Targeting Skin Neoplasms: A Review of Berberine’s Anticancer Properties
Abstract
1. Introduction
1.1. Epidemiology and Characteristics of Skin Cancers
1.2. Molecular Pathophysiology of Skin Cancers
2. Berberine and Its Anticancer Activity
2.1. Anticancer Effects of Berberine
2.2. Anticancer Effects of Berberine Derivatives
3. Berberine and Its Potential Anti-Melanoma Activity
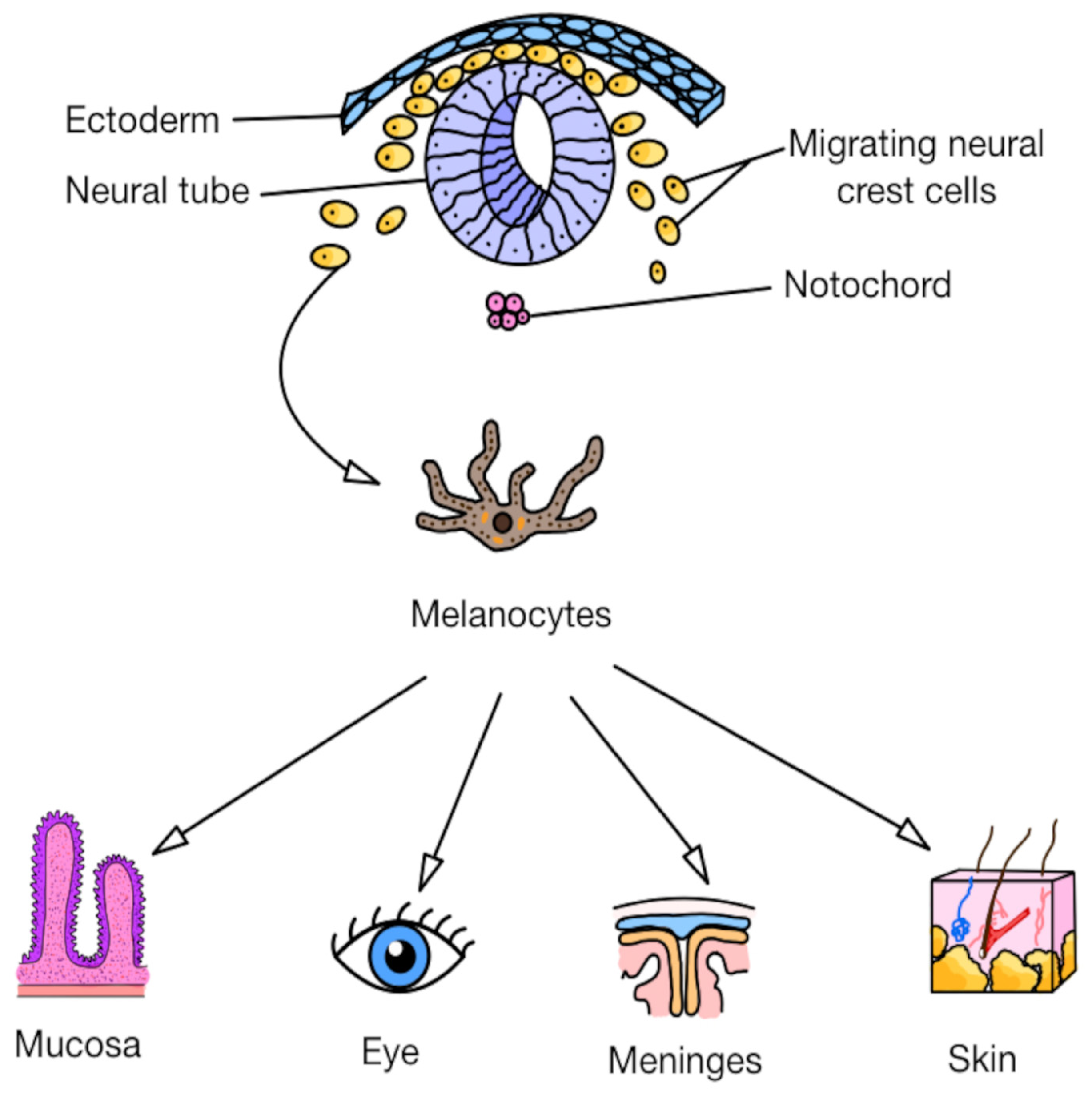
3.1. Primary Cutaneous and Noncutaneous Melanomas: Origin and Dissemination
3.2. In Vitro Studies
In Vitro Synergy of Combination Treatments
3.3. In Vitro and In Vivo Studies
In Vitro and In Vivo Synergy of Combination Treatments
4. Berberine’s Role in the Treatment and Prevention of Cutaneous Squamous Cell Carcinoma
4.1. Characteristics of Cutaneous Squamous Cell Carcinoma
4.2. In Vitro Studies
4.3. In Vitro and In Vivo Studies
5. Conclusions
6. Future Scope and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, S.; Ramam, M. Skin Tumours. J. Cutan. Aesthetic Surg. 2012, 5, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, H.; Schmidt, B.; Huang, J.T. Characteristics and Outcomes of Nonmelanoma Skin Cancer (NMSC) in Children and Young Adults. J. Am. Acad. Dermatol. 2015, 73, 785–790. [Google Scholar] [CrossRef]
- Roky, A.H.; Islam, M.M.; Ahasan, A.M.F.; Mostaq, M.S.; Mahmud, M.Z.; Amin, M.N.; Mahmud, M.A. Overview of Skin Cancer Types and Prevalence Rates across Continents. Cancer Pathog. Ther. 2024, 3, 89–100. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, B.; Badri, T.; Steele, R.B. Basal Cell Carcinoma; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Tow, R.; Hanoun, S.; Andresen, B.; Shahid, A.; Wang, J.; Kelly, K.M.; Meyskens, F.L.; Huang, Y. Recent Advances in Clinical Research for Skin Cancer Chemoprevention. Cancers 2023, 15, 3819. [Google Scholar] [CrossRef]
- Gailani, M.R.; Ståhle-Bäckdahl, M.; Leffell, D.J.; Glyn, M.; Zaphiropoulos, P.G.; Undén, A.B.; Dean, M.; Brash, D.E.; Bale, A.E.; Toftgård, R. The Role of the Human Homologue of Drosophila Patched in Sporadic Basal Cell Carcinomas. Nat. Genet. 1996, 14, 78–81. [Google Scholar] [CrossRef]
- Joshi, P.S.; Deshmukh, V.; Golgire, S. Gorlin-Goltz Syndrome. Dent. Res. J. 2012, 9, 100–106. [Google Scholar] [CrossRef]
- Darido, C.; Georgy, S.R.; Wilanowski, T.; Dworkin, S.; Auden, A.; Zhao, Q.; Rank, G.; Srivastava, S.; Finlay, M.J.; Papenfuss, A.T.; et al. Targeting of the Tumor Suppressor GRHL3 by a MiR-21-Dependent Proto-Oncogenic Network Results in PTEN Loss and Tumorigenesis. Cancer Cell 2011, 20, 635–648. [Google Scholar] [CrossRef]
- Chen, I.-P.; Henning, S.; Faust, A.; Boukamp, P.; Volkmer, B.; Greinert, R. UVA-Induced Epigenetic Regulation of P16INK4a in Human Epidermal Keratinocytes and Skin Tumor Derived Cells. Photochem. Photobiol. Sci. 2012, 11, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Dhull, A.K.; Atri, R.; Dhankhar, R.; Chauhan, A.K.; Kaushal, V. Major Risk Factors in Head and Neck Cancer: A Retrospective Analysis of 12-Year Experiences. World J. Oncol. 2018, 9, 80–84. [Google Scholar] [CrossRef]
- Gosman, L.M.; Țăpoi, D.-A.; Costache, M. Cutaneous Melanoma: A Review of Multifactorial Pathogenesis, Immunohistochemistry, and Emerging Biomarkers for Early Detection and Management. Int. J. Mol. Sci. 2023, 24, 15881. [Google Scholar] [CrossRef]
- Herlyn, M. Human Melanoma: Development and Progression. Cancer Metastasis Rev. 1990, 9, 101–112. [Google Scholar] [CrossRef]
- Di Raimondo, C.; Lozzi, F.; Di Domenico, P.P.; Campione, E.; Bianchi, L. The Diagnosis and Management of Cutaneous Metastases from Melanoma. Int. J. Mol. Sci. 2023, 24, 14535. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, L.; Lontano, A.; Merli, M.; Dika, E.; Nagore, E.; Quaglino, P.; Puig, S.; Ribero, S. Familial Melanoma and Susceptibility Genes: A Review of the Most Common Clinical and Dermoscopic Phenotypic Aspect, Associated Malignancies and Practical Tips for Management. J. Clin. Med. 2021, 10, 3760. [Google Scholar] [CrossRef]
- Heppt, M.V.; Siepmann, T.; Engel, J.; Schubert-Fritschle, G.; Eckel, R.; Mirlach, L.; Kirchner, T.; Jung, A.; Gesierich, A.; Ruzicka, T.; et al. Prognostic Significance of BRAF and NRAS Mutations in Melanoma: A German Study from Routine Care. BMC Cancer 2017, 17, 536. [Google Scholar] [CrossRef] [PubMed]
- Doan, H.; Silapunt, S.; Migden, M. Sonidegib, a Novel Smoothened Inhibitor for the Treatment of Advanced Basal Cell Carcinoma. OncoTargets Ther. 2016, 9, 5671–5678. [Google Scholar] [CrossRef]
- Trieu, K.G.; Tsai, S.-Y.; Eberl, M.; Ju, V.; Ford, N.C.; Doane, O.J.; Peterson, J.K.; Veniaminova, N.A.; Grachtchouk, M.; Harms, P.W.; et al. Basal Cell Carcinomas Acquire Secondary Mutations to Overcome Dormancy and Progress from Microscopic to Macroscopic Disease. Cell Rep. 2022, 39, 110779. [Google Scholar] [CrossRef]
- Atwood, S.X.; Li, M.; Lee, A.; Tang, J.Y.; Oro, A.E. GLI Activation by Atypical Protein Kinase C ι/λ Regulates the Growth of Basal Cell Carcinomas. Nature 2013, 494, 484–488. [Google Scholar] [CrossRef]
- Ikram, M.S.; Neill, G.W.; Regl, G.; Eichberger, T.; Frischauf, A.-M.; Aberger, F.; Quinn, A.; Philpott, M. GLI2 Is Expressed in Normal Human Epidermis and BCC and Induces GLI1 Expression by Binding to Its Promoter. J. Investig. Dermatol. 2004, 122, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Burren, R.; Scaletta, C.; Frenk, E.; Panizzon, R.G.; Applegate, L.A. Sunlight and Carcinogenesis: Expression Ofp53 and Pyrimidine Dimers in Human Skin Following UVA I, UVA I + II and Solar Simulating Radiations. Int. J. Cancer 1998, 76, 201–206. [Google Scholar] [CrossRef]
- Wang, N.J.; Sanborn, Z.; Arnett, K.L.; Bayston, L.J.; Liao, W.; Proby, C.M.; Leigh, I.M.; Collisson, E.A.; Gordon, P.B.; Jakkula, L.; et al. Loss-of-Function Mutations in Notch Receptors in Cutaneous and Lung Squamous Cell Carcinoma. Proc. Natl. Acad. Sci. USA 2011, 108, 17761–17766. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.G. Damage Removal and Gap Filling in Nucleotide Excision Repair. Enzymes 2019, 45, 59–97. [Google Scholar]
- Leung, A.K.; Barankin, B.; Lam, J.M.; Leong, K.F.; Hon, K.L. Xeroderma Pigmentosum: An Updated Review. Drugs Context 2022, 11, 2022-2-5. [Google Scholar] [CrossRef]
- Uribe, P.; Gonzalez, S. Epidermal Growth Factor Receptor (EGFR) and Squamous Cell Carcinoma of the Skin: Molecular Bases for EGFR-Targeted Therapy. Pathol.-Res. Pract. 2011, 207, 337–342. [Google Scholar] [CrossRef]
- White, A.C.; Tran, K.; Khuu, J.; Dang, C.; Cui, Y.; Binder, S.W.; Lowry, W.E. Defining the Origins of Ras/P53-Mediated Squamous Cell Carcinoma. Proc. Natl. Acad. Sci. USA 2011, 108, 7425–7430. [Google Scholar] [CrossRef]
- Doma, E.; Rupp, C.; Baccarini, M. EGFR-Ras-Raf Signaling in Epidermal Stem Cells: Roles in Hair Follicle Development, Regeneration, Tissue Remodeling and Epidermal Cancers. Int. J. Mol. Sci. 2013, 14, 19361–19384. [Google Scholar] [CrossRef]
- Leonardi, G.; Falzone, L.; Salemi, R.; Zanghì, A.; Spandidos, D.; Mccubrey, J.; Candido, S.; Libra, M. Cutaneous Melanoma: From Pathogenesis to Therapy (Review). Int. J. Oncol. 2018, 52, 1071–1080. [Google Scholar] [CrossRef]
- Paluncic, J.; Kovacevic, Z.; Jansson, P.J.; Kalinowski, D.; Merlot, A.M.; Huang, M.L.-H.; Lok, H.C.; Sahni, S.; Lane, D.J.R.; Richardson, D.R. Roads to Melanoma: Key Pathways and Emerging Players in Melanoma Progression and Oncogenic Signaling. Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Poulikakos, P.I.; Sullivan, R.J.; Yaeger, R. Molecular Pathways and Mechanisms of BRAF in Cancer Therapy. Clin. Cancer Res. 2022, 28, 4618–4628. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Flores, E.; Shannon, K. Targeting Oncogenic Ras. Genes Dev. 2007, 21, 1989–1992. [Google Scholar] [CrossRef]
- Hodis, E.; Watson, I.R.; Kryukov, G.V.; Arold, S.T.; Imielinski, M.; Theurillat, J.-P.; Nickerson, E.; Auclair, D.; Li, L.; Place, C.; et al. A Landscape of Driver Mutations in Melanoma. Cell 2012, 150, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Bernards, A.; Settleman, J. GAPs in Growth Factor Signalling. Growth Factors 2005, 23, 143–149. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Abdel-Malek, Z.A.; Ruwe, A.; Kavanagh-Starner, R.; Kadekaro, A.L.; Swope, V.; Haskell-Luevano, C.; Koikov, L.; Knittel, J.J. A-MSH Tripeptide Analogs Activate the Melanocortin 1 Receptor and Reduce UV-induced DNA Damage in Human Melanocytes. Pigment Cell Melanoma Res. 2009, 22, 635–644. [Google Scholar] [CrossRef]
- Scott, T.L.; Christian, P.A.; Kesler, M.V.; Donohue, K.M.; Shelton, B.; Wakamatsu, K.; Ito, S.; D’Orazio, J. Pigment-independent CAMP-mediated Epidermal Thickening Protects against Cutaneous UV Injury by Keratinocyte Proliferation. Exp. Dermatol. 2012, 21, 771–777. [Google Scholar] [CrossRef]
- Coppinger, C.; Pomales, B.; Movahed, M.R.; Marefat, M.; Hashemzadeh, M. Berberine: A Multi-Target Natural PCSK9 Inhibitor with the Potential to Treat Diabetes, Alzheimer’s, Cancer and Cardiovascular Disease. Curr. Rev. Clin. Exp. Pharmacol. 2024, 19, 312–326. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Xu, Q.; Ma, J.; Li, X.; Yan, J.; Tian, Y.; Wen, Y.; Chen, T. Berberine and Health Outcomes: An Umbrella Review. Phyther. Res. 2023, 37, 2051–2066. [Google Scholar] [CrossRef]
- Vlavcheski, F.; O’Neill, E.J.; Gagacev, F.; Tsiani, E. Effects of Berberine against Pancreatitis and Pancreatic Cancer. Molecules 2022, 27, 8630. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, J.; Chen, S.; Hu, S.; Shen, C.; Xiang, J.; Chen, N.; Wang, J.; Ma, X.; Zhang, Y.; et al. Berberine for Gastric Cancer Prevention and Treatment: Multi-Step Actions on the Correa’s Cascade Underlie Its Therapeutic Effects. Pharmacol. Res. 2022, 184, 106440. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, Z.; Sheikhsagha, E.; Behnam, M.; Nabavizadeh, F.; Ardestani, M.S.; Rahmati-Dehkordi, F.; Aschner, M.; Mirzaei, H.; Tamtaji, O.R. Berberine and Lung Cancer: From Pure Form to Its Nanoformulations. Asia Pac. J. Clin. Oncol. 2024. [Google Scholar] [CrossRef]
- Duda-Madej, A.; Viscardi, S.; Szewczyk, W.; Topola, E. Natural Alkaloids in Cancer Therapy: Berberine, Sanguinarine and Chelerythrine against Colorectal and Gastric Cancer. Int. J. Mol. Sci. 2024, 25, 8375. [Google Scholar] [CrossRef]
- Zhong, X.D.; Chen, L.J.; Xu, X.Y.; Liu, Y.J.; Tao, F.; Zhu, M.H.; Li, C.Y.; Zhao, D.; Yang, G.J.; Chen, J. Berberine as a Potential Agent for Breast Cancer Therapy. Front. Oncol. 2022, 12, 993775. [Google Scholar] [CrossRef]
- Carriero, F.; Martinelli, C.; Gabriele, F.; Barbieri, G.; Zanoletti, L.; Milanesi, G.; Casali, C.; Azzalin, A.; Manai, F.; Paolillo, M.; et al. Berberine Photo-activation Potentiates Cytotoxicity in Human Astrocytoma Cells through Apoptosis Induction. J. Pers. Med. 2021, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Feng, W.; Li, S.; Tang, H.; Qin, S.; Li, W.; Gong, Y.; Fang, Y.; Liu, Y.; Wang, S.; et al. Berberine Exerts Anti-Cancer Activity by Modulating Adenosine Monophosphate- Activated Protein Kinase (AMPK) and the Phosphatidylinositol 3-Kinase/Protein Kinase B (PI3K/AKT) Signaling Pathways. Curr. Pharm. Des. 2020, 27, 565–574. [Google Scholar] [CrossRef]
- Kaboli, P.J.; Rahmat, A.; Ismail, P.; Ling, K.H. Targets and Mechanisms of Berberine, a Natural Drug with Potential to Treat aCancer with Special Focus on Breast Cancer. Eur. J. Pharmacol. 2014, 740, 584–595. [Google Scholar] [CrossRef]
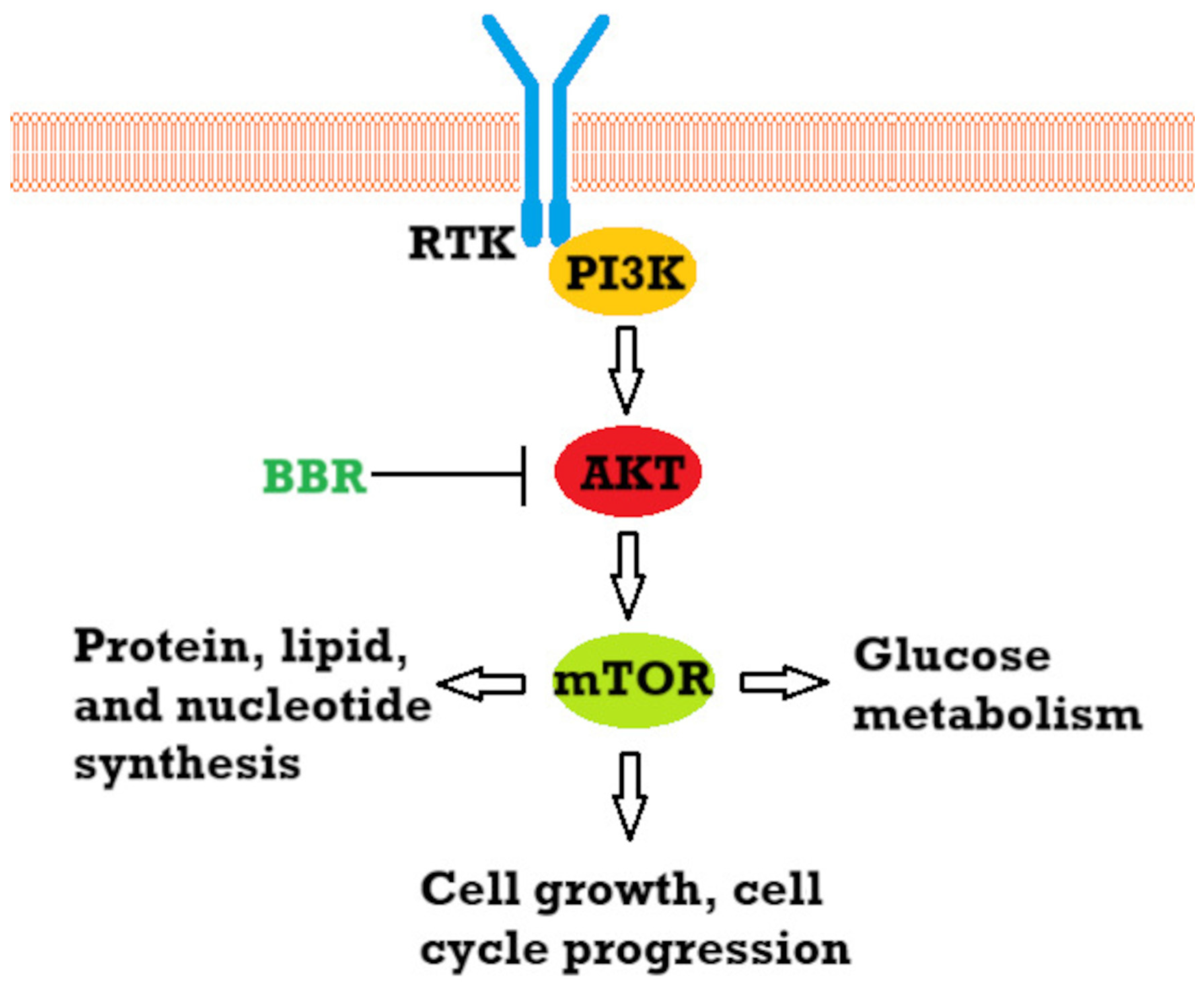
- Chamcheu, J.C.; Roy, T.; Uddin, M.B.; Banang-mbeumi, S.; Chamcheu, R.C.N.; Walker, A.L.; Liu, Y.Y.; Huang, S. Role and Therapeutic Targeting of the Pi3k/Akt/Mtor Signaling Pathway in Skin Cancer: A Review of Current Status and Future Trends on Natural and Synthetic Agents Therapy. Cells 2019, 8, 803. [Google Scholar] [CrossRef]
- Pópulo, H.; Lopes, J.M.; Soares, P. The MTOR Signalling Pathway in Human Cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef]
- Strickland, L.R.; Pal, H.C.; Elmets, C.A.; Afaq, F. Targeting Drivers of Melanoma with Synthetic Small Molecules and Phytochemicals. Cancer Lett. 2015, 359, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, X.; Cao, S.; Sun, Y.; He, X.; Jiang, B.; Yu, Y.; Duan, J.; Qiu, F.; Kang, N. Berberine Represses Human Gastric Cancer Cell Growth in Vitro and in Vivo by Inducing Cytostatic Autophagy via Inhibition of MAPK/MTOR/P70S6K and Akt Signaling Pathways. Biomed. Pharmacother. 2020, 128, 110245. [Google Scholar] [CrossRef] [PubMed]
- Okuno, K.; Garg, R.; Yuan, Y.C.; Tokunaga, M.; Kinugasa, Y.; Goel, A. Berberine and Oligomeric Proanthocyanidins Exhibit Synergistic Efficacy Through Regulation of PI3K-Akt Signaling Pathway in Colorectal Cancer. Front. Oncol. 2022, 12, 855860. [Google Scholar] [CrossRef]
- Lu, T.; Wang, Y.; Liu, F.; Zhang, L.; Huang, S.; Zhou, Y.; Wu, H.; Mao, Y.; Jin, C.; Song, W. Synergistic Inhibitory Effect of Berberine and Low-Temperature Plasma on Non-Small-Cell Lung Cancer Cells via PI3K-AKT-Driven Signaling Axis. Molecules 2023, 28, 7797. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. MTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Lin, H.; Lin, L.; Choi, Y.; Michniak-Kohn, B. Development and In-Vitro Evaluation of Co-Loaded Berberine Chloride and Evodiamine Ethosomes for Treatment of Melanoma. Int. J. Pharm. 2020, 581, 119278. [Google Scholar] [CrossRef]
- Lü, Y.; Han, B.; Yu, H.; Cui, Z.; Li, Z.; Wang, J. Berberine Regulates the MicroRNA-21-ITGB4-PDCD4 Axis and Inhibits Colon Cancer Viability. Oncol. Lett. 2018, 15, 5971–5976. [Google Scholar] [CrossRef]
- Lo, T.F.; Tsai, W.C.; Chen, S.T. MicroRNA-21-3p, a Berberine-Induced MiRNA, Directly Down-Regulates Human Methionine Adenosyltransferases 2A and 2B and Inhibits Hepatoma Cell Growth. PLoS ONE 2013, 8, e75628. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Shi, Y.; Cao, H.; Chaturvedi, R.; Calcutt, M.W.; Hu, T.; Ren, X.; Wilson, K.T.; Polk, D.B.; et al. Berberine Induces Caspase-Independent Cell Death in Colon Tumor Cells through Activation of Apoptosis-Inducing Factor. PLoS ONE 2012, 7, e36418. [Google Scholar] [CrossRef]
- Oberoi-Khanuja, T.K.; Murali, A.; Rajalingam, K. IAPs on the Move: Role of Inhibitors of Apoptosis Proteins in Cell Migration. Cell Death Dis. 2013, 4, e784. [Google Scholar] [CrossRef]
- Lopes, T.Z.; de Moraes, F.R.; Tedesco, A.C.; Arni, R.K.; Rahal, P.; Calmon, M.F. Berberine Associated Photodynamic Therapy Promotes Autophagy and Apoptosis via ROS Generation in Renal Carcinoma Cells. Biomed. Pharmacother. 2020, 123, 109794. [Google Scholar] [CrossRef] [PubMed]
- Gjorgieva Ackova, D.; Maksimova, V.; Smilkov, K.; Buttari, B.; Arese, M.; Saso, L. Alkaloids as Natural NRF2 Inhibitors: Chemoprevention and Cytotoxic Action in Cancer. Pharmaceuticals 2023, 16, 850. [Google Scholar] [CrossRef] [PubMed]
- Sajeev, A.; Sailo, B.; Unnikrishnan, J.; Talukdar, A.; Alqahtani, M.S.; Abbas, M.; Alqahtani, A.; Sethi, G.; Kunnumakkara, A.B. Unlocking the Potential of Berberine: Advancing Cancer Therapy through Chemosensitization and Combination Treatments. Cancer Lett. 2024, 597, 217019. [Google Scholar] [CrossRef]
- Li, S.Y.; Shi, C.J.; Fu, W.M.; Zhang, J.F. Berberine Inhibits Tumour Growth in Vivo and in Vitro through Suppressing the LincROR-Wnt/β-Catenin Regulatory Axis in Colorectal Cancer. J. Pharm. Pharmacol. 2023, 75, 129–138. [Google Scholar] [CrossRef]
- Huang, C.; Sun, Y.; Liao, S.R.; Chen, Z.X.; Lin, H.F.; Shen, W.Z. Suppression of Berberine and Probiotics (In Vitro and In Vivo) on the Growth of Colon Cancer With Modulation of Gut Microbiota and Butyrate Production. Front. Microbiol. 2022, 13, 869931. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Tao, C.; Wang, L.; Chen, Z.; Li, X.; Zeng, Q.; Ma, M.; Zhang, R.; Wu, Z. Berberine Chloride Suppresses Non-Small Cell Lung Cancer by Deregulating Sin3A/TOP2B Pathway In Vitro and In Vivo. Cancer Chemother. Pharmacol. 2020, 86, 151–161. [Google Scholar] [CrossRef]
- Li, Y.; Li, G.; Peng, C.; Shi, X.; Peng, F.; McGregor, A.; Xie, X. Berberrubine, an Attractive Derivative of Berberine with Multiple Pharmacological Activities. Arab. J. Chem. 2025, 18, 106045. [Google Scholar] [CrossRef]
- Chang, J.M.; Kam, K.H.; Chao, W.Y.; Zhao, P.W.; Chen, S.H.; Chung, H.C.; Li, Y.Z.; Wu, J.Y.; Lee, Y.R. Berberine Derivatives Suppress Cellular Proliferation and Tumorigenesis In Vitro in Human Non-Small-Cell Lung Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4218. [Google Scholar] [CrossRef]
- Guamán Ortiz, L.M.; Croce, A.L.; Aredia, F.; Sapienza, S.; Fiorillo, G.; Syeda, T.M.; Buzzetti, F.; Lombardi, P.; Scovassi, A.I. Effect of New Berberine Derivatives on Colon Cancer Cells. Acta Biochim. Biophys. Sin. 2015, 47, 824–833. [Google Scholar] [CrossRef]
- Lin, H.J.; Ho, J.H.; Tsai, L.C.; Yang, F.Y.; Yang, L.L.; Kuo, C.D.; Chen, L.G.; Liu, Y.W.; Wu, J.Y. Synthesis and In Vitro Photocytotoxicity of 9-/13-Lipophilic Substituted Berberine Derivatives as Potential Anticancer Agents. Molecule 2020, 25, 677. [Google Scholar] [CrossRef]
- Pierpaoli, E.; Arcamone, A.G.; Buzzetti, F.; Lombardi, P.; Salvatore, C.; Provinciali, M. Antitumor Effect of Novel Berberine Derivatives in Breast Cancer Cells. BioFactors 2013, 39, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.Y.; Hsu, L.C.; Chen, M.S.; Lin, Y.J.; Chen, L.G.; Kuo, C.D.; Wu, J.Y. Synthesis and Anticancer Activity of a Novel Series of 9-O-Substituted Berberine Derivatives: A Lipophilic Substitute Role. Bioorg. Med. Chem. Lett. 2013, 23, 305–309. [Google Scholar] [CrossRef]
- Xiong, Y.X.; Su, H.F.; Lv, P.; Ma, Y.; Wang, S.K.; Miao, H.; Liu, H.Y.; Tan, J.H.; Ou, T.M.; Gu, L.Q.; et al. A Newly Identified Berberine Derivative Induces Cancer Cell Senescence by Stabilizing Endogenous G-Quadruplexes and Sparking a DNA Damage Response at the Telomere Region. Oncotarget 2015, 6, 35625–35635. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous Melanoma. Lancet 2023, 402, 485–502. [Google Scholar] [CrossRef]
- Patel, D.R.; Blair, K.; Patel, B.C. Ocular Melanoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Sayan, M.; Mamidanna, S.; Oncel, D.; Jan, I.; Vergalasova, I.; Weiner, J.; Ohri, N.; Acikalin, B.; Chundury, A. Clinical Management of Uveal Melanoma: A Comprehensive Review with a Treatment Algorithm. Radiat. Oncol. J. 2020, 38, 162. [Google Scholar] [CrossRef] [PubMed]
- Kouvaras, S.; Rokkas, T.; Goga, H.; Gakiopoulou, H.; Arapantoni, P.; Haliotis, G.; Mantas, D. Multifocal Gastrointestinal Melanoma. J. Gastrointest. Liver Dis. 2019, 28, 237–240. [Google Scholar] [CrossRef]
- Kahl, A.R.; Gao, X.; Chioreso, C.; Goffredo, P.; Hassan, I.; Charlton, M.E.; Lin, C. Presentation, Management, and Prognosis of Primary Gastrointestinal Melanoma: A Population-Based Study. J. Surg. Res. 2021, 260, 46–55. [Google Scholar] [CrossRef]
- DePalo, D.K.; Elleson, K.M.; Carr, M.J.; Spiess, P.E.; Zager, J.S. Genitourinary Melanoma: An Overview for the Clinician. Asian J. Urol. 2022, 9, 407. [Google Scholar] [CrossRef]
- Rambhia, P.H.; Scott, J.F.; Vyas, R.; Gerstenblith, M.R. Genitourinary Melanoma. In Noncutaneous Melanoma; Exon Publications: Brisbane, Australia, 2018; pp. 61–81. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, W.; Su, X.; Tan, Q.; Sun, H.; Liu, Z.; Chen, N.; Gong, Q.; Yue, Q. Amelanotic Meningeal Melanoma with Leptomeningeal Dissemination: A Case Report and Systematic Literature Review. World Neurosurg. 2019, 122, 229–239. [Google Scholar] [CrossRef]
- Strashilov, S.; Yordanov, A. Aetiology and Pathogenesis of Cutaneous Melanoma: Current Concepts and Advances. Int. J. Mol. Sci. 2021, 22, 6395. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.K.; Lubner, M.G.; Menias, C.O.; Mellnick, V.M.; Kennedy, T.A.; Bhalla, S.; Pickhardt, P.J. Clinical and Imaging Features of Noncutaneous Melanoma. Am. J. Roentgenol. 2017, 208, 943–959. [Google Scholar] [CrossRef]
- Lerner, B.A.; Stewart, L.A.; Horowitz, D.P.; Carvajal, R.D. Mucosal Melanoma: New Insights and Therapeutic Options for a Unique and Aggressive Disease. Oncology 2017, 31, e23–e32. [Google Scholar]
- Heistein, J.B.; Acharya, U.; Mukkamalla, S.K.R. Malignant Melanoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Fang, J.; Huang, X.; Yang, Y.; Wang, X.; Liang, X.; Liu, J. Berberine-Photodynamic Induced Apoptosis by Activating Endoplasmic Reticulum Stress-Autophagy Pathway Involving CHOP in Human Malignant Melanoma Cells. Biochem. Biophys. Res. Commun. 2021, 552, 183–190. [Google Scholar] [CrossRef]
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- Lyden, D.; Ghajar, C.M.; Correia, A.L.; Aguirre-Ghiso, J.A.; Cai, S.; Rescigno, M.; Zhang, P.; Hu, G.; Fendt, S.M.; Boire, A.; et al. Metastasis. Cancer Cell 2022, 40, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Ye, L.; Sanders, A.J.; Lane, J.; Jiang, W.G. Cancer Invasion and Metastasis: Molecular and Cellular Perspective—Madame Curie Bioscience Database—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK164700/ (accessed on 22 January 2025).
- Liu, C.H.; Tang, W.C.; Sia, P.; Huang, C.C.; Yang, P.M.; Wu, M.H.; Lai, I.L.; Lee, K.H. Berberine Inhibits the Metastatic Ability of Prostate Cancer Cells by Suppressing Epithelial-to-Mesenchymal Transition (EMT)-Associated Genes with Predictive and Prognostic Relevance. Int. J. Med. Sci. 2015, 12, 63–71. [Google Scholar] [CrossRef]
- Liu, X.; Ji, Q.; Ye, N.; Sui, H.; Zhou, L.; Zhu, H.; Fan, Z.; Cai, J.; Li, Q. Berberine Inhibits Invasion and Metastasis of Colorectal Cancer Cells via COX-2/PGE2 Mediated JAK2/STAT3 Signaling Pathway. PLoS ONE 2015, 10, e0123478. [Google Scholar] [CrossRef]
- Liu, J.F.; Lai, K.C.; Peng, S.F.; Maraming, P.; Huang, Y.P.; Huang, A.C.; Chueh, F.S.; Huang, W.W.; Chung, J.G. Berberine Inhibits Human Melanoma A375.S2 Cell Migration and Invasion via Affecting the FAK, UPA, and NF-ΚB Signaling Pathways and Inhibits PLX4032 Resistant A375.S2 Cell Migration In Vitro. Molecules 2018, 23, 2019. [Google Scholar] [CrossRef]
- Boutros, A.; Croce, E.; Ferrari, M.; Gili, R.; Massaro, G.; Marconcini, R.; Arecco, L.; Tanda, E.T.; Spagnolo, F. The Treatment of Advanced Melanoma: Current Approaches and New Challenges. Crit. Rev. Oncol. Hematol. 2024, 196, 104276. [Google Scholar] [CrossRef]
- Khaddour, K.; Kurn, H.; Zito, P.M. Vemurafenib. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Durand, N.; Simsir, M.; Signetti, L.; Labbal, F.; Ballotti, R.; Mus-Veteau, I. Methiothepin Increases Chemotherapy Efficacy against Resistant Melanoma Cells. Molecules 2021, 26, 1867. [Google Scholar] [CrossRef] [PubMed]
- Fontana, R.; Mestre-Farrera, A.; Yang, J. Update on Epithelial-Mesenchymal Plasticity in Cancer Progression. Annu. Rev. Pathol. Mech. Dis. 2024, 19, 133–156. [Google Scholar] [CrossRef] [PubMed]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764. [Google Scholar] [CrossRef]
- Jonckheere, S.; Adams, J.; De Groote, D.; Campbell, K.; Berx, G.; Goossens, S. Epithelial-Mesenchymal Transition (EMT) as a Therapeutic Target. Cells Tissues Organs 2022, 211, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Li, L.; Li, H.; Tan, Y.; Li, B.; Wang, K.; Du, B. Berberine Suppressed Epithelial Mesenchymal Transition through Cross-Talk Regulation of PI3K/AKT and RARα/RARβ in Melanoma Cells. Biochem. Biophys. Res. Commun. 2016, 479, 290–296. [Google Scholar] [CrossRef]
- Colucci, M.; Zumerle, S.; Bressan, S.; Gianfanti, F.; Troiani, M.; Valdata, A.; D’Ambrosio, M.; Pasquini, E.; Varesi, A.; Cogo, F.; et al. Retinoic Acid Receptor Activation Reprograms Senescence Response and Enhances Anti-Tumor Activity of Natural Killer Cells. Cancer Cell 2024, 42, 646. [Google Scholar] [CrossRef]
- Olajossy, B.; Wronski, N.; Madej, E.; Komperda, J.; Szczygieł, M.; Wolnicka-Glubisz, A. RIPK4 Downregulation Reduces ABCG2 Expression, Increasing BRAF-Mutated Melanoma Cell Susceptibility to Cisplatin- and Doxorubicin-Induced Apoptosis. Biomolecules 2024, 14, 1573. [Google Scholar] [CrossRef]
- Weng, C.H.; Wu, C.S.; Wu, J.C.; Kung, M.L.; Wu, M.H.; Tai, M.H. Cisplatin-Induced Giant Cells Formation Is Involved in Chemoresistance of Melanoma Cells. Int. J. Mol. Sci. 2020, 21, 7892. [Google Scholar] [CrossRef]
- Wang, X.; Gong, Q.; Song, C.; Fang, J.; Yang, Y.; Liang, X.; Huang, X.; Liu, J. Berberine-Photodynamic Therapy Sensitizes Melanoma Cells to Cisplatin-Induced Apoptosis through ROS-Mediated P38 MAPK Pathways. Toxicol. Appl. Pharmacol. 2021, 418, 115484. [Google Scholar] [CrossRef]
- Moloudi, K.; Abrahamse, H.; George, B.P. Application of Liposomal Nanoparticles of Berberine in Photodynamic Therapy of A549 Lung Cancer Spheroids. Biochem. Biophys. Rep. 2024, 40, 101877. [Google Scholar] [CrossRef]
- Guan, X.; Zheng, X.; Vong, C.T.; Zhao, J.; Xiao, J.; Wang, Y.; Zhong, Z. Combined Effects of Berberine and Evodiamine on Colorectal Cancer Cells and Cardiomyocytes in Vitro. Eur. J. Pharmacol. 2020, 875, 173031. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Huang, S.; Zhang, Y.; Wang, H.; Li, L.; Ran, J.; Chen, D.; Li, X.; Li, J. Evodiamine Inhibits Growth of Vemurafenib Drug-Resistant Melanoma via Suppressing IRS4/PI3K/AKT Signaling Pathway. J. Nat. Med. 2024, 78, 342–354. [Google Scholar] [CrossRef]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune Checkpoint Inhibitors in Melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Sabbatino, F.; Liguori, L.; Pepe, S.; Ferrone, S. Immune Checkpoint Inhibitors for the Treatment of Melanoma. Expert Opin. Biol. Ther. 2022, 22, 563. [Google Scholar] [CrossRef] [PubMed]
- Ziogas, D.C.; Theocharopoulos, C.; Koutouratsas, T.; Haanen, J.; Gogas, H. Mechanisms of Resistance to Immune Checkpoint Inhibitors in Melanoma: What We Have to Overcome? Cancer Treat. Rev. 2023, 113, 102499. [Google Scholar] [CrossRef]
- Sebastiani, G.D.; Scirocco, C.; Galeazzi, M. Rheumatic Immune Related Adverse Events in Patients Treated with Checkpoint Inhibitors for Immunotherapy of Cancer. Autoimmun. Rev. 2019, 18, 805–813. [Google Scholar] [CrossRef]
- Luo, Z.; Li, Q.; He, S.; Liu, S.; Lei, R.; Kong, Q.; Wang, R.; Liu, X.; Wu, J. Berberine Sensitizes Immune Checkpoint Blockade Therapy in Melanoma by NQO1 Inhibition and ROS Activation. Int. Immunopharmacol. 2024, 142, 113031. [Google Scholar] [CrossRef]
- Khan, A.E.M.A.; Arutla, V.; Srivenugopal, K.S. Human NQO1 as a Selective Target for Anticancer Therapeutics and Tumor Imaging. Cells 2024, 13, 1272. [Google Scholar] [CrossRef]
- Iribarne, M. Editorial: Advances in Morphogenesis and Patterning: Zebrafish as a Model Organism. Front. Cell Dev. Biol. 2024, 12, 1376663. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as Tools for Drug Discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef]
- Ativui, S.; Danquah, C.A.; Ossei, P.P.S.; Ofori, M. Palmatine Attenuates Metastatic Lung Colonization of Triple Negative Breast Cancer Cells. Front. Pharmacol. 2022, 13, 853230. [Google Scholar] [CrossRef] [PubMed]
- Tuzimski, T.; Petruczynik, A.; Kaproń, B.; Plech, T.; Makuch-Kocka, A.; Janiszewska, D.; Sugajski, M.; Buszewski, B.; Szultka-Młyńska, M. In Vitro and In Silico of Cholinesterases Inhibition and In Vitro and In Vivo Anti-Melanoma Activity Investigations of Extracts Obtained from Selected Berberis Species. Molecules 2024, 29, 1048. [Google Scholar] [CrossRef] [PubMed]
- Licarete, E.; Rauca, V.F.; Luput, L.; Drotar, D.; Stejerean, I.; Patras, L.; Dume, B.; Toma, V.A.; Porfire, A.; Gherman, C.; et al. Overcoming Intrinsic Doxorubicin Resistance in Melanoma by Anti-Angiogenic and Anti-Metastatic Effects of Liposomal Prednisolone Phosphate on Tumor Microenvironment. Int. J. Mol. Sci. 2020, 21, 2968. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G.; Ahmed, K.A. The Protective Impact of Berberine against Doxorubicin-Induced Nephrotoxicity in Rats. Tissue Cell 2021, 73, 101612. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Gupta, P.; Bhatnagar, P.; Yadav, H.N.; Dinda, A.K. Solid Lipid Nanoformulation of Berberine Attenuates Doxorubicin Triggeredin Vitro Inflammation in H9c2 Rat Cardiomyocytes. Comb. Chem. High Throughput Screen. 2022, 25, 1695–1706. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G.; Ahmed, K.A. Neuroprotective Potential of Berberine Against Doxorubicin-Induced Toxicity in Rat’s Brain. Neurochem. Res. 2021, 46, 3247–3263. [Google Scholar] [CrossRef]
- Mittal, A.; Tabasum, S.; Singh, R.P. Berberine in Combination with Doxorubicin Suppresses Growth of Murine Melanoma B16F10 Cells in Culture and Xenograft. Phytomedicine 2014, 21, 340–347. [Google Scholar] [CrossRef]
- Pandesh, S.; Haghjooy Javanmard, S.; Shakeri-Zadeh, A.; Shokrani, P. Targeted Photothermal Therapy of Melanoma in C57BL/6 Mice Using Fe3O4@Au Core-Shell Nanoparticles and Near-Infrared Laser. J. Biomed. Phys. Eng. 2021, 11, 29. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, F.; Yan, J.; Luo, M.; Liu, K.; Liu, P.; Yan, G.; Li, C.; Yang, Y.; Zeng, Q.; et al. Photothermal Therapy Improves the Efficacy of Topical Immunotherapy against Melanoma. Photodiagnosis Photodyn. Ther. 2024, 49, 104290. [Google Scholar] [CrossRef]
- Li, Y.; Si, Y.; Yin, H. Nanomaterial-Mediated Photothermal Therapy Modulates Tumor-Associated Macrophages: Applications in Cancer Therapy. J. Mater. Chem. B 2024, 12, 11867–11886. [Google Scholar] [CrossRef]
- Shinde, V.R.; Thanekar, A.M.; Khatun, S.; Buddhiraju, H.S.; Bhattacharjee, B.; Rengan, A.K. Melanin-Ce6-Loaded Polydopamine Nanoparticles-Based Enhanced Phototherapy for B16 Melanoma Cancer Cells. Nanotechnology 2024, 35, 295101. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Shang, J.; Yang, L.; Deng, L.; Wu, S.; Chen, J.; Yang, J.; Wang, K.; Li, C.; Chen, J.; et al. Enhanced Anti-Tumor Efficacy of Berberine-Loaded Mesoporous Polydopamine Nanoparticles for Synergistic Chemotherapy and Photothermal Therapy. Int. J. Pharm. 2025, 670, 125151. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Armstrong, A.; Baum, C.; Bordeaux, J.S.; Brown, M.; Busam, K.J.; Eisen, D.B.; Iyengar, V.; Lober, C.; Margolis, D.J.; et al. Guidelines of Care for the Management of Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2018, 78, 560. [Google Scholar] [CrossRef]
- Zheng, S.; Yu, H.; Zhang, J.; Lau, W.C.; Chen, M.; Cheng, H.; Xian, H.; Ming, W.; Cheng, L.N.; He, Y.; et al. Prediction of Relative Survival Trends in Patients with Cutaneous Squamous Cell Carcinoma Using a Model-Based Period Analysis: A Retrospective Analysis of the Surveillance, Epidemiology, and End Results Database. BMJ Open 2024, 14, e086488. [Google Scholar] [CrossRef]
- Fania, L.; Didona, D.; Di Pietro, F.R.; Verkhovskaia, S.; Morese, R.; Paolino, G.; Donati, M.; Ricci, F.; Coco, V.; Ricci, F.; et al. Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Paolino, G.; Donati, M.; Didona, D.; Mercuri, S.; Cantisani, C. Histology of Non-Melanoma Skin Cancers: An Update. Biomedicines 2017, 5, 71. [Google Scholar] [CrossRef]
- Brantsch, K.D.; Meisner, C.; Schönfisch, B.; Trilling, B.; Wehner-Caroli, J.; Röcken, M.; Breuninger, H. Analysis of Risk Factors Determining Prognosis of Cutaneous Squamous-Cell Carcinoma: A Prospective Study. Lancet Oncol. 2008, 9, 713–720. [Google Scholar] [CrossRef]
- Stanganelli, I.; Spagnolo, F.; Argenziano, G.; Ascierto, P.A.; Bassetto, F.; Bossi, P.; Donato, V.; Massi, D.; Massone, C.; Patuzzo, R.; et al. The Multidisciplinary Management of Cutaneous Squamous Cell Carcinoma: A Comprehensive Review and Clinical Recommendations by a Panel of Experts. Cancers 2022, 14, 377. [Google Scholar] [CrossRef]
- Li, D.X.; Zhang, J.; Zhang, Y.; Zhao, P.W.; Yang, L.M. Inhibitory Effect of Berberine on Human Skin Squamous Cell Carcinoma A431 Cells. Genet. Mol. Res. 2015, 14, 10553–10568. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The Role of BCL-2 Family Proteins in Regulating Apoptosis and Cancer Therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Suraweera, C.D.; Hinds, M.G.; Kvansakul, M. Structural Investigation of Orf Virus Bcl-2 Homolog ORFV125 Interactions with BH3-Motifs from BH3-Only Proteins Puma and Hrk. Viruses 2021, 13, 1374. [Google Scholar] [CrossRef] [PubMed]
- Koolivand, Z.; Bahreini, F.; Rayzan, E.; Rezaei, N. Inducing Apoptosis in Acute Myeloid Leukemia; Mechanisms and Limitations. Heliyon 2025, 11, e41355. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Alam, S.; Shamsi, A.; Adnan, M.; Elasbali, A.M.; Al-Soud, W.A.; Alreshidi, M.; Hawsawi, Y.M.; Tippana, A.; Pasupuleti, V.R.; et al. Bax/Bcl-2 Cascade Is Regulated by the EGFR Pathway: Therapeutic Targeting of Non-Small Cell Lung Cancer. Front. Oncol. 2022, 12, 869672. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Asano, S. Pathophysiological Roles of Actin-Binding Scaffold Protein, Ezrin. Int. J. Mol. Sci. 2022, 23, 3246. [Google Scholar] [CrossRef]
- Hałas-Wiśniewska, M.; Arendt, W.; Grzanka, A.; Izdebska, M. Downregulation of Ezrin Suppresses Migration Potential in Cervical Cancer Cells. Pharmaceuticals 2024, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Buenaventura, R.G.M.; Merlino, G.; Yu, Y. Ez-Metastasizing: The Crucial Roles of Ezrin in Metastasis. Cells 2023, 12, 1620. [Google Scholar] [CrossRef]
- Lu, D.; Sun, L.; Li, Z.; Mu, Z. LncRNA EZR-AS1 Knockdown Represses Proliferation, Migration and Invasion of CSCC via the PI3K/AKT Signaling Pathway. Mol. Med. Rep. 2020, 23, 76. [Google Scholar] [CrossRef]
- Wang, S.-H.; Zhou, J.-D.; He, Q.-Y.; Yin, Z.-Q.; Cao, K.; Luo, C.-Q. MiR-199a Inhibits the Ability of Proliferation and Migration by Regulating CD44-Ezrin Signaling in Cutaneous Squamous Cell Carcinoma Cells. Int. J. Clin. Exp. Pathol. 2014, 7, 7131–7141. [Google Scholar]
- Kulshrestha, S.; Goel, A.; Banerjee, S.; Sharma, R.; Khan, M.R.; Chen, K.-T. Metabolomics and Network Pharmacology–Guided Analysis of TNF-α Expression by Argemone mexicana (Linn) Targeting NF-KB the Signalling Pathway in Cancer Cell Lines. Front. Oncol. 2024, 14, 1502819. [Google Scholar] [CrossRef]
- Xiao, W.; Li, Z.; Li, S.; Xia, Z.; Zhang, J.; Liu, H.; Chen, W. Effect of Gedunin on Cell Proliferation and Apoptosis in Skin Melanoma Cells A431 via the PI3K/JNK Signaling Pathway. Adv. Clin. Exp. Med. 2024, 34, 803–812. [Google Scholar] [CrossRef]
- Watt, S.A.; Purdie, K.J.; den Breems, N.Y.; Dimon, M.; Arron, S.T.; McHugh, A.T.; Xue, D.J.; Dayal, J.H.S.; Proby, C.M.; Harwood, C.A.; et al. Novel CARD11 Mutations in Human Cutaneous Squamous Cell Carcinoma Lead to Aberrant NF-ΚB Regulation. Am. J. Pathol. 2015, 185, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Yuan, X.; Tang, S.; Wang, T.; Liu, H.; Cao, Y. TOPK Affects Autophagy of Skin Squamous Cell Carcinoma by Regulating NF-KB Pathway through HDAC1. Dis. Markers 2022, 2022, 3771711. [Google Scholar] [CrossRef]
- Nawrocki, S.T.; Wang, W.; Carew, J.S. Autophagy: New Insights into Its Roles in Cancer Progression and Drug Resistance. Cancers 2020, 12, 3005. [Google Scholar] [CrossRef] [PubMed]
- Majidzadeh, H.; Araj-Khodaei, M.; Ghaffari, M.; Torbati, M.; Ezzati Nazhad Dolatabadi, J.; Hamblin, M.R. Nano-Based Delivery Systems for Berberine: A Modern Anti-Cancer Herbal Medicine. Colloids Surf. B Biointerfaces 2020, 194, 111188. [Google Scholar] [CrossRef]
- Solanki, R.; Parmar, B.; Jadav, M.; Pooja, D.; Kulhari, H.; Patel, S. Berberine Encapsulated Phenylboronic Acid-Conjugated Pullulan Nanoparticles: Synthesis, Characterization and Anticancer Activity Validated in A431 Skin Cancer Cells and 3D Spheroids. Int. J. Biol. Macromol. 2024, 273, 132737. [Google Scholar] [CrossRef]
- Cuan, X.; Yang, X.; Zhu, W.; Zhao, Y.; Luo, R.; Huang, Y.; Wang, X.; Sheng, J. Antitumor Effects of Erlotinib in Combination with Berberine in A431 Cells. BMC Pharmacol. Toxicol. 2023, 24, 29. [Google Scholar] [CrossRef]
- Kim, M.; Ryu, K.; Kim, H.; Lee, H.; Lim, J.; Kim, H.; Chang, M.J. Population Pharmacokinetics of Erlotinib in Patients with Non-Small Cell Lung Cancer (NSCLC): A Model-Based Meta-Analysis. Comput. Biol. Med. 2025, 186, 109682. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, D.; Zhang, C.; Huang, S.; Li, X.; Chen, Y.; Ma, Y.; Ju, S.; Ye, H.; Fan, W. EGFR-Targeting Oxygen-Saturated Nanophotosensitizers for Orchestrating Multifaceted Antitumor Responses by Counteracting Immunosuppressive Milieu. J. Control. Release 2024, 375, 127–141. [Google Scholar] [CrossRef]
- Hosseini, T.M.; Park, S.J.; Guo, T. The Mutational and Microenvironmental Landscape of Cutaneous Squamous Cell Carcinoma: A Review. Cancers 2024, 16, 2904. [Google Scholar] [CrossRef]
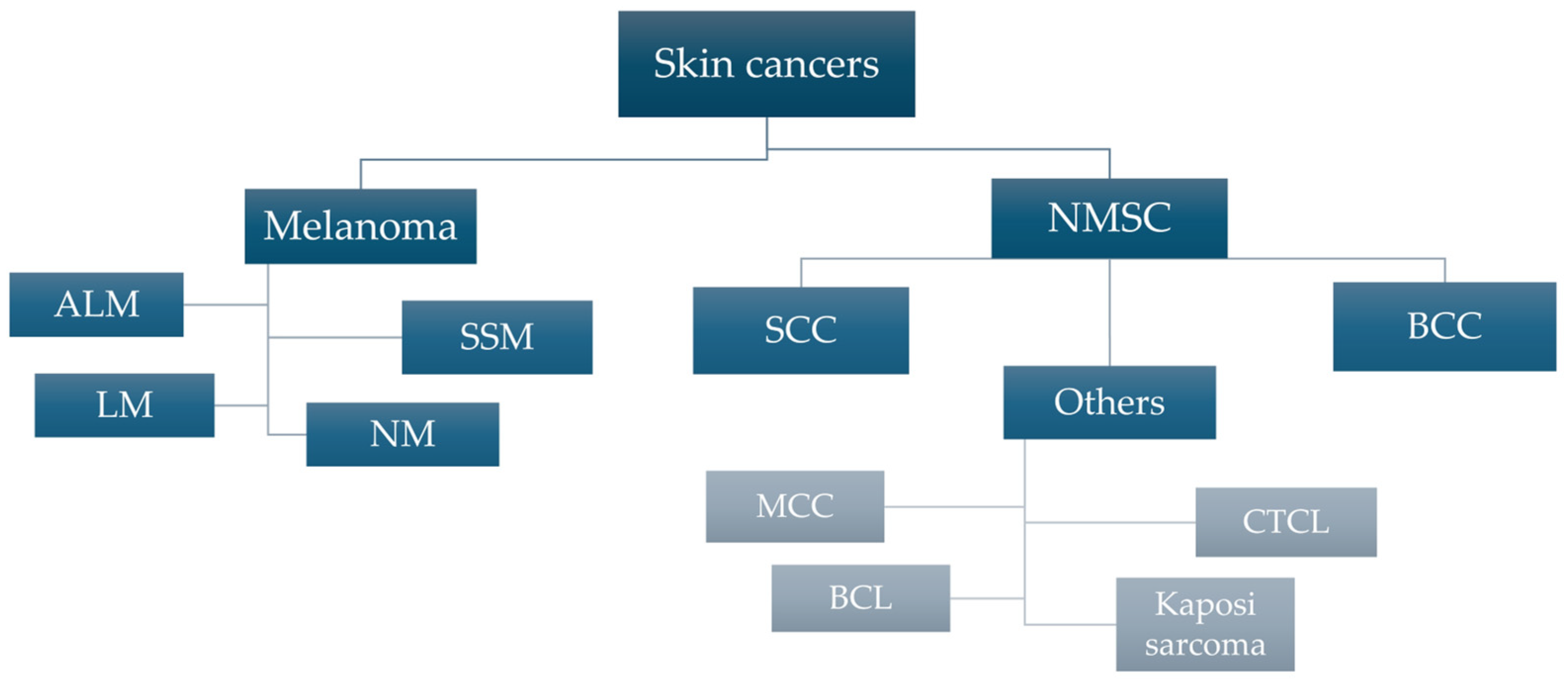
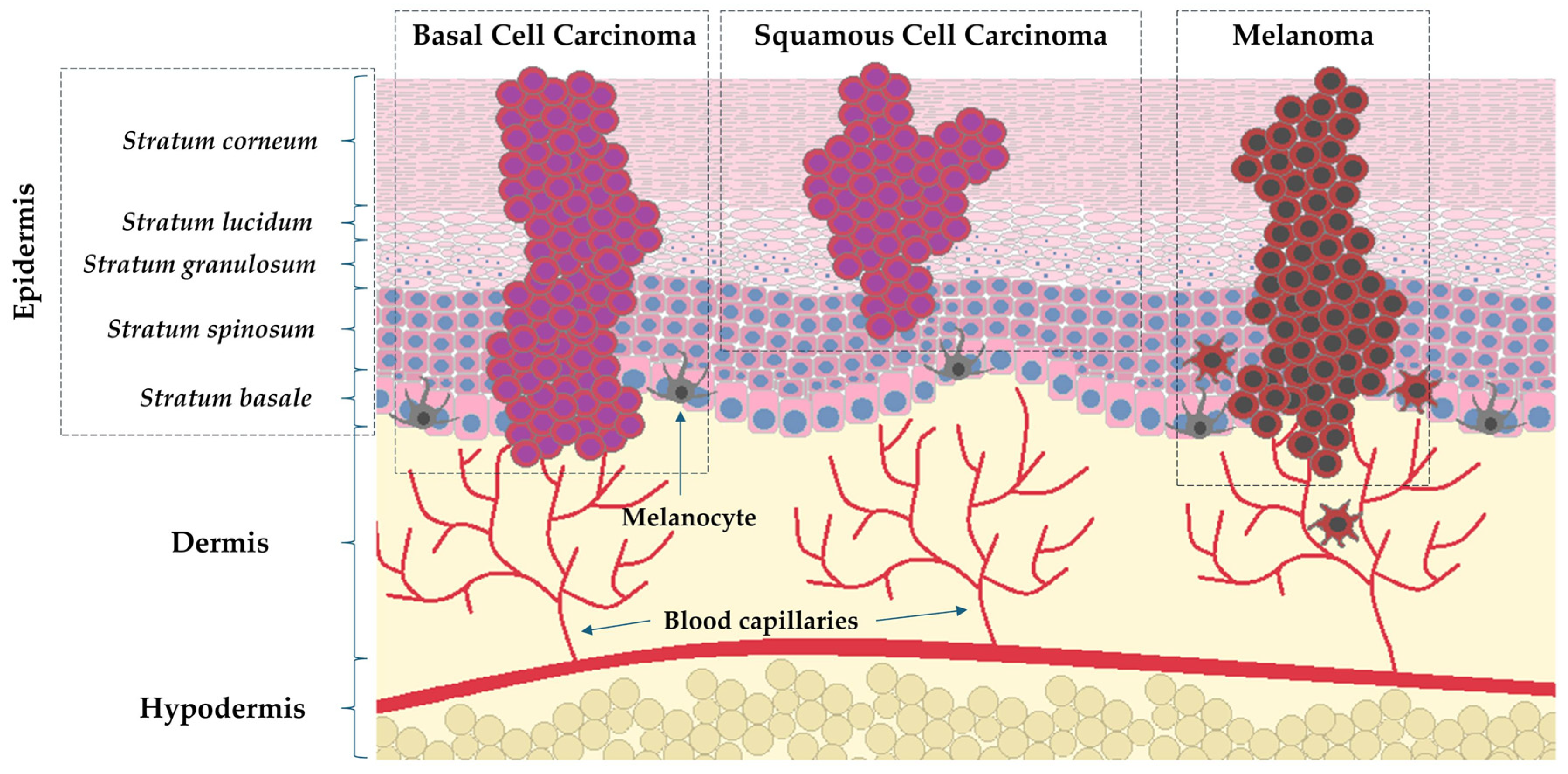
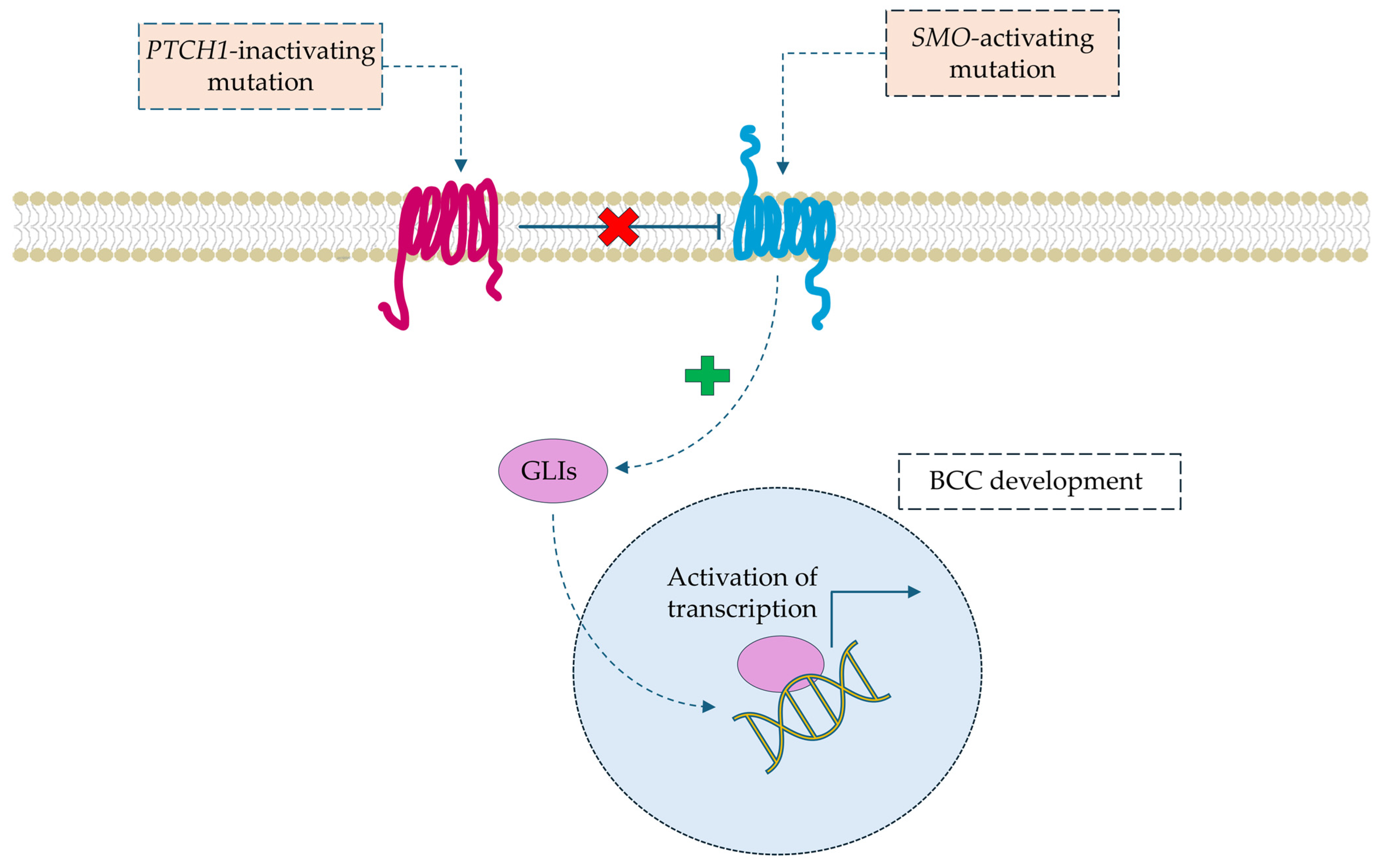

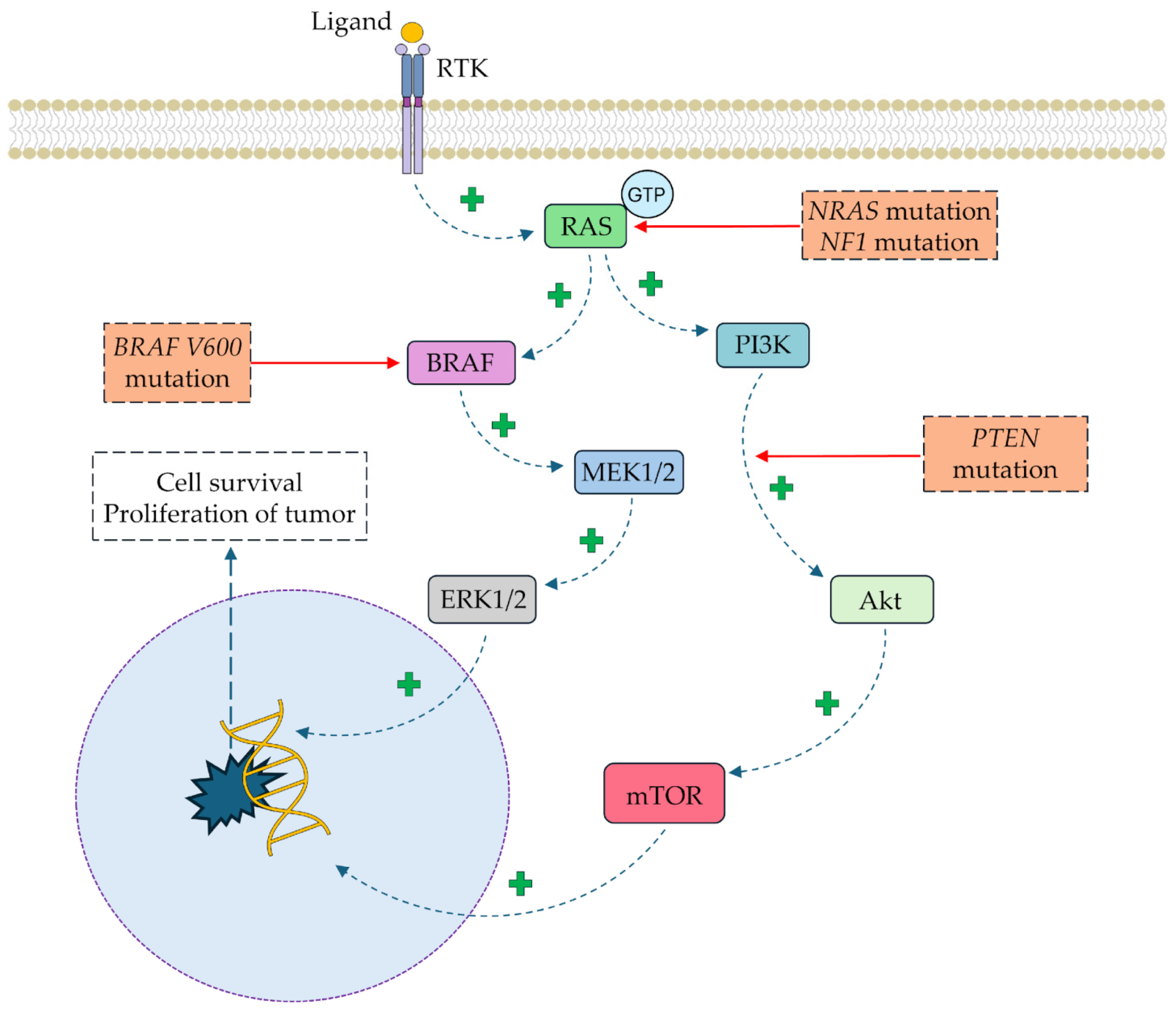
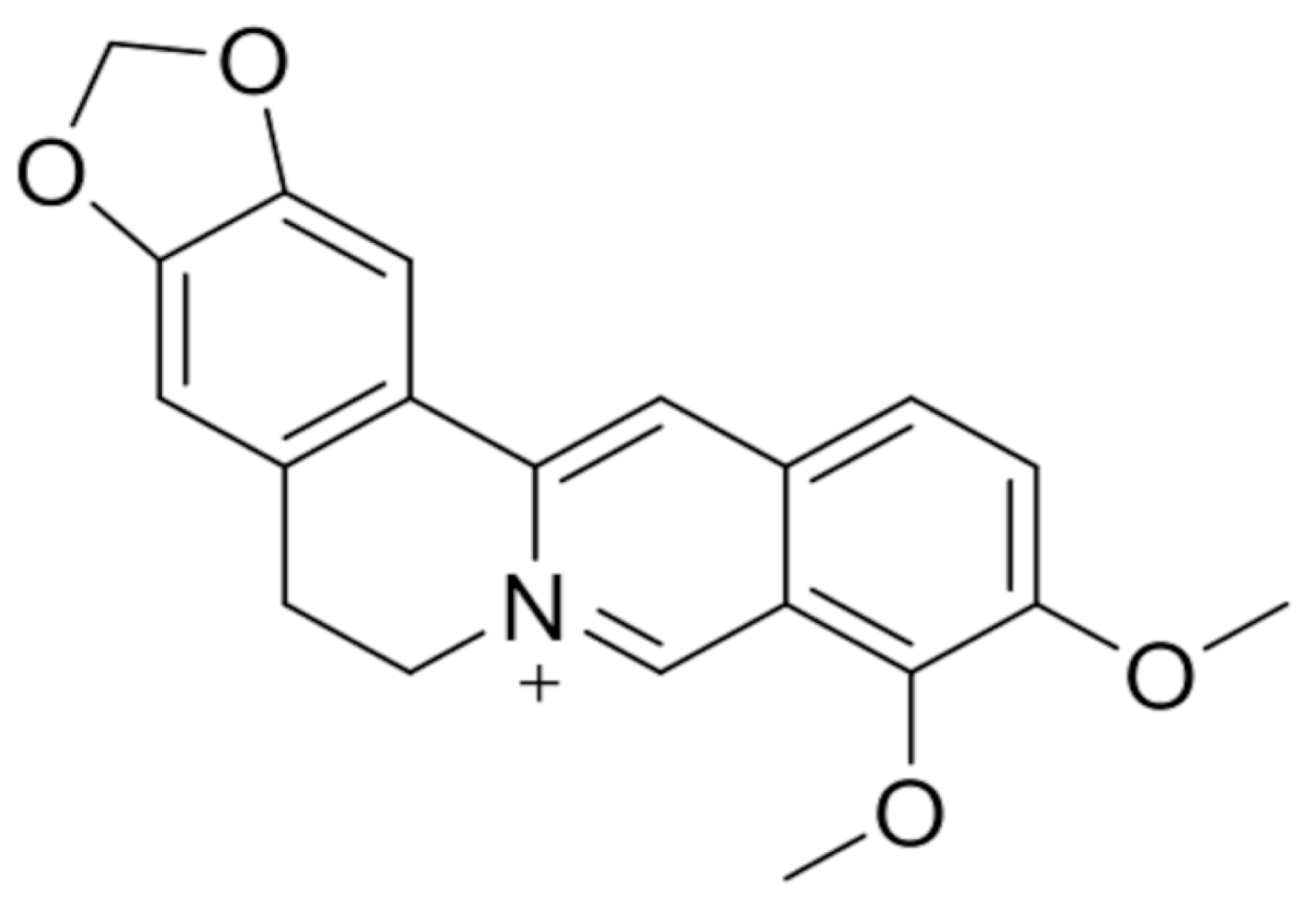


| BCC | SCC | Melanoma | |
|---|---|---|---|
| Prevalence | Most common skin cancer | 2nd most common skin cancer | 3rdmost common skin cancer |
| 80–90% of skin cancers 3.6 mln/annually (USA) | 15% of skin cancers 1.8 mln/annually (USA) | 5% ≥ of skin cancers 200,340 cases in 2024 (USA) | |
| Localization | Sun-exposed areas, e.g., face, ears, scalp, neck, shoulders, back | Various locations, often not exposed to sunlight | |
| Riskfactors | UV radiation | The main risk factors are identical to BCC | UV radiation |
| History of BCC/SCC | Burns in childhood | ||
| Age (median age of 68 years) | HPV 16/18/31/33/35 infections | Immunosuppression | |
| Male gender | Smoking | ||
| Fitzpatrick skin types I and II | Precancerous conditions, e.g., keratosis actinica and xeroderma pigmentosum Carcinoma in situ: Bowenoid papulosis and Erythroplasia of Queryrat | History of melanoma | |
| Immunosuppression | |||
| History of transplantation | Fitzpatrick skin types I and II | ||
| Genetic conditions, e.g., Gorlin–Goltz syndrome | Genetic conditions: atypical nevi, nevi congenitales, and atypical nevus syndrome | ||
| Growth characteristics | Slow growth, causing minimal damage to the surrounding tissue | Rapid growth, may cause local tissue destruction (especially SCC exulcerans) | Depends on the type: slow growth of LM, SMM and ALM, and rapid growth of NM |
| Metastases | Rarely metastasizes, mainly to regional lymph nodes, bones, lungs and skin | More frequent than BCC, mainly to lymph nodes | High risk of metastases, main locations: subcutaneous tissue, regional lymph nodes, lungs, CNS, and liver |
| References | [6,7,8,9,10] | [6,8,11,12,13] | [6,14,15,16,17,18] |
| Mechanism of the Activity | BBR’s Effect | Molecules Involved |
|---|---|---|
| Cyclin/Cdk levels | Suppression | Cyclin D1 |
| Growth ractor receptors | Suppression | EGFR, Her2/neu, PDGFR, VEGFR2 |
| Gene activators | Suppression | AP1, AP2, NF-κB |
| Tumor suppressors | Activation | p21, p27, p53 |
| Cell signaling pathways | Suppression | ERK/MAPK, PI3K/AKT, wnt/B-catenin |
| Apoptosis induction | Activation | Caspases, Bid, Bax, AIF, FasR, TNF-alpha |
| Apoptosis inhibition | Suppression | Bcl2, XIAP, IAP |
| microRNAs | Suppression/Activation | miR-21, miR-21-p |
| BBR Derivatives with the Highest Anticancer Activity Shown | Type of Cancer | Reference |
|---|---|---|
| 9-O-decylberberrubine bromide and 9-O-dodecylberberrubine bromide | Lung (NSCLC) | [69] |
| 13-(4,4-diphenylbutyl)-9,10-dimethoxy-5,6-dihydrobenzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium chloride | ||
| 13-(5,5-diphenylpentyl)-9,10-dimethoxy-5,6-dihydrobenzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium chloride | Colon (HCT116 and SW613-B3) | [70] |
| 9-O-dodecyl-, 13-dodecyl- and 13-O-dodecyl-berberine | Liver (HepG2) | [71] |
| 13-[3-(phenyl)propyl]berberine iodide 13-[2-(4-cholorophenyl)ethyl]berberine | Breast (SK-BR-3) | [72] |
| 9-O-farnesylberberine | Liver (HepG2) | [73] |
| 9-O-6-ammonia chloride hexylberberine | Cervix (Siha), Lung (A549), Promyelocytic leukemia (HL-60) | [74] |
| Melanoma | ||
|---|---|---|
| Actions of Berberine | Molecular Level | References |
| Reduced migration and invasion abilities | Downregulation of uPA Silencing of the FAK and NF-κB pathways | [94] |
| Reduced epithelial–mesenchymal transition | Downregulation of RARα/β Silencing of the PI3K/AKT pathway | [101] |
| Enhanced apoptosis rate of melanoma cells | Activation of the ROS-assisted p38/caspase pathway | [105] |
| Activation of autophagy and apoptosis | ROS-assisted endoplasmic reticulum stress Upregulation of the CHOP protein | [88] |
| Restoration of tumor immunogenicity | Downregulation of NQO1 expression | [113] |
| Squamous cell carcinoma | ||
| Enhanced apoptosis rate of SCC cells | Upregulation of BAX Downregulation of BCL-2 | [136] |
| Reduced migration and invasion abilities | Downregulation of the Ezrin protein | [140] |
| Suppression of tumor-promoting inflammation | Downregulation of TNF-α Silencing of the NF-κB pathway | [146] |
| Melanoma | |||
|---|---|---|---|
| Drug Combinations | Type of Study | Cell Lines Tested | References |
| BBR photodynamic therapy + cisplatin | in vitro | A375 SK-Mel-19 | [105] |
| BBR + evodiamine ethosome formulation | in vitro | B16 | [57] |
| BBR + doxorubicin | in vitro/in vivo | B16F10 | [123] |
| Squamous cell carcinoma | |||
| BBR + erlotinib | in vitro/in vivo | A431 | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duda-Madej, A.; Lipska, P.; Viscardi, S.; Bazan, H.; Sobieraj, J. Targeting Skin Neoplasms: A Review of Berberine’s Anticancer Properties. Cells 2025, 14, 1041. https://doi.org/10.3390/cells14141041
Duda-Madej A, Lipska P, Viscardi S, Bazan H, Sobieraj J. Targeting Skin Neoplasms: A Review of Berberine’s Anticancer Properties. Cells. 2025; 14(14):1041. https://doi.org/10.3390/cells14141041
Chicago/Turabian StyleDuda-Madej, Anna, Patrycja Lipska, Szymon Viscardi, Hanna Bazan, and Jakub Sobieraj. 2025. "Targeting Skin Neoplasms: A Review of Berberine’s Anticancer Properties" Cells 14, no. 14: 1041. https://doi.org/10.3390/cells14141041
APA StyleDuda-Madej, A., Lipska, P., Viscardi, S., Bazan, H., & Sobieraj, J. (2025). Targeting Skin Neoplasms: A Review of Berberine’s Anticancer Properties. Cells, 14(14), 1041. https://doi.org/10.3390/cells14141041







