Age-Related Mitochondrial Alterations Contribute to Myocardial Responses During Sepsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. CLP-Induced Sepsis Model
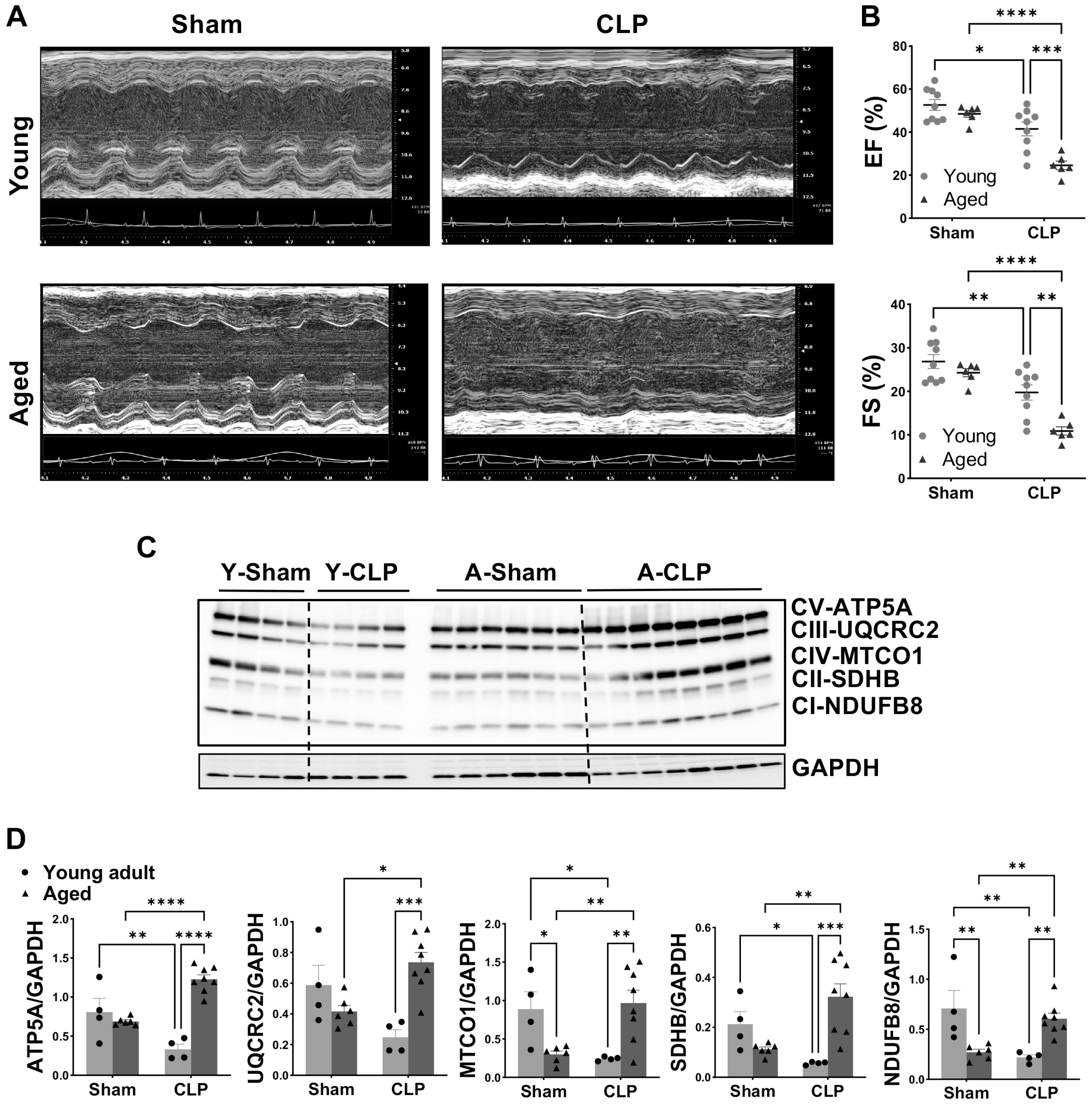
2.3. Echocardiographic Evaluation
2.4. Western Blotting
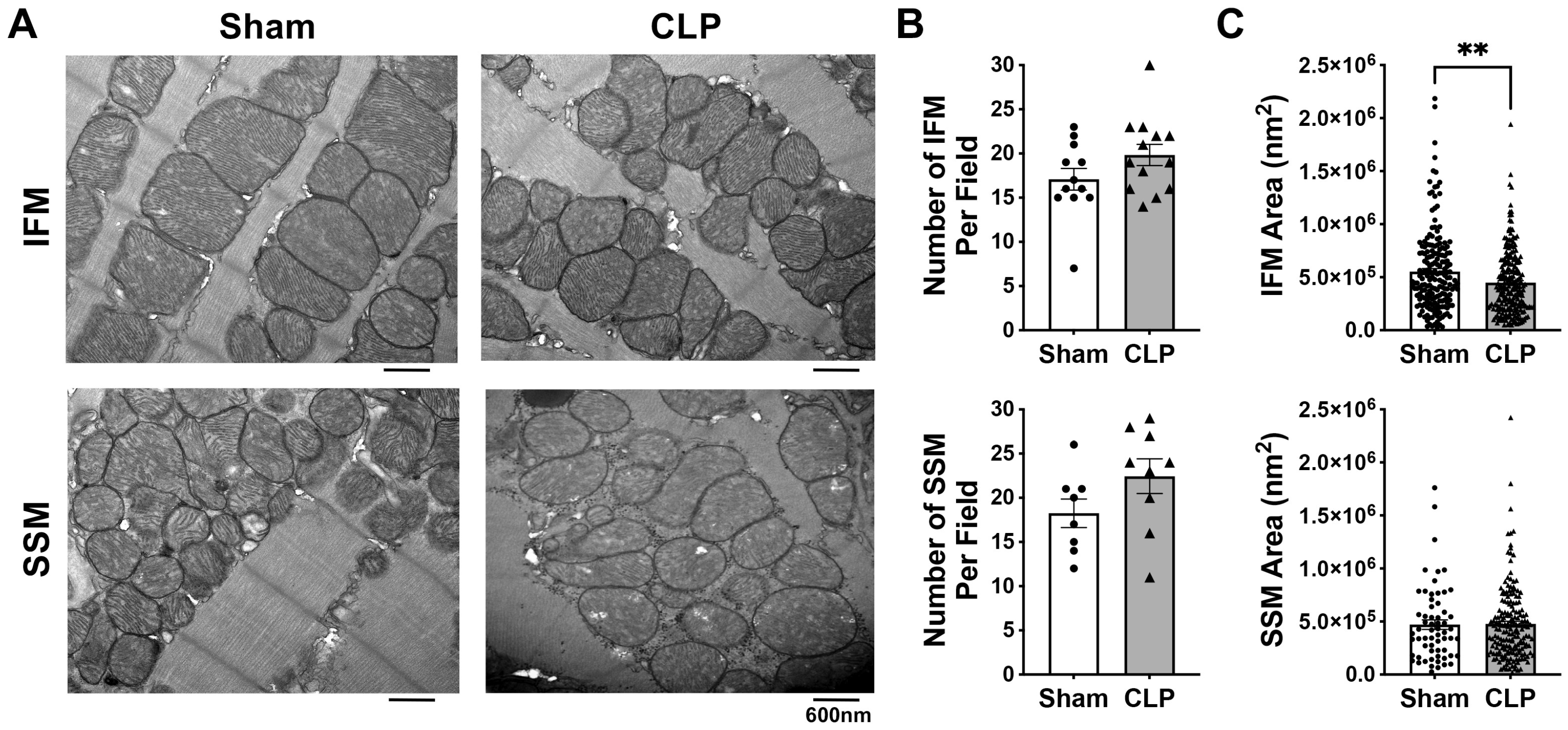
2.5. Transmission Electron Microscopy (TEM)
2.6. Mouse Cardiomyocyte Isolation
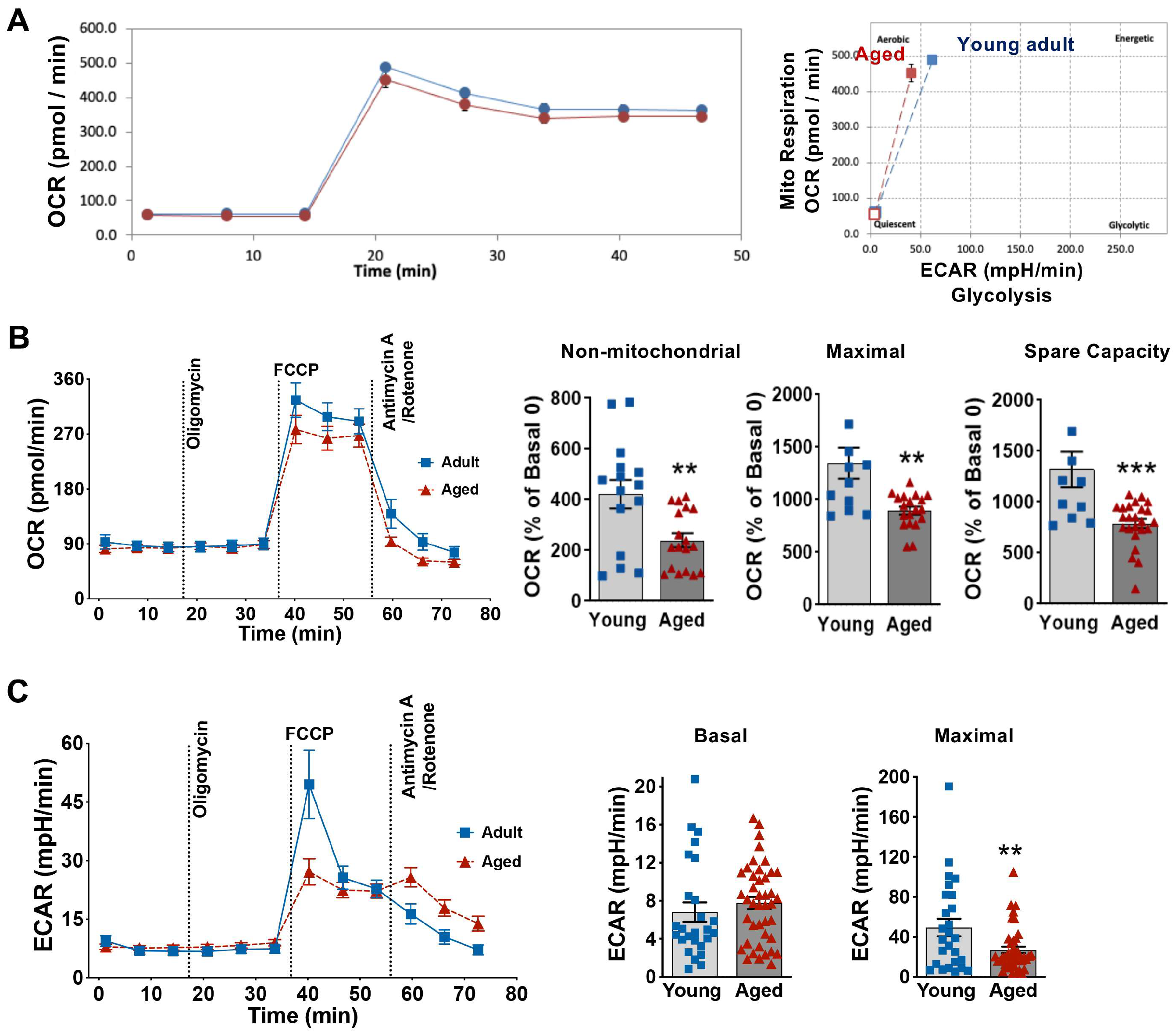
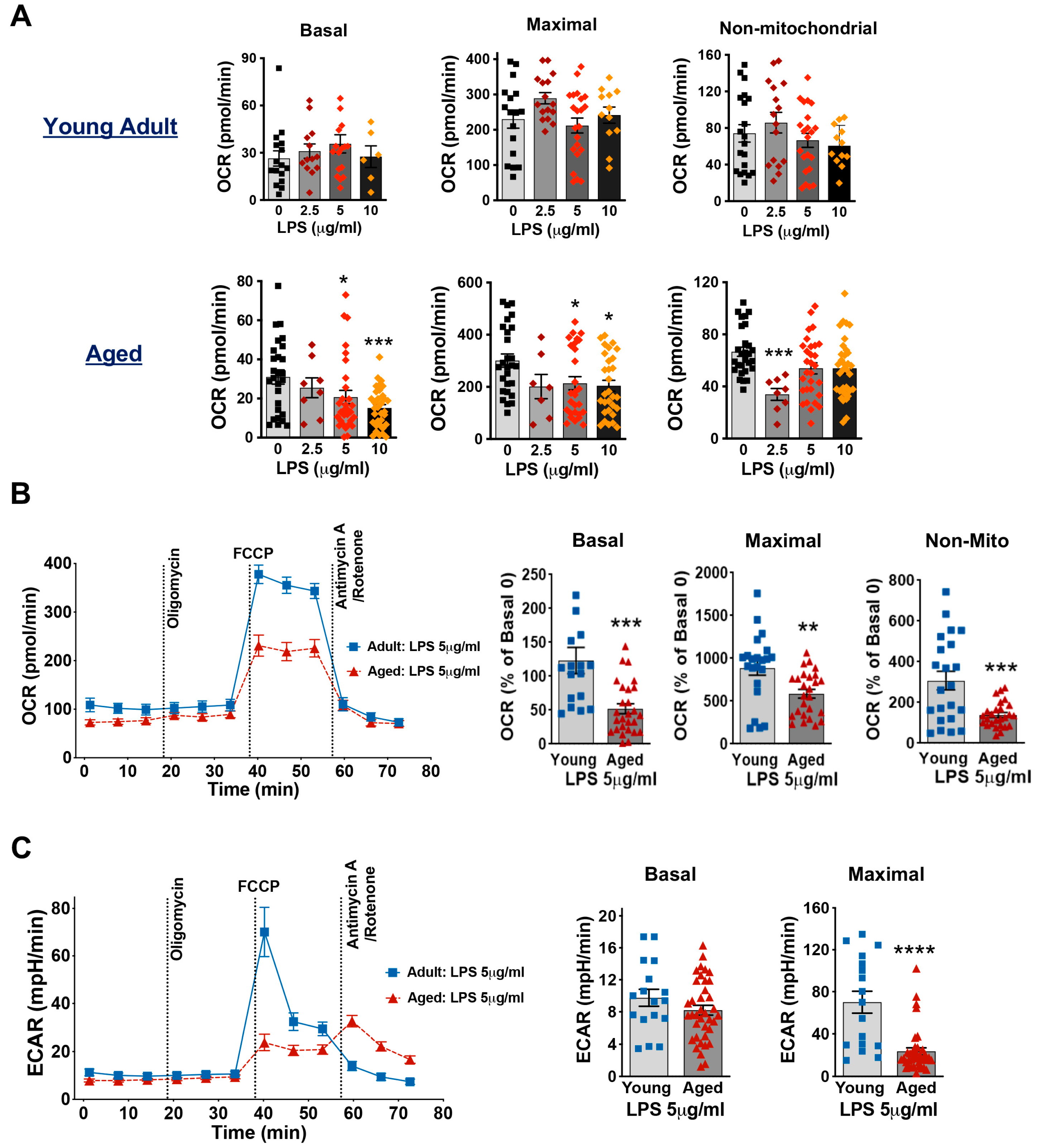
2.7. Measurement of Mitochondrial Bioenergetic Profiles
2.8. Detection of Mitochondrial Membrane Potential
2.9. Statistical Analysis
3. Results
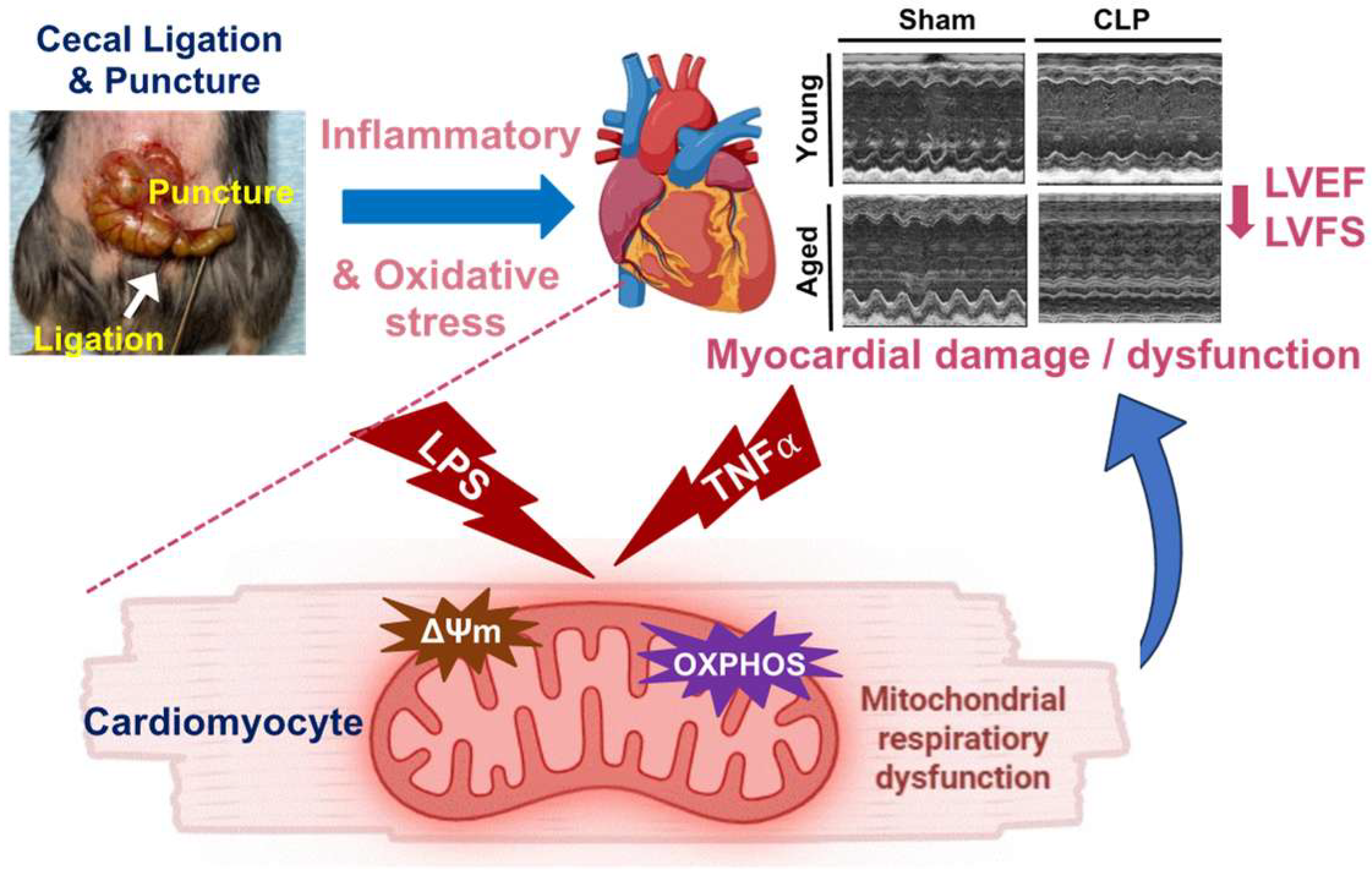
3.1. Worsened Cardiac Function and Altered Mitochondrial OXPHOS in Aged Mice Compared to Young Animals Following CLP
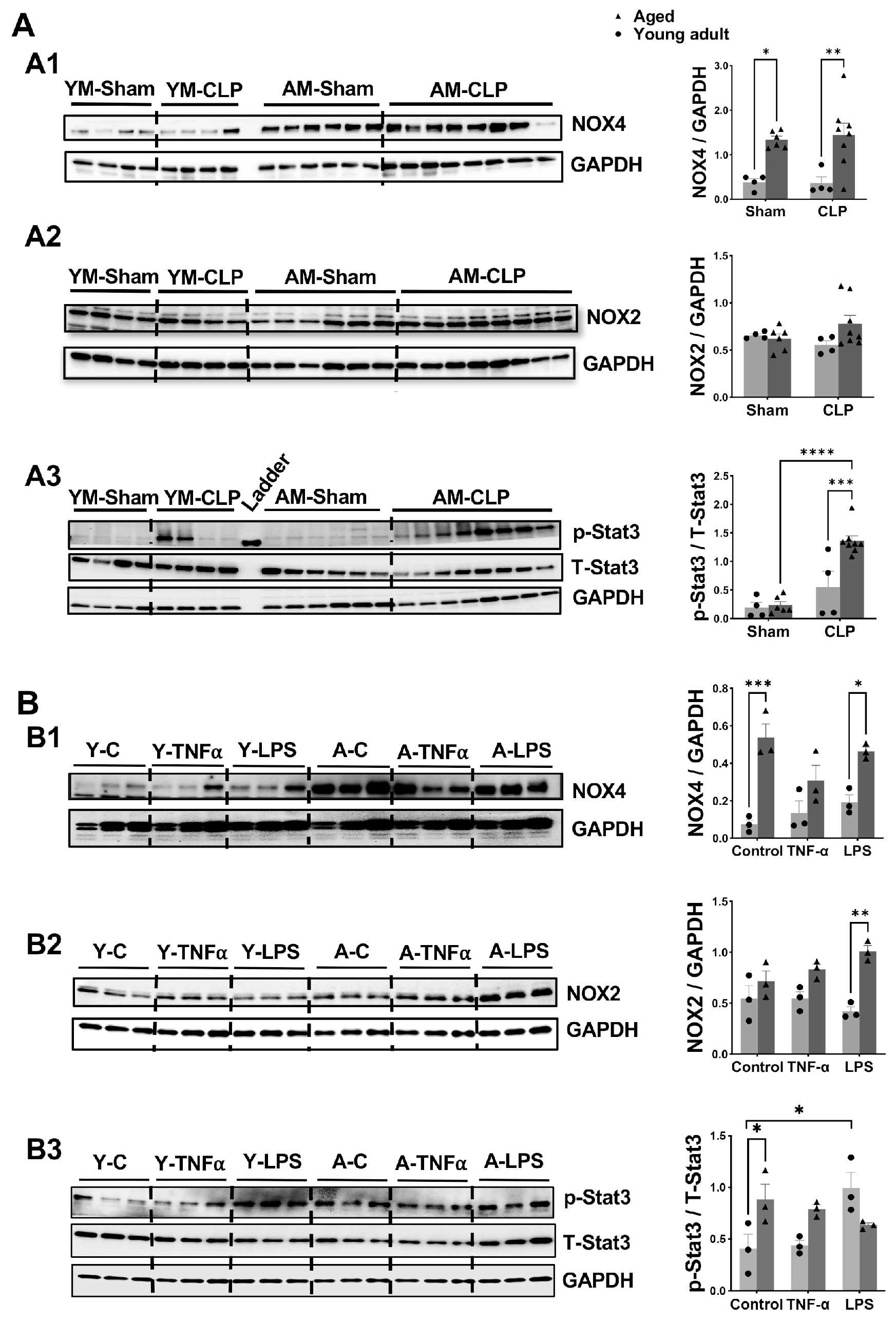
3.2. Effects of CLP on the Myocardial Mitochondrial Ultrastructure and Biogenesis in Aged Mice
3.3. Influence of Aging on Cardiomyocyte Energy Phenotype and Mitochondrial Metabolic Function
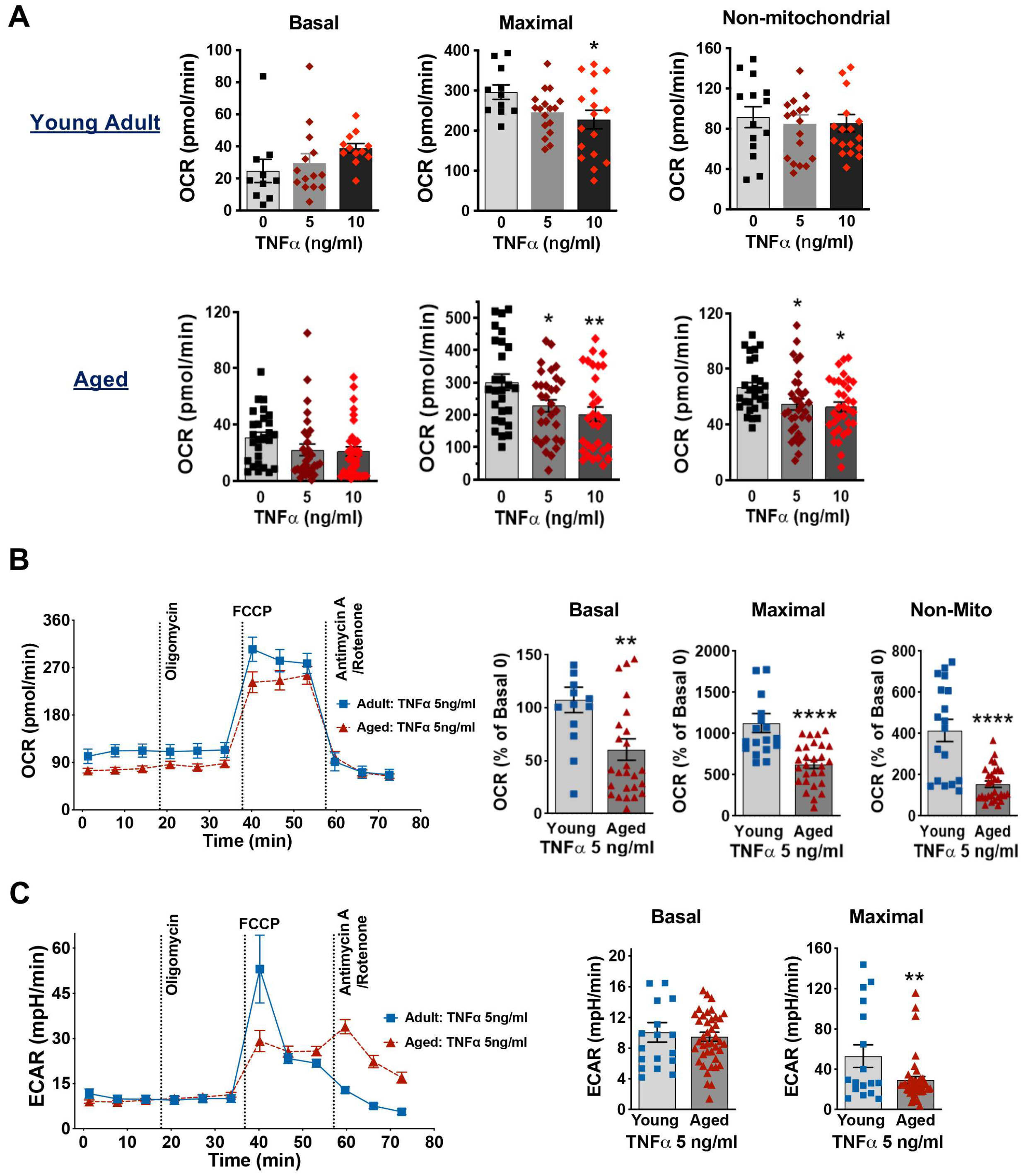
3.4. Aging Decreases Mitochondrial Respiration Function in Cardiomyocytes Exposed to LPS
3.5. Aging Worsens TNFα-Damaged Mitochondrial Respiratory Function in Cardiomyocytes
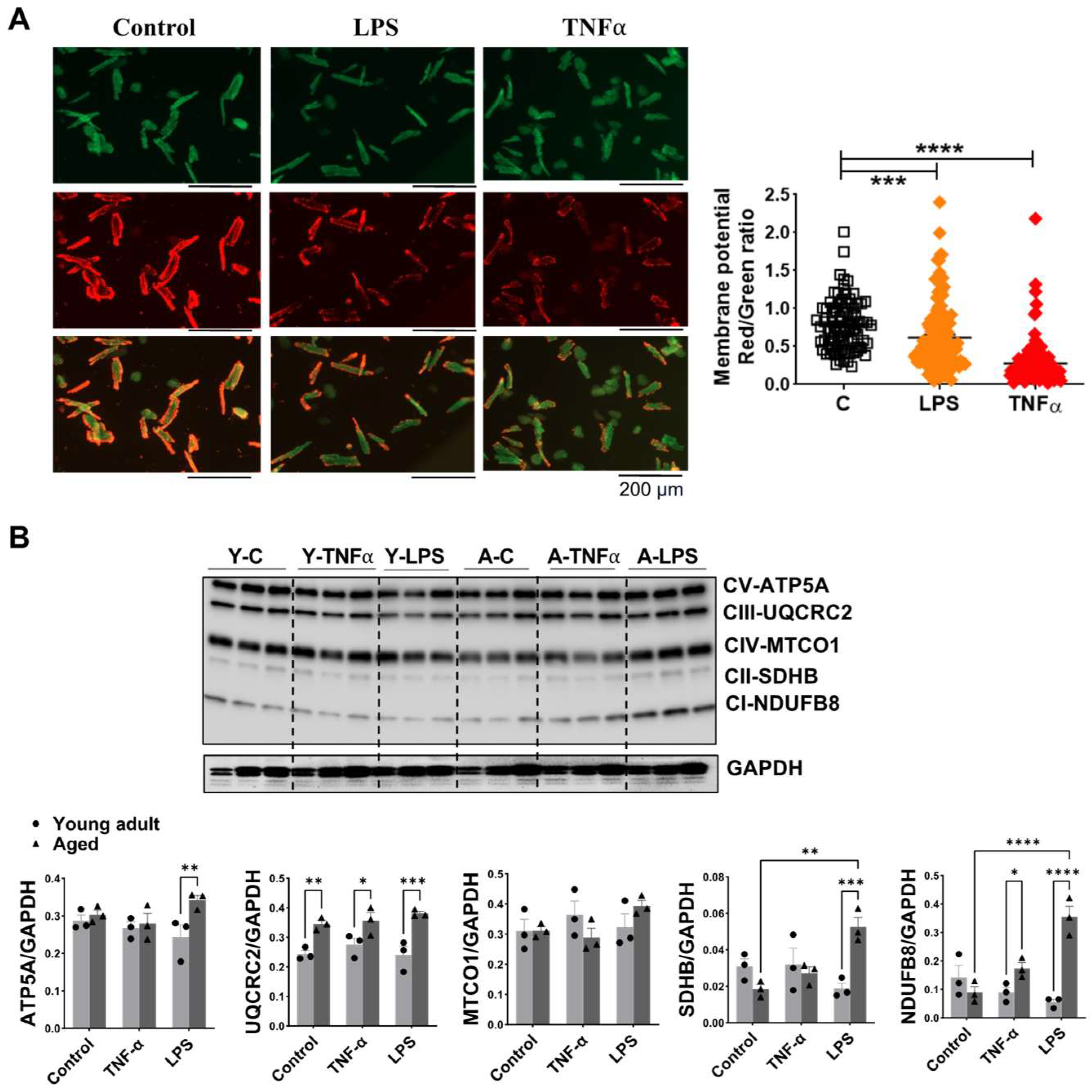
3.6. Impact of Aging on Mitochondrial Membrane Potential and OXPHOS in Mouse Cardiomyocytes Exposed to TNFα or LPS Stimulation
3.7. Changes in Signaling Molecules in Heart Tissue and Cardiomyocyte Following Inflammation in Aged Mice Compared to Young Adult Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLP | Cecal ligation and puncture |
| ECAR | Extracellular acidification rate |
| FCCP | Carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone |
| IFM | Interfibrillar mitochondria |
| I/R | Ischemia/reperfusion |
| LPS | lipopolysaccharide |
| LVEF | Left ventricular ejection fraction |
| LVFS | Left ventricular fractional shortening |
| MTCO1 | Mitochondrially encoded cytochrome c oxidase I |
| NDUFB8 | NADH–ubiquinone oxidoreductase subunit B8 |
| NOX | NADPH oxidases |
| OXPHOS | Oxidative phosphorylation |
| OCR | Oxygen consumption rate |
| ROS | Reactive oxygen species |
| SSM | Subsarcolemmal mitochondria |
| TEM | Transmission electron microscopy |
| TNFα | Tumor necrosis factor α |
| SDHB | Succinate Dehydrogenase iron-sulfur subunit |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| UQCRC2 | Ubiquinol–cytochrome c reductase core protein2 |
| ΔΨM | Mitochondrial membrane potential |
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Beesley, S.J.; Weber, G.; Sarge, T.; Nikravan, S.; Grissom, C.K.; Lanspa, M.J.; Shahul, S.; Brown, S.M. Septic Cardiomyopathy. Crit. Care Med. 2018, 46, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Derwall, M.; Al Zoubi, S.; Zechendorf, E.; Reuter, D.A.; Thiemermann, C.; Schuerholz, T. The Septic Heart: Current Understanding of Molecular Mechanisms and Clinical Implications. Chest 2019, 155, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Landesberg, G.; Gilon, D.; Meroz, Y.; Georgieva, M.; Levin, P.D.; Goodman, S.; Avidan, A.; Beeri, R.; Weissman, C.; Jaffe, A.S.; et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur. Heart. J. 2012, 33, 895–903. [Google Scholar] [CrossRef]
- Palmieri, V.; Innocenti, F.; Guzzo, A.; Guerrini, E.; Vignaroli, D.; Pini, R. Left Ventricular Systolic Longitudinal Function as Predictor of Outcome in Patients With Sepsis. Circ. Cardiovasc. Imaging 2015, 8, e003865. [Google Scholar] [CrossRef]
- Sanfilippo, F.; Corredor, C.; Fletcher, N.; Landesberg, G.; Benedetto, U.; Foex, P.; Cecconi, M. Diastolic dysfunction and mortality in septic patients: A systematic review and meta-analysis. Intensive Care Med. 2015, 41, 1004–1013. [Google Scholar] [CrossRef]
- Muller-Werdan, U.; Vogt, A.; Werdan, K. Septic cardiomyopathy-diagnosis and estimation of disease severity. Med. Klin. Intensive Notfmed. 2025, 120, 185–191. [Google Scholar]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Transduct. Target Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef]
- Protasoni, M.; Zeviani, M. Mitochondrial Structure and Bioenergetics in Normal and Disease Conditions. Int. J. Mol. Sci. 2021, 22, 586. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, R.; Jiang, X.; Lv, J.; Li, Y.; Ye, H.; Liu, W.; Wang, G.; Zhang, C.; Zheng, N.; et al. Toll-like receptor 4-induced ryanodine receptor 2 oxidation and sarcoplasmic reticulum Ca(2+) leakage promote cardiac contractile dysfunction in sepsis. J. Biol. Chem. 2018, 293, 794–807. [Google Scholar] [CrossRef]
- Bartz, R.R.; Suliman, H.B.; Piantadosi, C.A. Redox mechanisms of cardiomyocyte mitochondrial protection. Front Physiol. 2015, 6, 291. [Google Scholar] [CrossRef]
- Brealey, D.; Brand, M.; Hargreaves, I.; Heales, S.; Land, J.; Smolenski, R.; Davies, N.A.; Cooper, C.E.; Singer, M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002, 360, 219–223. [Google Scholar] [CrossRef]
- Pinto, B.B.; Dyson, A.; Umbrello, M.; Carre, J.E.; Ritter, C.; Clatworthy, I.; Duchen, M.R.; Singer, M. Improved Survival in a Long-Term Rat Model of Sepsis Is Associated With Reduced Mitochondrial Calcium Uptake Despite Increased Energetic Demand. Crit. Care Med. 2017, 45, e840–e848. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Martin, G.S.; Mannino, D.M.; Moss, M. The effect of age on the development and outcome of adult sepsis. Crit. Care Med. 2006, 34, 15–21. [Google Scholar] [CrossRef]
- Checchia, P.A.; Schierding, W.; Polpitiya, A.; Dixon, D.; Macmillan, S.; Muenzer, J.; Stromberg, P.; Coopersmith, C.M.; Buchman, T.G.; Cobb, J.P. Myocardial transcriptional profiles in a murine model of sepsis: Evidence for the importance of age. Pediatr. Crit. Care Med. 2008, 9, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Wieckowski, M.R.; Sinclair, D.A.; Kroemer, G.; Pinton, P.; Galluzzi, L. Targeting mitochondria for cardiovascular disorders: Therapeutic potential and obstacles. Nat. Rev. Cardiol. 2019, 16, 33–55. [Google Scholar] [CrossRef]
- Lucas, D.T.; Szweda, L.I. Cardiac reperfusion injury: Aging, lipid peroxidation, and mitochondrial dysfunction. Proc. Natl. Acad. Sci. USA 1998, 95, 510–514. [Google Scholar] [CrossRef]
- Shokolenko, I.; Venediktova, N.; Bochkareva, A.; Wilson, G.L.; Alexeyev, M.F. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 2009, 37, 2539–2548. [Google Scholar] [CrossRef]
- Salmonowicz, H.; Szczepanowska, K. The fate of mitochondrial respiratory complexes in aging. Trends Cell Biol. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.T.; Sokolowski, A.A.; Waldeck-Weiermair, M.; Malli, R.; Graier, W.F. Targeting Mitochondria to Counteract Age-Related Cellular Dysfunction. Genes 2018, 9, 165. [Google Scholar] [CrossRef]
- Remick, D.G.; Newcomb, D.E.; Bolgos, G.L.; Call, D.R. Comparison of the mortality and inflammatory response of two models of sepsis: Lipopolysaccharide vs. cecal ligation and puncture. Shock 2000, 13, 110–116. [Google Scholar]
- Deitch, E.A. Rodent models of intra-abdominal infection. Shock 2005, 24, 19–23. [Google Scholar] [CrossRef]
- Brianna, I.; Harvey, A.M.Y.; Monsivais, A.; Du, J.; Zadorozny, L.; Yu, Q.; Wang, M. Sex-Specific Differences in LPS-Induced Rapid Myocardial Dysfunction. Int. J. Mol. Sci. 2025, 26, 5963. [Google Scholar]
- Chen, J.; Chiazza, F.; Collino, M.; Patel, N.S.; Coldewey, S.M.; Thiemermann, C. Gender dimorphism of the cardiac dysfunction in murine sepsis: Signalling mechanisms and age-dependency. PLoS ONE 2014, 9, e100631. [Google Scholar] [CrossRef]
- Yang, K.; Fan, M.; Wang, X.; Xu, J.; Wang, Y.; Gill, P.S.; Ha, T.; Liu, L.; Hall, J.V.; Williams, D.L.; et al. Lactate induces vascular permeability via disruption of VE-cadherin in endothelial cells during sepsis. Sci. Adv. 2022, 8, eabm8965. [Google Scholar] [CrossRef]
- Wang, M.; Smith, K.; Yu, Q.; Miller, C.; Singh, K.; Sen, C.K. Mitochondrial connexin 43 in sex-dependent myocardial responses and estrogen-mediated cardiac protection following acute ischemia/reperfusion injury. Basic Res. Cardiol. 2019, 115, 1. [Google Scholar] [CrossRef]
- Scott, S.R.; Singh, K.; Yu, Q.; Sen, C.K.; Wang, M. Sex as Biological Variable in Cardiac Mitochondrial Bioenergetic Responses to Acute Stress. Int. J. Mol. Sci. 2022, 23, 9312. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Singh, J.; Scott, S.R.; Ellis, B.; Zorlutuna, P.; Wang, M. A Recombinant Dimethylarginine Dimethylaminohydrolase-1-Based Biotherapeutics to Pharmacologically Lower Asymmetric Dimethyl Arginine, thus Improving Postischemic Cardiac Function and Cardiomyocyte Mitochondrial Activity. Mol. Pharmacol. 2022, 101, 226–235. [Google Scholar] [CrossRef]
- Kim, M.; Nikouee, A.; Zou, R.; Ren, D.; He, Z.; Li, J.; Wang, L.; Djukovic, D.; Raftery, D.; Purcell, H.; et al. Age-Independent Cardiac Protection by Pharmacological Activation of Beclin-1 During Endotoxemia and Its Association With Energy Metabolic Reprograming in Myocardium-A Targeted Metabolomics Study. J. Am. Heart Assoc. 2022, 11, e025310. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, R.; Dai, Q.; Xu, G.; Zhang, G. The role and mechanism of the NLRP3-IL-1beta/IL-18 signaling axis in the progression of sepsis under an aging phenotype. Life Sci. 2025, 378, 123812. [Google Scholar] [CrossRef]
- Court, O.; Kumar, A.; Parrillo, J.E.; Kumar, A. Clinical review: Myocardial depression in sepsis and septic shock. Crit. Care 2002, 6, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Emelyanova, L.; Preston, C.; Gupta, A.; Viqar, M.; Negmadjanov, U.; Edwards, S.; Kraft, K.; Devana, K.; Holmuhamedov, E.; O’Hair, D.; et al. Effect of Aging on Mitochondrial Energetics in the Human Atria. J. Gerontol. A. Biol. Sci. Med. Sci. 2018, 73, 608–616. [Google Scholar] [CrossRef]
- Dickson, K.; Lehmann, C. Inflammatory Response to Different Toxins in Experimental Sepsis Models. Int. J. Mol. Sci. 2019, 20, 4341. [Google Scholar] [CrossRef]
- van der Poll, T.; van Deventer, S.J. Cytokines and anticytokines in the pathogenesis of sepsis. Infect. Dis. Clin. N. Am. 1999, 13, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Redl, H.; Schlag, G.; Adolf, G.R.; Natmessnig, B.; Davies, J. Tumor necrosis factor (TNF)-dependent shedding of the p55 TNF receptor in a baboon model of bacteremia. Infect. Immun. 1995, 63, 297–300. [Google Scholar] [CrossRef]
- Bhaskara, M.; Anjorin, O.; Yoniles, A.; Liu, J.; Wang, M. Importance of Per2 in cardiac mitochondrial protection during stress. Sci. Rep. 2024, 14, 1290. [Google Scholar] [CrossRef]
- Fu, X.Y. STAT3 in immune responses and inflammatory bowel diseases. Cell Res. 2006, 16, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker-Kleiner, D.; Hilfiker, A.; Drexler, H. Many good reasons to have STAT3 in the heart. Pharmacol. Ther. 2005, 107, 131–137. [Google Scholar] [CrossRef]
- Levy, D.E.; Darnell, J.E., Jr. Stats: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Sasai, T.; Tokioka, H.; Fukushima, T.; Mikane, T.; Oku, S.; Iwasaki, E.; Ishii, M.; Mieda, H.; Ishikawa, T.; Minami, E. Reliability of central venous pressure to assess left ventricular preload for fluid resuscitation in patients with septic shock. J. Intensive Care. 2014, 2, 58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, X.; Su, Y.; Liu, G. Prognostic value of echocardiography parameters, peripheral blood T lymphocyte subpopulations, NF-kappaB, and CD64 levels in neonatal sepsis. Am. J. Transl. Res. 2024, 16, 6140–6147. [Google Scholar] [CrossRef]
- Kang, R.; Laborde, C.; Savchenko, L.; Swiader, A.; Pizzinat, N.; Marsal, D.; Sainte-Marie, Y.; Boal, F.; Tronchere, H.; Roncalli, J.; et al. Age-Related Shift in Cardiac and Metabolic Phenotyping Linked to Inflammatory Cytokines and Antioxidant Status in Mice. Int. J. Mol. Sci. 2023, 24, 15841. [Google Scholar] [CrossRef]
- Haileselassie, B.; Mukherjee, R.; Joshi, A.U.; Napier, B.A.; Massis, L.M.; Ostberg, N.P.; Queliconi, B.B.; Monack, D.; Bernstein, D.; Mochly-Rosen, D. Drp1/Fis1 interaction mediates mitochondrial dysfunction in septic cardiomyopathy. J. Mol. Cell. Cardiol. 2019, 130, 160–169. [Google Scholar] [CrossRef]
- Park, C.B.; Larsson, N.G. Mitochondrial DNA mutations in disease and aging. J. Cell Biol. 2011, 193, 809–818. [Google Scholar] [CrossRef]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Argmann, C.; Houten, S.M.; Canto, C.; Jeninga, E.H.; Andreux, P.A.; Thomas, C.; Doenlen, R.; Schoonjans, K.; Auwerx, J. The metabolic footprint of aging in mice. Sci. Rep. 2011, 1, 134. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Ji, D.; Yin, J.Y.; Li, D.F.; Zhu, C.T.; Ye, J.P.; Pan, Y.Q. Effects of inflammatory and anti-inflammatory environments on the macrophage mitochondrial function. Sci. Rep. 2020, 10, 20324. [Google Scholar] [CrossRef]
- Navarro, A.; Boveris, A. The mitochondrial energy transduction system and the aging process. Am. J. Physiol. Cell Physiol. 2007, 292, C670–C686. [Google Scholar] [CrossRef]
- Andreu, A.L.; Arbos, M.A.; Perez-Martos, A.; Lopez-Perez, M.J.; Asin, J.; Lopez, N.; Montoya, J.; Schwartz, S. Reduced mitochondrial DNA transcription in senescent rat heart. Biochem. Biophys. Res. Commun. 1998, 252, 577–581. [Google Scholar] [CrossRef]
- Tatarkova, Z.; Kuka, S.; Racay, P.; Lehotsky, J.; Dobrota, D.; Mistuna, D.; Kaplan, P. Effects of aging on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol. Res. 2011, 60, 281–289. [Google Scholar] [CrossRef]
- Dai, D.F.; Chen, T.; Johnson, S.C.; Szeto, H.; Rabinovitch, P.S. Cardiac aging: From molecular mechanisms to significance in human health and disease. Antioxid. Redox Signal. 2012, 16, 1492–1526. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.F.; Santana, L.F.; Vermulst, M.; Tomazela, D.M.; Emond, M.J.; MacCoss, M.J.; Gollahon, K.; Martin, G.M.; Loeb, L.A.; Ladiges, W.C.; et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 2009, 119, 2789–2797. [Google Scholar] [CrossRef]
- Dai, D.F.; Chen, T.; Wanagat, J.; Laflamme, M.; Marcinek, D.J.; Emond, M.J.; Ngo, C.P.; Prolla, T.A.; Rabinovitch, P.S. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell 2010, 9, 536–544. [Google Scholar] [CrossRef]
- Sagar, S.; Gustafsson, A.B. Cardiovascular aging: The mitochondrial influence. J. Cardiovasc. Aging 2023, 3, 33. [Google Scholar] [CrossRef]
- Ramirez-Camacho, I.; Flores-Herrera, O.; Zazueta, C. The relevance of the supramolecular arrangements of the respiratory chain complexes in human diseases and aging. Mitochondrion 2019, 47, 266–272. [Google Scholar] [CrossRef]
- Chen, Q.; Thompson, J.; Hu, Y.; Lesnefsky, E.J. Aging-induced mitochondrial dysfunction: Two distinct populations of mitochondria versus a combined population. Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H385–H395. [Google Scholar] [CrossRef] [PubMed]
- Brandt, T.; Mourier, A.; Tain, L.S.; Partridge, L.; Larsson, N.G.; Kuhlbrandt, W. Changes of mitochondrial ultrastructure and function during ageing in mice and Drosophila. Elife 2017, 6, e24662. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yao, X.; Zhang, Q.J.; Zhu, M.; Liu, Z.P.; Ci, B.; Xie, Y.; Carlson, D.; Rothermel, B.A.; Sun, Y.; et al. Beclin-1-Dependent Autophagy Protects the Heart During Sepsis. Circulation 2018, 138, 2247–2262. [Google Scholar] [CrossRef]
- Kim, M.; Nikouee, A.; Sun, Y.; Zhang, Q.J.; Liu, Z.P.; Zang, Q.S. Evaluation of Parkin in the Regulation of Myocardial Mitochondria-Associated Membranes and Cardiomyopathy During Endotoxemia. Front. Cell Dev. Biol. 2022, 10, 796061. [Google Scholar] [CrossRef]
- Chen, X.S.; Cui, J.R.; Meng, X.L.; Wang, S.H.; Wei, W.; Gao, Y.L.; Shou, S.T.; Liu, Y.C.; Chai, Y.F. Angiotensin-(1-7) ameliorates sepsis-induced cardiomyopathy by alleviating inflammatory response and mitochondrial damage through the NF-kappaB and MAPK pathways. J. Transl. Med. 2023, 21, 2. [Google Scholar] [CrossRef]
- Drosatos, K.; Khan, R.S.; Trent, C.M.; Jiang, H.; Son, N.H.; Blaner, W.S.; Homma, S.; Schulze, P.C.; Goldberg, I.J. Peroxisome proliferator-activated receptor-gamma activation prevents sepsis-related cardiac dysfunction and mortality in mice. Circ. Heart Fail. 2013, 6, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Okada, M.; Ijiri, E.; Koga, D.; Watanabe, T.; Hayashi, K.; Kashiwagi, Y.; Fujita, S.; Hasebe, N. beta(3)-Adrenergic receptor blockade reduces mortality in endotoxin-induced heart failure by suppressing induced nitric oxide synthase and saving cardiac metabolism. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H283–H294. [Google Scholar] [CrossRef]
- Palmer, J.W.; Tandler, B.; Hoppel, C.L. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J. Biol. Chem. 1977, 252, 8731–8739. [Google Scholar] [CrossRef]
- Chen, Q.; Paillard, M.; Gomez, L.; Li, H.; Hu, Y.; Lesnefsky, E.J. Postconditioning modulates ischemia-damaged mitochondria during reperfusion. J. Cardiovasc. Pharmacol. 2012, 59, 101–108. [Google Scholar] [CrossRef]
- Joseph, L.C.; Reyes, M.V.; Lakkadi, K.R.; Gowen, B.H.; Hasko, G.; Drosatos, K.; Morrow, J.P. PKCdelta causes sepsis-induced cardiomyopathy by inducing mitochondrial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H778–H786. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, Y.T.; Teng, F.; Li, H.H.; Guo, S.B. S100a8/a9 contributes to sepsis-induced cardiomyopathy by activating ERK1/2-Drp1-mediated mitochondrial fission and respiratory dysfunction. Int. Immunopharmacol. 2023, 115, 109716. [Google Scholar] [CrossRef]
- Ago, T.; Kuroda, J.; Pain, J.; Fu, C.; Li, H.; Sadoshima, J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010, 106, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, J.; Ago, T.; Matsushima, S.; Zhai, P.; Schneider, M.D.; Sadoshima, J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. USA 2010, 107, 15565–15570. [Google Scholar] [CrossRef]
- Joseph, L.C.; Kokkinaki, D.; Valenti, M.C.; Kim, G.J.; Barca, E.; Tomar, D.; Hoffman, N.E.; Subramanyam, P.; Colecraft, H.M.; Hirano, M.; et al. Inhibition of NADPH oxidase 2 (NOX2) prevents sepsis-induced cardiomyopathy by improving calcium handling and mitochondrial function. JCI Insight 2017, 2, e94248. [Google Scholar] [CrossRef]
- Nikouee, A.; Yap, J.Q.; Rademacher, D.J.; Kim, M.; Zang, Q.S. An optimized Langendorff-free method for isolation and characterization of primary adult cardiomyocytes. BMC Cardiovasc. Disord. 2024, 24, 649. [Google Scholar] [CrossRef] [PubMed]
- Mondragon, R.R.; Wang, S.; Stevenson, M.D.; Lozhkin, A.; Vendrov, A.E.; Isom, L.L.; Runge, M.S.; Madamanchi, N.R. NOX4-driven mitochondrial oxidative stress in aging promotes myocardial remodeling and increases susceptibility to ventricular tachyarrhythmia. Free Radic. Biol. Med. 2025, 235, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Cao, Z.; Xu, X.; van Meir, E.G.; Lambeth, J.D. Homologs of gp91phox: Cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 2001, 269, 131–140. [Google Scholar] [CrossRef]
- Zhang, M.; Brewer, A.C.; Schroder, K.; Santos, C.X.; Grieve, D.J.; Wang, M.; Anilkumar, N.; Yu, B.; Dong, X.; Walker, S.J.; et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 18121–18126. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Jacoby, J.J.; Kalinowski, A.; Liu, M.G.; Zhang, S.S.; Gao, Q.; Chai, G.X.; Ji, L.; Iwamoto, Y.; Li, E.; Schneider, M.; et al. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc. Natl. Acad. Sci. USA 2003, 100, 12929–12934. [Google Scholar] [CrossRef]
- Boengler, K.; Buechert, A.; Heinen, Y.; Roeskes, C.; Hilfiker-Kleiner, D.; Heusch, G.; Schulz, R. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ. Res. 2008, 102, 131–135. [Google Scholar] [CrossRef]
- Yang, B.; Li, T.; Wang, Z.; Zhu, Y.; Niu, K.; Hu, S.; Lin, Z.; Zheng, X.; Jin, X.; Shen, C. Ruxolitinib-based senomorphic therapy mitigates cardiomyocyte senescence in septic cardiomyopathy by inhibiting the JAK2/STAT3 signaling pathway. Int. J. Biol. Sci. 2024, 20, 4314–4340. [Google Scholar] [CrossRef]
- Madonna, R.; Jiang, J.; Geng, Y.J. Attenuated expression of gelsolin in association with induction of aquaporin-1 and nitric oxide synthase in dysfunctional hearts of aging mice exposed to endotoxin. Int. J. Immunopathol. Pharmacol. 2012, 25, 911–922. [Google Scholar] [CrossRef]
- Saito, H.; Papaconstantinou, J. Age-associated differences in cardiovascular inflammatory gene induction during endotoxic stress. J. Biol. Chem. 2001, 276, 29307–29312. [Google Scholar] [CrossRef]
- Zhao, C.; Le, X.; Li, M.; Hu, Y.; Li, X.; Chen, Z.; Hu, G.; Hu, L.; Li, Q. Inhibition of Hsp110-STAT3 interaction in endothelial cells alleviates vascular remodeling in hypoxic pulmonary arterial Hypertension model. Respir. Res. 2023, 24, 289. [Google Scholar] [CrossRef]
- Li, N.; Wu, H.; Geng, R.; Tang, Q. Identification of Core Gene Biomarkers in Patients with Diabetic Cardiomyopathy. Dis. Markers. 2018, 2018, 6025061. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.J.; Yu, Q.; Chen, K.; Mahadev, K.; Zhang, S.X. Inhibition of reactive oxygen species by Lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: Role of NADPH oxidase 4. Diabetes 2010, 59, 1528–1538. [Google Scholar] [CrossRef]
- Manea, A.; Tanase, L.I.; Raicu, M.; Simionescu, M. Jak/STAT signaling pathway regulates nox1 and nox4-based NADPH oxidase in human aortic smooth muscle cells. Arter. Thromb. Vasc. Biol. 2010, 30, 105–112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Yu, Q.; Anjorin, O.E.; Wang, M. Age-Related Mitochondrial Alterations Contribute to Myocardial Responses During Sepsis. Cells 2025, 14, 1221. https://doi.org/10.3390/cells14151221
Du J, Yu Q, Anjorin OE, Wang M. Age-Related Mitochondrial Alterations Contribute to Myocardial Responses During Sepsis. Cells. 2025; 14(15):1221. https://doi.org/10.3390/cells14151221
Chicago/Turabian StyleDu, Jiayue, Qing Yu, Olufisayo E. Anjorin, and Meijing Wang. 2025. "Age-Related Mitochondrial Alterations Contribute to Myocardial Responses During Sepsis" Cells 14, no. 15: 1221. https://doi.org/10.3390/cells14151221
APA StyleDu, J., Yu, Q., Anjorin, O. E., & Wang, M. (2025). Age-Related Mitochondrial Alterations Contribute to Myocardial Responses During Sepsis. Cells, 14(15), 1221. https://doi.org/10.3390/cells14151221





