum amyloid A opposes lipoxin A(4) to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA 2012, 109, 935–940. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saqib, U.; Pandey, M.; Vyas, A.; Patidar, P.; Hajela, S.; Ali, A.; Tiwari, M.; Sarkar, S.; Yadav, N.; Patel, S.; et al. Lipoxins as Modulators of Diseases. Cells 2025, 14, 1244. https://doi.org/10.3390/cells14161244
Saqib U, Pandey M, Vyas A, Patidar P, Hajela S, Ali A, Tiwari M, Sarkar S, Yadav N, Patel S, et al. Lipoxins as Modulators of Diseases. Cells. 2025; 14(16):1244. https://doi.org/10.3390/cells14161244
Chicago/Turabian StyleSaqib, Uzma, Monika Pandey, Anjali Vyas, Preeti Patidar, Sumati Hajela, Asgar Ali, Meenakshi Tiwari, Sutripta Sarkar, Neelam Yadav, Shivani Patel, and et al. 2025. "Lipoxins as Modulators of Diseases" Cells 14, no. 16: 1244. https://doi.org/10.3390/cells14161244
APA StyleSaqib, U., Pandey, M., Vyas, A., Patidar, P., Hajela, S., Ali, A., Tiwari, M., Sarkar, S., Yadav, N., Patel, S., Shukla, D., Lienemann, G. N., White, F. A., García-Perdomo, H. A., Baig, M. S., Halade, G. V., Hajela, K., Sharma, S., & Obukhov, A. G. (2025). Lipoxins as Modulators of Diseases. Cells, 14(16), 1244. https://doi.org/10.3390/cells14161244







