Andrographis paniculata Extract Supports Skin Homeostasis by Enhancing Epidermal Stem Cell Function and Reinforcing Their Extracellular Niche
Abstract
1. Introduction
2. Materials and Methods
2.1. Andrographis paniculata Extract (APE) Preparation and Analysis
2.2. Human Keratinocyte Culture and Subpopulation Isolation
2.3. Human Fibroblast Culture
2.4. Human Skin Explant (HSE) Culture
2.5. Histo-Morphological Analysis of Skin Explants and Immunohistochemistry
2.6. Fluorescent Immunolabeling of Skin Explants
2.7. MTT Assay
2.8. Colony Forming Efficiency (CFE)
2.9. Co-Culture Model of DEJ Damage
2.10. ELISA Assay
2.11. Statistical Analysis
3. Results
3.1. Andrographolide Content in Andrographis paniculata Extract (APE)

3.2. Improvement of Dermo-Epidermal Junction Composition by APE in Skin Explants
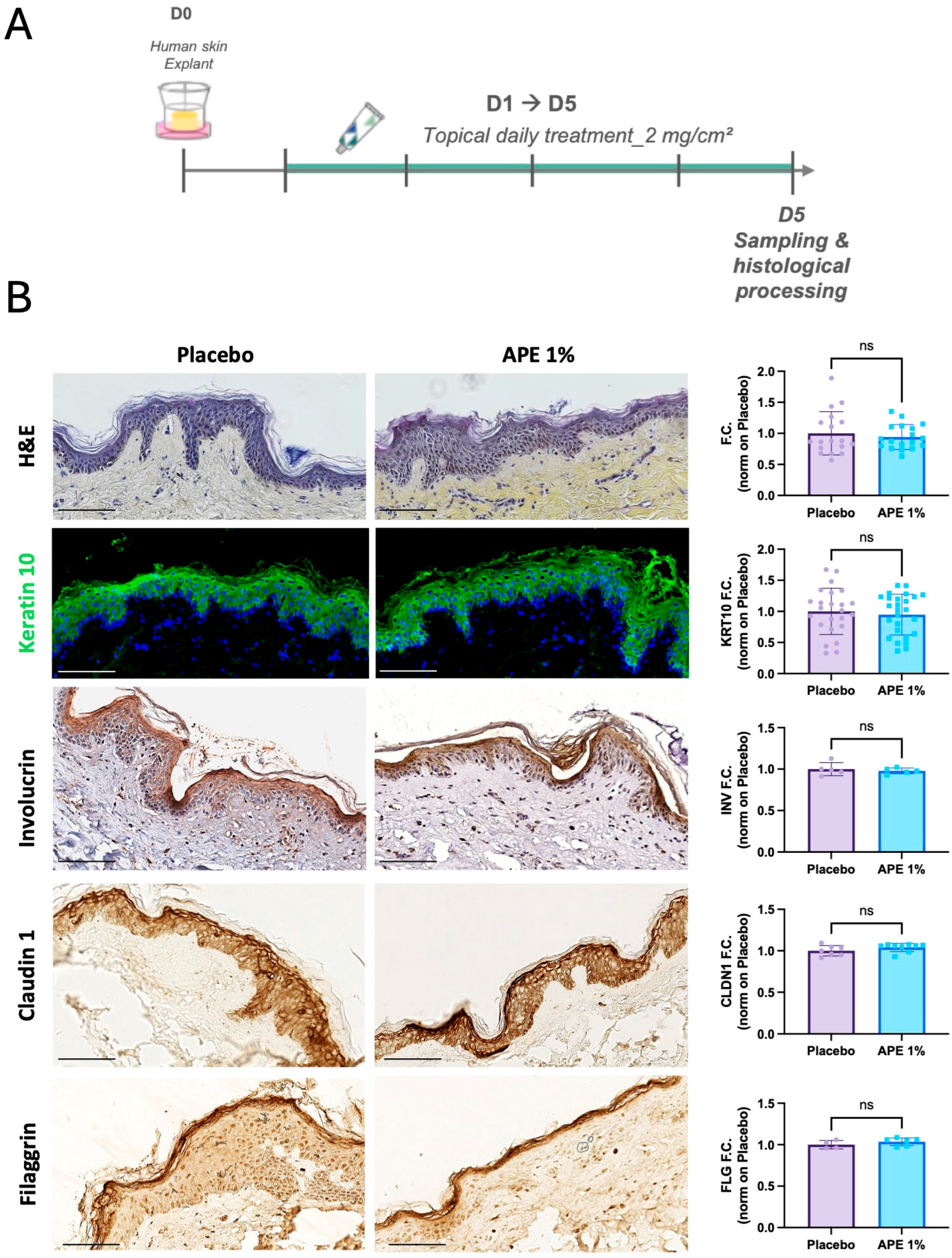

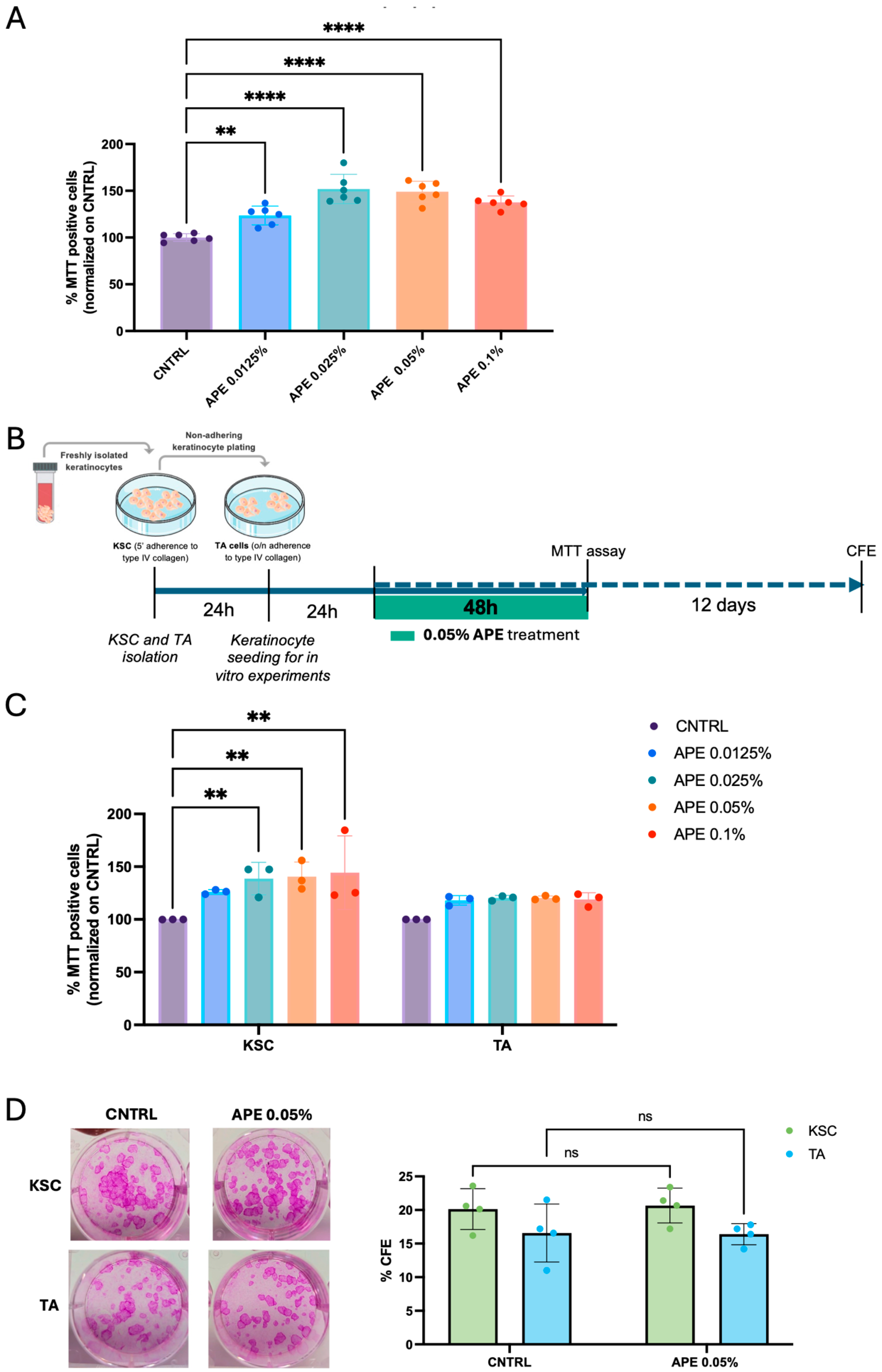
3.3. Stemness Potential Preservation and Stimulation of Skin Cell Proliferative Capability by APE in Skin Explant

3.4. Stimulation of Skin Cell Proliferative Capability by APE in Cell Cultures
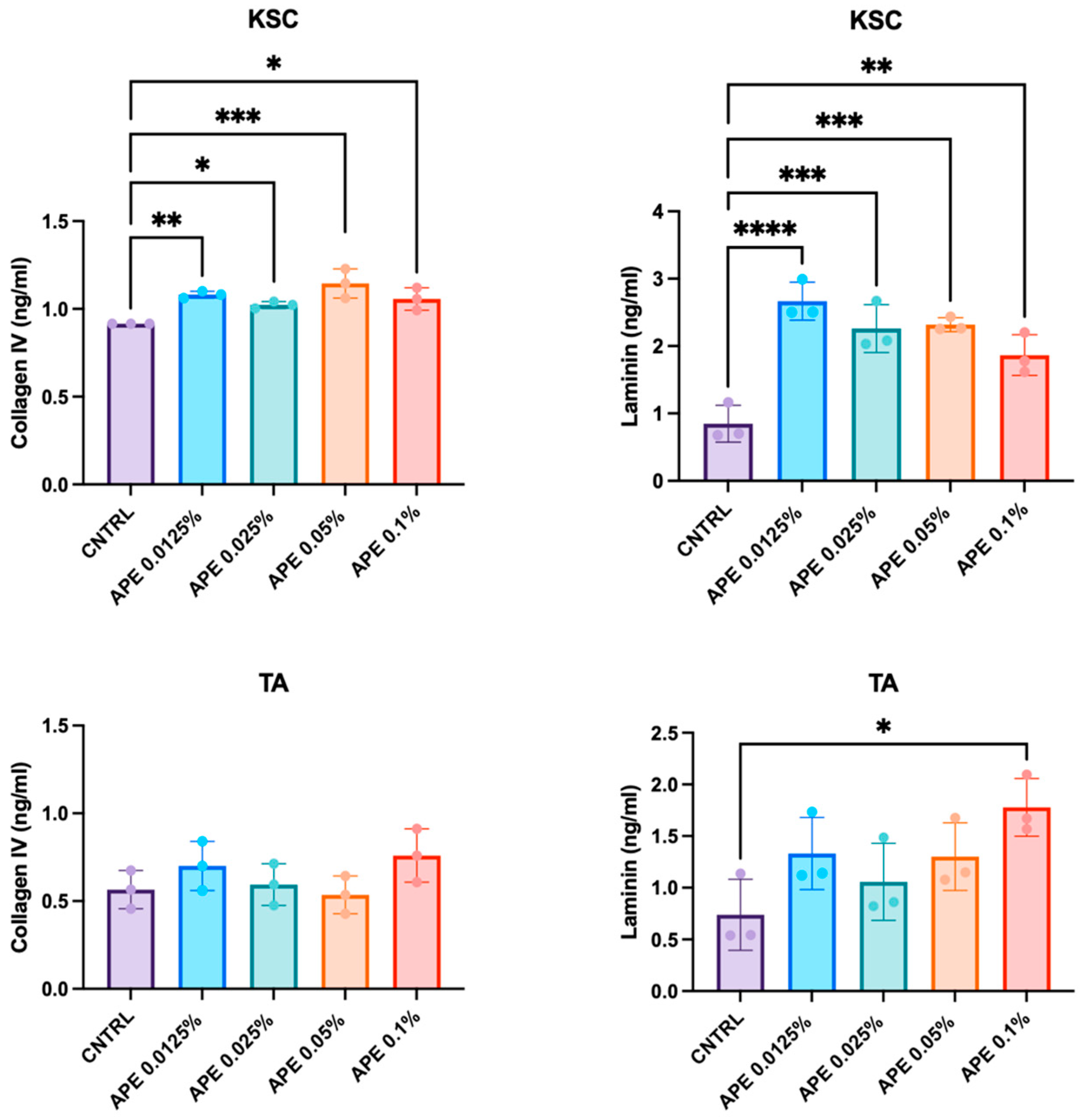
3.5. Stimulation of Skin Cell Laminin and Collagen Secretion by APE in Cell Cultures
3.6. APE Treatment Preserves KSC Capacity in Laminin Production in a DEJ Damaged Co-Culture Model

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| KSC | keratinocyte stem cells |
| TA | transient amplifying |
| ECM | extracellular matrix |
| DEJ | dermo-epidermal junction |
| EGF | epidermal growth factor |
| FAK | focal adhesion kinase |
| ERK | extracellular signal-regulated kinase |
| MMP | matrix metalloproteinase |
| APE | Andrographis paniculata extract |
| HF | human fibroblast |
| HSE | human skin explant |
| IHC | immunohistochemistry |
| IF | immunofluorescence |
| CFE | colony-forming efficiency |
| SD | standard deviation |
| KRT10 | Keratin 10 |
| INV | Involucrin |
| KRT15 | Keratin 15 |
| ITGB1 | Beta1-Integrin |
References
- Pincelli, C.; Marconi, A. Keratinocyte stem cells: Friends and foes. J. Cell Physiol. 2010, 225, 310–315. [Google Scholar] [CrossRef]
- Gadre, P.; Markova, P.; Ebrahimkutty, M.; Jiang, Y.; Bouzada, F.M.; Watt, F.M. Emergence and properties of adult mammalian epidermal stem cells. Dev. Biol. 2024, 515, 129–138. [Google Scholar] [CrossRef]
- Singh, R. Basal Cells in the Epidermis and Epidermal Differentiation. Stem Cell Rev. Rep. 2022, 18, 1883–1891. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Li, L.; Fuchs, E. Emerging interactions between skin stem cells and their niches. Nat. Med. 2014, 20, 847–856. [Google Scholar] [CrossRef]
- Fuchs, E. Finding one’s niche in the skin. Cell Stem Cell 2009, 4, 499–502. [Google Scholar] [CrossRef]
- Louis, B.; Tewary, M.; Bremer, A.W.; Philippeos, C.; Negri, V.A.; Zijl, S.; Gartner, Z.J.; Schaffer, D.V.; Watt, F.M. A reductionist approach to determine the effect of cell-cell contact on human epidermal stem cell differentiation. Acta Biomater. 2022, 150, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Russo, C.; Maugeri, G.; Musumeci, G.; Vicario, N.; Tibullo, D.; Giuffrida, R.; Parenti, R.; Lo Furno, D. Adult stem cell niches for tissue homeostasis. J. Cell Physiol. 2022, 237, 239–257. [Google Scholar] [CrossRef]
- Spradling, A.; Drummond-Barbosa, D.; Kai, T. Stem cells find their niche. Nature 2001, 414, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Blau, H. Tissue Stem Cells: Architects of Their Niches. Cell Stem Cell 2020, 27, 532–5564. [Google Scholar] [CrossRef] [PubMed]
- Mistry, K.; Richardson, G.; Vleminckx, S.; Smith, R.; Gevaert, E.; Lovat, P.E. Porcine-derived collagen peptides promote re-epithelialisation through activation of integrin signaling. Wound Repair. Reg. 2024, 32, 475–486. [Google Scholar] [CrossRef]
- Koivisto, L.; Heino, J.; Häkkinen, L.; Larjava, H. Integrins in wound healing. Adv. Wound Care 2014, 3, 762–783. [Google Scholar] [CrossRef]
- Jones, P.H.; Watt, F.M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 1993, 73, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Kim, D.H.; Noh, K.H.; Jung, C.-H.; Kang, H.M. The proliferative and multipotent epidermal progenitor cells for human skin reconstruction in vitro and in vivo. Cell Prolif. 2022, 55, e13284. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Bork, S.; Horn, P.; Krunic, D.; Walenda, T.; Diehlmann, A.; Benes, V.; Blake, J.; Huber, F.X.; Eckstein, V.; et al. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS ONE 2009, 4, e5846. [Google Scholar] [CrossRef]
- Herdenberg, C.; Hedman, H. HYPOTHESIS: Do LRIG Proteins Regulate Stem Cell Quiescence by Promoting BMP Signaling? Stem Cell Rev. Rep. 2023, 19, 59–66. [Google Scholar] [CrossRef]
- Palazzo, E.; Morandi, P.; Lotti, R.; Saltari, A.; Truzzi, F.; Schnebert, S.; Dumas, M.; Marconi, A.; Pincelli, C. Notch Cooperates with Survivin to Maintain Stemness and to Stimulate Proliferation in Human Keratinocytes during Aging. Int. J. Mol. Sci. 2015, 16, 26291–26302. [Google Scholar] [CrossRef]
- Marconi, A.; Dallaglio, K.; Lotti, R.; Vaschieri, C.; Truzzi, F.; Fantini, F.; Pincelli, C. Survivin identifies keratinocyte stem cells and is downregulated by anti-beta1 integrin during anoikis. Stem Cells 2007, 25, 149–155. [Google Scholar] [CrossRef]
- Webb, A.; Li, A.; Kaur, P. Location and phenotype of human adult keratinocyte stem cells of the skin. Differentiation 2004, 72, 387–395. [Google Scholar] [CrossRef]
- Iriyama, S.; Yasuda, M.; Nishikawa, S.; Takai, E.; Hosoi, J.; Amano, S. Decrease of laminin-511 in the basement membrane due to photoaging reduces epidermal stem/progenitor cells. Sci. Rep. 2020, 10, 12592. [Google Scholar] [CrossRef] [PubMed]
- Florian Labarrade 1, Jean-Marie Botto 1, Isabelle Marie Imbert 1miR-203 represses keratinocyte stemness by targeting survivin. J. Cosmet. Dermatol. 2022, 21, 6100–6108. [CrossRef]
- Liu, Y.; Lyle, S.; Yang, Z.; Cotsarelis, G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J. Investig. Dermatol. 2003, 121, 963–968. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Adjaye, J.; Akamatsu, H.; Moe-Behrens, G.; Niemann, C. Human skin stem cells and the aging process. Exp. Gerontol. 2008, 43, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Estrach, S.; Vivier, C.-M.; Féral, C.C. ECM and epithelial stem cells: The scaffold of destiny. Front. Cell Dev. Biol. 2024, 12, 1359585. [Google Scholar] [CrossRef]
- Hamazaki, J.; Murata, S. Relationships between protein degradation, cellular senescence, and organismal aging. J. Biochem. 2024, 175, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Rodríguez, S.; Folgueras, A.R.; López-Otín, C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef]
- Roig-Rosello, E.; Rousselle, P. The Human Epidermal Basement Membrane: A Shaped and Cell Instructive Platform That Aging Slowly Alters. Biomolecules 2020, 10, 1607. [Google Scholar] [CrossRef]
- Zorina, A.; Vadim Zorin, V.; Dmitry Kudlay, D.; Kopnin, P. Molecular Mechanisms of Changes in Homeostasis of the Dermal Extracellular Matrix: Both Involutional and Mediated by Ultraviolet Radiation. Int. J. Mol. Sci. 2022, 23, 6655. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K.A.; Nawi, A.F.M.; Zulkifli, N.; Barkat, A.; Hazrina Hadi, H. Aging and Wound Healing of the Skin: A Review of Clinical and Pathophysiological Hallmarks. Life 2022, 12, 2142. [Google Scholar] [CrossRef]
- Xu, H.; Lan, S.; Lin, S.; Wang, A.; Luo, Y.; Wang, J.; Yang, Z. Exploring the Active Constituents of Andrographis paniculata in Protecting the Skin Barrier and the Synergistic Effects with Collagen XVII. Antioxidants 2025, 14, 118. [Google Scholar] [CrossRef]
- You, J.; Roh, K.B.; Li, Z.; Liu, G.; Tang, J.; Shin, S.; Park, D.; Jung, E. The Antiaging Properties of Andrographis paniculata by Activation Epidermal Cell Stemness. Molecules 2015, 20, 17557–17569. [Google Scholar] [CrossRef]
- Li, X.; Yuan, W.; Wu, J.; Zhen, J.; Sun, Q.; Yu, M. Andrographolide, a natural anti-inflammatory agent: An Update. Front. Pharmacol. 2022, 13, 920435. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, F.; Saltari, A.; Palazzo, E.; Lotti, R.; Petrachi, T.; Dallaglio, K.; Gemelli, C.; Grisendi, G.; Dominici, M.; Pincelli, C.; et al. CD271 mediates stem cells to early progeny transition in human epidermis. J. Investig. Dermatol. 2015, 135, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Lotti, R.; Palazzo, E.; Quadri, M.; Dumas, M.; Schnebert, S.; Biondini, D.; Bianchini, M.A.; Nizard, C.; Pincelli, C.; Marconi, A. Isolation of an “Early” Transit Amplifying Keratinocyte Population in Human Epidermis: A Role for the Low Affinity Neurotrophin Receptor CD271. Stem Cells 2022, 40, 1149–1161. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, S.R.; Chai, L.; Zhao, J.; Wang, Y.; Wang, Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. 1), S17–S29. [Google Scholar] [CrossRef]
- Kaltchenko, M.V.; Chien, A.L. Photoaging: Current Concepts on Molecular Mechanisms, Prevention, and Treatment. Am. J. Clin. Dermatol. 2025, 26, 321–344. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.V.S.; Andrade, S.S. Decoding the impact of aging and environment stressors on skin cell communication. Biogerontology 2025, 26, 3. [Google Scholar] [CrossRef]
- Chao, W.W.; Lin, B.F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef]
- Zeng, B.; Wei, A.; Zhou, Q.; Yuan, M.; Lei, K.; Liu, Y.; Song, J.; Guo, L.; Ye, Q. Andrographolide: A review of its pharmacology, pharmacokinetics, toxicity and clinical trials and pharmaceutical researches. Phytother. Res. 2022, 36, 336–364. [Google Scholar] [CrossRef]
- Worakunphanich, W.; Thavorncharoensap, M.; Youngkong, S.; Thadanipon, K.; Thakkinstian, A. Safety of Andrographis paniculata: A systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2021, 30, 727–739. [Google Scholar] [CrossRef]
- Kalinovskii, A.P.; Logashina, Y.A.; Palikova, Y.A.; Palikov, V.A.; Osmakov, D.I.; Mineev, K.S.; Belozerova, O.A.; Shmygarev, V.I.; Kozlov, S.I.; Dyachenko, I.A.; et al. A Diterpenoid of the Medicinal Plant Andrographis paniculata Targets Cutaneous TRPV3 Channel and Relieves Itch. J. Nat. Prod. 2024, 87, 1852–1859. [Google Scholar] [CrossRef]
- Dharmasamitha, I.; Mas Rusyati, L.M.; Wati, D.K.; Gelgel Wirasuta, I.M.A. The Potential Anti-psoriatic Effects of Andrographolide: A Comparative Study to Topical Corticosteroids. Recent Adv. Inflamm. Allergy Drug Discov. 2025, 19, 46–70. [Google Scholar] [CrossRef] [PubMed]
- Aleemardani, M.; Trikić, M.Z.; Green, N.H.; Claeyssens, F. The Importance of Mimicking Dermal-Epidermal Junction for Skin Tissue Engineering: A Review. Bioengineering 2021, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.; Horsley, V. Home sweet home: Skin stem cell niches. Cell. Mol. Life Sci. 2012, 69, 2573–2582. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Iwasaki, A.; Chien, A.L.; Kang, S. UVB-mediated DNA damage induces matrix metalloproteinases to promote photoaging in an AhR- and SP1-dependent manner. JCI Insight 2022, 7, e156344. [Google Scholar] [CrossRef]
- Metral, E.; Bechetoille, N.; Demarne, F.; Damour, O.; Rachidi, W. Keratinocyte stem cells are more resistant to UVA radiation than their direct progeny. PLoS ONE 2018, 13, e0203863. [Google Scholar] [CrossRef]
- Dallaglio, K.; Palazzo, E.; Marconi, A.; Dumas, M.; Truzzi, F.; Lotti, R.; Bontè, F.; Pincelli, C. Endogenous survivin modulates survival and proliferation in UVB-treated human keratinocytes. Exp. Dermatol. 2009, 18, 464–471. [Google Scholar] [CrossRef]
- Notara, M.; Refaian, N.; Braun, G.; Steven, P.; Bock, F.; Cursiefen, C. Short-term UVB-irradiation leads to putative limbal stem cell damage and niche cell-mediated upregulation of macrophage recruiting cytokines. Stem Cell Res. 2015, 15, 643–654. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lotti, R.; Cattuzzato, L.; Huang, X.; Garandeau, D.; Palazzo, E.; Quadri, M.; Delluc, C.; Magdeleine, E.; Li, X.; Frechet, M.; et al. Andrographis paniculata Extract Supports Skin Homeostasis by Enhancing Epidermal Stem Cell Function and Reinforcing Their Extracellular Niche. Cells 2025, 14, 1176. https://doi.org/10.3390/cells14151176
Lotti R, Cattuzzato L, Huang X, Garandeau D, Palazzo E, Quadri M, Delluc C, Magdeleine E, Li X, Frechet M, et al. Andrographis paniculata Extract Supports Skin Homeostasis by Enhancing Epidermal Stem Cell Function and Reinforcing Their Extracellular Niche. Cells. 2025; 14(15):1176. https://doi.org/10.3390/cells14151176
Chicago/Turabian StyleLotti, Roberta, Laetitia Cattuzzato, Xuefeng Huang, David Garandeau, Elisabetta Palazzo, Marika Quadri, Cécile Delluc, Eddy Magdeleine, Xiaojing Li, Mathilde Frechet, and et al. 2025. "Andrographis paniculata Extract Supports Skin Homeostasis by Enhancing Epidermal Stem Cell Function and Reinforcing Their Extracellular Niche" Cells 14, no. 15: 1176. https://doi.org/10.3390/cells14151176
APA StyleLotti, R., Cattuzzato, L., Huang, X., Garandeau, D., Palazzo, E., Quadri, M., Delluc, C., Magdeleine, E., Li, X., Frechet, M., & Marconi, A. (2025). Andrographis paniculata Extract Supports Skin Homeostasis by Enhancing Epidermal Stem Cell Function and Reinforcing Their Extracellular Niche. Cells, 14(15), 1176. https://doi.org/10.3390/cells14151176








