Synthetic Small-Molecule Ligands Targeted to Adenosine Receptors: Is There Potential Towards Ischemic Heart Disease?
Abstract
1. Introduction
2. Adenosine
3. Historical Perspective of AR Targeting and ‘Cardioprotection’
4. Basic Summary of Adenosine Receptors (ARs) and Cell Biology Implications
5. AR Cross-Talks with Other Signalling Pathways
6. AR Synthetic Small-Molecule Ligands
7. Current Gaps in Clinical Translation of AR Ligands
8. Discussion
8.1. RPR749 (A1R Agonist)
8.2. LJ-1888 (A3R Antagonist)
8.3. IB-MECA (A3R Agonist)
8.4. GS-9667 (A1R Agonist)
8.5. LassBio-294 (A2AR Agonist)
8.6. CPA (A1R Agonist)
8.7. PSB-15826 (A2AR Agonist)
9. Insights into the Use of AR Synthetic Ligands in IHD
10. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, R.; Cai, M.; Wang, Z.; Liu, X.; Pei, J.; Zaid, M.; Xu, W.; Chowdhury, S.R. Incidence trends and specific risk factors of ischemic heart disease and stroke: An ecological analysis based on the Global Burden of Disease 2019. PLOS Glob. Public Health 2024, 4, e0003920. [Google Scholar] [CrossRef]
- Moran, A.E.; Forouzanfar, M.H.; Roth, G.A.; Mensah, G.A.; Ezzati, M.; Murray, C.J.; Naghavi, M. Temporal Trends in Ischemic Heart Disease Mortality in 21 World Regions, 1980 to 2010. Circulation 2014, 129, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, R.C.; Tong, X.; Khan, S.S.; Shah, N.S.; Jackson, S.L.; Loustalot, F.; Vaughan, A.S. Trends in Cardiovascular Disease Mortality Rates and Excess Deaths, 2010–2022. Am. J. Prev. Med. 2023, 66, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Conrad, N.; Molenberghs, G.; Verbeke, G.; Zaccardi, F.; Lawson, C.; Friday, J.M.; Su, H.; Jhund, P.S.; Sattar, N.; Rahimi, K.; et al. Trends in cardiovascular disease incidence among 22 million people in the UK over 20 years: Population based study. BMJ 2024, 385, e078523. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Guan, C.; Wu, S.; Xu, W.; Zhang, J. Global, regional, and national burden of ischaemic heart disease and its trends, 1990–2019. Public Health 2023, 223, 57–66. [Google Scholar] [CrossRef]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef]
- Santucci, A.; Riccini, C.; Cavallini, C. Treatment of stable ischaemic heart disease: The old and the new. Eur. Hear. J. Suppl. 2020, 22, E54–E59. [Google Scholar] [CrossRef]
- Cohn, P.F. Pharmacological treatment of ischaemic heart disease: Monotherapy vs combination therapy. Eur. Hear. J. 1997, 18, 27–34. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Hear. J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Vane, J.R.; Flower, R.J.; Botting, R.M. History of aspirin and its mechanism of action. Stroke 1990, 21, IV12–IV23. [Google Scholar]
- Damman, P.; Woudstra, P.; Kuijt, W.J.; de Winter, R.J.; James, S.K. P2Y12 platelet inhibition in clinical practice. J. Thromb. Thrombolysis 2011, 33, 143–153. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Fox, K.A.; Hacke, W.; Berger, P.B.; Black, H.R.; Boden, W.E.; Cacoub, P.; Cohen, E.A.; Creager, M.A.; Easton, J.D.; et al. Clopidogrel and Aspirin versus Aspirin Alone for the Prevention of Atherothrombotic Events. N. Engl. J. Med. 2006, 354, 1706–1717. [Google Scholar] [CrossRef]
- The ACTIVE Investigators. Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 360, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- D’AMario, D.; Galli, M.; Restivo, A.; Canonico, F.; Vergallo, R.; Migliaro, S.; Trani, C.; Burzotta, F.; Aurigemma, C.; Laborante, R.; et al. Ticagrelor enhances the cardioprotective effects of ischemic preconditioning in stable patients undergoing percutaneous coronary intervention: The TAPER-S randomized study. Eur. Hear. J.Cardiovasc. Pharmacother. 2023, 10, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiu, H.; Yan, L.; Guo, T.; Wang, Y.; Li, Y.; Zheng, J.; Tang, Y.; Xu, B.; Qiao, S.; et al. Efficacy and Safety of Ticagrelor and Clopidogrel in Patients with Stable Coronary Artery Disease Undergoing Percutaneous Coronary Intervention. J. Atheroscler. Thromb. 2021, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell. Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef]
- Li, S.; Schooling, C.M. Investigating the effects of statins on ischemic heart disease allowing for effects on body mass index: A Mendelian randomization study. Sci. Rep. 2022, 12, 3478. [Google Scholar] [CrossRef]
- Ambrosioni, E.; Borghi, C.; Magnani, B. The Effect of the Angiotensin-Converting–Enzyme Inhibitor Zofenopril on Mortality and Morbidity after Anterior Myocardial Infarction. N. Engl. J. Med. 1995, 332, 80–85. [Google Scholar] [CrossRef]
- Pitt, B.; Poole-Wilson, P.A.; Segal, R.; Martinez, F.A.; Dickstein, K.; Camm, A.J.; Konstam, M.A.; Riegger, G.; Klinger, G.H.; Neaton, J.; et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: Randomised trial—The Losartan Heart Failure Survival Study ELITE II. Lancet 2000, 355, 1582–1587. [Google Scholar] [CrossRef]
- McMurray, J.J.; Östergren, J.; Swedberg, K.; Granger, C.B.; Held, P.; Michelson, E.L.; Olofsson, B.; Yusuf, S.; Pfeffer, M.A. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: The CHARM-Added trial. Lancet 2003, 362, 767–771. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Hu, S.; Bai, X.; Li, X.; Li, J.; Zheng, X. Use of angiotensin receptor blocker is associated with improved 1 year mortality in heart failure with mid-range ejection fraction. ESC Hear. Fail. 2021, 8, 1438–1445. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Johansson, S.; Wang, Y.Q. Adenosine and the Regulation of Metabolism and Body Temperature. Adv. Pharmacol. 2011, 61, 77–94. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Arslan, G.; Halldner, L.; Kull, B.; Schulte, G.; Wasserman, W. Structure and function of adenosine receptors and their genes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000, 362, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, F.; Pasquini, S.; Contri, C.; Cappello, M.; Nigro, M.; Travagli, A.; Merighi, S.; Gessi, S.; Borea, P.A.; Varani, K. Pharmacology of Adenosine Receptors: Recent Advancements. Biomolecules 2023, 13, 1387. [Google Scholar] [CrossRef] [PubMed]
- Feoktistov, I.; Biaggioni, I.; Cronstein, B.N. Adenosine Receptors in Wound Healing, Fibrosis and Angiogenesis. Handb Exp Pharmacol. 2009, 193, 383–397. [Google Scholar]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Kubacka, M.; Mogilski, S.; Bednarski, M.; Pociecha, K.; Świerczek, A.; Nicosia, N.; Schabikowski, J.; Załuski, M.; Chłoń-Rzepa, G.; Hockemeyer, J.; et al. Antiplatelet Effects of Selected Xanthine-Based Adenosine A2A and A2B Receptor Antagonists Determined in Rat Blood. Int. J. Mol. Sci. 2023, 24, 13378. [Google Scholar] [CrossRef]
- Wolska, N.; Rozalski, M. Blood Platelet Adenosine Receptors as Potential Targets for Anti-Platelet Therapy. Int. J. Mol. Sci. 2019, 20, 5475. [Google Scholar] [CrossRef]
- Ely, S.W.; Berne, R.M. Protective effects of adenosine in myocardial ischemia. Circulation 1992, 85, 893–904. [Google Scholar] [CrossRef]
- Shneyvays, V.; Mamedova, L.K.; Leshem, D.; Korkus, A.; Shainberg, A. Insights into the cardioprotective function of aden-osine A1 and A3 receptors. Exp. Clin. Cardiol. 2002, 7, 138. [Google Scholar]
- Thourani, V.H.; Ronson, R.S.; Jordan, J.E.; Guyton, R.A.; Vinten-Johansen, J. Adenosine A3 pretreatment before cardioplegic arrest attenuates postischemic cardiac dysfunction. Ann. Thorac. Surg. 1999, 67, 1732–1737. [Google Scholar] [CrossRef]
- Lee, J.E.; Wilcox, K.; Jacobson, K.A.; Dichter, M.; Liang, B.T. Adenosine receptor subtypes and cardioprotection. Drug Dev. Res. 1998, 45, 394–401. [Google Scholar] [CrossRef]
- Liang, B.T.; Swierkosz, T.A.; Herrmann, H.C.; Kimmel, S.; Jacobson, K.A. Adenosine and ischemic preconditioning. Curr. Pharm. Des. 1999, 5, 1029. [Google Scholar] [CrossRef]
- Singh, L.; Kulshrestha, R.; Singh, N.; Jaggi, A.S. Mechanisms involved in adenosine pharmacological preconditioning-induced cardioprotection. Korean J. Physiol. Pharmacol. 2018, 22, 225–234. [Google Scholar] [CrossRef]
- Carr, C.S.; Hill, R.J.; Masamune, H.; Kennedy, S.P.; Knight, D.R.; Tracey, W.; Yellon, D.M. Evidence for a role for both the adenosine A1 and A3 receptors in protection of isolated human atrial muscle against simulated ischaemia. Cardiovasc. Res. 1997, 36, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Maddock, H.L.; Mocanu, M.M.; Yellon, D.M. Adenosine A3receptor activation protects the myocardium from reperfusion/reoxygenation injury. Am. J. Physiol. Circ. Physiol. 2002, 283, H1307–H1313. [Google Scholar] [CrossRef] [PubMed]
- Auchampach, J.A.; Rizvi, A.; Qiu, Y.; Tang, X.-L.; Maldonado, C.; Teschner, S.; Bolli, R. Selective Activation of A3Adenosine Receptors withN6-(3-Iodobenzyl)Adenosine-5′-N-Methyluronamide Protects Against Myocardial Stunning and Infarction Without Hemodynamic Changes in Conscious Rabbits. Circ. Res. 1997, 80, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Hendel, R.C.; Bateman, T.M.; Cerqueira, M.D.; Iskandrian, A.E.; Leppo, J.A.; Blackburn, B.; Mahmarian, J.J. Initial Clinical Experience With Regadenoson, a Novel Selective A2AAgonist for Pharmacologic Stress Single-Photon Emission Computed Tomography Myocardial Perfusion Imaging. JACC 2005, 46, 2069–2075. [Google Scholar] [CrossRef]
- Maurer, G. Adenosine as an adjunct to reperfusion in myocardial infarction. Eur. Hear. J. 2006, 27, 2376–2377. [Google Scholar] [CrossRef]
- Kloner, R.A.; Forman, M.B.; Gibbons, R.J.; Ross, A.M.; Alexander, R.W.; Stone, G.W. Impact of time to therapy and reperfusion modality on the efficacy of adenosine in acute myocardial infarction: The AMISTAD-2 trial. Eur. Hear. J. 2006, 27, 2400–2405. [Google Scholar] [CrossRef]
- Ross, A.M.; Gibbons, R.J.; Stone, G.W.; Kloner, R.A.; Alexander, R.W. A Randomized, Double-Blinded, Placebo-Controlled Multicenter Trial of Adenosine as an Adjunct to Reperfusion in the Treatment of Acute Myocardial Infarction (AMISTAD-II). J. Am. Coll. Cardiol. 2005, 45, 1775–1780. [Google Scholar] [CrossRef]
- Quintana, M.; Kahan, T.; Hjemdahl, P. Pharmacological Prevention of Reperfusion Injury in Acute Myocardial Infarction. Am. J. Cardiovasc. Drugs 2004, 4, 159–167. [Google Scholar] [CrossRef]
- Niccoli, G.; Rigattieri, S.; De Vita, M.R.; Valgimigli, M.; Corvo, P.; Fabbiocchi, F.; Romagnoli, E.; De Caterina, A.R.; La Torre, G.; Schiavo, P.L.; et al. Open-Label, Randomized, Placebo-Controlled Evaluation of Intracoronary Adenosine or Nitroprusside After Thrombus Aspiration During Primary Percutaneous Coronary Intervention for the Prevention of Microvascular Obstruction in Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2013, 6, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Quintana, M.; Hjemdahl, P.; Sollevi, A.; Kahan, T.; Edner, M.; Rehnqvist, N.; Swahn, E.; Kjerr, A.C.; Näsman, P. Left ventricular function and cardiovascular events following adjuvant therapy with adenosine in acute myocardial infarction treated with thrombolysis. Eur. J. Clin. Pharmacol. 2003, 59, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Critical Issues for the Translation of Cardioprotection. Circ. Res. 2017, 120, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, H.N.; Gupta, R.C.; Kohli, S.; Wang, M.; Rastogi, S.; Zhang, K.; Zimmermann, K.; Diedrichs, N.; Albrecht-Küpper, B.E. Chronic Therapy with a Partial Adenosine A1-Receptor Agonist Improves Left Ventricular Function and Remodeling in Dogs With Advanced Heart Failure. Circ. Hear. Fail. 2013, 6, 563–571. [Google Scholar] [CrossRef]
- Mangano, D.T.; Miao, Y.; Tudor, I.C.; Dietzel, C. Post-Reperfusion Myocardial Infarction. JACC 2006, 48, 206–214. [Google Scholar] [CrossRef]
- Bulluck, H.; Sirker, A.; Loke, Y.K.; Garcia-Dorado, D.; Hausenloy, D.J. Clinical benefit of adenosine as an adjunct to reperfusion in ST-elevation myocardial infarction patients: An updated meta-analysis of randomized controlled trials. Int. J. Cardiol. 2016, 202, 228–237. [Google Scholar] [CrossRef]
- Biber, K.; Klotz, K.-N.; Berger, M.; Gebicke-Härter, P.J.; van Calker, D. Adenosine A1Receptor-Mediated Activation of Phospholipase C in Cultured Astrocytes Depends on the Level of Receptor Expression. J. Neurosci. 1997, 17, 4956–4964. [Google Scholar] [CrossRef]
- Akbar, M.; Okajima, F.; Tomura, H.; Shimegi, S.; Kondo, Y. A single species of A1 adenosine receptor expressed in Chinese hamster ovary cells not only inhibits cAMP accumulation but also stimulates phospholipase C and arachidonate release. Mol. Pharmacol. 1994, 45, 1036–1042. [Google Scholar] [CrossRef]
- Lankford, A.R.; Cerniway, R.J.; Regan, S.E.; Crawford, M.M.; Byford, A.M.; Matherne, G.P. Mechanisms of cardiac protection with Overexpression of A1 adenosine receptors. Drug Dev. Res. 2003, 58, 439–446. [Google Scholar] [CrossRef]
- Bassi, R.; Heads, R.; Marber, M.; Clark, J. Targeting p38-MAPK in the ischaemic heart: Kill or cure? Curr. Opin. Pharmacol. 2008, 8, 141–146. [Google Scholar] [CrossRef]
- Shao, Z.; Bhattacharya, K.; Hsich, E.; Park, L.; Walters, B.; Germann, U.; Wang, Y.-M.; Kyriakis, J.; Mohanlal, R.; Kuida, K.; et al. c-Jun N-Terminal Kinases Mediate Reactivation of Akt and Cardiomyocyte Survival After Hypoxic Injury In Vitro and In Vivo. Circ. Res. 2006, 98, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.M.; Lindgren, E.; Yang, J.-N.; Herling, A.W.; Fredholm, B.B. Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue—Interactions with insulin. Eur. J. Pharmacol. 2008, 597, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K.; Nogowski, L. Effects of adenosine A1 receptor antagonism on lipogenesis and lipolysis in isolated rat adipocytes. Physiol. Res. 2009, 58, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Belardinelli, L.; Ozeck, M.J.; Shryock, J.C. Tonic activity of the rat adipocyte A1-adenosine receptor. Br. J. Pharmacol. 2002, 135, 1457–1466. [Google Scholar] [CrossRef]
- Yun, Y.; Chen, J.; Liu, R.; Chen, W.; Liu, C.; Wang, R.; Hou, Z.; Yu, Z.; Sun, Y.; Ijzerman, A.P.; et al. Long residence time adenosine A1 receptor agonists produce sustained wash-resistant antilipolytic effect in rat adipocytes. Biochem. Pharmacol. 2019, 164, 45–52. [Google Scholar] [CrossRef]
- Dhalla, A.K.; Chisholm, J.W.; Reaven, G.M.; Belardinelli, L. A1 Adenosine Receptor: Role in Diabetes and Obesity. In Adenosine Receptors in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2009; pp. 271–295. [Google Scholar] [CrossRef]
- Wojcik, M.; Zieleniak, A.; Wozniak, L.A. New Insight Into A1 Adenosine Receptors in Diabetes Treatment. Curr. Pharm. Des. 2010, 16, 4237–4242. [Google Scholar] [CrossRef]
- Chaptal, M.-C.; Maraninchi, M.; Musto, G.; Mancini, J.; Chtioui, H.; Dupont-Roussel, J.; Marlinge, M.; Fromonot, J.; Lalevee, N.; Mourre, F.; et al. Low Density Lipoprotein Cholesterol Decreases the Expression of Adenosine A2A Receptor and Lipid Rafts-Protein Flotillin-1: Insights on Cardiovascular Risk of Hypercholesterolemia. Cells 2024, 13, 488. [Google Scholar] [CrossRef]
- Leiva, A.; Guzmán-Gutiérrez, E.; Contreras-Duarte, S.; Fuenzalida, B.; Cantin, C.; Carvajal, L.; Salsoso, R.; Gutiérrez, J.; Pardo, F.; Sobrevia, L. Adenosine receptors: Modulators of lipid availability that are controlled by lipid levels. Mol. Asp. Med. 2017, 55, 26–44. [Google Scholar] [CrossRef]
- Pei, Y.; Li, H.; Cai, Y.; Zhou, J.; Luo, X.; Ma, L.; McDaniel, K.; Zeng, T.; Chen, Y.; Qian, X.; et al. Regulation of adipose tissue inflammation by adenosine 2A receptor in obese mice. J. Endocrinol. 2018, 239, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, A.; Carroll, S.H.; Johnston-Cox, H.; Farb, M.; Gokce, N.; Ravid, K. An Adenosine Receptor-Krüppel-like Factor 4 Protein Axis Inhibits Adipogenesis. J. Biol. Chem. 2014, 289, 21071–21081. [Google Scholar] [CrossRef] [PubMed]
- Abebe, W.; Mustafa, S.J. Effect of Low Density Lipoprotein on Adenosine Receptor-Mediated Coronary Vasorelaxation in Vitro. J. Pharmacol. Exp. Ther. 1997, 282, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Broadley, K.J. Drugs modulating adenosine receptors as potential therapeutic agents for cardiovascular diseases. Expert Opin. Ther. Patents 2000, 10, 1669–1692. [Google Scholar] [CrossRef]
- Dohadwala, M.M.; Givertz, M.M. Role of Adenosine Antagonism in the Cardiorenal Syndrome. Cardiovasc. Ther. 2008, 26, 276–286. [Google Scholar] [CrossRef]
- Eddy, M.T.; Lee, M.-Y.; Gao, Z.-G.; White, K.L.; Didenko, T.; Horst, R.; Audet, M.; Stanczak, P.; McClary, K.M.; Han, G.W.; et al. Allosteric Coupling of Drug Binding and Intracellular Signaling in the A2A Adenosine Receptor. Cell 2017, 172, 68–80.e12. [Google Scholar] [CrossRef]
- Kitagawa, K. CREB and cAMP response element-mediated gene expression in the ischemic brain. FEBS J. 2007, 274, 3210–3217. [Google Scholar] [CrossRef]
- Meller, R.; Minami, M.; Cameron, J.A.; Impey, S.; Chen, D.; Lan, J.-Q.; Henshall, D.C.; Simon, R.P. CREB-Mediated Bcl-2 Protein Expression after Ischemic Preconditioning. J. Cereb. Blood Flow Metab. 2005, 25, 234–246. [Google Scholar] [CrossRef]
- Peng, C.; Pei, H.; Wei, F.; Tian, X.; Deng, J.; Yan, C.; Li, Y.; Sun, M.; Zhang, J.; Liu, D.; et al. Cellular repressor of E1A-stimulated gene overexpression in bone mesenchymal stem cells protects against rat myocardial infarction. Int. J. Cardiol. 2015, 183, 232–241. [Google Scholar] [CrossRef]
- Stockwell, J.; Jakova, E.; Cayabyab, F.S. Adenosine A1 and A2A Receptors in the Brain: Current Research and Their Role in Neurodegeneration. Molecules 2017, 22, 676. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.B.; Most, D.; Blednov, Y.A.; Harris, R.A. PPAR agonists regulate brain gene expression: Relationship to their effects on ethanol consumption. Neuropharmacology 2014, 86, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Verzijl, D.; Ijzerman, A.P. Functional selectivity of adenosine receptor ligands. Purinergic Signal. 2011, 7, 171–192. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Halenda, S.P.; Sturek, M.; Wilden, P.A. Cell-Signaling Evidence for Adenosine Stimulation of Coronary Smooth Muscle Proliferation via the A1 Adenosine Receptor. Circ. Res. 2005, 97, 574–582. [Google Scholar] [CrossRef]
- Mustafa, S.J.; Morrison, R.R.; Teng, B.; Pelleg, A. Adenosine receptors and the heart: Role in regulation of coronary blood flow and cardiac electrophysiology. Adenosine Recept. Health Dis. 2009, 193, 161–188. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Duncker, D.J.; Zhang, J.; Bache, R.J. ATP-Sensitive K+Channels, Adenosine, and Nitric Oxide–Mediated Mechanisms Account for Coronary Vasodilation During Exercise. Circ. Res. 1998, 82, 346–359. [Google Scholar] [CrossRef]
- Duncker, D.J.; van Zon, N.S.; Pavek, T.J.; Herrlinger, S.K.; Bache, R.J. Endogenous adenosine mediates coronary vasodilation during exercise after K(ATP)+ channel blockade. J. Clin. Investig. 1995, 95, 285–295. [Google Scholar] [CrossRef]
- Headrick, J.P.; Ashton, K.J.; Rose’MEyer, R.B.; Peart, J.N. Cardiovascular adenosine receptors: Expression, actions and interactions. Pharmacol. Ther. 2013, 140, 92–111. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Bonney, S.K.; Eckle, T. Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends Mol. Med. 2013, 19, 345–354. [Google Scholar] [CrossRef]
- Dixon, A.K.; Gubitz, A.K.; Sirinathsinghji, D.J.; Richardson, P.J.; Freeman, T.C. Tissue distribution of adenosine receptor mRNAs in the rat. Br. J. Pharmacol. 1996, 118, 1461–1468. [Google Scholar] [CrossRef]
- Mutafova-Yambolieva, V.N.; Keef, K.D. Adenosine-induced hyperpolarization in guinea pig coronary artery involves A2breceptors and KATPchannels. Am. J. Physiol. Circ. Physiol. 1997, 273, H2687–H2695. [Google Scholar] [CrossRef]
- Ongini, E.; Dionisotti, S.; Gessi, S.; Irenius, E.; Fredholm, B.B. Comparison of CGS 15943, ZM 241385 and SCH 58261 as antagonists at human adenosine receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1999, 359, 7–10. [Google Scholar] [CrossRef]
- Ongini, E.; Fredholm, B.B. Pharmacology of adenosine A2A receptors. Trends Pharmacol. Sci. 1996, 17, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Headrick, J.P.; Gauthier, N.S.; Morrison, R.; Matherne, G.P. Cardioprotection by KATPchannels in wild-type hearts and hearts overexpressing A1-adenosine receptors. Am. J. Physiol. Circ. Physiol. 2000, 279, H1690–H1697. [Google Scholar] [CrossRef] [PubMed]
- Peart, J.N.; Gross, G.J. Cardioprotection following adenosine kinase inhibition in rat hearts. Basic Res. Cardiol. 2005, 100, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Bättig, K.; Holmén, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar] [CrossRef]
- Miura, T.; Miki, T. GSK-3.BETA., a Therapeutic Target for Cardiomyocyte Protection. Circ. J. 2009, 73, 1184–1192. [Google Scholar] [CrossRef]
- Tanaka, T.; Saotome, M.; Katoh, H.; Satoh, T.; Hasan, P.; Ohtani, H.; Satoh, H.; Hayashi, H.; Maekawa, Y. Glycogen synthase kinase-3β opens mitochondrial permeability transition pore through mitochondrial hexokinase II dissociation. J. Physiol. Sci. 2018, 68, 865–871. [Google Scholar] [CrossRef]
- Miura, T.; Nishihara, M.; Miki, T. Drug Development Targeting the Glycogen Synthase Kinase-3β (GSK-3β)-Mediated Signal Transduction Pathway: Role of GSK-3β in Myocardial Protection Against Ischemia/Reperfusion Injury. J. Pharmacol. Sci. 2009, 109, 162–167. [Google Scholar] [CrossRef]
- Talukder, M.A.H.; Morrison, R.R.; Jacobson, M.A.; Jacobson, K.A.; Ledent, C.; Mustafa, S.J. Targeted deletion of adenosine A3receptors augments adenosine-induced coronary flow in isolated mouse heart. Am. J. Physiol. Circ. Physiol. 2002, 282, H2183–H2189. [Google Scholar] [CrossRef]
- Wan, T.C.; Ge, Z.-D.; Tampo, A.; Mio, Y.; Bienengraeber, M.W.; Tracey, W.R.; Gross, G.J.; Kwok, W.-M.; Auchampach, J.A. The A3 Adenosine Receptor Agonist CP-532,903 [N6-(2,5-Dichlorobenzyl)-3′-aminoadenosine-5′-N-methylcarboxamide] Protects against Myocardial Ischemia/Reperfusion Injury via the Sarcolemmal ATP-Sensitive Potassium Channel. J. Pharmacol. Exp. Ther. 2008, 324, 234–243. [Google Scholar] [CrossRef]
- Shaikh, G.; Cronstein, B. Signaling pathways involving adenosine A2A and A2B receptors in wound healing and fibrosis. Purinergic Signal. 2016, 12, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Scuruchi, M.; Mannino, F.; Imbesi, C.; Pallio, G.; Vermiglio, G.; Bagnato, G.; Minutoli, L.; Bitto, A.; Squadrito, F.; Irrera, N. Biglycan Involvement in Heart Fibrosis: Modulation of Adenosine 2A Receptor Improves Damage in Immortalized Cardiac Fibroblasts. Int. J. Mol. Sci. 2023, 24, 1784. [Google Scholar] [CrossRef] [PubMed]
- Ernens, I.; Léonard, F.; Vausort, M.; Rolland-Turner, M.; Devaux, Y.; Wagner, D.R. Adenosine up-regulates vascular endothelial growth factor in human macrophages. Biochem. Biophys. Res. Commun. 2010, 392, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; Hodge, J.W. Targeting Immunosuppressive Adenosine Signaling: A Review of Potential Immunotherapy Combination Strategies. Int. J. Mol. Sci. 2023, 24, 8871. [Google Scholar] [CrossRef]
- Mazziotta, C.; Rotondo, J.C.; Lanzillotti, C.; Campione, G.; Martini, F.; Tognon, M. Cancer biology and molecular genetics of A3 adenosine receptor. Oncogene 2021, 41, 301–308. [Google Scholar] [CrossRef]
- Kanno, T.; Gotoh, A.; Fujita, Y.; Nakano, T.; Nishizaki, T. A3Adenosine Receptor Mediates Apoptosis in 5637 Human Bladder Cancer Cells by GqProtein/PKC-Dependent AIF Upregulation. Cell. Physiol. Biochem. 2012, 30, 1159–1168. [Google Scholar] [CrossRef]
- Marucci, G.; Santinelli, C.; Buccioni, M.; Navia, A.M.; Lambertucci, C.; Zhurina, A.; Yli-Harja, O.; Volpini, R.; Kandhavelu, M. Anticancer activity study of A 3 adenosine receptor agonists. Life Sci. 2018, 205, 155–163. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Buccioni, M. Current Understanding of the Role of Adenosine Receptors in Cancer. Molecules 2024, 29, 3501. [Google Scholar] [CrossRef]
- Bar Yehuda, S.; Stemmer, S.; Madi, L.; Castel, D.; Ochaion, A.; Cohen, S.; Barer, F.; Zabutti, A.; Perez-Liz, G.; Del Valle, L. CF102 an A3 adenosine receptor agonist induces in vivo apoptosis of Hepatocellular carci-noma. Cancer Res. 2008, 68, 5712. [Google Scholar]
- Azambuja, J.H.; Ludwig, N.; Braganhol, E.; Whiteside, T.L. Inhibition of the Adenosinergic Pathway in Cancer Rejuvenates Innate and Adaptive Immunity. Int. J. Mol. Sci. 2019, 20, 5698. [Google Scholar] [CrossRef]
- Coombs, M.R.P.; Belderbos, M.E.; Gallington, L.C.; Bont, L.; Levy, O. Adenosine modulates Toll-like receptor function: Basic mechanisms and translational opportunities. Expert Rev. Anti-Infect. Ther. 2011, 9, 261–269. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, W.; Zhang, G.X.; Chen, J.C.; Wang, Q.L.; Mao, M.Y.; Deng, S.C.; Jin, L.P.; Liu, H.; Kuang, Y.H. Adenosine A2A receptor activation regulates the M1 macrophages activation to initiate innate and adaptive immunity in psoriasis. Clin. Immunol. 2024, 266, 110309. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Ross, W.G.; Agbai, O.N.; Frazier, R.; Figler, R.A.; Rieger, J.; Linden, J.; Ernst, P.B. The A2B Adenosine Receptor Impairs the Maturation and Immunogenicity of Dendritic Cells. J. Immunol. 2009, 182, 4616–4623. [Google Scholar] [CrossRef] [PubMed]
- Methner, C.; Schmidt, K.; Cohen, M.V.; Downey, J.M.; Krieg, T. Both A2a and A2b adenosine receptors at reperfusion are necessary to reduce infarct size in mouse hearts. Am. J. Physiol. Circ. Physiol. 2010, 299, H1262–H1264. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, C.; Veth, J.; Sukurova, L.; Zuiderwijk-Sick, E.A.; Simonetti, E.; Koenen, H.J.P.; Burm, S.M.; van Noort, J.M.; Ijzerman, A.P.; van Hijum, S.A.F.T.; et al. TLR-Induced IL-12 and CCL2 Production by Myeloid Cells Is Dependent on Adenosine A3 Receptor–Mediated Signaling. J. Immunol. 2019, 202, 2421–2430. [Google Scholar] [CrossRef]
- Sun, D.; Shao, H.; Kaplan, H.J. TLR ligand ligation switches adenosine receptor usage of BMDCs leading to augmented Th17 responses in experimental autoimmune uveitis. Curr. Res. Immunol. 2022, 3, 73–84. [Google Scholar] [CrossRef]
- Jung, M.; Ma, Y.; Iyer, R.P.; DeLeon-Pennell, K.Y.; Yabluchanskiy, A.; Garrett, M.R.; Lindsey, M.L. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res. Cardiol. 2017, 112, 33. [Google Scholar] [CrossRef]
- Zhang, J.J.; Rizk, R.; Li, X.; Lee, B.G.; Matthies, M.L.; Bietz, K.A.; Kim, K.; Huard, J.; Wang, Y.; Chen, W.C.W. Interleukin-10 exhibit dose-dependent effects on macrophage phenotypes and cardiac remodeling after myocardial infarction. Front. Physiol. 2025, 15, 1481460. [Google Scholar] [CrossRef]
- Glover, D.K.; Riou, L.M.; Ruiz, M.; Sullivan, G.W.; Linden, J.; Rieger, J.M.; Macdonald, T.L.; Watson, D.D.; Beller, G.A. Reduction of infarct size and postischemic inflammation from ATL-146e, a highly selective adenosine A2Areceptor agonist, in reperfused canine myocardium. Am. J. Physiol. Circ. Physiol. 2005, 288, H1851–H1858. [Google Scholar] [CrossRef]
- Patel, R.A.G.; Glover, D.K.; Broisat, A.; Kabul, H.K.; Ruiz, M.; Goodman, N.C.; Kramer, C.M.; Meerdink, D.J.; Linden, J.; Beller, G.A. Reduction in myocardial infarct size at 48 hours after brief intravenous infusion of ATL-146e, a highly selective adenosine A2Areceptor agonist. Am. J. Physiol. Circ. Physiol. 2009, 297, H637–H642. [Google Scholar] [CrossRef]
- Rork, T.H.; Wallace, K.L.; Kennedy, D.P.; Marshall, M.A.; Lankford, A.R.; Linden, J. Adenosine A2Areceptor activation reduces infarct size in the isolated, perfused mouse heart by inhibiting resident cardiac mast cell degranulation. Am. J. Physiol. Circ. Physiol. 2008, 295, H1825–H1833. [Google Scholar] [CrossRef] [PubMed]
- Timperi, E.; Barnaba, V. CD39 Regulation and Functions in T Cells. Int. J. Mol. Sci. 2021, 22, 8068. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N.; Sitkovsky, M. Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat. Rev. Rheumatol. 2016, 13, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yu-Jing, L.; Ma, T. The immunomodulatory function of adenosine in sepsis. Front. Immunol. 2022, 13, 936547. [Google Scholar] [CrossRef]
- Layland, J.; Carrick, D.; Lee, M.; Oldroyd, K.; Berry, C. Adenosine. JACC Cardiovasc. Interv. 2014, 7, 581–591. [Google Scholar] [CrossRef]
- Chandrasekaran, B.; Samarneh, S.; Jaber, A.M.Y.; Kassab, G.; Agrawal, N. Therapeutic Potentials of A2B Adenosine Receptor Ligands: Current Status and Perspectives. Curr. Pharm. Des. 2019, 25, 2741–2771. [Google Scholar] [CrossRef]
- Guieu, R.; Deharo, J.-C.; Maille, B.; Crotti, L.; Torresani, E.; Brignole, M.; Parati, G. Adenosine and the Cardiovascular System: The Good and the Bad. J. Clin. Med. 2020, 9, 1366. [Google Scholar] [CrossRef]
- Strååt, E.; Henriksson, P.; Edlund, A. Adenosine provokes myocardial ischaemia in patients with ischaemic heart disease without increasing cardiac work. J. Intern. Med. 1991, 230, 319–323. [Google Scholar] [CrossRef]
- Ruan, W.; Ma, X.; Bang, I.H.; Liang, Y.; Muehlschlegel, J.D.; Tsai, K.-L.; Mills, T.W.; Yuan, X.; Eltzschig, H.K. The Hypoxia-Adenosine Link during Myocardial Ischemia—Reperfusion Injury. Biomedicines 2022, 10, 1939. [Google Scholar] [CrossRef]
- Marchi, E.; Muraca, I.; Berteotti, M.; Gori, A.M.; Valenti, R.; Marcucci, R. Adenosine in Interventional Cardiology: Physiopathologic and Pharmacologic Effects in Coronary Artery Disease. Int. J. Mol. Sci. 2024, 25, 5852. [Google Scholar] [CrossRef]
- Lasley, R.D. Adenosine Receptor-Mediated Cardioprotection—Current Limitations and Future Directions. Front. Pharmacol. 2018, 9, 310. [Google Scholar] [CrossRef]
- Hori, M.; Kitakaze, M. Adenosine, the heart, and coronary circulation. Hypertension 1991, 18, 565–574. [Google Scholar] [CrossRef]
- Zhang, Y.; Wernly, B.; Cao, X.; Mustafa, S.J.; Tang, Y.; Zhou, Z. Adenosine and adenosine receptor-mediated action in coronary microcirculation. Basic Res. Cardiol. 2021, 116, 22. [Google Scholar] [CrossRef] [PubMed]
- Sadigh, B.; Quintana, M.; Sylvén, C.; Berglund, M.; Brodin, L.Å. The ischemic preconditioning effect of adenosine in patients with ischemic heart disease. Cardiovasc. Ultrasound 2009, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh-Narbat, M.; Annabi, N.; Tamayol, A.; Oklu, R.; Ghanem, A.; Khademhosseini, A. Adenosine-associated delivery systems. J. Drug Target. 2015, 23, 580–596. [Google Scholar] [CrossRef] [PubMed]
- May, L.T.; Chuo, C.H.; Vecchio, E.A.; Wang, B.H.; Kompa, A.R.; Christopoulos, A.; Scammells, P.J.; White, P.J.; Baltos, J.-A. Adenosine G Protein-Coupled Receptor Biased Agonism to Treat Ischemic Heart Disease. FASEB J. 2018, 32, 555.19. [Google Scholar] [CrossRef]
- A Vecchio, E.; Baltos, J.; Nguyen, A.T.N.; Christopoulos, A.; White, P.J.; May, L.T. New paradigms in adenosine receptor pharmacology: Allostery, oligomerization and biased agonism. Br. J. Pharmacol. 2018, 175, 4036–4046. [Google Scholar] [CrossRef]
- Chuo, C.H.; Devine, S.M.; Scammells, P.J.; Krum, H.; Christopoulos, A.; May, L.T.; White, P.J.; Wang, B.H. VCP746, a novel A1 adenosine receptor biased agonist, reduces hypertrophy in a rat neonatal cardiac myocyte model. Clin. Exp. Pharmacol. Physiol. 2016, 43, 976–982. [Google Scholar] [CrossRef]
- Cooper, S.L.; March, J.; Sabbatini, A.R.; Hill, S.J.; Jörg, M.; Scammells, P.J.; Woolard, J. The effect of two selective A1-receptor agonists and the bitopic ligand VCP746 on heart rate and regional vascular conductance in conscious rats. Br. J. Pharmacol. 2019, 177, 346–359. [Google Scholar] [CrossRef]
- Navarro, G.; Gonzalez, A.; Campanacci, S.; Rivas-Santisteban, R.; Reyes-Resina, I.; Casajuana-Martin, N.; Cordomí, A.; Pardo, L.; Franco, R. Experimental and computational analysis of biased agonism on full-length and a C-terminally truncated adenosine A2A receptor. Comput. Struct. Biotechnol. J. 2020, 18, 2723–2732. [Google Scholar] [CrossRef]
- Pottie, E.; Tosh, D.K.; Gao, Z.-G.; Jacobson, K.A.; Stove, C.P. Assessment of biased agonism at the A3 adenosine receptor using β-arrestin and miniGαi recruitment assays. Biochem. Pharmacol. 2020, 177, 113934. [Google Scholar] [CrossRef]
- Müller, C.E.; Jacobson, K.A. Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim Biophys Acta. 2011, 1808, 1290–1308. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Van Galen, P.J.M.; Williams, M. Adenosine receptors: Pharmacology, structure-activity relationships, and therapeutic potential. J. Med. Chem. 1992, 35, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, M.P.; Vergelli, C.; Cilibrizzi, A.; Crocetti, L.; Biancalani, C.; Graziano, A.; Piaz, V.D.; Loza, M.I.; Cadavid, M.I.; Díaz, J.L. Pyrazolo [1′,5′:1,6]pyrimido [4,5-d]pyridazin-4(3H)-ones as selective human A1 adenosine receptor ligands. Bioorg. Med. Chem. 2010, 18, 7890–7899. [Google Scholar] [CrossRef] [PubMed]
- Graziano, A.; Giovannoni, M.P.; Cilibrizzi, A.; Crocetti, L.; Piaz, V.D.; Vergelli, C.; Trincavelli, M.L.; Martini, C.; Giacomelli, C. New Pyrazolo [1’,5’:1,6]pyrimido [4,5-d]pyridazin-4(3H)-ones Fluoroderivatives as Human A1 Adenosine Receptor Lig-ands. Acta Chim. Slov. 2012, 59, 648–655. [Google Scholar]
- Giovannoni, M.P.; Ciciani, G.; Cilibrizzi, A.; Crocetti, L.; Daniele, S.; Mannelli, L.D.C.; Ghelardini, C.; Giacomelli, C.; Guerrini, G.; Martini, C.; et al. Further studies on pyrazolo [1′,5′:1,6]pyrimido [4,5-d]pyridazin-4(3H)-ones as potent and selective human A1 adenosine receptor antagonists. Eur. J. Med. Chem. 2015, 89, 32–41. [Google Scholar] [CrossRef]
- Suresh, R.R.; Gao, Z.-G.; Salmaso, V.; Chen, E.; Campbell, R.G.; Poe, R.B.; Liston, T.E.; Jacobson, K.A. Selective A3 Adenosine Receptor Antagonist Radioligand for Human and Rodent Species. ACS Med. Chem. Lett. 2022, 13, 623–631. [Google Scholar] [CrossRef]
- Crocetti, L.; Pearce, A.; Vege, V.S.; Xu, Q.; Xu, J.; Buthmann, H.; Giovannoni, M.P.; Guerrini, G.; Catarzi, F.; Selleri, S.; et al. New heterocyclic A1/A3 adenosine receptor ligands through molecular simplification strategies. Eur. J. Med. Chem. Rep. 2025, 13, 100253. [Google Scholar] [CrossRef]
- Stampelou, M.; Suchankova, A.; Tzortzini, E.; Dhingra, L.; Barkan, K.; Lougiakis, N.; Marakos, P.; Pouli, N.; Ladds, G.; Kolocouris, A. Dual A1/A3 Adenosine Receptor Antagonists: Binding Kinetics and Structure−Activity Relationship Studies Using Mutagenesis and Alchemical Binding Free Energy Calculations. J. Med. Chem. 2022, 65, 13305–13327. [Google Scholar] [CrossRef]
- Suchankova, A.; Stampelou, M.; Koutsouki, K.; Pousias, A.; Dhingra, L.; Barkan, K.; Pouli, N.; Marakos, P.; Tenta, R.; Kolocouris, A.; et al. Discovery of a High Affinity Adenosine A1/A3 Receptor Antagonist with a Novel 7-Amino-pyrazolo [3,4-d]pyridazine Scaffold. ACS Med. Chem. Lett. 2022, 13, 923–934. [Google Scholar] [CrossRef]
- Yang, X.; Heitman, L.H.; Ijzerman, A.P.; van der Es, D. Molecular probes for the human adenosine receptors. Purinergic Signal. 2020, 17, 85–108. [Google Scholar] [CrossRef]
- Federico, S.; Lassiani, L.; Spalluto, G. Chemical Probes for the Adenosine Receptors. Pharmaceuticals 2019, 12, 168. [Google Scholar] [CrossRef]
- Nielsen, C.D.-T.; Dhasmana, D.; Floresta, G.; Wohland, T.; Cilibrizzi, A. Illuminating the Path to Target GPCR Structures and Functions. Biochemistry 2020, 59, 3783–3795. [Google Scholar] [CrossRef]
- Geldenhuys, W.J.; Hanif, A.; Yun, J.; Nayeem, M.A. Exploring Adenosine Receptor Ligands: Potential Role in the Treatment of Cardiovascular Diseases. Molecules 2017, 22, 917. [Google Scholar] [CrossRef]
- Moukeila, M.B.Y.; Thokerunga, E.; He, F.; Bongolo, C.C.; Xia, Y.; Wang, F.; Gado, A.F.; Mamoudou, H.; Khan, S.; Ousseina, B.; et al. Adenosine 2 receptor regulates autophagy and apoptosis to alleviate ischemia reperfusion injury in type 2 diabetes via IRE-1 signaling. BMC Cardiovasc. Disord. 2023, 23, 154. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Cheirif, J.; Kleiman, N.S.; Verani, M.S.; Trakhtenbroit, A. Diagnosis of ischemic heart disease with adenosine echocardiography. JACC 1991, 18, 1271–1279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garnock-Jones, K.P.; Curran, M.P. Regadenoson. Am. J. Cardiovasc. Drugs 2010, 10, 65–71. [Google Scholar] [CrossRef]
- Yao, Z.; Gross, G.J. A comparison of adenosine-induced cardioprotection and ischemic preconditioning in dogs. Efficacy, time course, and role of KATP channels. Circulation 1994, 89, 1229–1236. [Google Scholar] [CrossRef]
- Yang, Z.; Linden, J.; Berr, S.S.; Kron, I.L.; Beller, G.A.; French, B.A. Timing of adenosine 2A receptor stimulation relative to reperfusion has differential effects on infarct size and cardiac function as assessed in mice by MRI. Am. J. Physiol. Circ. Physiol. 2008, 295, H2328–H2335. [Google Scholar] [CrossRef]
- Heide, R.S.V.; Steenbergen, C. Cardioprotection and Myocardial Reperfusion. Circ. Res. 2013, 113, 464–477. [Google Scholar] [CrossRef]
- Solenkova, N.V.; Solodushko, V.; Cohen, M.V.; Downey, J.M. Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. Am. J. Physiol. Circ. Physiol. 2006, 290, H441–H449. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, B.; Guo, Y.; Zheng, F. Efficacy of Adenosine in Patients With Acute Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Medicine 2015, 94, e1279. [Google Scholar] [CrossRef] [PubMed]
- Laborante, R.; Bianchini, E.; Restivo, A.; Ciliberti, G.; Galli, M.; Vergallo, R.; Rodolico, D.; Zito, A.; Princi, G.; Leone, A.M.; et al. Adenosine as adjunctive therapy in acute coronary syndrome: A meta-analysis of randomized controlled trials. Eur. Heart J. 2022, 9, 173–182. [Google Scholar] [CrossRef]
- Desmet, W.; Bogaert, J.; Dubois, C.; Sinnaeve, P.; Adriaenssens, T.; Pappas, C.; Ganame, J.; Dymarkowski, S.; Janssens, S.; Belmans, A.; et al. High-dose intracoronary adenosine for myocardial salvage in patients with acute ST-segment elevation myocardial infarction. Eur. Hear. J. 2010, 32, 867–877. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Tosh, D.K.; Jain, S.; Gao, Z.-G. Historical and Current Adenosine Receptor Agonists in Preclinical and Clinical Development. Front. Cell. Neurosci. 2019, 13, 124. [Google Scholar] [CrossRef]
- Giblett, J.P.; Bulluck, H. Cardioprotection for Acute MI in Light of the CONDI2/ERIC-PPCI Trial: New Targets Needed. Interv. Cardiol. Rev. 2020, 15. [Google Scholar] [CrossRef]
- Ragosta, M. Adenosine as Adjunctive Therapy for Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2015, 8, 2000–2002. [Google Scholar] [CrossRef][Green Version]
- Hosey, M.M.; McMahon, K.K.; Green, R.D. Inhibitory adenosine receptors in the heart: Characterization by ligand binding studies and effects on β-adrenergic receptor stimulated adenylate cyclase and membrane protein phosphorylation. J. Mol. Cell. Cardiol. 1984, 16, 931–942. [Google Scholar] [CrossRef]
- Gomes, C.V.; Kaster, M.P.; Tomé, A.R.; Agostinho, P.M.; Cunha, R.A. Adenosine receptors and brain diseases: Neuroprotection and neurodegeneration. Biochim. Biophys. Acta (BBA)-Biomembr. 2011, 1808, 1380–1399. [Google Scholar] [CrossRef]
- Shaw, S.; Uniyal, A.; Gadepalli, A.; Tiwari, V.; Belinskaia, D.A.; Shestakova, N.N.; Venugopala, K.N.; Deb, P.K. Adenosine receptor signalling: Probing the potential pathways for the ministration of neuropathic pain. Eur. J. Pharmacol. 2020, 889, 173619. [Google Scholar] [CrossRef]
- Jung, S.-M.; Peyton, L.; Essa, H.; Choi, D.-S. Adenosine receptors: Emerging non-opioids targets for pain medications. Neurobiol. Pain 2022, 11, 100087. [Google Scholar] [CrossRef]
- Li, D.; Huo, X.; Shen, L.; Qian, M.; Wang, J.; Mao, S.; Chen, W.; Li, R.; Zhu, T.; Zhang, B.; et al. Astrocyte heterogeneity in ischemic stroke: Molecular mechanisms and therapeutic targets. Neurobiol. Dis. 2025, 209, 106885. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Oliva, P.; Suresh, R.R. A3 Adenosine Receptor Ligands: From Discovery to Clinical Trials. In Purinergic Receptors and their Modulators; Colotta, V., Supuran, C.T., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 157–177. [Google Scholar]
- Rizzolio, F.; La Montagna, R.; Tuccinardi, T.; Russo, G.; Caputi, M.; Giordano, A. Adenosine Receptor Ligands in Clinical Trials. Curr. Top. Med. Chem. 2010, 10, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Kutryb-Zając, B.; Kawecka, A.; Nasadiuk, K.; Braczko, A.; Stawarska, K.; Caiazzo, E.; Koszałka, P.; Cicala, C. Drugs targeting adenosine signaling pathways: A current view. Biomed. Pharmacother. 2023, 165, 115184. [Google Scholar] [CrossRef]
- Deb, P.K.; Maity, P.; Sarkar, B.; Venugopala, K.N.; Tekade, R.K.; Batra, S. Insights from Clinical Trials on A2A Adenosine Receptor Antagonists for Cancer Treatment. ACS Pharmacol. Transl. Sci. 2025, 8, 1498–1512. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Bax, J.J.; Hernandez, A.F.; Wirtz, A.B.; Pap, A.F.; Ferreira, A.C.; Senni, M.; van der Laan, M.; Butler, J.; PANTHEON Investigators. Safety and efficacy of the partial adenosine A1 receptor agonist neladenoson bialanate in patients with chronic heart failure with reduced ejection fraction: A phase IIb, randomized, double-blind, placebo-controlled trial. Eur. J. Hear. Fail. 2019, 21, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Tendera, M.; Gaszewska-Żurek, E.; Parma, Z.; Ponikowski, P.; Jankowska, E.; Kawecka-Jaszcz, K.; Czarnecka, D.; Krzemińska-Pakuła, M.; Bednarkiewicz, Z.; Sosnowski, M.; et al. The new oral adenosine A1 receptor agonist capadenoson in male patients with stable angina. Clin. Res. Cardiol. 2012, 101, 585–591. [Google Scholar] [CrossRef]
- Parichatikanond, W.; Duangrat, R.; Nuamnaichati, N.; Mangmool, S. Role of A1 adenosine receptor in cardiovascular diseases: Bridging molecular mechanisms with therapeutic opportunities. Exp. Mol. Pathol. 2025, 141, 104952. [Google Scholar] [CrossRef]
- Shah, B.; Rohatagi, S.; Natarajan, C.; Kirkesseli, S.; Baybutt, R.; Jensen, B.K. Pharmacokinetics, Pharmacodynamics, and Safety of a Lipid-Lowering Adenosine A1 Agonist, RPR749, in Healthy Subjects. Am. J. Ther. 2004, 11, 175–189. [Google Scholar] [CrossRef]
- Park, J.-G.; Jeong, S.-J.; Yu, J.; Kim, G.; Jeong, L.S.; Oh, G.T. LJ-1888, a selective antagonist for the A3 adenosine receptor, ameliorates the development of atherosclerosis and hypercholesterolemia in apolipoprotein E knock-out mice. BMB Rep. 2018, 51, 520–525. [Google Scholar] [CrossRef]
- Dorotea, D.; Cho, A.; Lee, G.; Kwon, G.; Lee, J.; Sahu, P.K.; Jeong, L.S.; Cha, D.R.; Ha, H. Orally active, species-independent novel A3 adenosine receptor antagonist protects against kidney injury in db/db mice. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Auchampach, J.A.; Ge, Z.-D.; Wan, T.C.; Moore, J.; Gross, G.J. A3adenosine receptor agonist IB-MECA reduces myocardial ischemia-reperfusion injury in dogs. Am. J. Physiol. Circ. Physiol. 2003, 285, H607–H613. [Google Scholar] [CrossRef]
- Staehr, P.M.; Dhalla, A.K.; Zack, J.; Wang, X.; Ho, Y.L.; Bingham, J.; Belardinelli, L. Reduction of Free Fatty Acids, Safety, and Pharmacokinetics of Oral GS-9667, an A1 Adenosine Receptor Partial Agonist. J. Clin. Pharmacol. 2013, 53, 385–392. [Google Scholar] [CrossRef]
- da Silva, J.S.; Gabriel-Costa, D.; Sudo, R.T.; Wang, H.; Groban, L.; Ferraz, E.B.; Nascimento, J.H.M.; Fraga, C.A.M.; Barreiro, E.J.; Zapata-Sudo, G. Adenosine A2A receptor agonist prevents cardiac remodeling and dysfunction in spontaneously hypertensive male rats after myocardial infarction. Drug Des. Devel. Ther. 2017, ume11, 553–562. [Google Scholar] [CrossRef]
- Joosen, M.J.A.; Bueters, T.J.H.; van Helden, H.P.M. Cardiovascular effects of the adenosine A1 receptor agonist N 6-cyclopentyladenosine (CPA) decisive for its therapeutic efficacy in sarin poisoning. Arch. Toxicol. 2003, 78, 34–39. [Google Scholar] [CrossRef]
- Fuentes, E.; Fuentes, M.; Caballero, J.; Palomo, I.; Hinz, S.; El-Tayeb, A.; Müller, C.E. Adenosine A2Areceptor agonists with potent antiplatelet activity. Platelets 2017, 29, 292–300. [Google Scholar] [CrossRef]
- Jeong, L.S.; Choe, S.A.; Gunaga, P.; Kim, H.O.; Lee, H.W.; Lee, S.K.; Tosh, D.K.; Patel, A.; Palaniappan, K.K.; Gao, Z.-G.; et al. Discovery of a New Nucleoside Template for Human A3 Adenosine Receptor Ligands: D-4‘-Thioadenosine Derivatives without 4‘-Hydroxymethyl Group as Highly Potent and Selective Antagonists. J. Med. Chem. 2007, 50, 3159–3162. [Google Scholar] [CrossRef] [PubMed]
- Kiesman, W.F.; Elzein, E.; Zablocki, J. A1 Adenosine Receptor Antagonists, Agonists, and Allosteric Enhancers. In Adenosine Receptors in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2009; Volume 193, pp. 25–58. [Google Scholar] [CrossRef]
- Schoelch, C.; Kuhlmann, J.; Gossel, M.; Mueller, G.; Neumann-Haefelin, C.; Belz, U.; Kalisch, J.; Biemer-Daub, G.; Kramer, W.; Juretschke, H.-P.; et al. Characterization of Adenosine-A1 Receptor–Mediated Antilipolysis in Rats by Tissue Microdialysis, 1H-Spectroscopy, and Glucose Clamp Studies. Diabetes 2004, 53, 1920–1926. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, A.; Chitalia, S.V.; Ravid, K. Bone Marrow and Adipose Tissue Adenosine Receptors Effect on Osteogenesis and Adipogenesis. Int. J. Mol. Sci. 2020, 21, 7470. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, W.; Zhou, J.; Dong, X. Bidirectional causal relationship between hypercholesterolemia and ischemic heart disease: A Mendelian randomization study. Front. Cardiovasc. Med. 2023, 10, 1302282. [Google Scholar] [CrossRef]
- Gunia-Krzyżak, A.; Żelaszczyk, D.; Rapacz, A.; Żesławska, E.; Waszkielewicz, A.M.; Pańczyk, K.; Słoczyńska, K.; Pękala, E.; Nitek, W.; Filipek, B.; et al. Structure-anticonvulsant activity studies in the group of (E)-N-cinnamoyl aminoalkanols derivatives monosubstituted in phenyl ring with 4-Cl, 4-CH3 or 2-CH3. Bioorg. Med. Chem. 2017, 25, 471–482. [Google Scholar] [CrossRef]
- Zhu, R.; Frazier, C.R.; Linden, J.; Macdonald, T.L. N6-Ethyl-2-alkynyl NECAs, selective human A3 adenosine receptor agonists. Bioorganic Med. Chem. Lett. 2006, 16, 2416–2418. [Google Scholar] [CrossRef]
- Minetti, P.; Tinti, M.O.; Carminati, P.; Castorina, M.; Di Cesare, M.A.; Di Serio, S.; Gallo, G.; Ghirardi, O.; Giorgi, F.; Giorgi, L.; et al. 2-n-Butyl-9-methyl-8-[1,2,3]triazol-2-yl-9H-purin-6-ylamine and Analogues as A2A Adenosine Receptor Antagonists. Design, Synthesis, and Pharmacological Characterization. J. Med. Chem. 2005, 48, 6887–6896. [Google Scholar] [CrossRef] [PubMed]
- Germain, A.R.; Carmody, L.C.; Nag, P.P.; Morgan, B.; VerPlank, L.; Fernandez, C.; Donckele, E.; Feng, Y.; Perez, J.R.; Dandapani, S.; et al. Cinnamides as selective small-molecule inhibitors of a cellular model of breast cancer stem cells. Bioorg. Med. Chem. Lett. 2013, 23, 1834–1838. [Google Scholar] [CrossRef] [PubMed]
- Stambaugh, K.; Jacobson, K.A.; Jiang, J.L.; Liang, B.T.; Robin, E.; Marcillac, F.; Raddatz, E.; Headrick, J.P.; Hack, B.; Ashton, K.J.; et al. A novel cardioprotective function of adenosine A1 and A3 receptors during prolonged simulated ischemia. Am. J. Physiol. Circ. Physiol. 1997, 273, H501–H505. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shneyvays, V.; Jacobson, K.; Li, A.-H.; Nawrath, H.; Zinman, T.; Isaac, A.; Shainberg, A. Induction of Apoptosis in Rat Cardiocytes by A3 Adenosine Receptor Activation and Its Suppression by Isoproterenol. Exp. Cell Res. 2000, 257, 111–126. [Google Scholar] [CrossRef]
- Ge, Z.-D.; Peart, J.N.; Kreckler, L.M.; Wan, T.C.; Jacobson, M.A.; Gross, G.J.; Auchampach, J.A. Cl-IB-MECA [2-Chloro-N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide] Reduces Ischemia/Reperfusion Injury in Mice by Activating the A Adenosine Receptor. J. Pharmacol. Exp. Ther. 2006, 319, 1200–1210. [Google Scholar] [CrossRef]
- Ge, Z.-D.; van der Hoeven, D.; Maas, J.E.; Wan, T.C.; Auchampach, J.A. A3 adenosine receptor activation during reperfusion reduces infarct size through actions on bone marrow-derived cells. J. Mol. Cell. Cardiol. 2010, 49, 280–286. [Google Scholar] [CrossRef]
- Tian, Y.; Marshall, M.; French, B.A.; Linden, J.; Yang, Z. The infarct-sparing effect of IB-MECA against myocardial ischemia/reperfusion injury in mice is mediated by sequential activation of adenosine A3 and A2A receptors. Basic Res. Cardiol. 2015, 110, 16. [Google Scholar] [CrossRef]
- Hofer, M.; Pospíšil, M.; Dušek, L.; Hoferová, Z.; Komůrková, D. Agonist of the adenosine A3 receptor, IB-MECA, and inhibitor of cyclooxygenase-2, meloxicam, given alone or in a combination early after total body irradiation enhance survival of γ-irradiated mice. Radiat. Environ. Biophys. 2013, 53, 211–215. [Google Scholar] [CrossRef]
- Panjehpour, M.; Karami-Tehrani, F. An adenosine analog (IB-MECA) inhibits anchorage-dependent cell growth of various human breast cancer cell lines. Int. J. Biochem. Cell Biol. 2004, 36, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, A.K.; Wong, M.Y.; Voshol, P.J.; Belardinelli, L.; Reaven, G.M. A1adenosine receptor partial agonist lowers plasma FFA and improves insulin resistance induced by high-fat diet in rodents. Am. J. Physiol. Metab. 2007, 292, E1358–E1363. [Google Scholar] [CrossRef] [PubMed]
- Fatholahi, M.; Xiang, Y.; Wu, Y.; Li, Y.; Wu, L.; Dhalla, A.K.; Belardinelli, L.; Shryock, J.C. A Novel Partial Agonist of the A1 -Adenosine Receptor and Evidence of Receptor Homogeneity in Adipocytes. J. Pharmacol. Exp. Ther. 2006, 317, 676–684. [Google Scholar] [CrossRef]
- Elzein, E.; Zablocki, J. A1adenosine receptor agonists and their potential therapeutic applications. Expert Opin. Investig. Drugs 2008, 17, 1901–1910. [Google Scholar] [CrossRef]
- Dhalla, A.K.; Santikul, M.; Smith, M.; Wong, M.-Y.; Shryock, J.C.; Belardinelli, L. Antilipolytic Activity of a Novel Partial A1 Adenosine Receptor Agonist Devoid of Cardiovascular Effects: Comparison with Nicotinic Acid. J. Pharmacol. Exp. Ther. 2007, 321, 327–333. [Google Scholar] [CrossRef]
- Carroll, S.H.; Wigner, N.A.; Kulkarni, N.; Johnston-Cox, H.; Gerstenfeld, L.C.; Ravid, K. A2B Adenosine Receptor Promotes Mesenchymal Stem Cell Differentiation to Osteoblasts and Bone Formation in Vivo. J. Biol. Chem. 2012, 287, 15718–15727. [Google Scholar] [CrossRef]
- Ham, J.; Evans, B.A.J. An emerging role for adenosine and its receptors in bone homeostasis. Front. Endocrinol. 2012, 3, 30507. [Google Scholar] [CrossRef]
- Kara, F.M.; Chitu, V.; Sloane, J.; Axelrod, M.; Fredholm, B.B.; Stanley, E.R.; Cronstein, B.N. Adenosine A1receptors (A1Rs) play a critical role in osteoclast formation and function. FASEB J. 2010, 24, 2325–2333. [Google Scholar] [CrossRef]
- Carvalho, V.F.; Ferreira, T.P.T.; de Arantes, A.C.S.; Noël, F.; Tesch, R.; Sant’aNna, C.M.R.; Barreiro, E.J.L.; Fraga, C.A.M.; e Silva, P.M.R.; Martins, M.A. LASSBio-897 Reduces Lung Injury Induced by Silica Particles in Mice: Potential Interaction with the A2A Receptor. Front. Pharmacol. 2017, 8, 778. [Google Scholar] [CrossRef]
- Pedreira, J.G.B.; Silva, R.R.; Noël, F.G.; Barreiro, E.J. Effect of S–Se Bioisosteric Exchange on Affinity and Intrinsic Efficacy of Novel N-acylhydrazone Derivatives at the Adenosine A2A Receptor. Molecules 2021, 26, 7364. [Google Scholar] [CrossRef]
- Silva, C.L.M.; Noël, F.; Barreiro, E.J. Cyclic GMP-dependent vasodilatory properties of LASSBio 294 in rat aorta. Br. J. Pharmacol. 2002, 135, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Mazzacuva, F.; Crocetti, L.; Giovannoni, M.P.; Cilibrizzi, A. PDE4 Inhibitors: Profiling Hits through the Multitude of Structural Classes. Int. J. Mol. Sci. 2023, 24, 11518. [Google Scholar] [CrossRef] [PubMed]
- Sudo, R.T.; Zapata-Sudo, G.; Barreiro, E.J. The new compound, LASSBio 294, increases the contractility of intact and saponin-skinned cardiac muscle from Wistar rats. Br. J. Pharmacol. 2001, 134, 603–613. [Google Scholar] [CrossRef]
- Costa, D.G.; da Silva, J.S.; Kümmerle, A.E.; Sudo, R.T.; Landgraf, S.S.; Caruso-Neves, C.; Fraga, C.A.; Barreiro, E.J.d.L.; Zapata-Sudo, G. LASSBio-294, A Compound With Inotropic and Lusitropic Activity, Decreases Cardiac Remodeling and Improves Ca2+ Influx Into Sarcoplasmic Reticulum After Myocardial Infarction. Am. J. Hypertens. 2010, 23, 1220–1227. [Google Scholar] [CrossRef]
- Rocha, B.d.S.; da Silva, J.S.; Pedreira, J.G.B.; Montagnoli, T.L.; Barreiro, E.J.; Zapata-Sudo, G. Efeito Anti-Hipertensivo de Novos Agonistas do Receptor de Adenosina em Ratos Espontaneamente Hipertensos. Arq. Bras. Cardiol. 2024, 121, e20230405. [Google Scholar] [CrossRef]
- da Silva, J.S.; Pereira, S.L.; Maia, R.D.C.; Landgraf, S.S.; Caruso-Neves, C.; Kümmerle, A.E.; Fraga, C.A.M.; Barreiro, E.J.; Sudo, R.T.; Zapata-Sudo, G. N-acylhydrazone improves exercise intolerance in rats submitted to myocardial infarction by the recovery of calcium homeostasis in skeletal muscle. Life Sci. 2014, 94, 30–36. [Google Scholar] [CrossRef]
- Alencar, A.K.; Pereira, S.L.; da Silva, F.E.; Mendes, L.V.; Cunha, V.D.M.; Lima, L.M.; Montagnoli, T.L.; Caruso-Neves, C.; Ferraz, E.B.; Tesch, R.; et al. N-acylhydrazone derivative ameliorates monocrotaline-induced pulmonary hypertension through the modulation of adenosine AA2R activity. Int. J. Cardiol. 2014, 173, 154–162. [Google Scholar] [CrossRef]
- Alencar, A.K.N.; Pereira, S.L.; Montagnoli, T.L.; Maia, R.C.; Kümmerle, A.E.; Landgraf, S.S.; Caruso-Neves, C.; Ferraz, E.B.; Tesch, R.; Nascimento, J.H.M.; et al. Beneficial effects of a novel agonist of the adenosine A2A receptor on monocrotaline-induced pulmonary hypertension in rats. Br. J. Pharmacol. 2013, 169, 953–962. [Google Scholar] [CrossRef]
- Silva, A.G.; Zapata-Sudo, G.; Kummerle, A.E.; Fraga, C.A.; Barreiro, E.J.; Sudo, R.T. Synthesis and vasodilatory activity of new N-acylhydrazone derivatives, designed as LASSBio-294 analogues. Bioorganic Med. Chem. 2005, 13, 3431–3437. [Google Scholar] [CrossRef]
- Alencar, A.K.N.; Montes, G.C.; Barreiro, E.J.; Sudo, R.T.; Zapata-Sudo, G. Adenosine Receptors As Drug Targets for Treatment of Pulmonary Arterial Hypertension. Front. Pharmacol. 2017, 8, 858. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Ijzerman, A.P.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001, 53, 527–552. [Google Scholar] [CrossRef]
- Klotz, K.-N. Adenosine receptors and their ligands. Naunyn-Schmiedebergs Arch. Pharmacol. 2000, 362, 382–391. [Google Scholar] [CrossRef]
- Lohse, M.J.; Klotz, K.-N.; Schwabe, U.; Cristalli, G.; Vittori, S.; Grifantini, M. 2-Chloro-N6-cyclopentyladenosine: A highly selective agonist at A1 adenosine receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1988, 337, 687–689. [Google Scholar] [CrossRef]
- Gao, Z.-G.; Jacobson, K.A. 2-Chloro-N6-cyclopentyladenosine, adenosine A1 receptor agonist, antagonizes the adenosine A3 receptor. Eur. J. Pharmacol. 2002, 443, 39–42. [Google Scholar] [CrossRef]
- Trivedi, B.K.; Blankley, C.J.; Bristol, J.A.; Hamilton, H.W.; Patt, W.C.; Kramer, W.J.; Johnson, S.A.; Bruns, R.F.; Cohen, D.M.; Ryan, M.J. N6-Substituted adenosine receptor agonists: Potential antihypertensive agents. J. Med. Chem. 1991, 34, 1043–1049. [Google Scholar] [CrossRef]
- Shewale, S.V.; Anstadt, M.P.; Horenziak, M.; Izu, B.; Morgan, E.E.; Lucot, J.B.; Morris, M. Sarin Causes Autonomic Imbalance and Cardiomyopathy. J. Cardiovasc. Pharmacol. 2012, 60, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Bolli, R.; Black, R.G.; Kodani, E.; Tang, X.-L.; Yang, Z.; Bhattacharya, S.; Auchampach, J.A. A1or A3Adenosine Receptors Induce Late Preconditioning Against Infarction in Conscious Rabbits by Different Mechanisms. Circ. Res. 2001, 88, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, A.; Liu, G.S.; Wilborn, W.H.; Downey, J.M. Pretreatment with the adenosine A1 selective agonist, 2-chloro-N6-cyclopentyladenosine (CCPA), causes a sustained limitation of infarct size in rabbits. Cardiovasc. Res. 1993, 27, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Bueters, T.J.; Groen, B.; Danhof, M.; Ijzerman, A.P.; van Helden, H.P. Therapeutic efficacy of the adenosine A 1 receptor agonist N 6 -cyclopentyladenosine (CPA) against organophosphate intoxication. Arch. Toxicol. 2002, 76, 650–656. [Google Scholar] [CrossRef]
- Martire, A.; Lambertucci, C.; Pepponi, R.; Ferrante, A.; Benati, N.; Buccioni, M.; Dal Ben, D.; Marucci, G.; Klotz, K.-N.; Volpini, R.; et al. Neuroprotective potential of adenosine A1 receptor partial agonists in experimental models of cerebral ischemia. J. Neurochem. 2019, 149, 211–230. [Google Scholar] [CrossRef]
- Headrick, J.P.; Peart, J.N.; Reichelt, M.E.; Haseler, L.J. Adenosine and its receptors in the heart: Regulation, retaliation and adaptation. Biochim. Biophys. Acta (BBA)-Biomembr. 2011, 1808, 1413–1428. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.Y.; Stone, R.A.; Civan, M.M. Knockout of A3 adenosine receptors reduces mouse intraocular pressure. Invest. Ophthalmol. Vis. Sci. 2002, 43, 3021–3026. [Google Scholar] [PubMed]
- Sawynok, J. Adenosine receptor activation and nociception. Eur. J. Pharmacol. 1998, 347, 1–11. [Google Scholar] [CrossRef]
- El-Tayeb, A.; Michael, S.; Abdelrahman, A.; Behrenswerth, A.; Gollos, S.; Nieber, K.; Müller, C.E. Development of Polar Adenosine A2AReceptor Agonists for Inflammatory Bowel Disease: Synergism with A2BAntagonists. ACS Med. Chem. Lett. 2011, 2, 890–895. [Google Scholar] [CrossRef]
- Mazzon, E.; Esposito, E.; Impellizzeri, D.; DI Paola, R.; Melani, A.; Bramanti, P.; Pedata, F.; Cuzzocrea, S. CGS 21680, an Agonist of the Adenosine (A2A) Receptor, Reduces Progression of Murine Type II Collagen-induced Arthritis. J. Rheumatol. 2011, 38, 2119–2129. [Google Scholar] [CrossRef]
- De Filippo, E.; Hinz, S.; Pellizzari, V.; Deganutti, G.; El-Tayeb, A.; Navarro, G.; Franco, R.; Moro, S.; Schiedel, A.C.; Müller, C.E. A2A and A2B adenosine receptors: The extracellular loop 2 determines high (A2A) or low affinity (A2B) for adenosine. Biochem. Pharmacol. 2020, 172, 113718. [Google Scholar] [CrossRef]
- Boncler, M.; Bartczak, K.; Rozalski, M. Potential for modulation of platelet function via adenosine receptors during inflammation. Br. J. Pharmacol. 2023, 181, 547–563. [Google Scholar] [CrossRef]
- Fuentes, E.; Badimon, L.; Caballero, J.; Padró, T.; Vilahur, G.; Alarcón, M.; Pérez, P.; Palomo, I. Protective mechanisms of adenosine 5′-monophosphate in platelet activation and thrombus formation. Thromb. Haemost. 2014, 112, 491–507. [Google Scholar] [CrossRef]
- Gnad, T.; Scheibler, S.; von Kügelgen, I.; Scheele, C.; Kilić, A.; Glöde, A.; Hoffmann, L.S.; Reverte-Salisa, L.; Horn, P.; Mutlu, S.; et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 2014, 516, 395–399. [Google Scholar] [CrossRef]
- Volpini, R.; Costanzi, S.; Lambertucci, C.; Portino, F.R.; Taffi, S.; Vittori, S.; Klotz, K.-N.; Cristalli, G.; Zhdankin, V.V. Aden-osine receptor agonists: Synthesis and binding affinity of 2-(aryl)alkylthioadenosine derivatives. Arkivoc 2004, 5, 301–311. [Google Scholar] [CrossRef]
- El-Tayeb, A.; Iqbal, J.; Behrenswerth, A.; Romio, M.; Schneider, M.; Zimmermann, H.; Schrader, J.; Müller, C.E. Nucleoside-5′-monophosphates as Prodrugs of Adenosine A2AReceptor Agonists Activated by ecto-5′-Nucleotidase†Contribution to celebrate the 100th anniversary of the Division of Medicinal Chemistry of the American Chemical Society. J. Med. Chem. 2009, 52, 7669–7677. [Google Scholar] [CrossRef]
- Ripley, D.P.; Jenkins, N.P.; Thomas, H.E. The Use of Adenosine in the Assessment of Stable Coronary Heart Disease. J. R. Coll. Physicians Edinb. 2019, 49, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, M.; Hori, M. It is time to ask what adenosine can do for cardioprotection. Hear. Vessel. 1998, 13, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Lütjens, R.; Perry, B.; Schelshorn, D.; Rocher, J.-P. New technologies enabling the industrialization of allosteric modulator discovery. Drug Discov. Today Technol. 2013, 10, e253–e260. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A. Structure-Based Approaches to Ligands for G-Protein-Coupled Adenosine and P2Y Receptors, from Small Molecules to Nanoconjugates. J. Med. Chem. 2013, 56, 3749–3767. [Google Scholar] [CrossRef]
- Qin, C.; Yang, Y.H.; May, L.; Gao, X.; Stewart, A.G.; Tu, Y.; Woodman, O.L.; Ritchie, R.H. Cardioprotective potential of annexin-A1 mimetics in myocardial infarction. Pharmacol. Ther. 2015, 148, 47–65. [Google Scholar] [CrossRef]
- Marshall, S.A.; Qin, C.X.; Jelinic, M.; O’Sullivan, K.; Deo, M.; Walsh, J.; Li, M.; Parry, L.J.; Ritchie, R.H.; Leo, C.H. The Novel Small-molecule Annexin-A1 Mimetic, Compound 17b, Elicits Vasoprotective Actions in Streptozotocin-induced Diabetic Mice. Int. J. Mol. Sci. 2020, 21, 1384. [Google Scholar] [CrossRef]
- García, R.A.; Ito, B.R.; Lupisella, J.A.; Carson, N.A.; Hsu, M.-Y.; Fernando, G.; Heroux, M.; Bouvier, M.; Dierks, E.; Kick, E.K.; et al. Preservation of Post-Infarction Cardiac Structure and Function via Long-Term Oral Formyl Peptide Receptor Agonist Treatment. JACC Basic Transl. Sci. 2019, 4, 905–920. [Google Scholar] [CrossRef]
- Cilibrizzi, A. Correspondence: Compound 17b and formyl peptide receptor biased agonism in relation to cardioprotective effects in ischaemia-reperfusion injury. Nat. Commun. 2018, 9, 531. [Google Scholar] [CrossRef]
- Studley, W.R.; Lamanna, E.; Martin, K.A.; Nold-Petry, C.A.; Royce, S.G.; Woodman, O.L.; Ritchie, R.H.; Qin, C.X.; Bourke, J.E. The small-molecule formyl peptide receptor biased agonist, compound 17b, is a vasodilator and anti-inflammatory in mouse precision-cut lung slices. Br. J. Pharmacol. 2023, 181, 2287–2301. [Google Scholar] [CrossRef]
- Nusca, A.; Patti, G. Platelet Function and Inhibition in Ischemic Heart Disease. Curr. Cardiol. Rep. 2012, 14, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Authi, K.S.; Kirpotina, L.N.; Schepetkin, I.A.; Quinn, M.T.; Cilibrizzi, A. Development of small-molecule fluorescent probes targeting neutrophils via N-formyl peptide receptors. RSC Med. Chem. 2025, 16, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Fernando, H.; McFadyen, J.D.; Wang, X.; Shaw, J.; Stub, D.; Peter, K. P2Y12 Antagonists in Cardiovascular Disease—Finding the Best Balance Between Preventing Ischemic Events and Causing Bleeding. Front. Cardiovasc. Med. 2022, 9, 854813. [Google Scholar] [CrossRef]
- Storey, R.F. The P2Y 12 receptor as a therapeutic target in cardiovascular disease. Platelets 2001, 12, 197–209. [Google Scholar] [CrossRef]
- Addis, P.; Bali, U.; Baron, F.; Campbell, A.; Harborne, S.; Jagger, L.; Milne, G.; Pearce, M.; Rosethorne, E.M.; Satchell, R.; et al. Key aspects of modern GPCR drug discovery. SLAS Discov. Adv. Sci. Drug Discov. 2023, 29, 1–22. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, T.; Lu, X.; Lan, X.; Chen, Z.; Lu, S. G protein-coupled receptors (GPCRs): Advances in structures, mechanisms and drug discovery. Signal Transduct. Target. Ther. 2024, 9, 88. [Google Scholar] [CrossRef]
- Campbell, A.P.; Smrcka, A.V. Targeting G protein-coupled receptor signalling by blocking G proteins. Nat. Rev. Drug Discov. 2018, 17, 789–803. [Google Scholar] [CrossRef]
- Pasquini, S.; Contri, C.; Cappello, M.; Borea, P.A.; Varani, K.; Vincenzi, F. Update on the recent development of allosteric modulators for adenosine receptors and their therapeutic applications. Front. Pharmacol. 2022, 13, 1030895. [Google Scholar] [CrossRef]
- Jiang, K.; Wu, J.; Wang, Q.; Chen, X.; Zhang, Y.; Gu, X.; Tang, K. Nanoparticles targeting the adenosine pathway for cancer immunotherapy. J. Mater. Chem. B 2024, 12, 5787–5811. [Google Scholar] [CrossRef]
- Cao, L.; Li, Y.; Yang, S.; Li, G.; Zhou, Q.; Sun, J.; Xu, T.; Yang, Y.; Liao, R.; Shi, Y.; et al. The adenosine analog prodrug ATV006 is orally bioavailable and has preclinical efficacy against parental SARS-CoV-2 and variants. Sci. Transl. Med. 2022, 14, eabm7621. [Google Scholar] [CrossRef] [PubMed]
- Simonetto, C.; Rospleszcz, S.; Kaiser, J.C.; Furukawa, K. Heterogeneity in coronary heart disease risk. Sci. Rep. 2022, 12, 10131. [Google Scholar] [CrossRef]
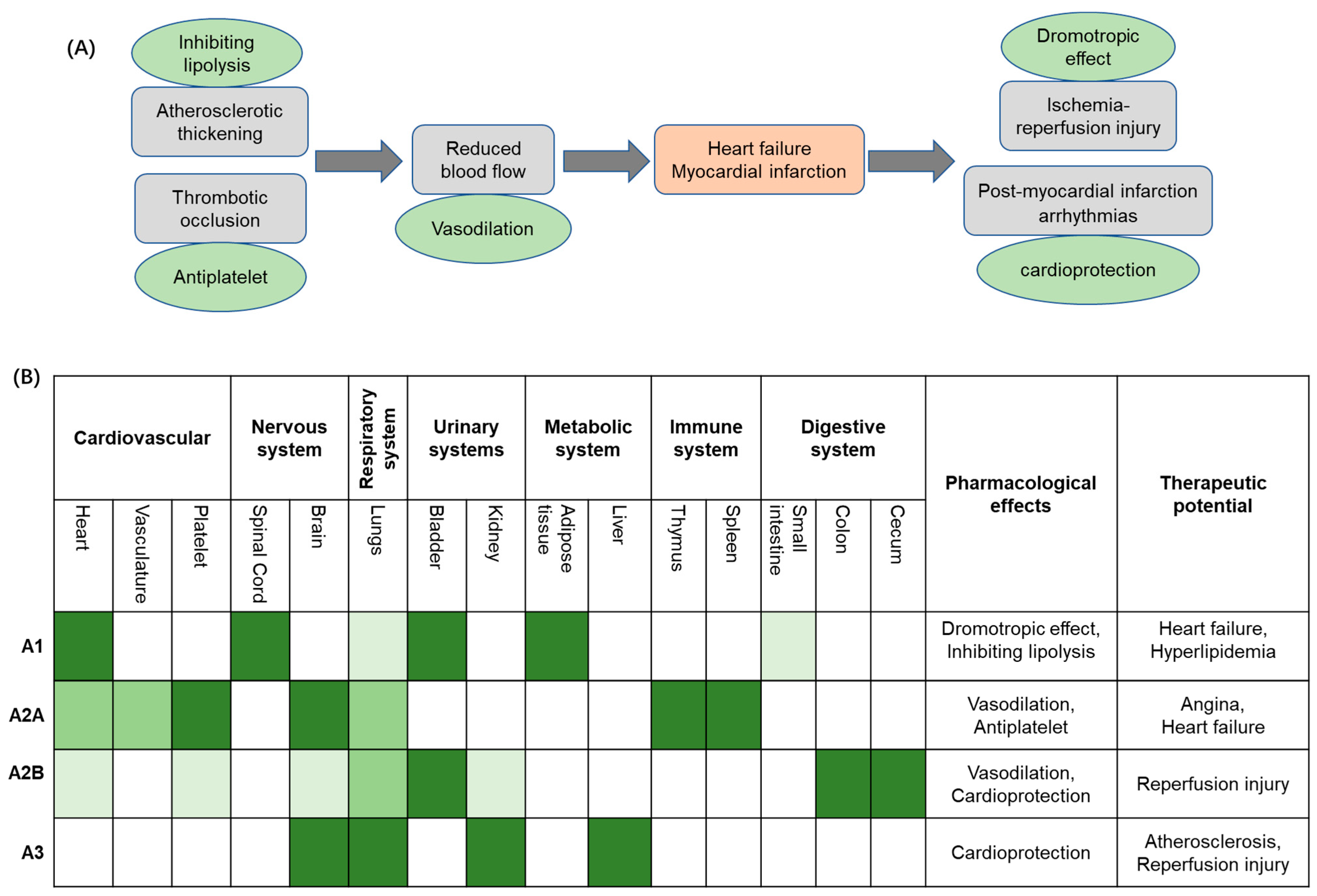
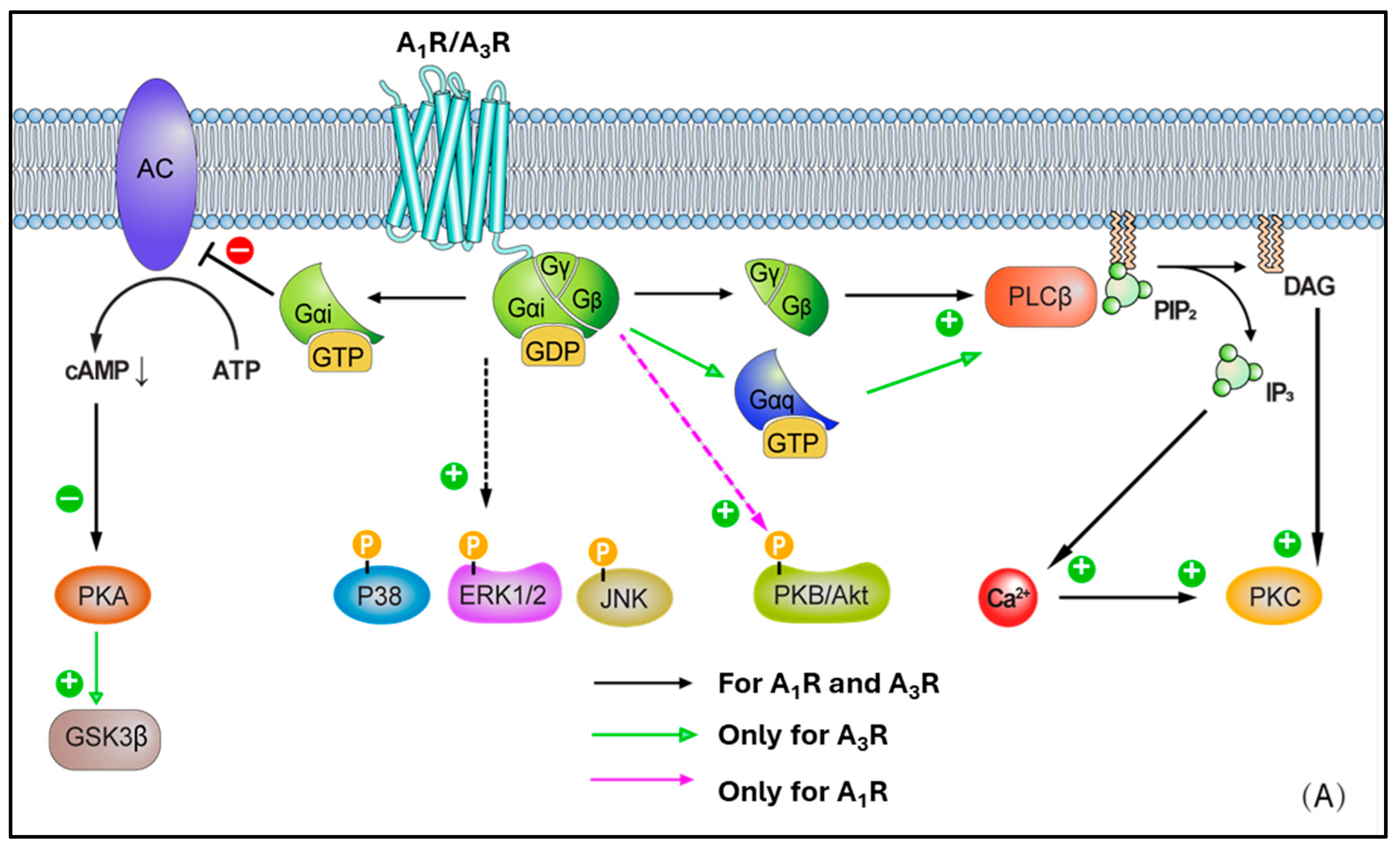
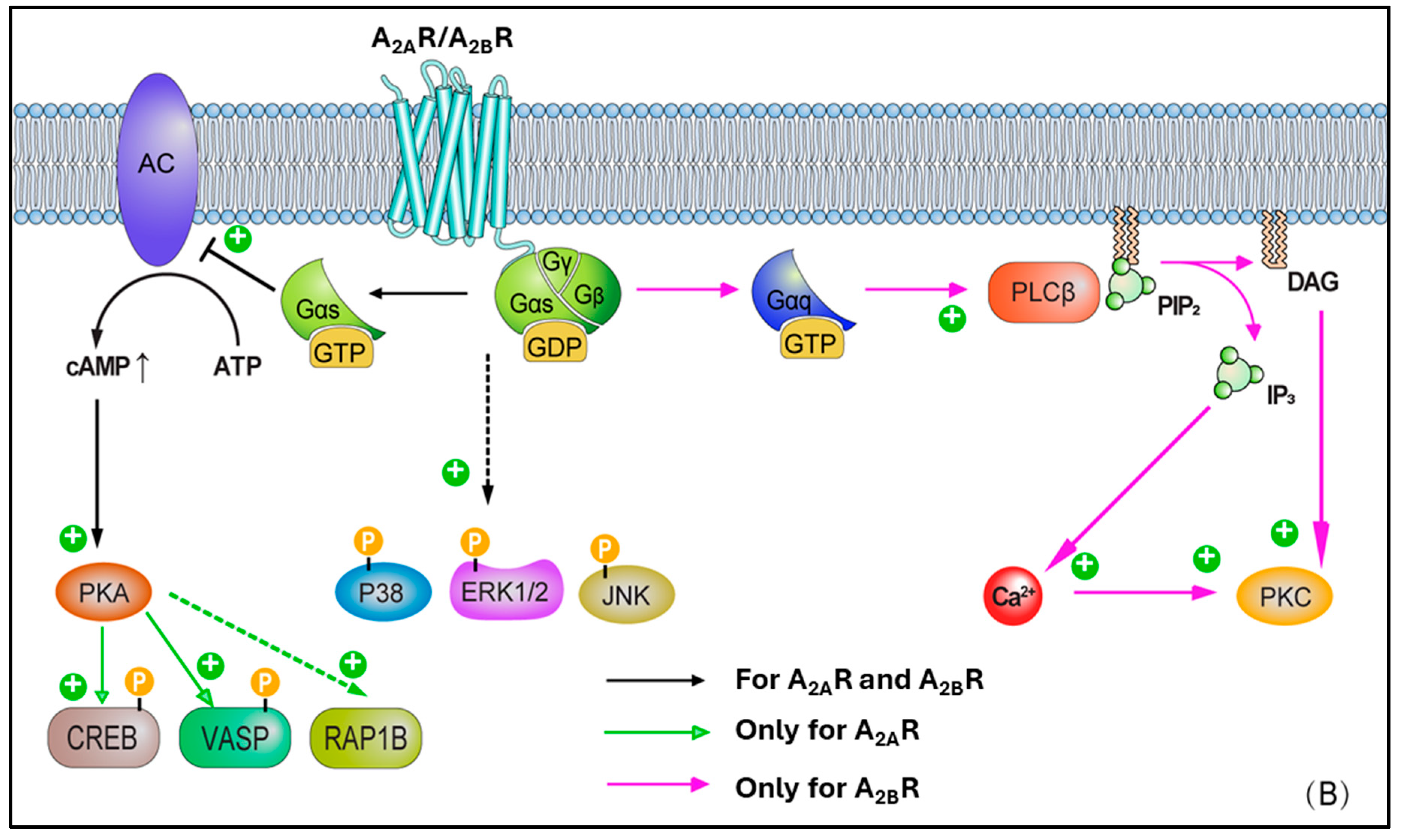
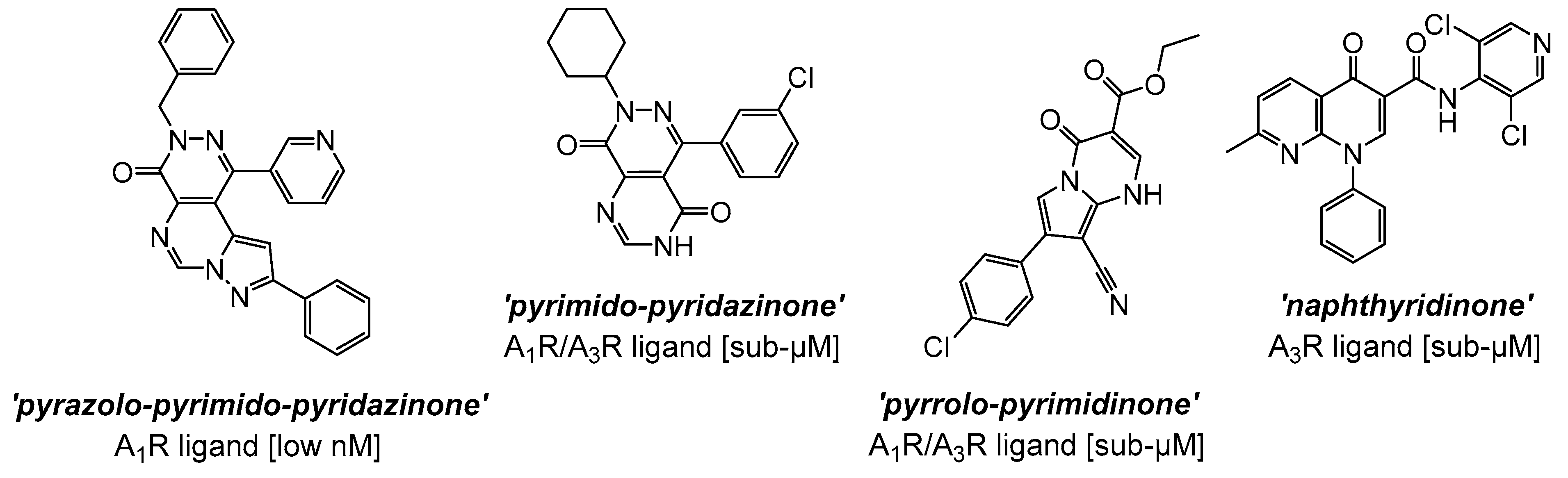
| Effect | ARs | Mechanism | Outcome |
|---|---|---|---|
| Bradycardia | A1 | Gi → ↓ cAMP → ↑ K+ efflux | Persistently slows heart rate |
| Atrioventricular node block | A1 | Gi → ↓ cAMP → ↑ K+ efflux | Interrupts reentrant tachycardia |
| Coronary vasodilation | A2A, A2B | Gs → ↑ cAMP in smooth muscle NO ↑, ↑ K+ efflux | ↑ Coronary flow |
| Hypotension | A2A | Systemic vasodilation | ↓ BP |
| Anti-platelet | A2A | Gs → ↑ cAMP in platelets | ↓ Aggregation |
| Ischemic protection | A1, A2A, A3 | Multiple | ↓ Injury during MI |
| Immune Cell Type | ARs | Effect |
|---|---|---|
| T cells | A2A, A2B | ↓ T cell activation, ↓ IL-2 production, ↓ proliferation |
| Macrophages | A2A, A2B | ↓ Pro-inflammatory cytokines (e.g., TNF-α, IL-6), ↑ IL-10 |
| Neutrophils | A2A | ↓ Chemotaxis, ↓ degranulation, ↓ reactive oxygen species (ROS) |
| Dendritic cells | A2A | ↓ Maturation and antigen presentation |
| Mast cells | A2B, A3 | ↑ Histamine release (context-dependent), ↑ pro-inflammatory cytokines |
| Natural Killer (NK) cells | A2A | ↓ Cytotoxicity and cytokine secretion |
| AR Ligand | Structure | AR | Objective | Potency |
|---|---|---|---|---|
| RPR-749 [172] (clinical stage) | structure not available (Aventis, 1429209-67-5) | A1 | lipid concentration activity | low-nM Ki |
| LJ-1888 [173,174] (9d in [180]) (pre-clinical stage) | 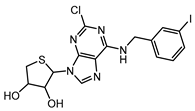 | A3 | cardioprotection | Ki ≈ 10–30 nM |
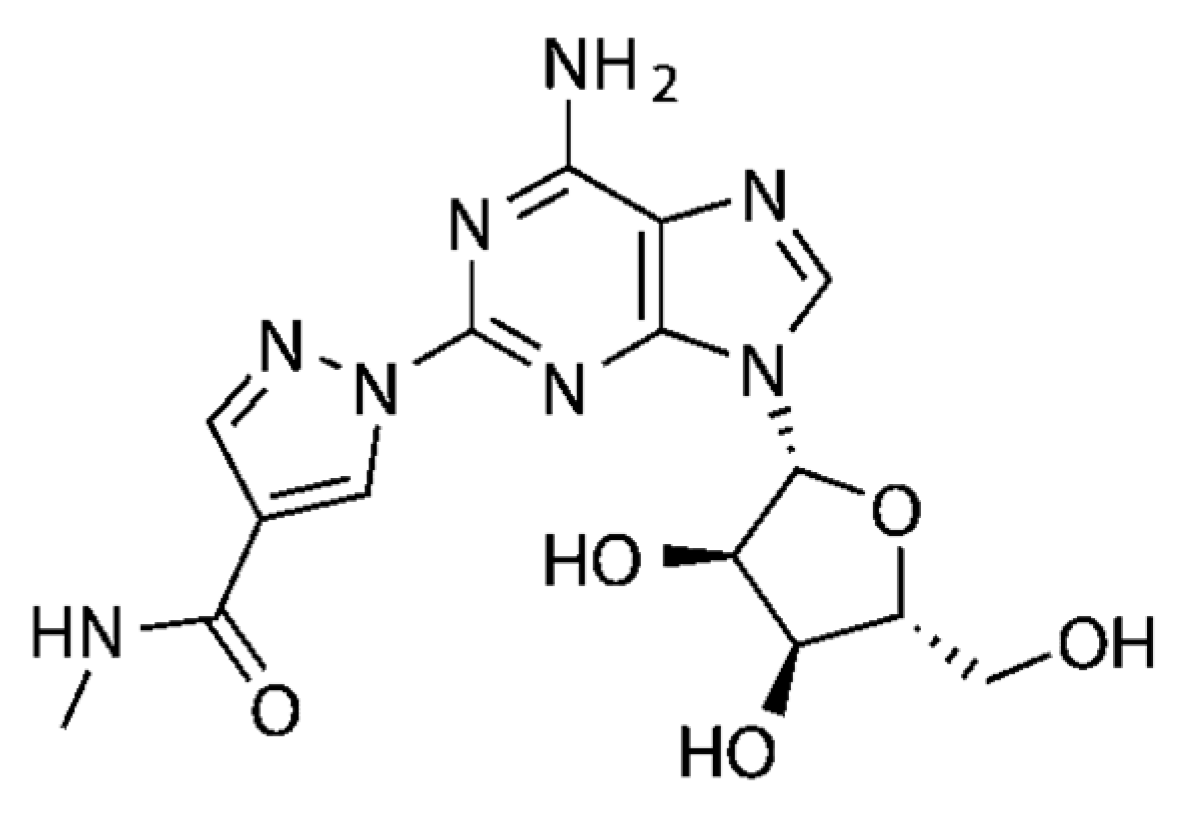
| IB-MECA [175] (pre-clinical stage) |  | A3 | cardioprotection | Ki ≈ 1.1 nM |
| GS-9667 [176] (clinical stage) | 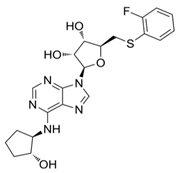 | A1 | lipid concentration activity | Ki ≈ 55 nM |
| LassBio-294 [177] (pre-clinical stage) | 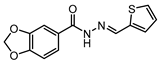 | A2A | cardioprotection | µM EC50 |
| CPA [178] (clinical stage) | 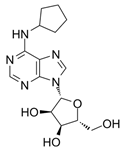 | A1 | cardiovascular effects | sub- to low-nM Ki |
| PSB-15826 [179] (clinical stage) | 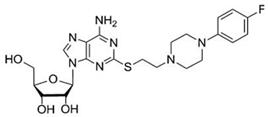 | A2A | antiplatelet activity | sub-µM EC50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Opoku, Y.N.; Authi, K.S.; Cilibrizzi, A. Synthetic Small-Molecule Ligands Targeted to Adenosine Receptors: Is There Potential Towards Ischemic Heart Disease? Cells 2025, 14, 1219. https://doi.org/10.3390/cells14151219
Xu Q, Opoku YN, Authi KS, Cilibrizzi A. Synthetic Small-Molecule Ligands Targeted to Adenosine Receptors: Is There Potential Towards Ischemic Heart Disease? Cells. 2025; 14(15):1219. https://doi.org/10.3390/cells14151219
Chicago/Turabian StyleXu, Qi, Yaw Nana Opoku, Kalwant S. Authi, and Agostino Cilibrizzi. 2025. "Synthetic Small-Molecule Ligands Targeted to Adenosine Receptors: Is There Potential Towards Ischemic Heart Disease?" Cells 14, no. 15: 1219. https://doi.org/10.3390/cells14151219
APA StyleXu, Q., Opoku, Y. N., Authi, K. S., & Cilibrizzi, A. (2025). Synthetic Small-Molecule Ligands Targeted to Adenosine Receptors: Is There Potential Towards Ischemic Heart Disease? Cells, 14(15), 1219. https://doi.org/10.3390/cells14151219






