Evaluating the Causal Effects of ADHD and Autism on Cardiovascular Diseases and Vice Versa: A Systematic Review and Meta-Analysis of Mendelian Randomization Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Data Extraction
2.2. Study Quality
2.3. Analysis
3. Results
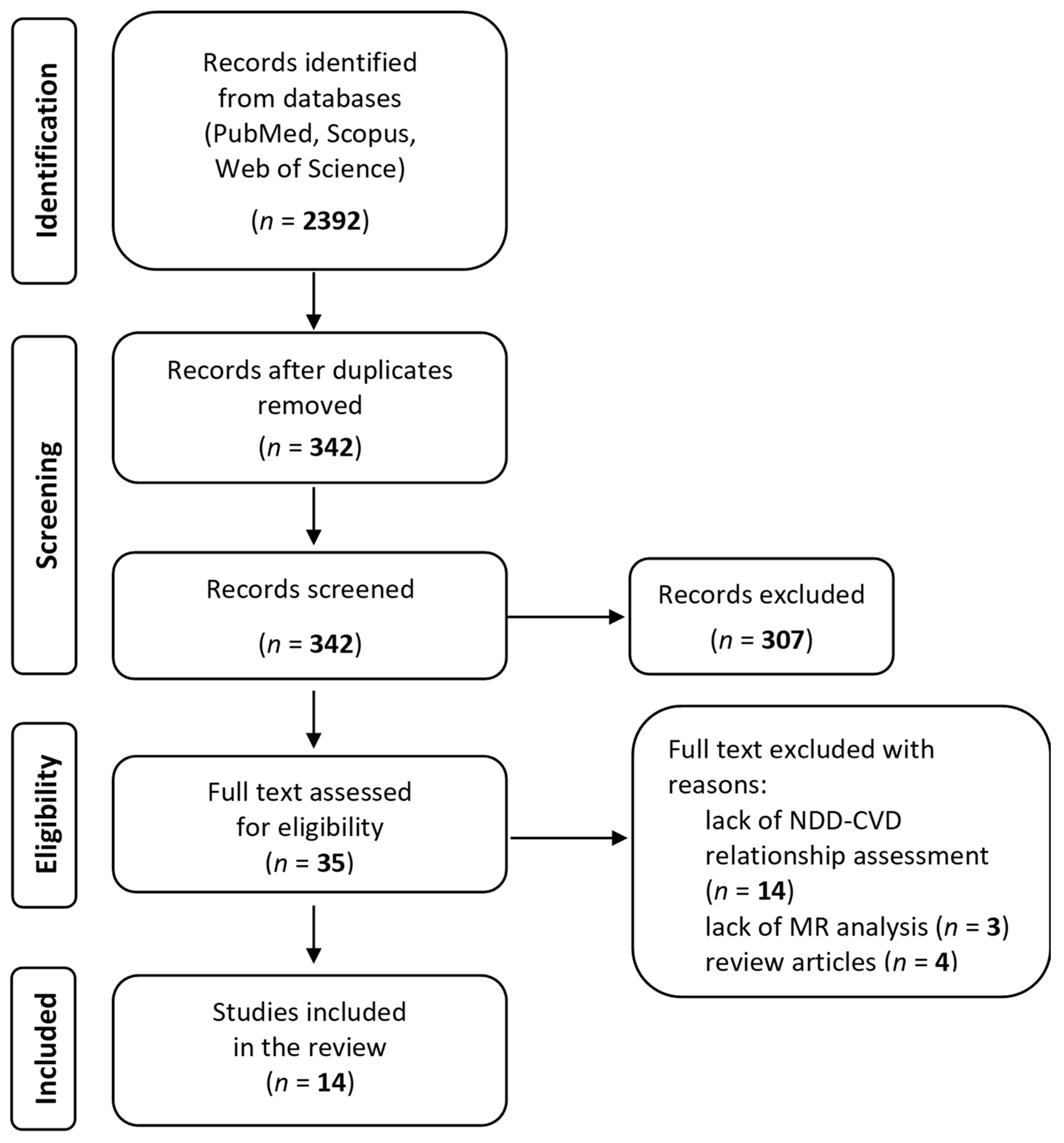
3.1. Data Search
3.2. Quality and Bias
3.3. Study Characteristics
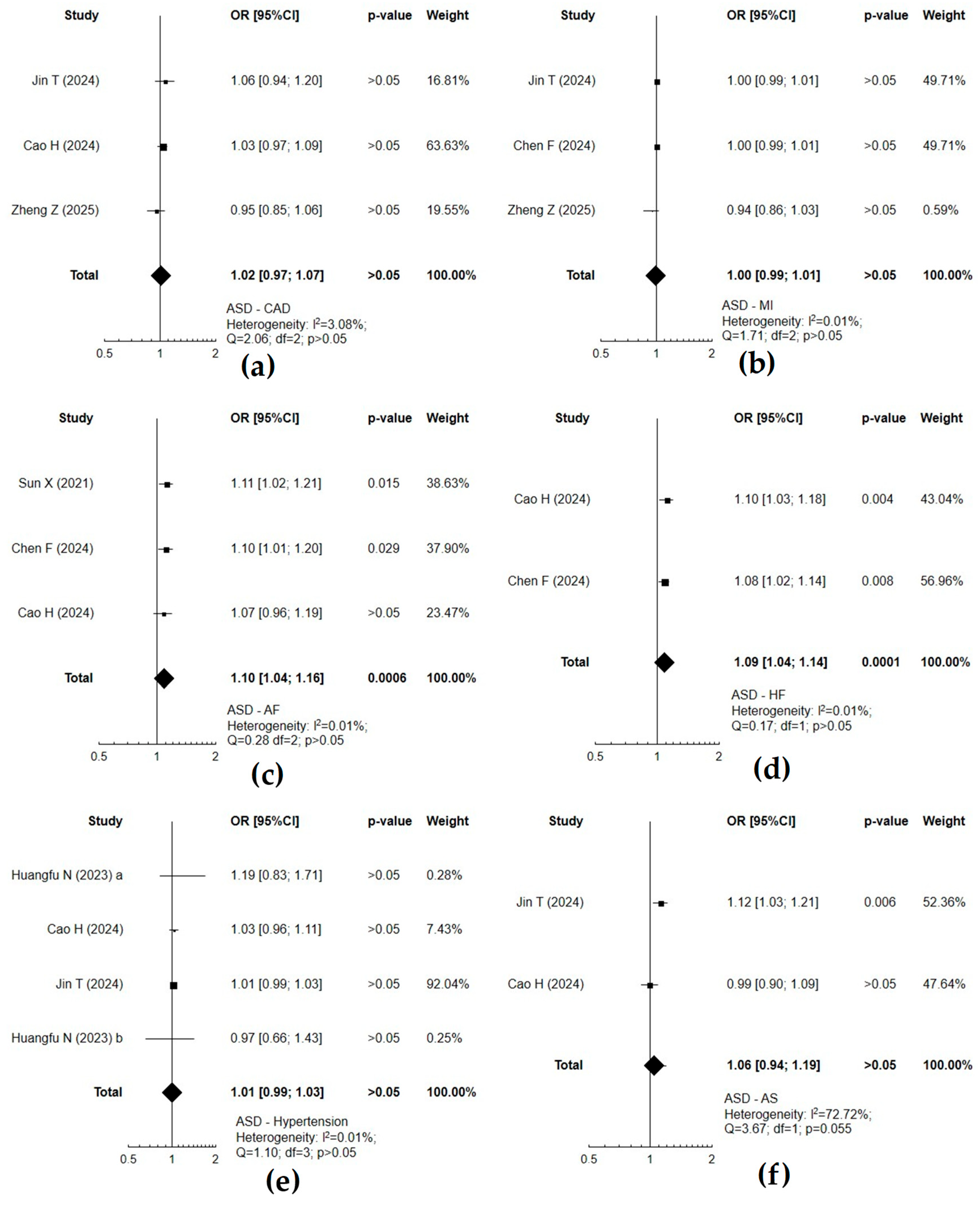
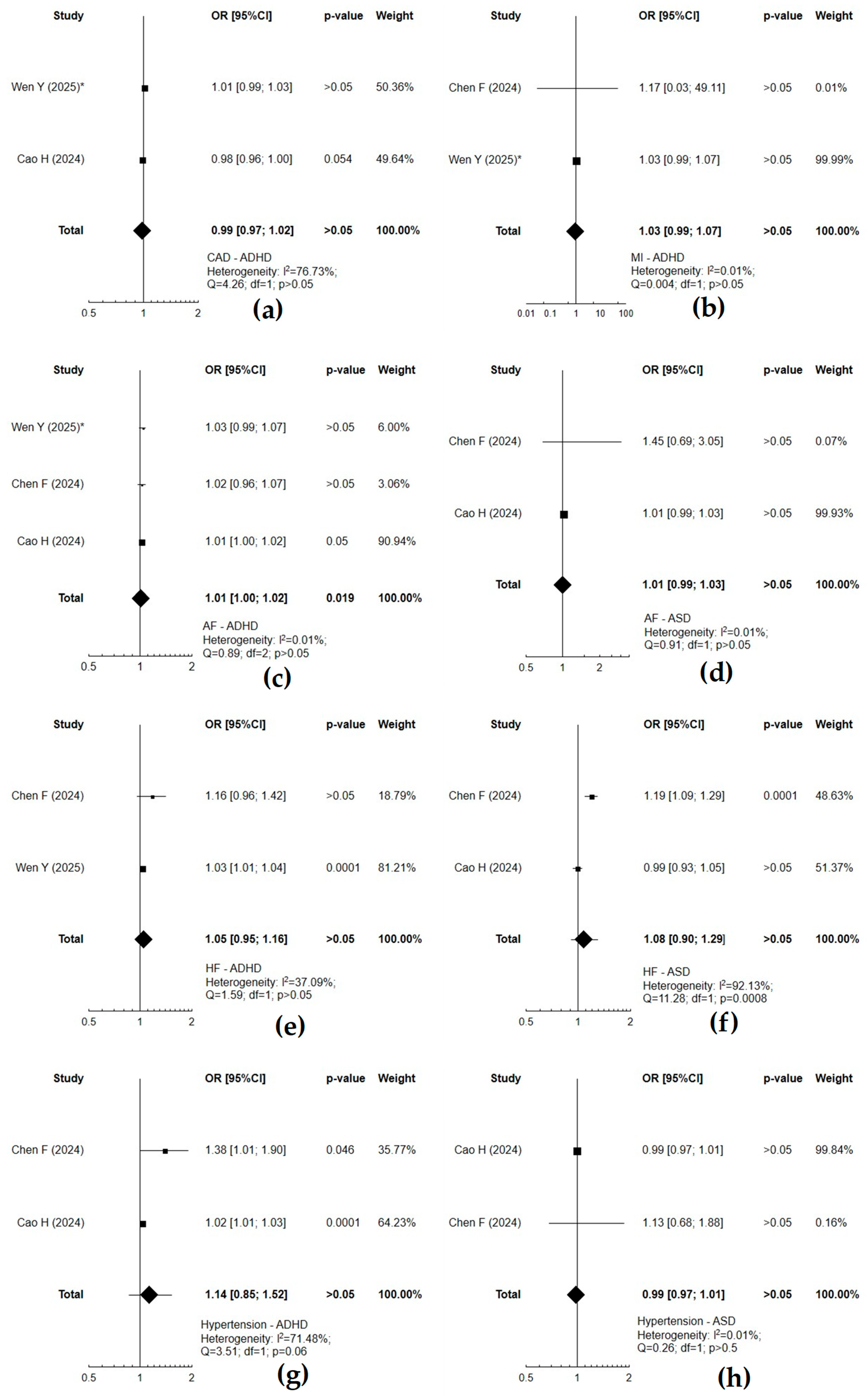
3.4. The Relationship Between Neurodevelopmental and Cardiovascular Disorders
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADHD | attention deficit hyperactivity disorder |
| AF | atrial fibrillation |
| AIS | any ischemic stroke |
| AS | any stroke |
| ASD | autism spectrum disorder |
| CAD | coronary artery disease |
| CES | cardioembolic stroke |
| CVD | cardiovascular disease |
| GWASs | genome-wide association studies |
| HF | heart failure |
| IVW | inverse variance weighted |
| LAS | large-artery atherosclerotic stroke |
| MI | myocardial infarction |
| MR | Mendelian randomization |
| MW-IVW | variance weighted with modified weights |
| PDs | psychiatric disorders |
| PGC | the Psychiatric Genomics Consortium |
| SNPs | single nucleotide polymorphisms |
| SSGAC | Social Science Genetics Association Consortium |
| SVS | small-vessel stroke |
References
- Antolini, G.; Colizzi, M. Where Do Neurodevelopmental Disorders Go? Casting the Eye Away from Childhood towards Adulthood. Healthcare 2023, 11, 1015. [Google Scholar] [CrossRef]
- Khouzam, N.R.; Khouzam, S.R.; Khouzam, R.N. Heartfelt Minds: Uncovering the Intricate yet Overlooked Connection Between Psychiatric Disorders and Cardiology. Curr. Probl. Cardiol. 2024, 49, 102006. [Google Scholar] [CrossRef]
- Global Burden of Disease Study Autism Spectrum, C. The global epidemiology and health burden of the autism spectrum: Findings from the Global Burden of Disease Study 2021. Lancet Psychiatry 2025, 12, 111–121. [Google Scholar] [CrossRef]
- Song, P.; Zha, M.; Yang, Q.; Zhang, Y.; Li, X.; Rudan, I.; Global Health Epidemiology Reference, G. The prevalence of adult attention-deficit hyperactivity disorder: A global systematic review and meta-analysis. J. Glob. Health 2021, 11, 04009. [Google Scholar] [CrossRef]
- Li, L.; Yao, H.; Zhang, L.; Garcia-Argibay, M.; Du Rietz, E.; Brikell, I.; Solmi, M.; Cortese, S.; Ramos-Quiroga, J.A.; Ribases, M.; et al. Attention-deficit/hyperactivity disorder is associated with increased risk of cardiovascular diseases: A systematic review and meta-analysis. JCPP Adv. 2023, 3, e12158. [Google Scholar] [CrossRef]
- Bishop, L.; Charlton, R.A.; McLean, K.J.; McQuaid, G.A.; Lee, N.R.; Wallace, G.L. Cardiovascular disease risk factors in autistic adults: The impact of sleep quality and antipsychotic medication use. Autism Res. 2023, 16, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Snetselaar, L.G.; Strathearn, L.; Ryckman, K.; Nothwehr, F.; Torner, J. Association between history of attention-deficit/hyperactivity disorder diagnosis and cardiovascular disease in U.S. adults. Health Psychol. 2022, 41, 693–700. [Google Scholar] [CrossRef]
- Li, L.; Chang, Z.; Sun, J.; Garcia-Argibay, M.; Du Rietz, E.; Dobrosavljevic, M.; Brikell, I.; Jernberg, T.; Solmi, M.; Cortese, S.; et al. Attention-deficit/hyperactivity disorder as a risk factor for cardiovascular diseases: A nationwide population-based cohort study. World Psychiatry 2022, 21, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.K.; Riglin, L.; Blakey, R.; Collishaw, S.; Davey Smith, G.; Stergiakouli, E.; Tilling, K.; Thapar, A. Childhood attention-deficit hyperactivity disorder problems and mid-life cardiovascular risk: Prospective population cohort study. Br. J. Psychiatry 2023, 223, 472–477. [Google Scholar] [CrossRef]
- Smari, U.J.; Valdimarsdottir, U.A.; Aspelund, T.; Hauksdottir, A.; Thordardottir, E.B.; Hartman, C.A.; Andell, P.; Larsson, H.; Zoega, H. Psychiatric comorbidities in women with cardiometabolic conditions with and without ADHD: A population-based study. BMC Med. 2023, 21, 450. [Google Scholar] [CrossRef]
- Hand, B.N.; Angell, A.M.; Harris, L.; Carpenter, L.A. Prevalence of physical and mental health conditions in Medicare-enrolled, autistic older adults. Autism 2020, 24, 755–764. [Google Scholar] [CrossRef]
- Chang, H.W.; Hsu, M.J.; Chien, L.N.; Chi, N.F.; Yu, M.C.; Chen, H.C.; Lin, Y.F.; Hu, C.J. Role of the Autism Risk Gene Shank3 in the Development of Atherosclerosis: Insights from Big Data and Mechanistic Analyses. Cells 2023, 12, 2546. [Google Scholar] [CrossRef] [PubMed]
- Thom, R.P.; Palumbo, M.L.; Keary, C.J.; Hooker, J.M.; McDougle, C.J.; Ravichandran, C.T. Prevalence and factors associated with overweight, obesity, and hypertension in a large clinical sample of adults with autism spectrum disorder. Sci. Rep. 2022, 12, 9737. [Google Scholar] [CrossRef]
- Jenabi, E.; Ayubi, E.; Farashi, S.; Bashirian, S.; Mehri, F. Neonatal risk factors associated with attention-deficit/hyperactivity disorder: An umbrella review. Clin. Exp. Pediatr. 2023, 66, 441–446. [Google Scholar] [CrossRef]
- Hasan, M.T.; Shaban Abdelgalil, M.; Elbadawy, M.A.; Mahmoud Elrosasy, A.; Elkhadragy, A.; El Garhy, M.; Awad, A.K. Are congenital heart defects connected to more severe attention-deficit/hyperactivity disorder?: A systematic review and meta-analysis. Medicine 2023, 102, e36193. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, Q.Q.; Pang, X.Y.; Huang, X.B.; Yang, G.M.; Zhao, S. Association of congenital heart disease and neurodevelopmental disorders: An observational and Mendelian randomization study. Ital. J. Pediatr. 2024, 50, 63. [Google Scholar] [CrossRef]
- Gu, S.; Katyal, A.; Zhang, Q.; Chung, W.; Franciosi, S.; Sanatani, S. The Association Between Congenital Heart Disease and Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Pediatr. Cardiol. 2023, 44, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekara, C.S.; Ancona, D.; Cortes, L.; Hu, A.; Rimu, A.H.; Robohm-Leavitt, C.; Payne, D.; Wakefield, S.M.; Mastergeorge, A.M.; Kahathuduwa, C.N. Association Between Autism Spectrum Disorders and Cardiometabolic Diseases: A Systematic Review and Meta-analysis. JAMA Pediatr. 2023, 177, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Weir, E.; Allison, C.; Warrier, V.; Baron-Cohen, S. Increased prevalence of non-communicable physical health conditions among autistic adults. Autism 2021, 25, 681–694. [Google Scholar] [CrossRef]
- Sigmon, E.R.; Kelleman, M.; Susi, A.; Nylund, C.M.; Oster, M.E. Congenital Heart Disease and Autism: A Case-Control Study. Pediatrics 2019, 144, e20184114. [Google Scholar] [CrossRef]
- Larsson, S.C.; Butterworth, A.S.; Burgess, S. Mendelian randomization for cardiovascular diseases: Principles and applications. Eur. Heart J. 2023, 44, 4913–4924. [Google Scholar] [CrossRef] [PubMed]
- Demontis, D.; Walters, R.K.; Martin, J.; Mattheisen, M.; Als, T.D.; Agerbo, E.; Baldursson, G.; Belliveau, R.; Bybjerg-Grauholm, J.; Baekvad-Hansen, M.; et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 2019, 51, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Byrne, E.M.; Zhu, Z.; Qi, T.; Skene, N.G.; Bryois, J.; Pardinas, A.F.; Stahl, E.; Smoller, J.W.; Rietschel, M.; Bipolar Working Group of the Psychiatric Genomics, C.; et al. Conditional GWAS analysis to identify disorder-specific SNPs for psychiatric disorders. Mol. Psychiatry 2021, 26, 2070–2081. [Google Scholar] [CrossRef]
- Laxmi; Golmei, P.; Srivastava, S.; Kumar, S. Single nucleotide polymorphism-based biomarker in primary hypertension. Eur. J. Pharmacol. 2024, 972, 176584. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Tada, H.; Yamagishi, M. The genetics of atrial fibrillation. Curr. Opin. Cardiol. 2017, 32, 10–16. [Google Scholar] [CrossRef]
- Lahm, H.; Jia, M.; Dressen, M.; Wirth, F.; Puluca, N.; Gilsbach, R.; Keavney, B.D.; Cleuziou, J.; Beck, N.; Bondareva, O.; et al. Congenital heart disease risk loci identified by genome-wide association study in European patients. J. Clin. Investig. 2021, 131, e141837. [Google Scholar] [CrossRef]
- Benn, M.; Nordestgaard, B.G. From genome-wide association studies to Mendelian randomization: Novel opportunities for understanding cardiovascular disease causality, pathogenesis, prevention, and treatment. Cardiovasc. Res. 2018, 114, 1192–1208. [Google Scholar] [CrossRef]
- Elhage, K.G.; Kranyak, A.; Jin, J.Q.; Haran, K.; Spencer, R.K.; Smith, P.L.; Davis, M.S.; Hakimi, M.; Bhutani, T.; Liao, W. Mendelian Randomization Studies in Atopic Dermatitis: A Systematic Review. J. Investig. Dermatol. 2024, 144, 1022–1037. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef]
- Birney, E. Mendelian Randomization. Cold Spring Harb. Perspect. Med. 2022, 12, a041302. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Baranova, A.; Zhao, Q.; Zhang, F. Bidirectional associations between mental disorders, antidepressants and cardiovascular disease. BMJ Ment. Health 2024, 27, e300975. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, W.; Pang, M.; Zhu, B.; Liu, H.; Hu, D.; Luo, Y.; Wang, S.; Wu, S.; He, J.; et al. The effects of psychiatric disorders on the risk of chronic heart failure: A univariable and multivariable Mendelian randomization study. Front. Public Health 2024, 12, 1306150. [Google Scholar] [CrossRef]
- Chen, F.; Dong, X.; Yu, Z.; Zhang, Y.; Shi, Y. The brain-heart axis: Integrative analysis of the shared genetic etiology between neuropsychiatric disorders and cardiovascular disease. J. Affect. Disord. 2024, 355, 147–156. [Google Scholar] [CrossRef]
- Du, R.; Zhou, Y.; You, C.; Liu, K.; King, D.A.; Liang, Z.S.; Ranson, J.M.; Llewellyn, D.J.; Huang, J.; Zhang, Z. Attention-deficit/hyperactivity disorder and ischemic stroke: A Mendelian randomization study. Int. J. Stroke 2023, 18, 346–353. [Google Scholar] [CrossRef]
- Leppert, B.; Riglin, L.; Wootton, R.E.; Dardani, C.; Thapar, A.; Staley, J.R.; Tilling, K.; Davey Smith, G.; Thapar, A.; Stergiakouli, E. The Effect of Attention Deficit/Hyperactivity Disorder on Physical Health Outcomes: A 2-Sample Mendelian Randomization Study. Am. J. Epidemiol. 2021, 190, 1047–1055. [Google Scholar] [CrossRef]
- Sui, X.; Liu, T.; Liang, Y.; Zhang, B. Psychiatric disorders and cardiovascular diseases: A mendelian randomization study. Heliyon 2023, 9, e20754. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, X.; Deng, L.; Zhu, G.; Si, X.; Gao, X.; Lu, X.; Wang, T. Genetic evidence of the causal relationships between psychiatric disorders and cardiovascular diseases. J. Psychosom. Res. 2025, 189, 112029. [Google Scholar] [CrossRef]
- Xiang, W.; Shen, Y.; Li, Y.; Chen, S.; Cao, Q.; Xu, L. Causal association between mental disorders and cerebrovascular diseases: Evidence from Mendelian randomization study. J. Affect. Disord. 2025, 368, 461–470. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, X.; Wu, J.; Hu, G.; Bai, S.; Yu, R. A Mendelian randomization study of the effect of mental disorders on cardiovascular disease. Front. Cardiovasc. Med. 2024, 11, 1329463. [Google Scholar] [CrossRef]
- Zheng, Z.; Cai, D. Causality Between ADHD, ASD, and CVDs: A Two-Step, Two-Sample Mendelian Randomization Investigation. J. Atten. Disord. 2025, 29, 3–13. [Google Scholar] [CrossRef]
- Jin, T.; Huang, W.; Pang, Q.; Cao, Z.; Xing, D.; Guo, S.; Zhang, T. Genetically identified mediators associated with increased risk of stroke and cardiovascular disease in individuals with autism spectrum disorder. J. Psychiatr. Res. 2024, 174, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, L.; Wang, Z.; Lu, Y.; Chen, M.; He, Y.; Xu, H.; Zheng, L. Association of Autism Spectrum Disorder, Neuroticism, and Subjective Well-Being With Cardiovascular Diseases: A Two-Sample Mendelian Randomization Study. Front. Cardiovasc. Med. 2021, 8, 676030. [Google Scholar] [CrossRef]
- Huangfu, N.; Lu, Y.; Ma, H.; Hu, Z.; Cui, H.; Yang, F. Genetic liability to mental disorders in relation to the risk of hypertension. Front. Cardiovasc. Med. 2023, 10, 1087251. [Google Scholar] [CrossRef]
- Nelson, C.P.; Goel, A.; Butterworth, A.S.; Kanoni, S.; Webb, T.R.; Marouli, E.; Zeng, L.; Ntalla, I.; Lai, F.Y.; Hopewell, J.C.; et al. Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat. Genet. 2017, 49, 1385–1391. [Google Scholar] [CrossRef]
- Jiang, L.; Zheng, Z.; Qi, T.; Kemper, K.E.; Wray, N.R.; Visscher, P.M.; Yang, J. A resource-efficient tool for mixed model association analysis of large-scale data. Nat. Genet. 2019, 51, 1749–1755. [Google Scholar] [CrossRef]
- Shah, S.; Henry, A.; Roselli, C.; Lin, H.; Sveinbjornsson, G.; Fatemifar, G.; Hedman, A.K.; Wilk, J.B.; Morley, M.P.; Chaffin, M.D.; et al. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat. Commun. 2020, 11, 163. [Google Scholar] [CrossRef]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipila, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Roselli, C.; Chaffin, M.D.; Weng, L.C.; Aeschbacher, S.; Ahlberg, G.; Albert, C.M.; Almgren, P.; Alonso, A.; Anderson, C.D.; Aragam, K.G.; et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat. Genet. 2018, 50, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.G.; Tsao, N.L.; Singhal, P.; Liu, C.; Vy, H.M.T.; Paranjpe, I.; Backman, J.D.; Bellomo, T.R.; Bone, W.P.; Biddinger, K.J.; et al. Genome-wide association and multi-trait analyses characterize the common genetic architecture of heart failure. Nat. Commun. 2022, 13, 6914. [Google Scholar] [CrossRef]
- Hartiala, J.A.; Han, Y.; Jia, Q.; Hilser, J.R.; Huang, P.; Gukasyan, J.; Schwartzman, W.S.; Cai, Z.; Biswas, S.; Tregouet, D.A.; et al. Genome-wide analysis identifies novel susceptibility loci for myocardial infarction. Eur. Heart J. 2021, 42, 919–933. [Google Scholar] [CrossRef]
- van der Harst, P.; Verweij, N. Identification of 64 Novel Genetic Loci Provides an Expanded View on the Genetic Architecture of Coronary Artery Disease. Circ. Res. 2018, 122, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Chauhan, G.; Traylor, M.; Sargurupremraj, M.; Okada, Y.; Mishra, A.; Rutten-Jacobs, L.; Giese, A.K.; van der Laan, S.W.; Gretarsdottir, S.; et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet. 2018, 50, 524–537. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Thorolfsdottir, R.B.; Fritsche, L.G.; Zhou, W.; Skov, M.W.; Graham, S.E.; Herron, T.J.; McCarthy, S.; Schmidt, E.M.; Sveinbjornsson, G.; et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat. Genet. 2018, 50, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Donertas, H.M.; Fabian, D.K.; Valenzuela, M.F.; Partridge, L.; Thornton, J.M. Common genetic associations between age-related diseases. Nat. Aging 2021, 1, 400–412. [Google Scholar] [CrossRef]
- Gao, X.; Qin, Y.; Jiao, S.; Hao, J.; Zhao, J.; Wang, J.; Wen, Y.; Wang, T. Genetic evidence for the causal relations between metabolic syndrome and psychiatric disorders: A Mendelian randomization study. Transl. Psychiatry 2024, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, J.; Heng, P.; Luo, J. Mitochondrial related Mendelian randomization identifies causal associations between metabolic disorders and childhood neurodevelopmental disorders. Medicine 2024, 103, e40481. [Google Scholar] [CrossRef]
- Zhu, H.; Ni, H.; Yang, Q.; Ni, J.; Ji, J.; Yang, S.; Peng, F. Evaluating the Bidirectional Causal Effects of Alzheimer’s Disease Across Multiple Conditions: A Systematic Review and Meta-Analysis of Mendelian Randomization Studies. Int. J. Mol. Sci. 2025, 26, 3589. [Google Scholar] [CrossRef]
- Liu, N.; Tan, J.S.; Liu, L.; Li, H.; Wang, Y.; Yang, Y.; Qian, Q. Roles of obesity in mediating the causal effect of attention-deficit/hyperactivity disorder on diabetes. Epidemiol. Psychiatr. Sci. 2023, 32, e32. [Google Scholar] [CrossRef]
- Garcia-Argibay, M.; du Rietz, E.; Lu, Y.; Martin, J.; Haan, E.; Lehto, K.; Bergen, S.E.; Lichtenstein, P.; Larsson, H.; Brikell, I. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl. Psychiatry 2022, 12, 152. [Google Scholar] [CrossRef]
- Tang, J.; Ou, J.; Chen, Y.; Li, L.; Liu, H.; Sun, M.; Luo, M.; Zhong, T.; Wang, T.; Wei, J.; et al. The risk of attention-deficit hyperactivity disorder among children with congenital heart disease: A systematic review and meta-analysis. Child Care Health Dev. 2024, 50, e13174. [Google Scholar] [CrossRef] [PubMed]
- Jenabi, E.; Bashirian, S.; Fariba, F.; Naghshtabrizi, B. The association between congenital heart disease and the risk of Autism spectrum disorders or attention-deficit/hyperactivity disorder among children: A meta-analysis. Eur. J. Psychiatry 2022, 36, 71–76. [Google Scholar] [CrossRef]
- Chieh, A.Y.; Bryant, B.M.; Kim, J.W.; Li, L. Systematic review investigating the relationship between autism spectrum disorder and metabolic dysfunction. Res. Autism Spectr. Disord. 2021, 86, 101821. [Google Scholar] [CrossRef]
- Joynt, K.E.; Whellan, D.J.; O'Connor, C.M. Depression and cardiovascular disease: Mechanisms of interaction. Biol. Psychiatry 2003, 54, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hartman, C.A.; Kuja-Halkola, R.; Faraone, S.V.; Almqvist, C.; Larsson, H. Attention-deficit/hyperactivity disorder and clinically diagnosed obesity in adolescence and young adulthood: A register-based study in Sweden. Psychol. Med. 2019, 49, 1841–1849. [Google Scholar] [CrossRef]
- Cortese, S.; Sun, S.; Zhang, J.; Sharma, E.; Chang, Z.; Kuja-Halkola, R.; Almqvist, C.; Larsson, H.; Faraone, S.V. Association between attention deficit hyperactivity disorder and asthma: A systematic review and meta-analysis and a Swedish population-based study. Lancet Psychiatry 2018, 5, 717–726. [Google Scholar] [CrossRef]
- Lee, S.S.; Humphreys, K.L.; Flory, K.; Liu, R.; Glass, K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clin. Psychol. Rev. 2011, 31, 328–341. [Google Scholar] [CrossRef]
- Ding, H.; Ouyang, M.; Wang, J.; Xie, M.; Huang, Y.; Yuan, F.; Jia, Y.; Zhang, X.; Liu, N.; Zhang, N. Shared genetics between classes of obesity and psychiatric disorders: A large-scale genome-wide cross-trait analysis. J. Psychosom. Res. 2022, 162, 111032. [Google Scholar] [CrossRef]
- Soler Artigas, M.; Sánchez-Mora, C.; Rovira, P.; Vilar-Ribó, L.; Ramos-Quiroga, J.A.; Ribasés, M. Mendelian randomization analysis for attention deficit/hyperactivity disorder: Studying a broad range of exposures and outcomes. Int. J. Epidemiol. 2023, 52, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Iob, E.; Pingault, J.B.; Munafo, M.R.; Stubbs, B.; Gilthorpe, M.S.; Maihofer, A.X.; Psychiatric Genomics Consortium Posttraumatic Stress Disorder Working Group; Danese, A. Testing the causal relationships of physical activity and sedentary behaviour with mental health and substance use disorders: A Mendelian randomisation study. Mol. Psychiatry 2023, 28, 3429–3443. [Google Scholar] [CrossRef] [PubMed]
- Dennison, C.A.; Legge, S.E.; Bracher-Smith, M.; Menzies, G.; Escott-Price, V.; Smith, D.J.; Doherty, A.R.; Owen, M.J.; O'Donovan, M.C.; Walters, J.T.R. Association of genetic liability for psychiatric disorders with accelerometer-assessed physical activity in the UK Biobank. PLoS ONE 2021, 16, e0249189. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, J.; Nordstrom, P. HPA axis hyperactivity and cardiovascular mortality in mood disorder inpatients. J. Affect. Disord. 2009, 116, 88–92. [Google Scholar] [CrossRef]
- Raedler, T.J. Inflammatory mechanisms in major depressive disorder. Curr. Opin. Psychiatry 2011, 24, 519–525. [Google Scholar] [CrossRef] [PubMed]

| Study | Ethnicity | Cohort/ Dataset | Type of Analysis | Exposure | Sample Size | Outcome | Sample Size | Odds Ratio ¥ (OR) 95% CI | Conclusion | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | ||||||||
| Liu et al. (2024) [16] | European | PGC (ADHD); iPSYCH-PGC (ASD); FinnGen (CHD) | two-sample MR | ADHD | 20,183 | 35,191 | CHD | 3459 | 39,040 | 0.98 (0.91–1.06) | Children with CHD are at greater risk of developing ADHD |
| ASD | 18,382 | 27,969 | 0.96 (0.84–1.11) | ||||||||
| Cao et al. (2024) [33] | European (mostly) | PGC (ADHD); iPSYCH-PGC (ASD); Nelson et al. (2017) [46] (CAD); Jiang et al. (2019) [47] (hypertension); Shah et al. (2020) [48] (HF); Kurki et al. (2023) [49] (AF, arrhythmias, stroke) | MR and multivariable MR | ADHD | 38,691 | 275,986 | Arrhythmias | 59,182 | 204,429 | 1.15 (0.92–1.44) | ADHD plays significant roles in elevating the chances of CVD |
| AF | 40,594 | 168,000 | 1.38 (1.01–1.88) * | ||||||||
| CAD | 71,602 | 260,875 | 1.35 (1.21–1.51) * | ||||||||
| HF | 47,309 | 930,014 | 1.41 (1.18–1.7) * | ||||||||
| Hypertension | 122,620 | 332,683 | 0.91 (0.76–1.08) | ||||||||
| Stroke | 34,560 | 249,480 | 1.12 (0.94–1.34) | ||||||||
| ASD | 18,381 | 27,969 | Arrhythmias | 59,182 | 204,429 | 1.01 (0.93–1.10) | |||||
| AF | 40,594 | 168,000 | 1.07 (0.95–1.21) | ||||||||
| CAD | 71,602 | 260,875 | 1.03 (0.97–1.09) | ||||||||
| HF | 47,309 | 930,014 | 1.1 (1.03–1.18) * | ||||||||
| Hypertension | 122,620 | 332,683 | 1.03 (0.96–1.10) | ||||||||
| Stroke | 34,560 | 249,480 | 0.99 (0.9–1.09) | ||||||||
| Arrhythmias | 59,182 | 204,429 | ADHD | 38,691 | 275,986 | 1.02 (1.01–1.04) * | |||||
| AF | 40,594 | 168,000 | 1.01 (1.00–1.02) | ||||||||
| CAD | 71,602 | 260,875 | 0.98 (0.96–1.01) | ||||||||
| HF | 47,309 | 930,014 | 1.00 (0.95–1.06) | ||||||||
| Hypertension | 122,620 | 332,683 | 1.02 (1.01–1.03) * | ||||||||
| Stroke | 34,560 | 249,480 | 1.01 (0.99–1.03) | ||||||||
| Arrhythmias | 59,182 | 204,429 | ASD | 18,381 | 27,969 | 1.04 (1.00–1.07) | |||||
| AF | 40,594 | 168,000 | 1.01 (0.99–1.03) | ||||||||
| CAD | 71,602 | 260,875 | 0.96 (0.92–1.00) | ||||||||
| HF | 47,309 | 930,014 | 0.99 (0.93–1.07) | ||||||||
| Hypertension | 122,620 | 332,683 | 0.99 (0.97–1.02) | ||||||||
| Stroke | 34,560 | 249,480 | 1.01 (0.97–1.06) | ||||||||
| Chen Y. et al. (2024) [34] | European | PGC (ADHD); iPSYCH-PGC (ASD); Shah et al. (2020) [48] (HF) | univariable and multivariable two-sample MR | ADHD | 20,183 | 35,191 | HF | 47,309 | 930,014 | 1.12 (1.04–1.2) * | ADHD and ASD may have a causal relationship with an increased risk of HF |
| ASD | 18,382 | 27,969 | HF | 47,309 | 930,014 | 1.29 (1.07–1.56) *,& | |||||
| Chen F. et al. (2024) [35] | European | PGC (ADHD); iPSYCH-PGC (ASD); UK Biobank (hypertension); Roselli et al. (2018) [50] (AF); Nelson et al. (2017) [46] (CAD); Levin et al. (2022) [51] (HF); Hartiala et al. (2021) [52] (MI) | bidirectional MR | ADHD | 38,691 | 186,843 | AF | 65,446 | 588,190 | 1.088 (1.026–1.153) * | Further studies are needed for the shared genetic etiology |
| CAD | 60,801 | 123,504 | 1.187 (1.087–1.297) * | ||||||||
| Cardiomyopathy | 361,194 | 1.000 (0.999–1.001) | |||||||||
| HF | 115,150 | 1,550,331 | 1.097 (1.032–1.165) * | ||||||||
| Hypertension | 361,194 | 1.007 (0.994–1.021) | |||||||||
| MI | 61,000 | 578,000 | 1.005 (1.001–1.009) * | ||||||||
| ASD | 18,381 | 27,969 | AF | 65,446 | 588,190 | 1.099 (1.011–1.195) * | |||||
| CAD | 60,801 | 123,504 | 0.999 (0.994–1.004) | ||||||||
| Cardiomyopathy | 361,194 | 1.000 (0.999–1.006) | |||||||||
| HF | 115,150 | 1,550,331 | 1.077 (1.019–1.139) * | ||||||||
| Hypertension | 361,194 | 1.001 (0.986–1.015) | |||||||||
| MI | 61,000 | 578,000 | 0.999 (0.995–1.003) | ||||||||
| AF | 65,446 | 588,190 | ADHD | 38,691 | 186,843 | 1.017 (0.964–1.074) | |||||
| CAD | 60,801 | 123,504 | 0.973 (0.911–1.039) | ||||||||
| HF | 115,150 | 1,550,331 | 1.165 (0.956–1.419) | ||||||||
| Hypertension | 361,194 | 1.385 (1.006–1.907) * | |||||||||
| MI | 61,000 | 578,000 | 1.169 (0.028–49.112) | ||||||||
| AF | 65,446 | 588,190 | ASD | 18,381 | 27,969 | 1.458 (0.696–3.056) | |||||
| CAD | 60,801 | 123,504 | 0.958 (0.899–1.021) | ||||||||
| HF | 115,150 | 1,550,331 | 1.188 (1.091–1.294) * | ||||||||
| Hypertension | 361,194 | 1.134 (0.676–1.904) | |||||||||
| MI | 61,000 | 578,000 | 1.241 (0.011–141.915) | ||||||||
| Du et al. (2023) [36] | European | Demontis et al. (2019) [22] (ADHD); MEGASTROKE (CVDs) | two-sample MR | ADHD | 19,099 | 34,194 | AIS | 40,585 | 406,111 | 0.96 (0.67–1.38) | Genetic predisposition to ADHD was associated with an enhanced risk of AIS, particularly LAS |
| LAS | 40,585 | 406,111 | 1.4 (1.10–1.76) * | ||||||||
| CES | 40,585 | 406,111 | 1.20 (1.02–1.41) * | ||||||||
| SVS | 40,585 | 406,111 | 1.05 (0.84–1.31) | ||||||||
| CAD | 40,585 | 406,111 | 1.11 (1.01–1.22) * | ||||||||
| Leppert et al. (2021) [37] | European | Demontis et al. (2019) [22] (ADHD); CARDIoGRAMplusC4D (CAD, MI); UK Biobank (hypertension) | bidirectional two-sample MR | ADHD | 19,099 | 34,194 | CAD | 60,801 | 123,504 | 1.11 (1.03–1.19) * | The findings support a causal relationship between ADHD and CAD |
| MI | 43,676 | 128,199 | 1.06 (0.97–1.16) | ||||||||
| Hypertension | 87,690 | 249,469 | 1.05 (0.97–1.13) | ||||||||
| Sui et al. (2023) [38] | European | PGC (ADHD); van der Harst and Verweij (2018) [53] (CAD); Shah et al. (2020) [48] (HF); Roselli et al. (2018) [50] (AF); Malik et al. (2018) [54] (AIS) | two-sample MR | ADHD | 20,183 | 35,191 | CAD | 122,733 | 424,528 | 0.99 (0.93–1.06) | ADHD is associated with an increased risk of HF, AF, and IS |
| AF | 55,114 | 482,295 | 1.08 (1.02–1.15) * | ||||||||
| HF | 47,309 | 930,014 | 1.12 (1.04–1.20) * | ||||||||
| IS | 440,328 | 1.15 (1.05–1.25) * | |||||||||
| Wen et al. (2025) [39] | European | PGC (ADHD); CARDIo-GRAM (CAD, MI); Shah et al. (2020) [48] (HF); Nielsen et al. (2018) [55] (AF) | bidirectional two-sample MR | ADHD | 38,691 | 186,843 | AF | 60,620 | 970,216 | 1.011 (1.009–1.030) *,MW | There are bidirectional causal relationships between HF and ADHD |
| CAD | 22,233 | 64,762 | 1.032 (0.994–1.070) MW | ||||||||
| HF | 47,309 | 930,014 | 1.027 (1.014–1.039) *,MW | ||||||||
| MI | 42,335 | 78,240 | 1.039 (1.025–1.051) *,MW | ||||||||
| AF | 60,620 | 970,216 | ADHD | 38,691 | 186,843 | 1.029 (0.991–1.067) MW | |||||
| CAD | 22,233 | 64,762 | 1.010 (0.985–1.035) MW | ||||||||
| HF | 47,309 | 930,014 | 1.025 (1.013–1.038) * | ||||||||
| MI | 42,335 | 78,240 | 1.032 (0.991–1.073) MW | ||||||||
| Xiang et al. (2025) [40] | European | PGC (ADHD); MEGASTROKE (CVDs) | bidirectional two-sample MR | ADHD | 38,691 | 186,843 | AS | 40,585 | 1.118 (1.047–1.195) * | Genetically predicted ADHD increases the risk of LAS; ASD but not ADHD is causally linked to CVD. | |
| AIS | 34,217 | 1.118 (1.035–1.206) * | |||||||||
| LAS | 4373 | 1.206 (1.023–1.422) * | |||||||||
| CES | 7197 | 1.023 (0.876–1.195) | |||||||||
| SVS | 5386 | 0.980 (0.843–1.138) | |||||||||
| Yu et al. (2024) [41] | European | UK Biobank (ADHD, ASD); FinnGen (CVDs) | MR | ADHD | 55,374 | CVD | 377,277 | 1.02 (0.99–1.06) | ASD but not ADHD is causally linked to CVD | ||
| ASD | 46,351 | 1.05 (1.00–1.09) | |||||||||
| Zheng and Cai (2025) [42] | European | PGC (ADHD); iPSYCH-PGC (ASD); CARDIoGRAMplusC4D (MI, CAD); HERMES (HF); MEGASTROKE (LAS, CES, SVS); Nielsen et al. (2018) [55] (AF) | two-sample MR | ADHD | 20,183 | 35,181 | MI | 43,676 | 128,188 | 1.062 (0.971–1.162) | Cardiovascular monitoring in individuals with ADHD or ASD is crucial to prevent associated risk factors |
| AF | 60,620 | 970,216 | 1.042 (0.896–1.101) | ||||||||
| HF | 47,309 | 930,014 | 1.139 (1.065–1.218) * | ||||||||
| CAD | 60,801 | 123,304 | 1.115 (1.029–1.209) * | ||||||||
| LAS | 7193 | 406,111 | 1.345 (1.092–1.656) * | ||||||||
| CES | 4373 | 406,111 | 1.144 (0.973–1.345) | ||||||||
| SVS | 5386 | 406,111 | 1.088 (0.896–1.322) | ||||||||
| ASD | 18,381 | 27,969 | MI | 43,676 | 128,188 | 0.939 (0.857–1.029) | |||||
| AF | 60,620 | 970,216 | 1.089 (1.026–1.155) * | ||||||||
| HF | 47,309 | 930,014 | 1.112 (1.035–1.194) * | ||||||||
| CAD | 60,801 | 123,304 | 0.953 (0.849–1.069) | ||||||||
| LAS | 7193 | 406,111 | 1.13 (0.911–1.403) * | ||||||||
| CES | 4373 | 406,111 | 1.038 (0.877–1.228) | ||||||||
| SVS | 5386 | 406,111 | 1.084 (0.888–1.324) | ||||||||
| Jin et al. (2024) [43] | European | iPSYCH-PGC (ASD); Nielsen et al. (2018) [55] (AF); Malik et al. (2018) [54] (AS, AIS, LAS, CES, SVS); van der Harst and Verweij (2018) [53] (CAD); Dönertaş et al. (2021) [56] (MI, hypertension) | two-sample MR | ASD | 18,382 | 27,969 | AF | 60,620 | 970,216 | 1.082 (1.0019–1.1684) * | Causal relationships between ASD and AS, IS, LAS, and HF |
| HF | 47,309 | 930,014 | 1.102 (1.001–1.213) * | ||||||||
| CAD | 122,733 | 424,528 | 1.059 (0.943–1.189) | ||||||||
| MI | 11,081 | 473,517 | 1.001 (0.9980–1.004) | ||||||||
| Hypertension | 129,909 | 354,689 | 1.01 (0.99–1.02) | ||||||||
| AS | 40,585 | 406,111 | 1.118 (1.032–1.214) * | ||||||||
| IS | 34,217 | 406,111 | 1.116 (1.024–1.216) * | ||||||||
| LAS | 47,309 | 406,111 | 1.290 (1.039–1.601) * | ||||||||
| CES | 122,733 | 406,111 | 0.994 (0.830–1.191) | ||||||||
| SVS | 11,081 | 406,111 | 1.205 (0.975–1.488) | ||||||||
| Sun et al. (2021) [44] | European (mostly) | iPSYCH-PGC (ASD); CARDIoGRAMplusC4D (CAD, MI); HERMES (HF); Nielsen et al. (2018) [55] (AF) | two-sample MR | ASD | 18,381 | 27,969 | CAD | 60,801 | 123,504 | 0.997 (0.897–1.106) | Genetic predisposition to ASD was associated with a higher risk of AF and HF |
| MI | 0.993 (0.883–1.117) | ||||||||||
| AF | 60,620 | 970,216 | 1.109 (1.023–1.201) * | ||||||||
| HF | 47,309 | 930,014 | 1.138 (1.036–1.251) * | ||||||||
| Huangfu et al. (2023) [45] | European | PGC (ADHD); iPSYCH-PGC (ASD); FinnGen and UK Biobank (hypertension) | two-sample MR | ADHD | 20,183 | 35,191 | Hypertension | 42,857 | 162,837 | 0.98 (0.91–1.07) | No links were identified between genetic predisposition to ASD or ADHD and the risk of hypertension |
| 54,358 | 408,652 | 1.10 (1.00–1.19) | |||||||||
| ASD | 18,381 | 27,969 | 42,857 | 162,837 | 1.19 (0.83–1.71) | ||||||
| 54,358 | 408,652 | 0.97 (0.66–1.42) | |||||||||
| Exposure(s) | Outcomes | MR | Exposure(s) | Outcomes | MR | Observational Studies |
|---|---|---|---|---|---|---|
| ADHD | CAD | + | CAD | ADHD | − | ADHD increases the risk of CAD [7] |
| MI | − | MI | − | no data | ||
| AF | − | AF | + | no data | ||
| HF | + | HF | − | ADHD increases the risk of HF [61] | ||
| CHD | n.d. | CHD | + 1 | CHD in children increases the risk of ADHD [5,14,15,16,17,62,63] | ||
| hypertension | − | hypertension | − | ADHD is associated (but not significantly) with a higher risk of hypertension [5] | ||
| AS | + | AS | n.d. | ADHD increases the risk of stroke [7], including ischemic [12] and hemorrhagic [8] | ||
| AIS | + | AIS | n.d. | |||
| CES | − | CES | n.d. | |||
| LAS | + | LAS | n.d. | |||
| SVS | − | SVS | n.d. | |||
| ASD | CAD | − | CAD | ASD | n.d. | heart diseases have greater odds in older autistic adults [11] |
| MI | − | MI | − 1 | no data | ||
| AF | + | AF | − | patients with ASD are more predisposed to arrhythmias; no data regarding AF [19] | ||
| arrythmias | − 1 | arrythmias | − 1 | |||
| HF | + | HF | − | adults with ASD are at a higher risk of HF [60] | ||
| CHD | n.d. | CHD | − 1 | children with CHD have an increased risk of ASD [5,14,15,16,17,62,63] | ||
| hypertension | − | hypertension | − | within ASD populations: (1) higher prevalence of hypertension [64] or its modest increase [13]; (2) no significant increase in its risk [18]; or (3) lower blood pressure [19] | ||
| AS | − | AS | n.d. | ASD is not associated with an increased risk of stroke [18] | ||
| AIS | n.d. | AIS | n.d. | |||
| CES | n.d. | CES | n.d. | |||
| LAS | n.d. | LAS | n.d. | |||
| SVS | n.d. | SVS | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryszkiewicz, P.; Malinowska, B.; Jasińska-Stroschein, M. Evaluating the Causal Effects of ADHD and Autism on Cardiovascular Diseases and Vice Versa: A Systematic Review and Meta-Analysis of Mendelian Randomization Studies. Cells 2025, 14, 1180. https://doi.org/10.3390/cells14151180
Ryszkiewicz P, Malinowska B, Jasińska-Stroschein M. Evaluating the Causal Effects of ADHD and Autism on Cardiovascular Diseases and Vice Versa: A Systematic Review and Meta-Analysis of Mendelian Randomization Studies. Cells. 2025; 14(15):1180. https://doi.org/10.3390/cells14151180
Chicago/Turabian StyleRyszkiewicz, Piotr, Barbara Malinowska, and Magdalena Jasińska-Stroschein. 2025. "Evaluating the Causal Effects of ADHD and Autism on Cardiovascular Diseases and Vice Versa: A Systematic Review and Meta-Analysis of Mendelian Randomization Studies" Cells 14, no. 15: 1180. https://doi.org/10.3390/cells14151180
APA StyleRyszkiewicz, P., Malinowska, B., & Jasińska-Stroschein, M. (2025). Evaluating the Causal Effects of ADHD and Autism on Cardiovascular Diseases and Vice Versa: A Systematic Review and Meta-Analysis of Mendelian Randomization Studies. Cells, 14(15), 1180. https://doi.org/10.3390/cells14151180






