Tension Force Stress Downregulates the Expression of Osteogenic Markers and Mineralization in Embryonic Stem-Cell-Derived Embryoid Bodies
Abstract
1. Introduction
2. Materials and Methods
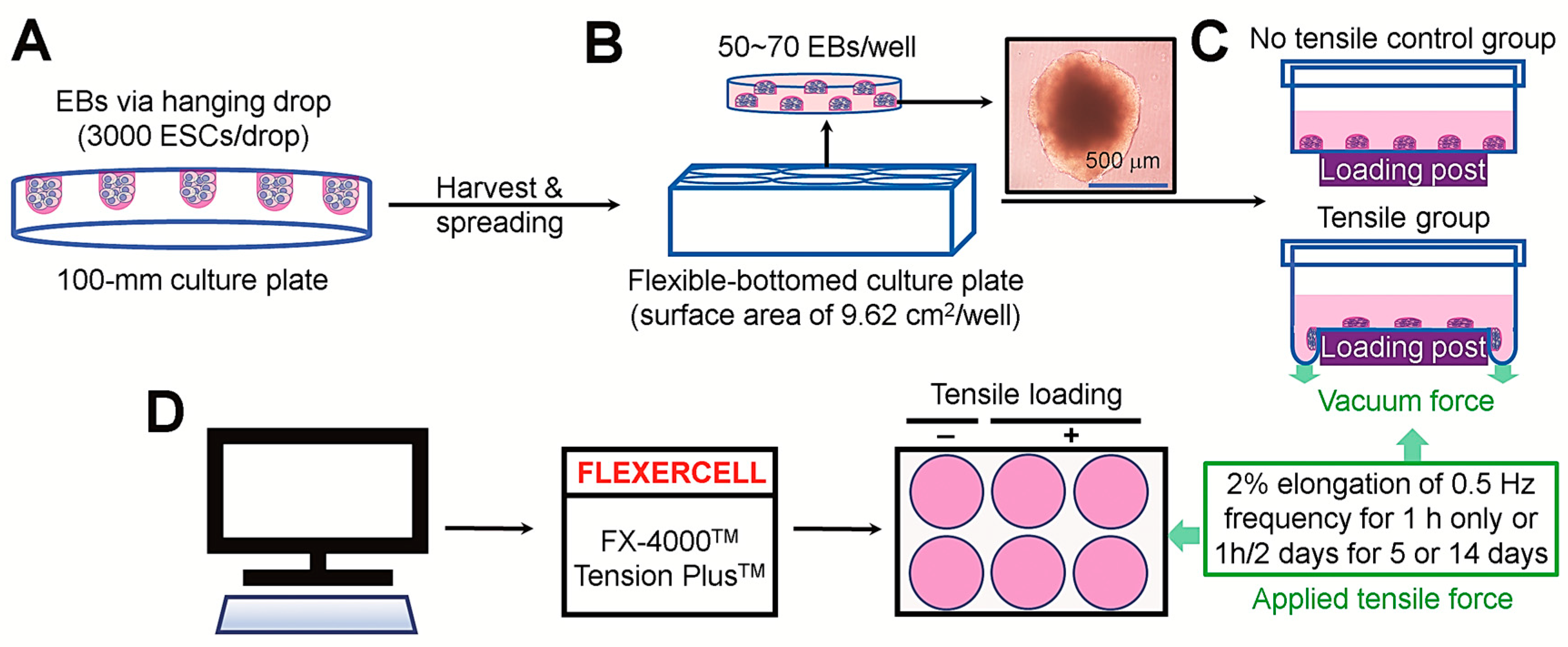
2.1. Cells, Chemicals, and Laboratory Wares
2.2. Cell Culture and EB Production
2.3. Immunofluorescence Assay
2.4. Application of Tensile Stress
2.5. siRNA Transfection and Pharmacological Inhibition of MAPKs
2.6. Mineralization Assay
2.7. Polymerase Chain Reaction (PCR) Assay
2.8. Preparation of Protein Lysates
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
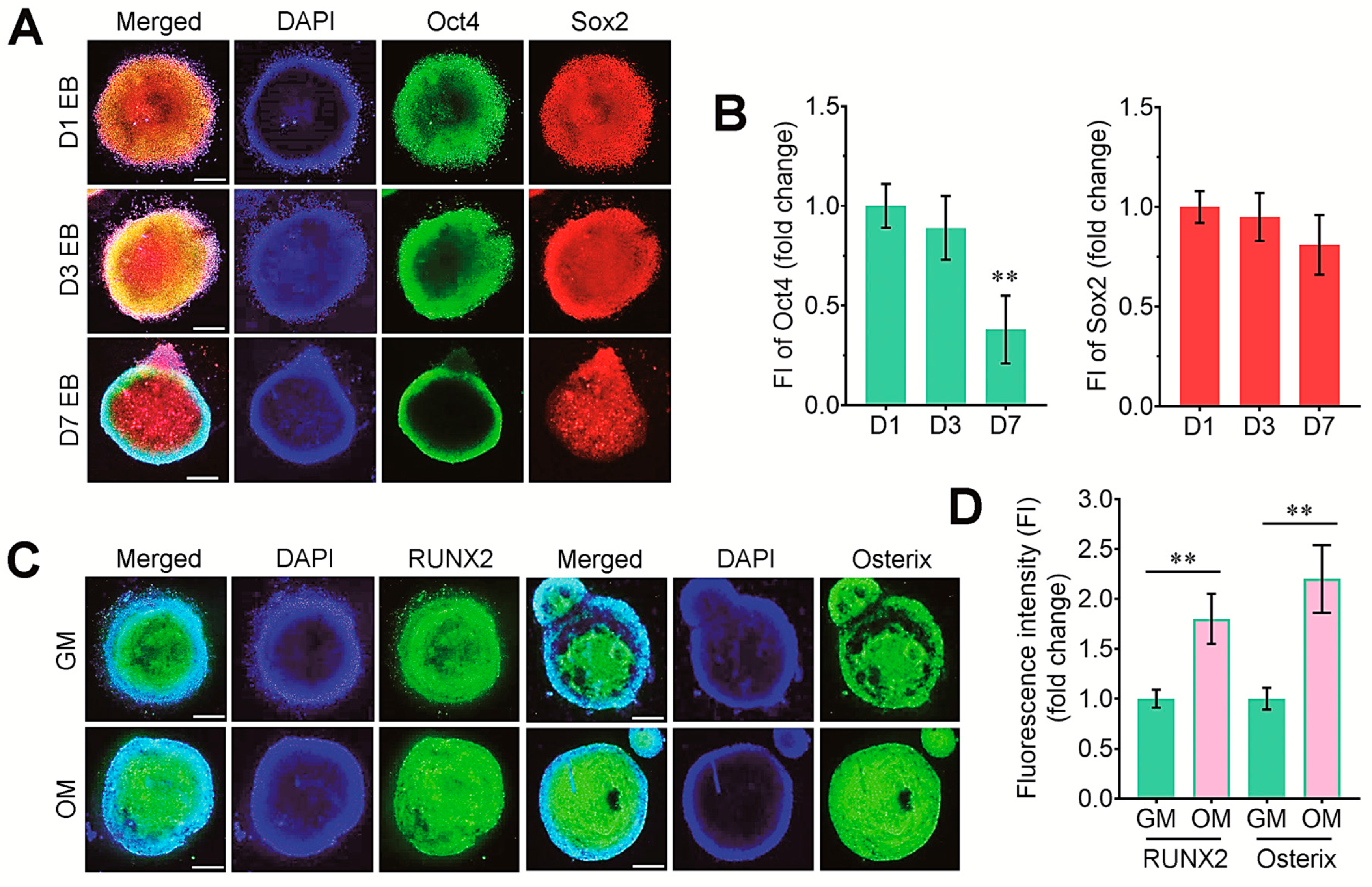
3.1. Experimental Design and Characterization of Undifferentiated and Osteogenic Marker Expression in EBs in the Presence and Absence of DAG
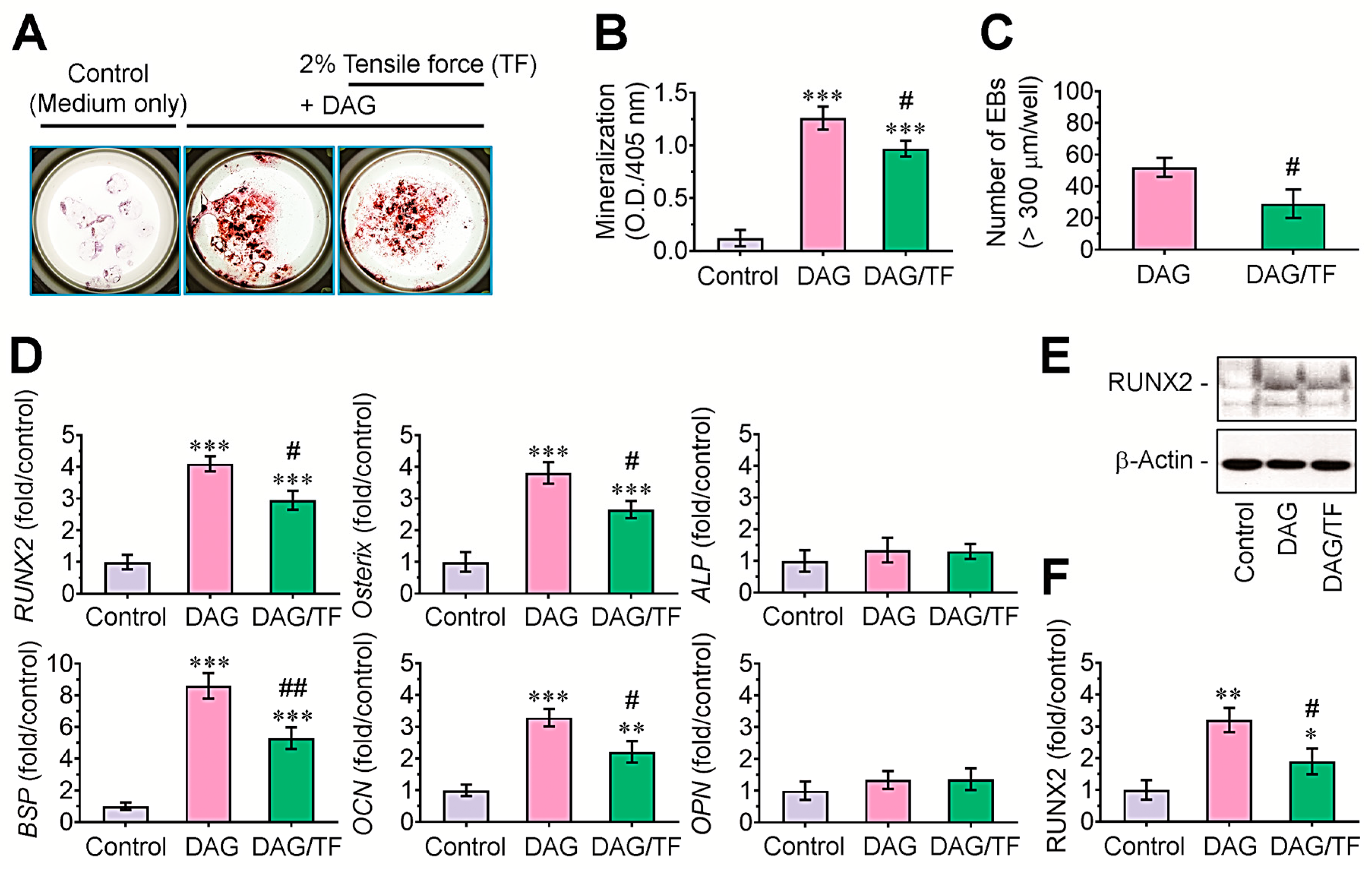
3.2. Cyclic Tensile Stress Diminishes DAG-Induced Mineralization of EBs, and This Is Orchestrated by the Downregulation of Osteogenic Regulatory Molecules
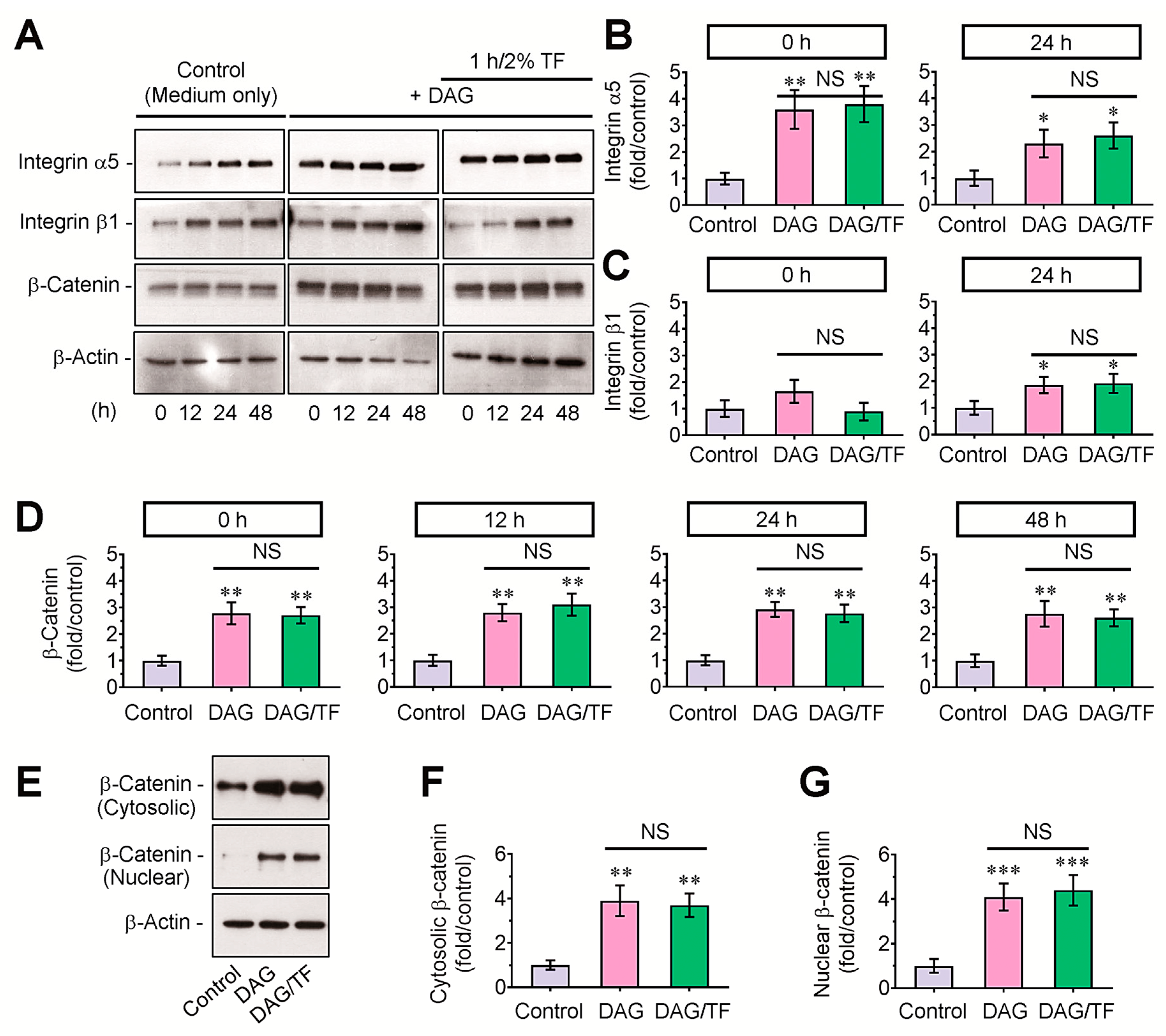
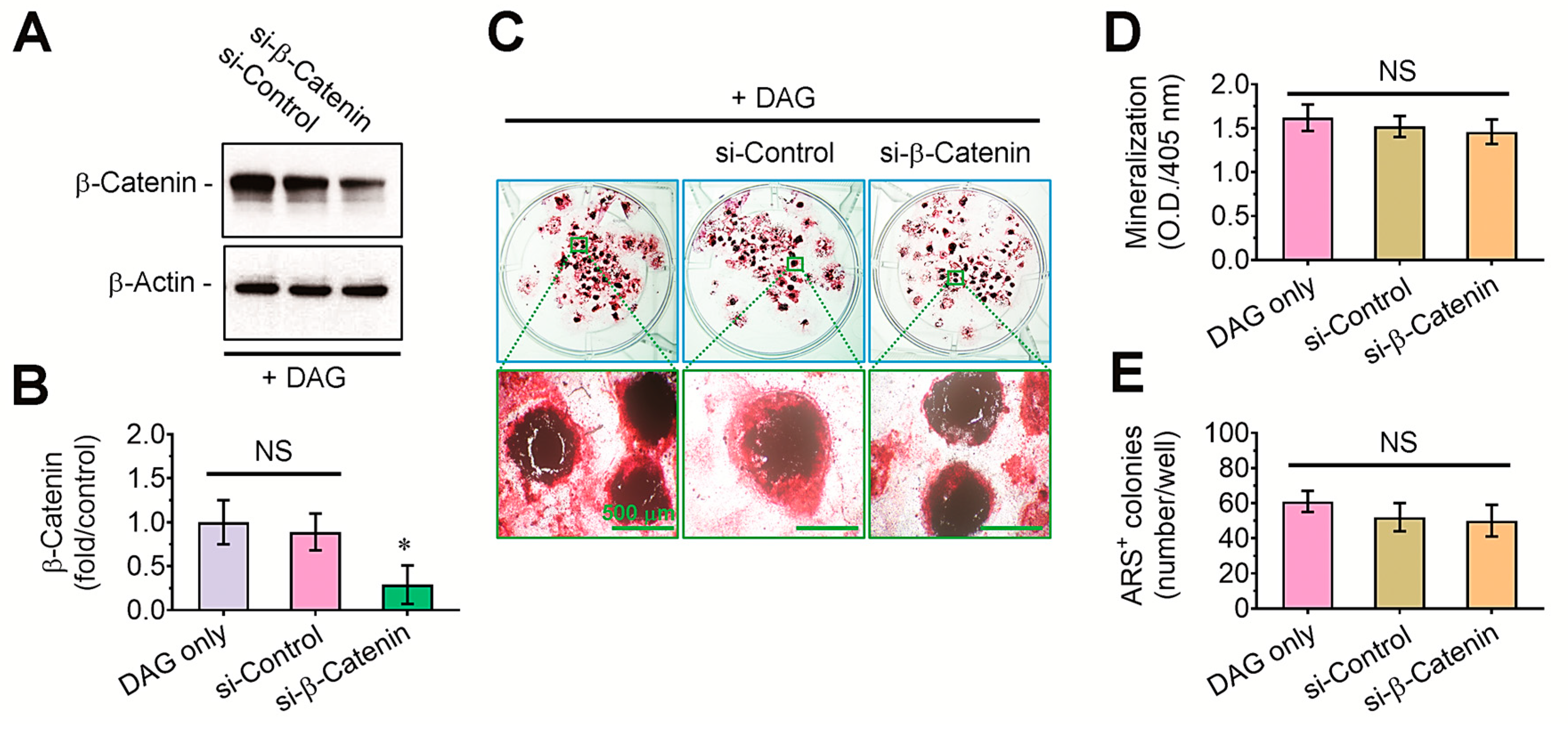
3.3. DAG-Stimulated Mineralization of EBs and Its Inhibition by Tensile Stress Are Not Directly Affected by β-Catenin-Mediated Signaling
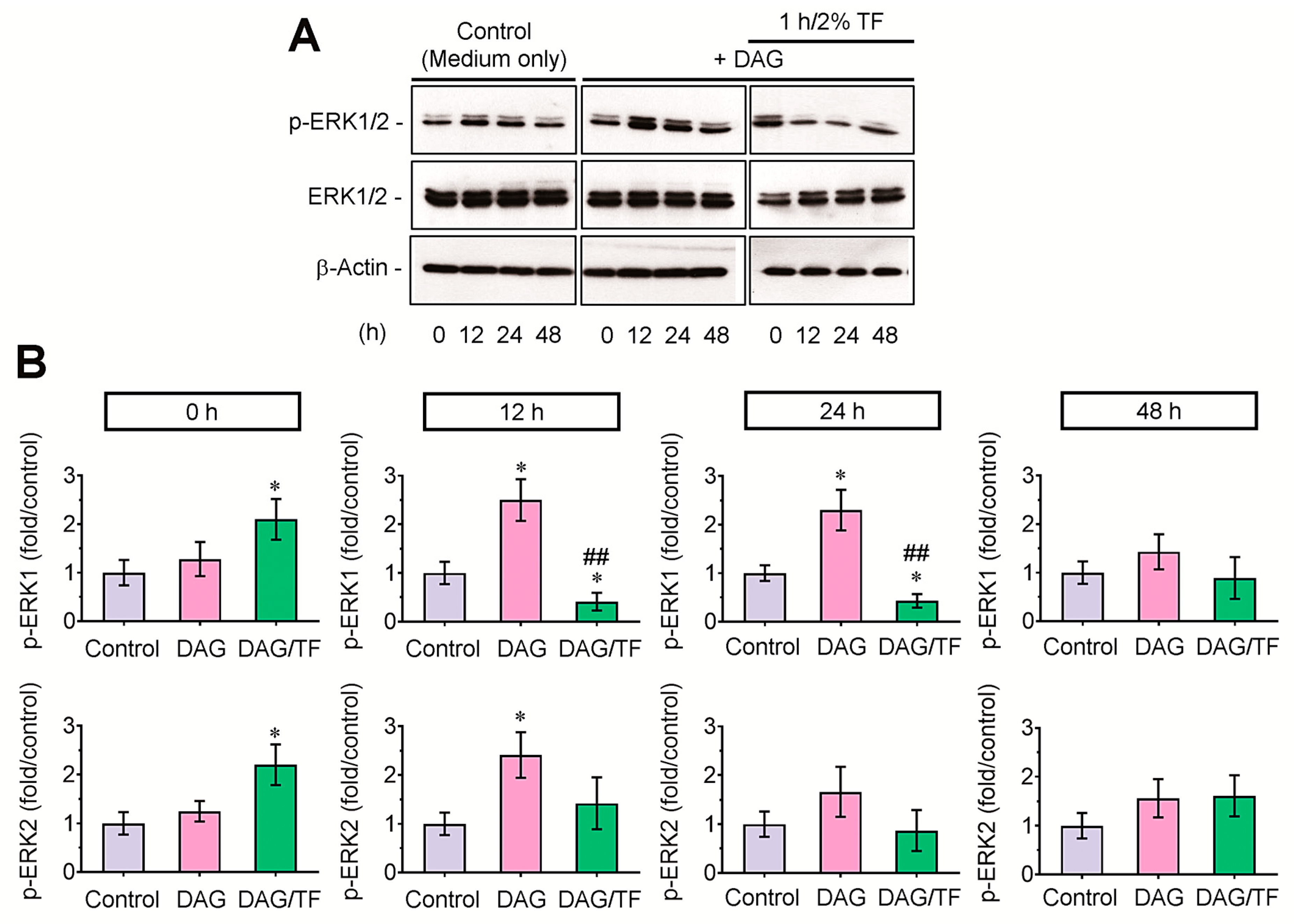
3.4. Tensile Stress Diminishes Phosphorylation of ERK1 in DAG-Stimulated EBs
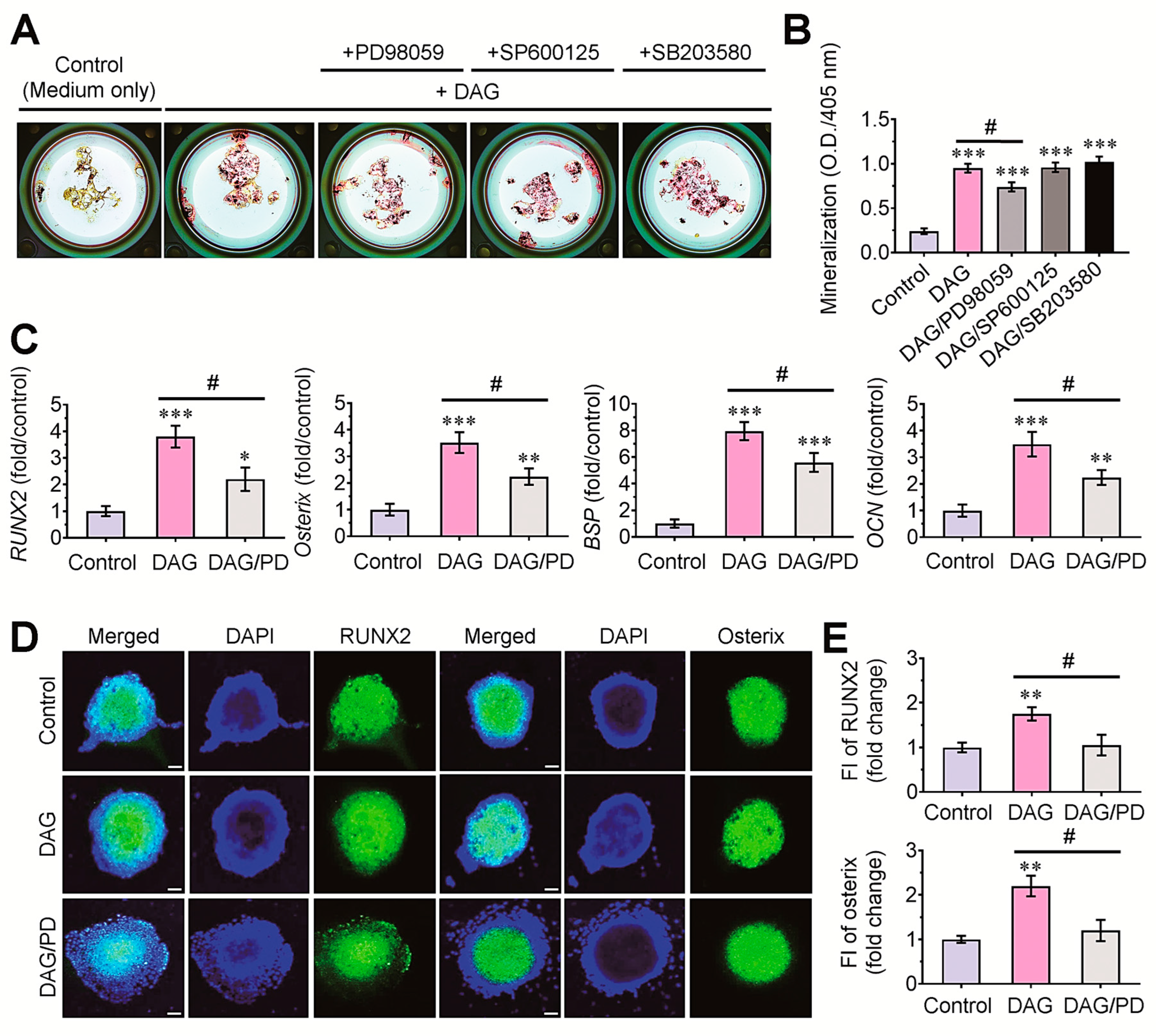
3.5. Pharmacological ERK Inhibitor Attenuates DAG-Mediated Mineralization of EBs via the Downregulation of Osteogenic Marker Genes
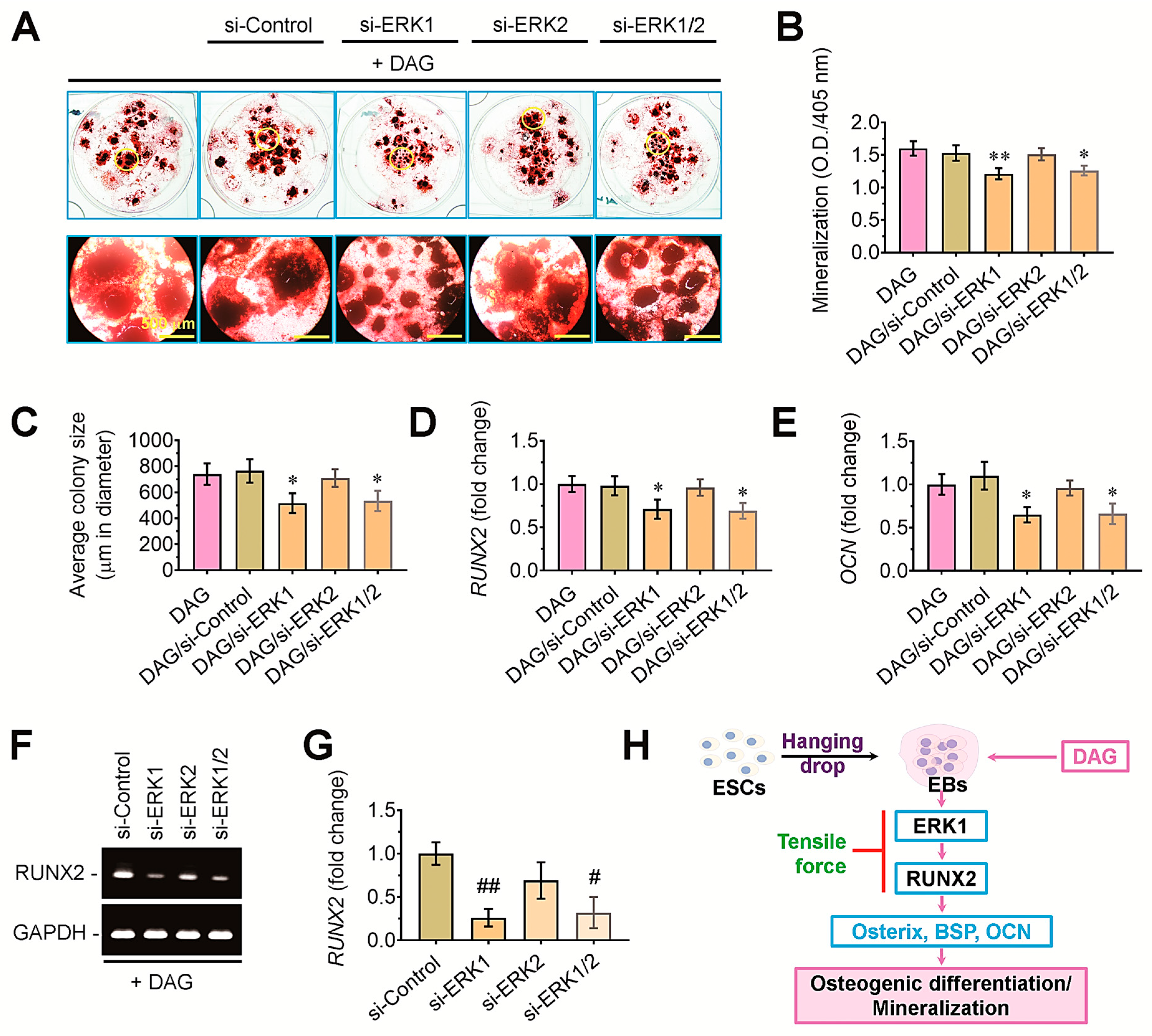
3.6. ERK1-Mediated Signaling Acts as an Upstream Effector of RUNX2 in DAG-Stimulated EBs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pettinato, G.; Wen, X.; Zhang, N. Formation of Well-defined Embryoid Bodies from Dissociated Human Induced Pluripotent Stem Cells using Microfabricated Cell-repellent Microwell Arrays. Sci. Rep. 2014, 4, 7402. [Google Scholar] [CrossRef]
- Dang, S.M.; Gerecht-Nir, S.; Chen, J.; Itskovitz-Eldor, J.; Zandstra, P.W. Controlled, Scalable Embryonic Stem Cell Differentiation Culture. Stem Cells 2004, 22, 275–282. [Google Scholar] [CrossRef]
- Goh, S.K.; Olsen, P.; Banerjee, I. Extracellular Matrix Aggregates from Differentiating Embryoid Bodies as a Scaffold to Support ESC Proliferation and Differentiation. PLoS ONE 2013, 8, e61856. [Google Scholar] [CrossRef]
- Rutledge, K.; Cheng, Q.; Pryzhkova, M.; Harris, G.M.; Jabbarzadeh, E. Enhanced Differentiation of Human Embryonic Stem Cells on Extracellular Matrix-Containing Osteomimetic Scaffolds for Bone Tissue Engineering. Tissue Eng. Part C 2014, 20, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Hackett, T.-L.; Vriesde, N.R.T.F.; AL-Fouadi, M.; Mostaco-Guidolin, L.; Maftoun, D.; Hsieh, A.; Coxson, N.; Usman, K.; Sin, D.D.; Booth, S.; et al. The Role of the Dynamic Lung Extracellular Matrix Environment on Fibroblast Morphology and Inflammation. Cells 2022, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bahng, J.H.; Kotov, N.A. Three-dimensional biomimetic scaffolds for hepatic differentiation of size-controlled embryoid bodies. J. Mater. Res. 2019, 34, 1371–1380. [Google Scholar] [CrossRef]
- Kook, S.H.; Son, Y.O.; Hwang, J.M.; Kim, E.M.; Lee, C.B.; Jeon, Y.M.; Kim, J.G.; Lee, J.C. Mechanical force inhibits osteoclastogenic potential of human periodontal ligament fibroblasts through OPG production and ERK-mediated signaling. J. Cell. Biochem. 2009, 106, 1010–1019. [Google Scholar] [CrossRef]
- Maul, T.M.; Chew, D.W.; Nieponice, A.; Vorp, D.A. Mechanical stimuli differentially control stem cell behavior: Morphology, proliferation, and differentiation. Biomech. Model Mechanobiol. 2011, 10, 939–953. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Chi, J.; Wang, M.; Chen, J.; Hu, L.; Chen, Z.; Backman, L.J.; Zhang, W. Topographic Orientation of Scaffolds for Tissue Regeneration: Recent Advances in Biomaterial Design and Applications. Biomimetics 2022, 7, 131. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, J.Y.; Kook, S.H.; Lee, J.C. A novel electrospinning method for self-assembled tree-like fibrous scaffolds: Microenvironment-associated regulation of MSC behavior and bone regeneration. J. Mater. Sci. Technol. 2022, 115, 52–70. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Wang, T. How the mechanical microenvironment of stem cell growth affects their differentiation: A review. Stem Cell Res. Ther. 2022, 13, 415. [Google Scholar] [CrossRef] [PubMed]
- Kook, S.H.; Jang, Y.S.; Lee, J.C. Human periodontal ligament fibroblasts stimulate osteoclastogenesis in response to compression force through TNF-α-mediated activation of CD4+ T cells. J. Cell. Biochem. 2011, 112, 2891–2901. [Google Scholar] [CrossRef]
- Kook, S.H.; Jang, Y.S.; Lee, J.C. Involvement of JNK-AP-1 and ERK-NF-κB signaling in tension-stimulated expression of type I collagen and MMP-1 in human periodontal ligament fibroblasts. J. Appl. Physiol. 2011, 111, 1575–1583. [Google Scholar] [CrossRef]
- Cha, B.; Geng, K.; Mahamud, R.M.; Fu, J.; Mukherjee, A.; Kim, Y.; Jho, E.H.; Kim, T.H.; Kahn, M.L.; Xia, L.; et al. Mechanotransduction activates canonical Wnt/β-catenin signaling to promote lymphatic vascular patterning and the development of lymphatic and lymphovenous valves. Genes Dev. 2016, 30, 1454–1469. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.O.; Jang, Y.S.; Heo, J.S.; Chung, W.T.; Choi, K.C.; Lee, J.C. Apoptosis-inducing factor plays a critical role in caspase independent, pyknotic cell death in hydrogen peroxide-exposed cells. Apoptosis 2009, 14, 796–808. [Google Scholar] [CrossRef]
- Yu, H.S.; Kim, J.J.; Kim, H.W.; Lewis, M.P.; Wall, I. Impact of mechanical stretch on the cell behaviors of bone and surrounding tissues. J. Tissue Eng. 2016, 7, 2041731415618342. [Google Scholar] [CrossRef]
- Jang, H.K.; Kim, B.S. Modulation of Stem Cell Differentiation with Biomaterials. Int. J. Stem Cells 2010, 3, 80–84. [Google Scholar] [CrossRef]
- Steward, A.J.; Wagner, D.R.; Kelly, D.J. Exploring the roles of integrin binding and cytoskeletal reorganization during mesenchymal stem cell mechanotransduction in soft and stiff hydrogels subjected to dynamic compression. J. Mech. Behav. Biomed. Mater. 2014, 38, 174–182. [Google Scholar] [CrossRef]
- Zeevaert, K.; Mabrouk, M.H.E.; Wagner, W.; Goetzke, R. Cell Mechanics in Embryoid Bodies. Cells 2020, 9, 2270. [Google Scholar] [CrossRef]
- Heo, J.S.; Lee, J.C. β-Catenin mediates cyclic strain-stimulated cardiomyogenesis in mouse embryonic stem cells through ROS-dependent and integrin-mediated PI3K/Akt pathways. J. Cell. Biochem. 2011, 112, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Kook, S.H.; Lee, J.C. Tensile Force Inhibits the Proliferation of Human Periodontal Ligament Fibroblasts Through Ras-p38 MAPK Up-Regulation. J. Cell. Physiol. 2012, 227, 1098–1106. [Google Scholar] [CrossRef]
- Brembeck, F.H.; Rosário, M.; Birchmeier, W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr. Opin. Genet. Dev. 2006, 16, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Case, N.; Ma, M.; Sen, B.; Xie, Z.; Gross, T.S.; Rubin, J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J. Biol. Chem. 2008, 283, 29196–29205. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Ikegame, M.; Tabuchi, Y.; Furusawa, Y.; Kawai, M.; Hattori, A.; Kondo, T.; Yamamoto, T. Tensile stress stimulates the expression of osteogenic cytokines/growth factors and matricellular proteins in the mouse cranial suture at the site of osteoblast differentiation. Biomed. Res. 2016, 37, 117–126. [Google Scholar] [CrossRef]
- Liedert, A.; Kaspar, D.; Blakytny, R.; Claes, L.; Ignatius, A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem. Biophys. Res. Commun. 2006, 349, 1–5. [Google Scholar] [CrossRef]
- Komori, T. Regulation of osteoblast differentiation by Runx2. Adv. Exp. Med. Biol. 2010, 658, 43–49. [Google Scholar]
- Hojo, H.; Saito, T.; He, X.; Guo, Q.; Onodera, S.; Azuma, T.; Koebis, M.; Nakao, K.; Aiba, A.; Seki, M.; et al. Runx2 regulates chromatin accessibility to direct the osteoblast program at neonatal stages. Cell Rep. 2022, 40, 111315. [Google Scholar] [CrossRef]
- Truong, L.H.; Kuliwaba, J.S.; Tsangari, H.; Fazzalari, N. Differential gene expression of bone anabolic factors and trabecular bone architectural changes in the proximal femoral shaft of primary hip osteoarthritis patients. Arthritis Res. Ther. 2006, 8, R188. [Google Scholar] [CrossRef] [PubMed]
- Štefková, K.; Procházková, J.; Pacherník, J. Alkaline Phosphatase in Stem Cells. Stem Cells Int. 2015, 2015, 628368. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Cao, B.; Heazlewood, C.K.; Domingues, M.; Sun, X.; Debele, E.; McGregor, N.E.; Sims, N.A.; Heazlewood, S.Y.; Nilsson, S.K. Osteopontin is An Important Regulative Component of the Fetal Bone Marrow Hematopoietic Stem Cell Niche. Cells 2019, 8, 985. [Google Scholar] [CrossRef]
- Rabenstein, M.; Hucklenbroich, J.; Willuweit, A.; Ladwig, A.; Fink, G.R.; Schroeter, M.; Langen, K.J.; Rueger, M.A. Osteopontin mediates survival, proliferation and migration of neural stem cells through the chemokine receptor CXCR4. Stem Cell Res. Ther. 2015, 6, 99. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, J.-H.; Kim, C.-C.; Lee, J.; Kim, J.; Lee, J.-C.; Kook, S.-H. Tension Force Stress Downregulates the Expression of Osteogenic Markers and Mineralization in Embryonic Stem-Cell-Derived Embryoid Bodies. Cells 2025, 14, 991. https://doi.org/10.3390/cells14130991
An J-H, Kim C-C, Lee J, Kim J, Lee J-C, Kook S-H. Tension Force Stress Downregulates the Expression of Osteogenic Markers and Mineralization in Embryonic Stem-Cell-Derived Embryoid Bodies. Cells. 2025; 14(13):991. https://doi.org/10.3390/cells14130991
Chicago/Turabian StyleAn, Ju-Hyeon, Chun-Choo Kim, Junil Lee, Junhyeok Kim, Jeong-Chae Lee, and Sung-Ho Kook. 2025. "Tension Force Stress Downregulates the Expression of Osteogenic Markers and Mineralization in Embryonic Stem-Cell-Derived Embryoid Bodies" Cells 14, no. 13: 991. https://doi.org/10.3390/cells14130991
APA StyleAn, J.-H., Kim, C.-C., Lee, J., Kim, J., Lee, J.-C., & Kook, S.-H. (2025). Tension Force Stress Downregulates the Expression of Osteogenic Markers and Mineralization in Embryonic Stem-Cell-Derived Embryoid Bodies. Cells, 14(13), 991. https://doi.org/10.3390/cells14130991





