Central Insulin-Like Growth Factor-1-Induced Anxiolytic and Antidepressant Effects in a Rat Model of Sporadic Alzheimer’s Disease Are Associated with the Peripheral Suppression of Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
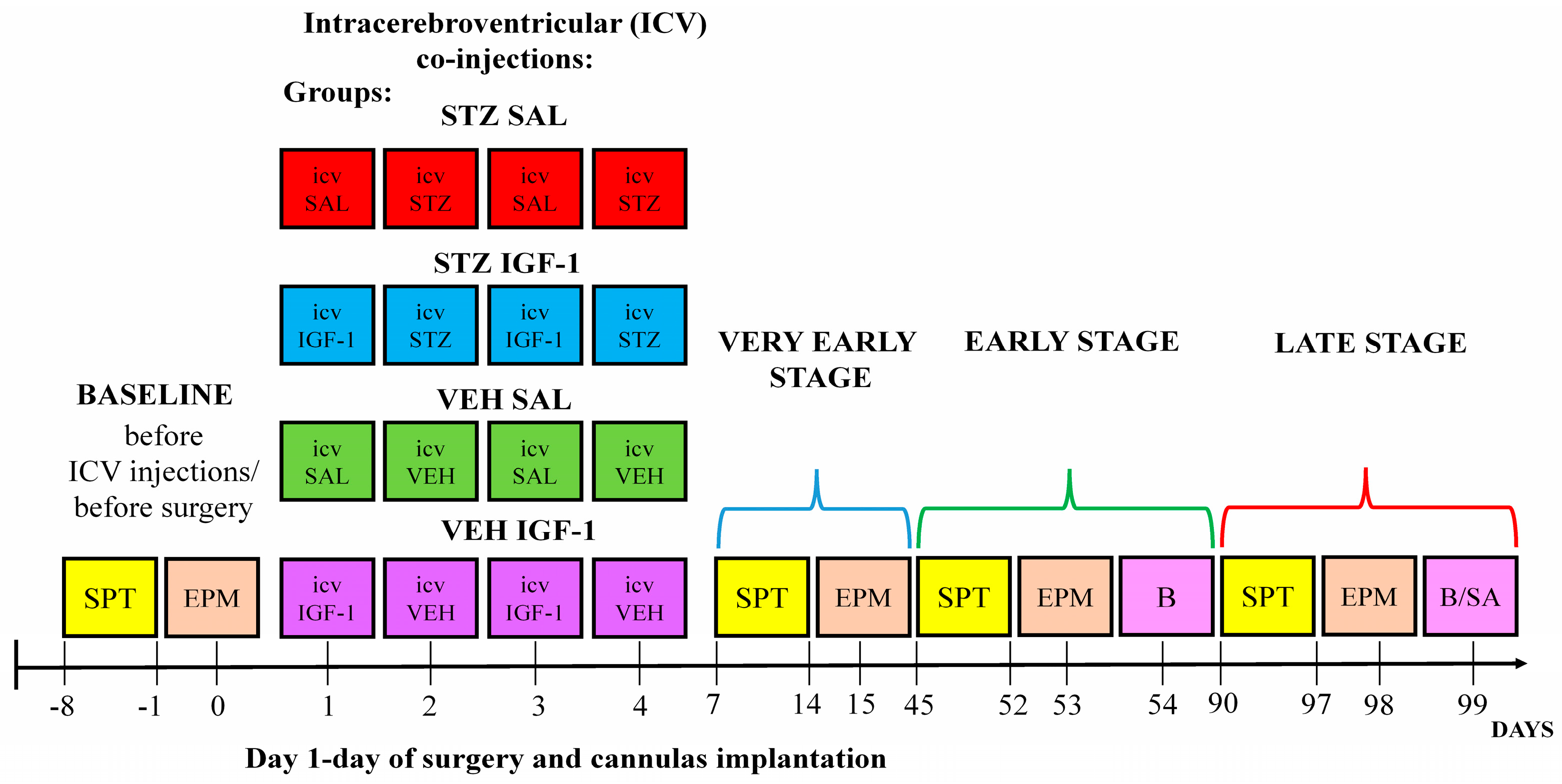
2.2. Induction of Sporadic Alzheimer’s Disease Model and Treatment with Insulin-Like Growth Factor-1 (IGF-1)
2.3. Sucrose Preference Test (SPT)
2.4. Elevated Plus Maze (EPM)
2.5. Determination of Hematological and Immunological Parameters in the Blood and Spleen
2.6. Detemination of Anti-Inflammatory Interleukin-10 (IL-10) and Pro-Inflammatory Interleukin-6 (IL-6) Concentrations in Plasma
2.7. Determination of Corticosterone (CORT) Concentration in Plasma
2.8. Sacrifice of Animal and Collection of Tissues and Organs
2.9. Data Analysis
3. Results
3.1. Behavioral Activity Associated with Anhedonia in the Sucrose Preference Test (SPT)
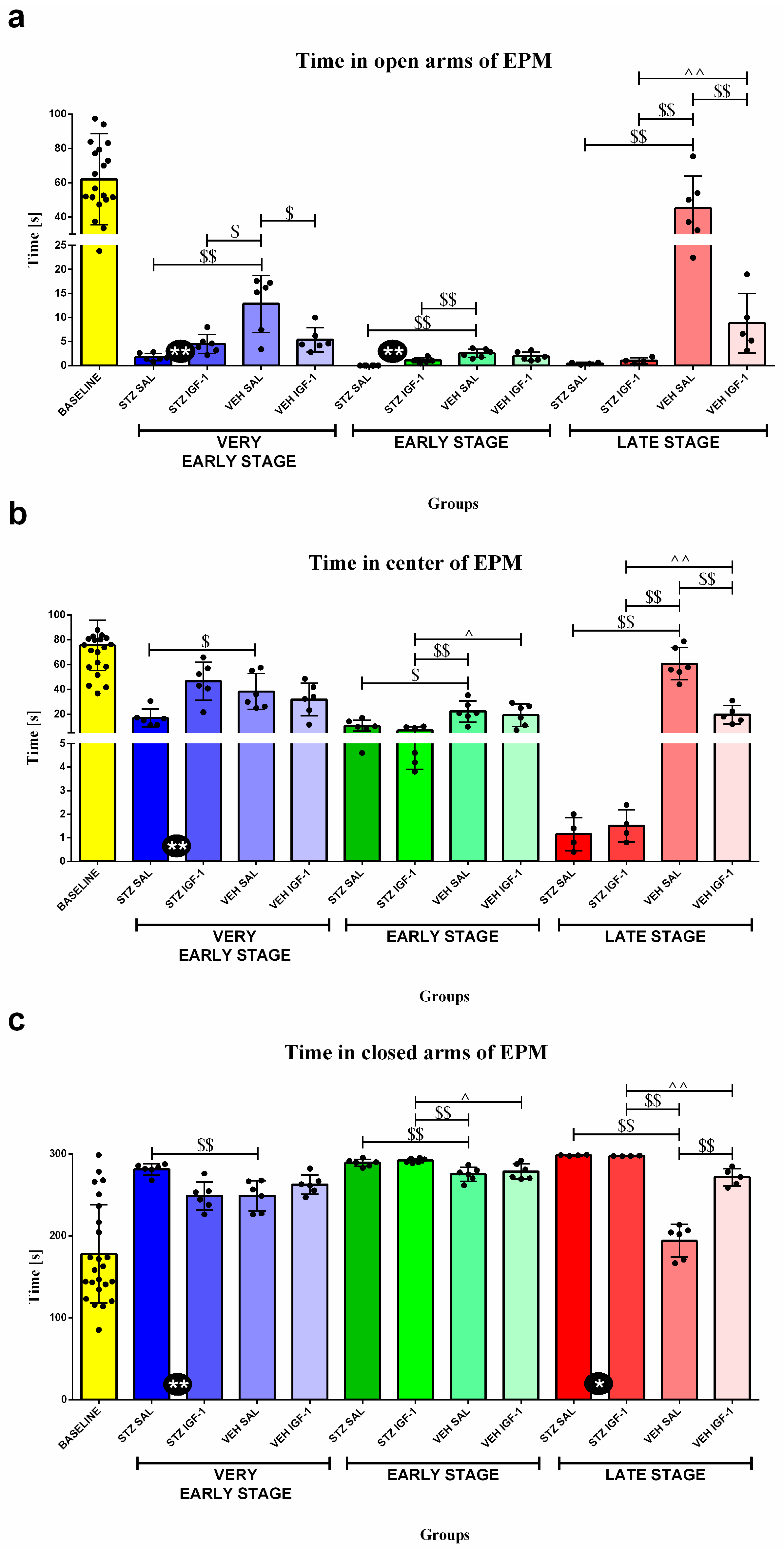
3.2. Behavioral Activity Associated with Anxiety in the Elevated Plus Maze (EPM)
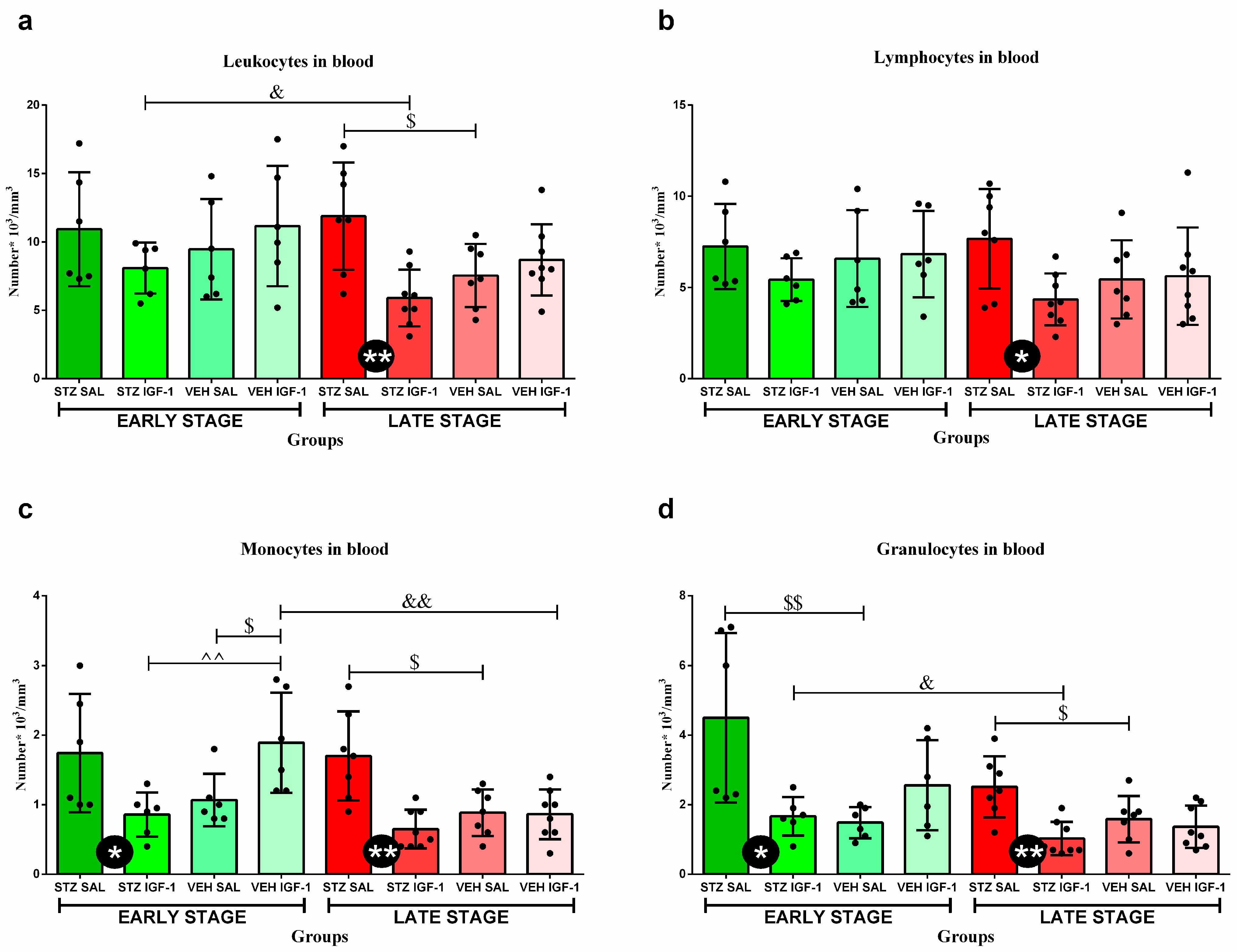
3.3. Peripheral Blood Leukocytes and Their Subpopulations’ Numbers and Percentages
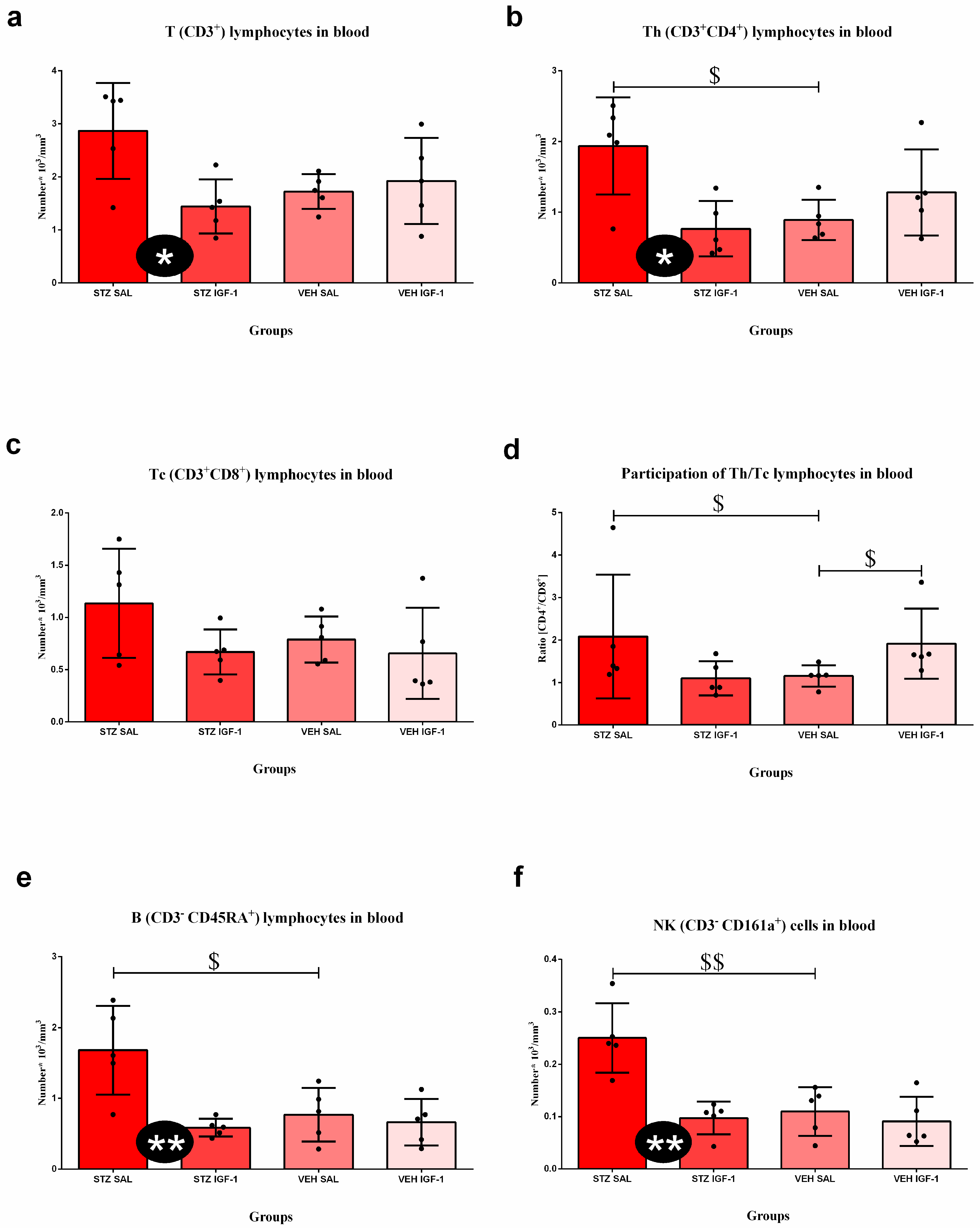
3.4. Peripheral Blood and Spleen Lymphocytes and Their Subpopulations’ Numbers and Percentages
3.5. Relative Weights of the Spleen and Thymus
3.6. Hematological Parameters
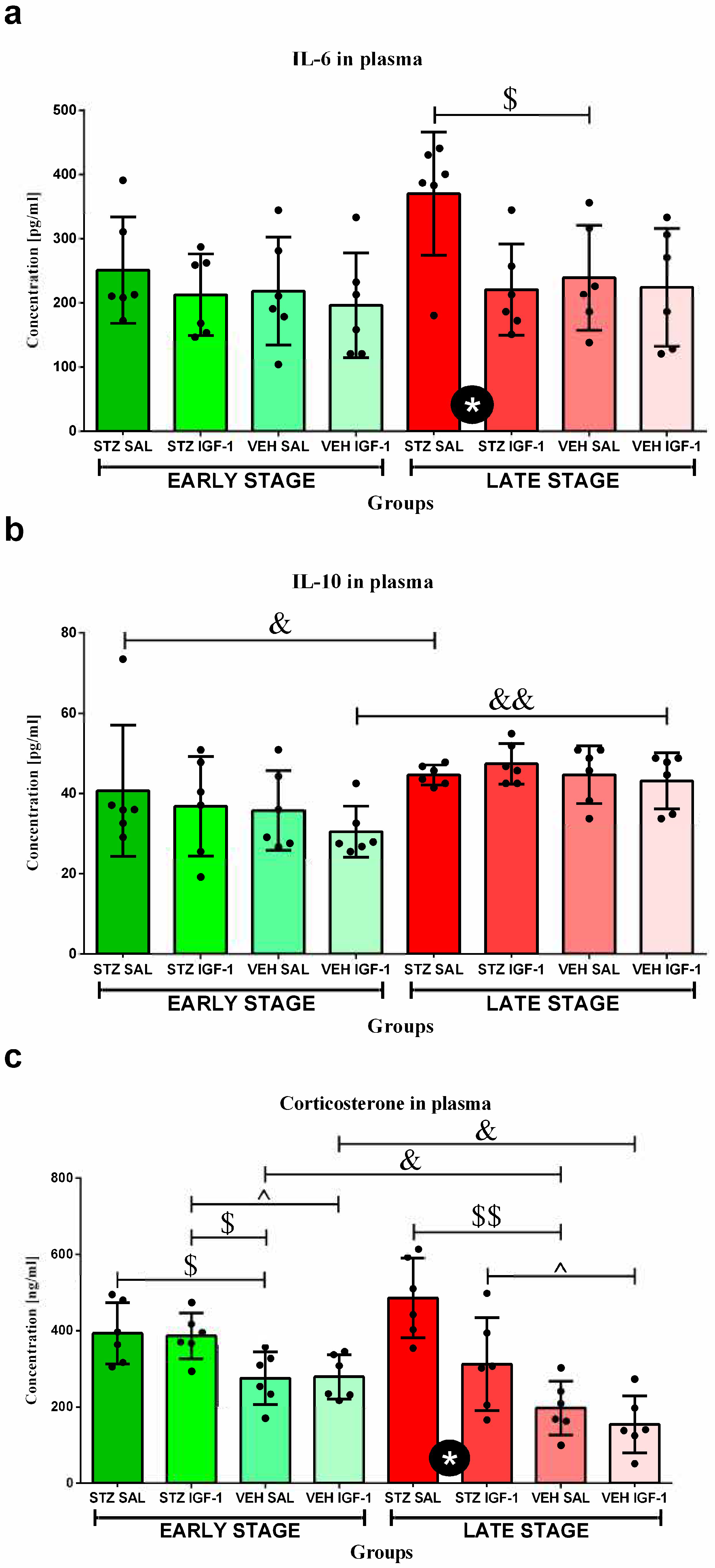
3.7. The Concentrations of Interleukin 6 (IL-6) and Interleukin 10 (IL-10) in Plasma
3.8. Determination of Plasma Corticosterone (CORT) Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IGF-1 | Insulin-like growth factor-1 |
| AD | Alzheimer’s disease |
| sAD | Sporadic Alzheimer’s disease |
| STZ | Streptozotocin |
| VEH | Vehicle |
| SAL | Saline |
| ICV | Intracerebroventricular injection |
| VES | Very early state of sAD progression |
| ES | Early state of sAD progression |
| LS | Late state of sAD progression |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| IL-17 | Interleukin 17 |
| EPM | Elevated plus maze |
| SPT | Sucrose preference test |
| GC | Glicocorticoid |
| BBB | Brain–blood barrier |
| ROS | Reactive oxygen species |
| CORT | Corticosterone |
| Th | T helper lymphocytes |
| Tc | T cytotoxic lymphocytes |
| Aβ | Beta amyloid |
| IRBS | Insulin-resistant brain state |
| HPA axis | Hypothalamic–pituitary–adrenal axis |
| PBS | Phosphate-buffered saline |
| RBC | Red blood cell |
| HGB | Hemoglobin concentration |
| MCHC | Mean hemoglobin concentration in the red blood cells |
| MCH | Mean mass of the hemoglobin in the red blood cells |
| MCV | Mean corpuscular volume |
| HCT | Hematocrit |
| RDW | Red cell distribution width |
| PLT | Platelet |
| MPV | Mean platelet volume |
| PCT | Platelecrit |
References
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Rabinovici, G.D. Late-onset Alzheimer Disease. Contin. Lifelong Learn. Neurol. 2019, 25, 14–33. [Google Scholar] [CrossRef]
- Correia, S.C.; Santos, R.X.; Perry, G.; Zhu, X.; Moreira, P.I.; Smith, M.A. Insulin-resistant brain state: The culprit in sporadic Alzheimer’s disease? Ageing Res. Rev. 2011, 10, 264–273. [Google Scholar] [CrossRef]
- Buccellato, F.R.; D’Anca, M.; Serpente, M.; Arighi, A.; Galimberti, D. The role of glymphatic system in Alzheimer’s and Parkinson’s disease pathogenesis. Biomedicines 2022, 10, 2261. [Google Scholar] [CrossRef]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef]
- Balin, B.J.; Hudson, A.P. Etiology and pathogenesis of late-onset Alzheimer’s disease. Curr. Allergy Asthma Rep. 2014, 14, 417. [Google Scholar] [CrossRef]
- Mravec, B.; Horvathova, L.; Padova, A. Brain under stress and Alzheimer’s disease. Cell. Mol. Neurobiol. 2018, 38, 73–84. [Google Scholar] [CrossRef]
- Armstrong, R.A. Risk factors for Alzheimer’s disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.; Abondio, P.; Bruno, R.; Ceraudo, L.; Paparazzo, E.; Citrigno, L.; Luiselli, D.; Bruni, A.C.; Passarino, G.; Colao, R.; et al. Alzheimer’s disease as a viral disease: Revisiting the infectious hypothesis. Ageing Res. Rev. 2023, 91, 102068. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhao, G.; Zhao, Y. Dysregulation of energy metabolism in Alzheimer’s disease. J. Neurol. 2024, 272, 2. [Google Scholar] [CrossRef] [PubMed]
- Sharan, P.; Vellapandian, C. Hypothalamic-Pituitary-Adrenal (HPA) axis: Unveiling the potential mechanisms involved in stress-induced Alzheimer’s disease and depression. Cureus 2024, 16, e67595. [Google Scholar] [CrossRef]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s disease: Treatment strategies and their limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef]
- Vitek, G.E.; Decourt, B.; Sabbagh, M.N. Lecanemab (BAN2401): An anti-beta-amyloid monoclonal antibody for the treatment of Alzheimer disease. Expert. Opin. Investig. Drugs 2023, 32, 89–94. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Tota, S.K.; Kumar, A.; Ahmad, A.S. Streptozotocin intracerebroventricular-induced neurotoxicity and brain insulin resistance: A therapeutic intervention for treatment of sporadic Alzheimer’s disease (sAD)-like pathology. Mol. Neurobiol. 2016, 53, 4548–4562. [Google Scholar] [CrossRef]
- Titisari, N.; Ahmad, H.; Samsulrizal, N.; Fauzi, A.; Abdul Razak, I.S. The mechanism underlying streptozotocin injection for the development of a nontransgenic Alzheimer’s disease animal model. Open Vet. J. 2025, 15, 594–600. [Google Scholar] [CrossRef]
- Salkovic-Petrisic, M.; Hoyer, S. Central insulin resistance as a trigger for sporadic Alzheimer-like pathology: An experimental approach. J. Neural. Transm. Suppl. 2007, 72, 217–233. [Google Scholar] [CrossRef]
- Mehla, J.; Pahuja, M.; Gupta, Y.K. Streptozotocin-induced sporadic Alzheimer’s disease: Selection of appropriate dose. J. Alzheimer Dis. 2013, 33, 17–21. [Google Scholar] [CrossRef]
- Majkutewicz, I.; Kurowska, E.; Podlacha, M.; Myślińska, D.; Grembecka, B.; Ruciński, J.; Plucińska, K.; Jerzemowska, G.; Wrona, D. Dimethyl fumarate attenuates intracerebroventricular streptozotocin-induced spatial memory impairment and hippocampal neurodegeneration in rats. Behav. Brain Res. 2016, 308, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Majkutewicz, I.; Kurowska, E.; Podlacha, M.; Myślińska, D.; Grembecka, B.; Ruciński, J.; Pierzynowska, K.; Wrona, D. Age-dependent effects of dimethyl fumarate on cognitive and neuropathological features in the streptozotocin-induced rat model of Alzheimer’s disease. Brain Res. 2018, 1686, 19–33. [Google Scholar] [CrossRef]
- Dunacka, J.; Świątek, G.; Wrona, D. High behavioral reactivity to novelty as a susceptibility factor for memory and anxiety disorders in streptozotocin-induced neuroinflammation as a rat model of Alzheimer’s disease. Int. J. Mol. Sci. 2024, 25, 11562. [Google Scholar] [CrossRef] [PubMed]
- Naghibi, S.; Shariatzadeh Joneydi, M.; Barzegari, A.; Davoodabadi, A.; Ebrahimi, A.; Eghdami, E.; Fahimpour, N.; Ghorbani, M.; Mohammadikia, E.; Rostami, M.; et al. Treadmill exercise sex-dependently alters susceptibility to depression-like behaviour, cytokines and BDNF in the hippocampus and prefrontal cortex of rats with sporadic Alzheimer-like disease. Physiol. Behav. 2021, 241, 113595. [Google Scholar] [CrossRef]
- Knezovic, A.; Osmanovic-Barilar, J.; Curlin, M.; Hof, P.R.; Simic, G.; Riederer, P.; Salkovic-Petrisic, M. Staging of cognitive deficits and neuropathological and ultrastructural changes in streptozotocin-induced rat model of Alzheimer’s disease. J. Neural Transm. 2015, 122, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Spiers, J.G.; Chen, H.J.; Sernia, C.; Lavidis, N.A. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front. Neurosci. 2015, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Rocamora-Reverte, L.; Villunger, A.; Wiegers, G.J. Cell-Specific Immune regulation by glucocorticoids in murine models of infection and inflammation. Cells 2022, 11, 2126. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Topete, D.; Cidlowski, J.A. One hormone, two actions: Anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation 2015, 22, 20–32. [Google Scholar] [CrossRef]
- Vyas, S.; Rodrigues, A.J.; Silva, J.M.; Tronche, F.; Almeida, O.F.; Sousa, N.; Sotiropoulos, I. Chronic stress and glucocorticoids: From neuronal plasticity to neurodegeneration. Neural Plast. 2016, 2016, 6391686. [Google Scholar] [CrossRef]
- Hu, P.; Lu, Y.; Pan, B.X.; Zhang, W.H. New insights into the pivotal role of the amygdala in inflammation-related depression and anxiety disorder. Int.J. Mol. Sci. 2022, 23, 11076. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, G.; Luo, Y.; Jiang, L.; Chi, H.; Tian, G. Neuroinflammation in Alzheimer’s disease: Insights from peripheral immune cells. Immun. Ageing 2024, 21, 38. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, M.; Hao, W.; Wang, Y.; Zhang, T.; Liu, C. Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression. Transl. Psychiatry 2023, 13, 5. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Costa-Besada, M.A.; Labandeira, C.M.; Villar-Cheda, B.; Rodríguez-Perez, A. Insulin-like growth factor-1 and neuroinflammation. Front. Aging Neurosci. 2017, 9, 365. [Google Scholar] [CrossRef]
- Gray, S.C.; Kinghorn, K.J.; Woodling, N.S. Shifting equilibriums in Alzheimer’s disease: The complex roles of microglia in neuroinflammation, neuronal survival and neurogenesis. Neural Regen. Res. 2020, 15, 1208–1219. [Google Scholar] [CrossRef]
- Carlson, S.W.; Saatman, K.E. Central infusion of insulin-like growth factor-1 increases hippocampal neurogenesis and improves neurobehavioral function after traumatic brain injury. J. Neurotrauma 2018, 35, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Lackey, B.R.; Gray, S.L.; Henricks, D.M. Actions and interactions of the IGF system in Alzheimer’s disease: Review and hypotheses. Growth Horm. IGF Res. 2000, 10, 1–13. [Google Scholar] [CrossRef]
- Herrera, M.L.; Camparini, L.G.; Oliveros, A.L.; Bellini, M.J.; Herenu, C.B. Potentialities of IGF-1 for regulating oxidative stress in neuroinflammation and neurodegeneration: Theoretical review. Explor. Neuroprot. Ther. 2024, 4, 442–458. [Google Scholar] [CrossRef]
- Basta-Kaim, A.; Szczesny, E.; Glombik, K.; Stachowicz, K.; Slusarczyk, J.; Nalepa, I.; Zelek-Molik, A.; Rafa-Zablocka, K.; Budziszewska, B.; Kubera, M.; et al. Prenatal stress affects insulin-like growth factor-1 (IGF-1) level and IGF-1 receptor phosphorylation in the brain of adult rats. Eur. Neuropsychopharmacol. 2014, 24, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Dunacka, J.; Grembecka, B.; Majkutewicz, I.; Wrona, D. Central insulin-like growth factor-1 treatment enhances working and reference memory by reducing neuroinflammation and amyloid beta deposition in a rat model of sporadic Alzheimer’s disease. Pharmaceuticals 2025, 18, 527. [Google Scholar] [CrossRef]
- Westwood, A.J.; Beiser, A.; Decarli, C.; Harris, T.B.; Chen, T.C.; He, X.M.; Roubenoff, R.; Pikula, A.; Au, R.; Braverman, L.E.; et al. Insulin-like growth factor-1 and risk of Alzheimer dementia and brain atrophy. Neurology 2014, 82, 1613–1619. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2007; ISBN 978-0-0804-7515-8. [Google Scholar]
- Malberg, J.E.; Platt, B.; Rizzo, S.J.; Ring, R.H.; Lucki, I.; Schechter, L.E.; Rosenzweig-Lipson, S. Increasing the levels of insulin-like growth factor-I by an IGF binding protein inhibitor produces anxiolytic and antidepressant-like effects. Neuropsychopharmacology 2007, 11, 2360–2368. [Google Scholar] [CrossRef]
- Berry, A.; Bellisario, V.; Capoccia, S.; Tirassa, P.; Calza, A.; Alleva, E.; Cirulli, F. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology 2012, 37, 762–772. [Google Scholar] [CrossRef]
- Fonseca-Rodrigues, D.; Gonçalves, J.; Laranjeira, I.; Almeida, A.; Pinto-Ribeiro, F. Sucrose intake and preference by Wistar Han rats are not influenced by sex or food/water deprivation. Pharmacol. Biochem. Behav. 2022, 216, 73387. [Google Scholar] [CrossRef] [PubMed]
- Podlacha, M.; Glac, W.; Listowska, M.; Grembecka, B.; Majkutewicz, I.; Myślińska, D.; Plucińska, K.; Jerzemowska, G.; Grzybowska, M.; Wrona, D. Medial septal NMDA glutamate Receptors are involved in modulation of blood natural killer cell activity in rats. J. Neuroimmune Pharmacol. 2016, 11, 121–132. [Google Scholar] [CrossRef]
- Wrona, D.; Majkutewicz, I.; Świątek, G.; Dunacka, J.; Grembecka, B.; Glac, W. Dimethyl fumarate as the peripheral blood inflammatory mediators inhibitor in prevention of streptozotocin-induced neuroinflammation in aged rats. J. Inflamm. Res. 2022, 15, 33–52. [Google Scholar] [CrossRef]
- Listowska, M.; Glac, W.; Grembecka, B.; Grzybowska, M.; Wrona, D. Change in blood CD4+ T and CD8+ T lymphocytes in stressed rats pretreated chronically with desipramine are more pronounced after chronic open field stress challenge. J. Neuroimmunol. 2015, 282, 54–62. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawisk, J.; et al. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Stomrud, E.; Lindberg, O.; Westman, E.; Johansson, P.M.; van Westen, D.; Mattsson, N.; Hansson, O. Apathy and anxiety are early markers of Alzheimer’s disease. Neurobiol. Aging 2020, 85, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Swann, P.; Mirza-Davies, A.; O’Brien, J. Associations between neuropsychiatric symptoms and inflammation in neurodegenerative dementia: A systematic review. J. Inflamm. Res. 2024, 17, 6113–6141. [Google Scholar] [CrossRef]
- Palmqvist, S.; Schöll, M.; Strandberg, O.; Mattsson, N.; Stomrud, E.; Zetterberg, H.; Blennow, K.; Landau, S.; Jagust, W.; Hansson, O. Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat. Commun. 2017, 8, 1214. [Google Scholar] [CrossRef]
- Rosenberg, P.B.; Nowrangi, M.A.; Lyketsos, C.G. Neuropsychiatric symptoms in Alzheimer’s disease: What might be associated brain circuits? Mol. Asp. Med. 2015, 43–44, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Theleritis, C.; Politis, A.; Siarkos, K.; Lyketsos, C.G. A review of neuroimaging findings of apathy in Alzheimer’s disease. Int. Psychogeriatr. 2014, 26, 195–207. [Google Scholar] [CrossRef]
- Hopperton, K.E.; Mohammad, D.; Trépanier, M.O.; Giuliano, V.; Bazinet, R.P. Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: A systematic review. Mol. Psychiatry 2018, 23, 177–198. [Google Scholar] [CrossRef]
- Minett, T.; Classey, J.; Matthews, F.E.; Fahrenhold, M.; Taga, M.; Brayne, C.; Ince, P.G.; Nicoll, J.A.R.; Boche, D.; MRC CFAS. Microglial immunophenotype in dementia with Alzheimer’s pathology. J. Neuroinflamm. 2016, 13, 135. [Google Scholar] [CrossRef]
- Lee, J.R.; Suh, S.W.; Han, J.W.; Byun, S.; Kwon, S.J.; Lee, K.H.; Kwak, K.P.; Kim, B.J.; Kim, S.G.; Kim, J.L.; et al. Anhedonia and dysphoria are differentially associated with the risk of dementia in the cognitively normal elderly individuals: A prospective cohort study. Psychiatry Investig. 2019, 16, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Botto, R.; Callai, N.; Cermelli, A.; Causarano, L.; Rainero, I. Anxiety and depression in Alzheimer’s disease: A systematic review of pathogenetic mechanisms and relation to cognitive decline. Neurol. Sci. 2022, 43, 4107–4124. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Silva, D.; Carrettiero, D.C.; Oliveira, A.S.A.; Rodrigues, S.; Dos Santos-Lopes, J.; Canas, P.M.; Cunha, R.A.; Almeida, M.C.; Ferreira, T.L. Anandamide effects in a streptozotocin-induced Alzheimer’s disease-like sporadic dementia in rats. Front. Neurosci. 2018, 12, 653. [Google Scholar] [CrossRef]
- Roy, A.; Sharma, S.; Nag, T.C.; Katyal, J.; Gupta, Y.K.; Jain, S. Cognitive dysfunction and anxiety resulting from synaptic downscaling, hippocampal atrophy, and ventricular enlargement with intracerebroventricular streptozotocin injection in male Wistar rats. Neurotox. Res. 2022, 40, 2179–2202. [Google Scholar] [CrossRef]
- Kleinridders, A.; Cai, W.; Cappellucci, L.; Ghazarian, A.; Collins, W.R.; Vienberg, S.G.; Pothos, E.N.; Kahn, C.R. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc. Natl. Acad. Sci. USA 2015, 112, 3463–3468. [Google Scholar] [CrossRef] [PubMed]
- Maglio, L.E.; Noriega-Prieto, J.A.; Maroto, I.B.; Martin-Cortecero, J.; Muñoz-Callejas, A.; Callejo-Móstoles, M.; Fernández de Sevilla, D. IGF-1 facilitates extinction of conditioned fear. eLife 2021, 10, e67267. [Google Scholar] [CrossRef]
- Bake, S.; Selvamani, A.; Cherry, J.; Sohrabji, F. Blood brain barrier and neuroinflammation are critical targets of IGF-1-mediated neuroprotection in stroke for middle-aged female rats. PLoS ONE 2014, 9, e91427. [Google Scholar] [CrossRef]
- Galle, S.A.; Geraedts, I.K.; Deijen, J.B.; Milders, M.V.; Drent, M.L. The interrelationship between insulin-like growth factor 1, apolipoprotein E ε4, lifestyle factors, and the aging body and brain. J. Prev. Alzheimers Dis. 2020, 7, 265–273. [Google Scholar] [CrossRef]
- Schurmann, A.; Spencer, G.S.; Berry, C.J.; Decuypere, E.; Goddeeris, B. Evidence for suppression of immune function by insulin-like growth factor-1 in dwarf rats in vivo. Experientia 1996, 52, 55–59. [Google Scholar] [CrossRef]
- Shin, J.H.; Seong, J.K.; Yi, S.S. Sequential alterations of glucocorticoid receptors in the hippocampus of STZ-treated type 1 diabetic rats. J. Vet. Sci. 2014, 15, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Savino, W.; Mendes-da-Cruz, D.A.; Lepletier, A.; Dardenne, M. Hormonal control of T-cell development in health and disease. Nat. Rev. Endocrinol. 2016, 12, 77–89. [Google Scholar] [CrossRef]
- Chu, Y.W.; Schmitz, S.; Choudhury, B.; Telford, W.; Kapoor, V.; Garfield, S.; Howe, D.; Gress, R.E. Exogenous insulin-like growth factor 1 enhances thymopoiesis predominantly through thymic epithelial cell expansion. Blood 2008, 112, 2836–2846. [Google Scholar] [CrossRef]
- Unger, M.S.; Li, E.; Scharnagl, L.; Poupardin, R.; Altendorfer, B.; Mrowetz, H.; Hutter-Paier, B.; Weiger, T.M.; Heneka, M.T.; Attems, J.; et al. CD8+ T-cells infiltrate Alzheimer’s disease brains and regulate neuronal- and synapse-related gene expression in APP-PS1 transgenic mice. Brain Behav. Immun. 2020, 89, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Bake, S.; Okoreeh, A.K.; Alaniz, R.C.; Sohrabji, F. Insulin-like growth factor (IGF)-I modulates endothelial blood-brain barrier function in ischemic middle-aged female rats. Endocrinology 2016, 157, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Bake, S.; Okoreeh, A.; Khosravian, H.; Sohrabji, F. Insulin-like Growth Factor (IGF)-1 treatment stabilizes the microvascular cytoskeleton under ischemic conditions. Exp. Neurol. 2019, 311, 162–172. [Google Scholar] [CrossRef]
- El-Hakim, Y.; Mani, K.K.; Pickle, K.A.; Akbari, Z.; Samiya, N.; Pham, C.; Salas, G.; Pilla, R.; Sohrabji, F. Peripheral, but not central, IGF-1 treatment attenuates stroke-induced cognitive impairment in middle-aged female Sprague Dawley rats: The gut as a therapeutic target. Brain Behav. Immun. 2024, 122, 150–166. [Google Scholar] [CrossRef]
- Clark, R.; Strasser, J.; McCabe, S.; Robbins, K.; Jardieu, P. Insulin-like growth factor-1 stimulation of lymphopoiesis. J. Clin. Investig. 1993, 92, 540–548. [Google Scholar] [CrossRef]
- Bilbao, D.; Luciani, L.; Johannesson, B.; Piszczek, A.; Rosenthal, N. Insulin-like growth factor-1 stimulates regulatory T cells and suppresses autoimmune disease. EMBO Mol. Med. 2014, 6, 1423–1435. [Google Scholar] [CrossRef]
- Kuang, W.H.; Dong, Z.Q.; Tian, L.T.; Li, J. IGF-1 defends against chronic-stress induced depression in rat models of chronic unpredictable mild stress through the PI3K/Akt/FoxO3a pathway. Kaohsiung J. Med. Sci. 2018, 34, 370–376. [Google Scholar] [CrossRef]
- Guo, N.; Wang, X.; Xu, M.; Bai, J.; Yu, H.; Zhang, L. PI3K/AKT signaling pathway: Molecular mechanisms and therapeutic potential in depression. Pharmacol. Res. 2024, 206, 107300. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, A.; Sah, D.K.; Woo, M.; Song, J. Identification of the molecular mechanism of insulin-like growth factor-1 (IGF-1): A promising therapeutic target for neurodegenerative diseases associated with metabolic syndrome. Cell Biosci. 2023, 13, 16. [Google Scholar] [CrossRef] [PubMed]
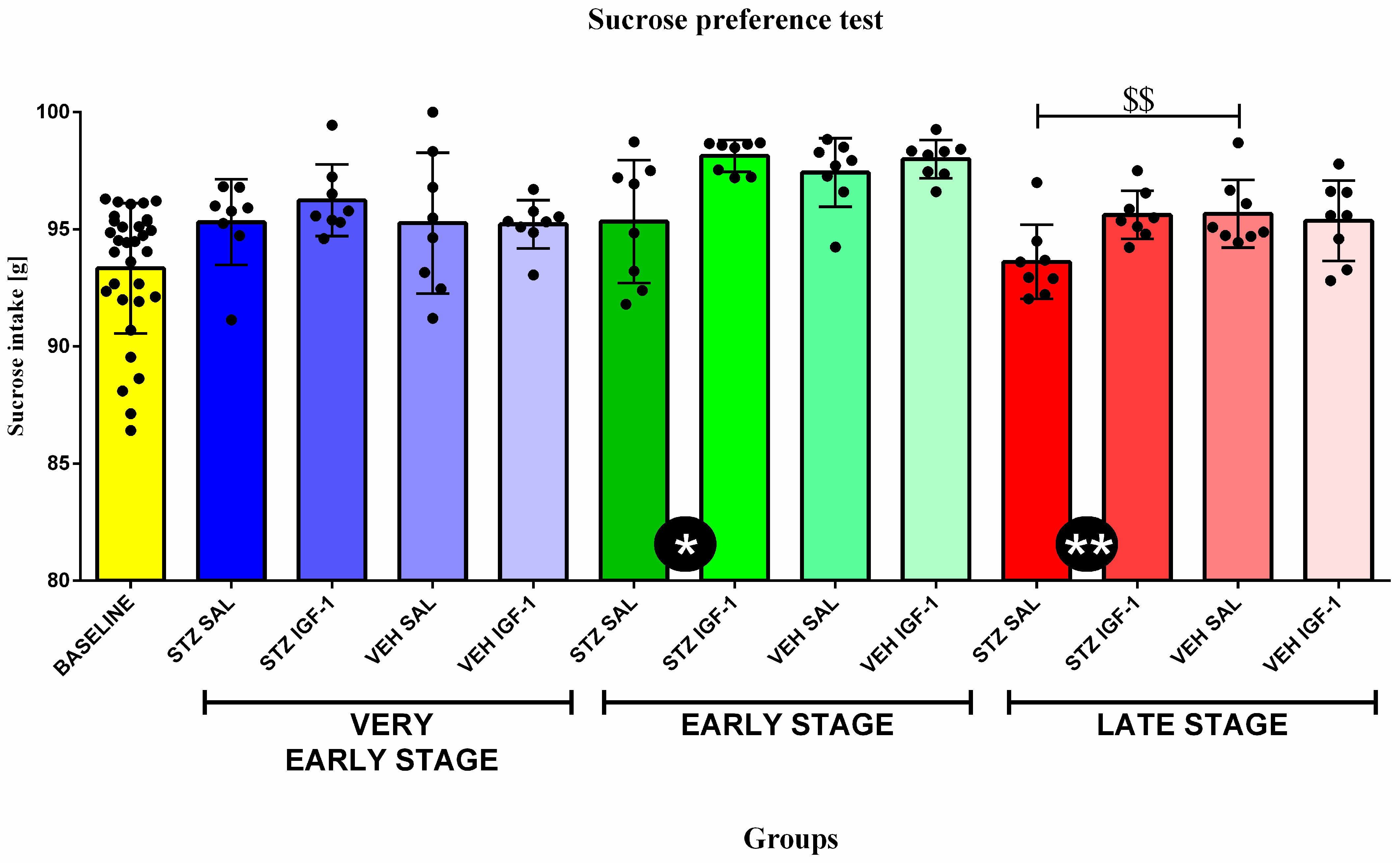
| Group | Phase | Sucrose Intake (g) |
|---|---|---|
| BASELINE | Baseline | 93.34 ± 2.78 |
| STZ SAL | Very early stage | 95.31 ± 1.82 @ |
| STZ IGF-1 | 96.24 ± 1.53 @@ | |
| VEH SAL | 95.26 ± 3.01 | |
| VEH IGF-1 | 95.21 ± 1.03 | |
| STZ SAL | Early stage | 95.33 ± 2.62 |
| STZ IGF-1 | 98.13 ± 0.68 @@@% | |
| VEH SAL | 97.42 ± 1.47 @@@ | |
| VEH IGF-1 | 98.00 ± 0.82 @@@%%% | |
| STZ SAL | Late stage | 93.61 ± 1.59 |
| STZ IGF-1 | 95.62 ± 1.03 @&& | |
| VEH SAL | 95.67 ± 1.45 @ | |
| VEH IGF-1 | 95.36 ± 1.71 @&& |
| Group | Phase | Time (s) | ||
|---|---|---|---|---|
| a. Open Arms | b. Center | c. Closed Arms | ||
| BASELINE | Baseline | 62.04 ± 26.48 | 75.55 ± 20.26 | 178.03 ± 60 |
| STZ SAL | Very early stage | 1.77 ± 0.77 @@@ | 16.93 ± 7.17 @@@ | 281.3 ± 7.04 @@@ |
| STZ IGF-1 | 4.47 ± 2 @@@ | 46.77 ± 15.37 @@ | 248.77 ± 17.03 @ | |
| VEH SAL | 12.83 ± 5.95 @@@ | 38.30 ± 14.48 @@@ | 248.87 ± 18.44 @ | |
| VEH IGF-1 | 5.37 ± 2.52 @@@ | 31.93 ± 13.19 @@@ | 262.70 ± 11.77 @@ | |
| STZ SAL | Early stage | 0 ± 0 @@@%% | 10.77 ± 4.36 @@@% | 289.23 ± 4.36 @@@% |
| STZ IGF-1 | 1.07 ± 0.53 @@@%% | 6.9 ± 3 @@@%% | 292.03 ± 2.7 @@@%% | |
| VEH SAL | 2.53 ± 0.88 @@@%% | 22.17 ± 8.58 @@@% | 275.3 ± 8.51 @@%% | |
| VEH IGF-1 | 1.9 ± 0.9 @@@%% | 19.33 ± 8.95 @@@ | 278.77 ± 9.49 @@% | |
| STZ SAL | Late stage | 0.45 ± 0.19 @@%%&& | 1.15 ± 0.7 @@%%&& | 299 ± 0.71 @@%&& |
| STZ IGF-1 | 1 ± 0.59 @@@%% | 1.5 ± 0.68 @@%%&& | 298.5 ± 1.11 @@%%&& | |
| VEH SAL | 45.27 ± 18.77 %%&& | 60.70 ± 12.98 %&& | 194.03 ± 19.98 %%&& | |
| VEH IGF-1 | 8.8 ± 6.21 @@@%% | 19.68 ± 7.35 @@@ | 271.52 ± 10.52 @@ | |
| Group | Phase | Percentage of Lymphocytes [%] | Percentage of Monocytes [%] | Percentage of Granulocytes [%] | |
|---|---|---|---|---|---|
| a. | STZ SAL | EARLY STAGE | 67.93 ± 5.09 | 14.03 ± 3.43 | 18.05 ± 1.86 |
| STZ IGF-1 | 68.28 ± 7.53 | 10.78 ± 2.14 ^ | 20.94 ± 6.73 | ||
| VEH SAL | 73.17 ± 6.99 | 11.37 ± 2.33 | 15.47 ± 5.75 | ||
| VEH IGF-1 | 62.45 ± 7.96 $ | 14.03 ± 2.51 | 23.53 ± 6.52 $ | ||
| b. | STZ SAL | LATE STAGE | 67.64 ± 7.51 | 13.89 ± 3.20 | 21.47 ± 6.30 |
| STZ IGF-1 | 74.38 ± 9.41 | 10.76 ± 2.79 | 13.61 ± 5.19 *& | ||
| VEH SAL | 73.17 ± 6.99 | 11.37 ± 2.33 | 15.47 ± 5.75 | ||
| VEH IGF-1 | 62.45 ± 7.96 | 14.03 ± 2.51 | 23.53 ± 6.52 |
| a. | Group | Percentage of Blood T (CD3+) Lymphocytes [%] | Percentage of Blood Th (CD3+CD4+) Lymphocytes [%] | Percentage of Blood Tc (CD3+CD8+) Lymphocytes [%] | Percentage of Blood B (CD45RA+) Lymphocytes [%] | Percentage of Blood NK (CD161a+) Cells [%] |
| STZ SAL | 35.81 ± 5.97 | 23.88 ± 5.58 | 14.12 ± 4.42 | 20.47 ± 1.61 | 3.48 ± 1.67 | |
| STZ IGF-1 | 41.19 ± 8.35 | 21.99 ± 9.12 | 19.15 ± 3.05 * | 17.35 ± 3.76 | 2.96 ± 1.28 | |
| VEH SAL | 37.60 ± 4.01 | 17.28 ± 3.38 | 16.95 ± 5.83 | 13.80 ± 5.89 | 2.17 ± 0.94 | |
| VEH IGF-1 | 34.28 ± 7.27 | 28.74 ± 9.51 $ | 13.84 ± 5.05 | 14.66 ± 5.32 | 2.11 ± 0.95 | |
| b. | Group | Percentage of Spleen T (CD3+) Lymphocytes [%] | Percentage of Spleen B (CD45RA+) Lymphocytes [%] | Percentage of Spleen NK (CD161a+) cells [%] | ||
| STZ SAL | 22.70 ± 10.76 | 8.96 ± 3.88 | 2.29 ± 1.06 | |||
| STZ IGF-1 | 43.59 ± 10.55 *$$ | 18.58 ± 3.88 *$$ | 4.91 ± 1.51 *$$ | |||
| VEH SAL | 16.82 ± 2.97 | 11.12 ± 1.39 | 1.42 ± 0.64 | |||
| VEH IGF-1 | 39.34 ± 15.33 $$ | 14.54 ± 2.66 $ | 3.64 ± 1.66 $ | |||
| c. | Group | Relative Weight of Spleen (mg/kg b.w.) | Relative Weight of Thymus (mg/kg b.w.) | |||
| STZ SAL | 178.07 ± 23.40 | 55.69 ± 12.42 $ | ||||
| STZ IGF-1 | 187.56 ± 25.46 | 52.56 ± 14.91 | ||||
| VEH SAL | 175.35 ± 20.16 | 43.15 ± 10.58 | ||||
| VEH IGF-1 | 180.60 ± 9.93 | 44.97 ± 12.97 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunacka, J.; Grembecka, B.; Wrona, D. Central Insulin-Like Growth Factor-1-Induced Anxiolytic and Antidepressant Effects in a Rat Model of Sporadic Alzheimer’s Disease Are Associated with the Peripheral Suppression of Inflammation. Cells 2025, 14, 1189. https://doi.org/10.3390/cells14151189
Dunacka J, Grembecka B, Wrona D. Central Insulin-Like Growth Factor-1-Induced Anxiolytic and Antidepressant Effects in a Rat Model of Sporadic Alzheimer’s Disease Are Associated with the Peripheral Suppression of Inflammation. Cells. 2025; 14(15):1189. https://doi.org/10.3390/cells14151189
Chicago/Turabian StyleDunacka, Joanna, Beata Grembecka, and Danuta Wrona. 2025. "Central Insulin-Like Growth Factor-1-Induced Anxiolytic and Antidepressant Effects in a Rat Model of Sporadic Alzheimer’s Disease Are Associated with the Peripheral Suppression of Inflammation" Cells 14, no. 15: 1189. https://doi.org/10.3390/cells14151189
APA StyleDunacka, J., Grembecka, B., & Wrona, D. (2025). Central Insulin-Like Growth Factor-1-Induced Anxiolytic and Antidepressant Effects in a Rat Model of Sporadic Alzheimer’s Disease Are Associated with the Peripheral Suppression of Inflammation. Cells, 14(15), 1189. https://doi.org/10.3390/cells14151189






