GIGYF2: A Multifunctional Regulator at the Crossroads of Gene Expression, mRNA Surveillance, and Human Disease
Abstract
1. Introduction
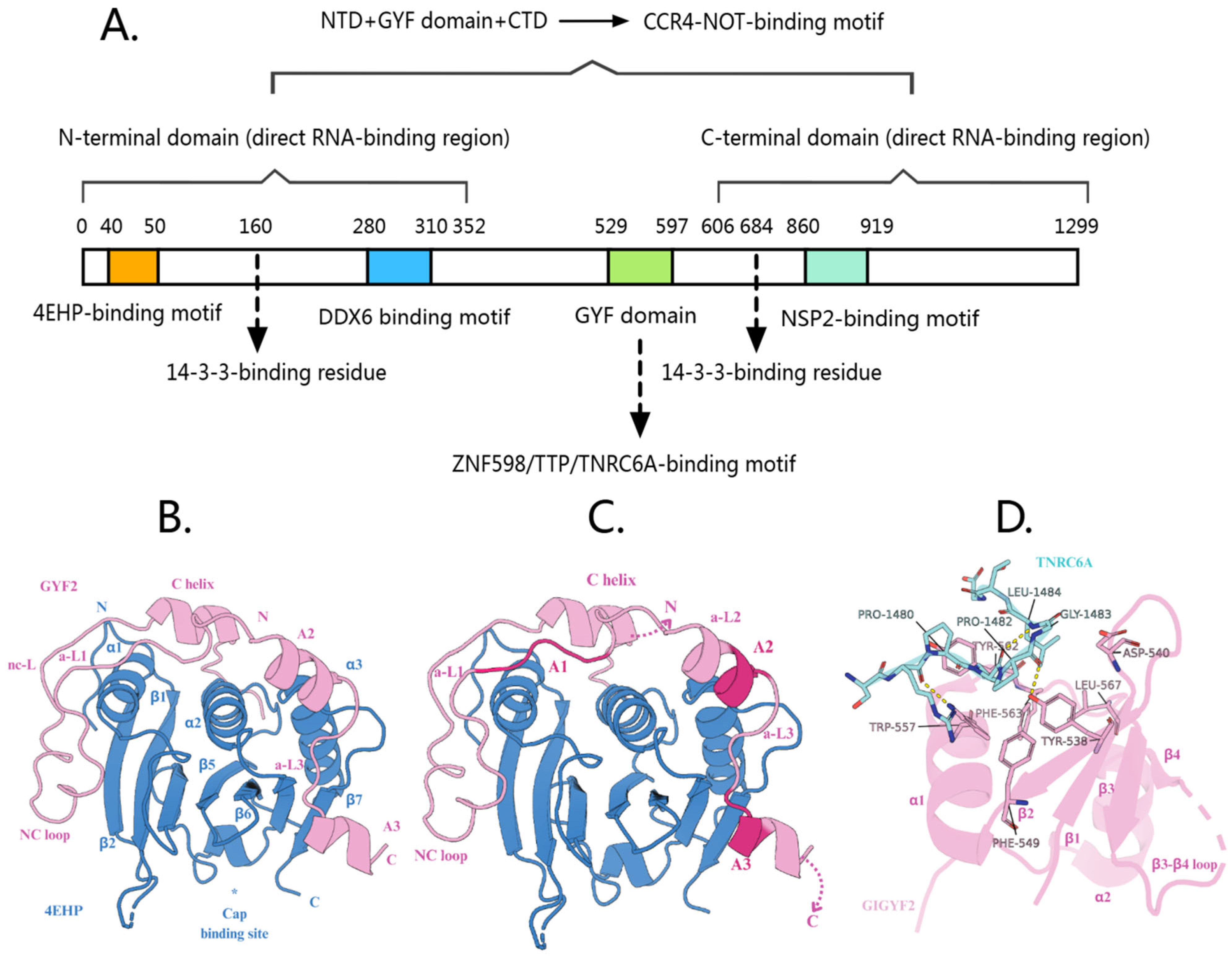
2. The Structure of GIGYF2
2.1. N-Terminal Domain
2.1.1. 4EHP-Binding Motif
2.1.2. DDX6-Binding Motif
| Interaction Proteins | GIGYF2 Components | GIGYF2 Residues | Interaction Partners | References |
|---|---|---|---|---|
| 4EHP (Human) | Canonical Motif | Y41 | P55 | [28] |
| M48/L49/Y43 | 4EHP dorsal surface | |||
| F52 | W95/P78 | |||
| Noncanonical Loop | F67 | Y64/K83/I85 | ||
| I70 | Y64 | |||
| I70/Q72 | H100/V102 | |||
| Linker | I58/D55 | H100 | ||
| P59 | F97 | |||
| A1 | L79/A80 | E149 | ||
| L79/V82 | R146 | |||
| P77 | R103 | |||
| A3 | S98 | R138 | ||
| S98-OH | E177 | |||
| V101/L102 | R138/I211 | |||
| Me31B/DDX6 (Dm) | MBM Motif | P347/E348 /W349 | V283/L310/L311 /F370 | [23] |
| F361/F367 | A275/H284/C285 /L289/I421 | |||
| D362 | H368 | |||
| G365/F367 | F276 | |||
| F361 | K423 | |||
| TNRC6A (Human) | GYF Domain | Y538/F549 /W557/Y562 | P1481/P1482 | [37] |
2.2. GYF Domain: Interactions with ZNF598/TTP/TNRC6A
2.3. C-Terminal Domain Interactions: Cooperative Roles of Multiple Structural Elements
3. Transcriptional Regulation by GIGYF2
4. GIGYF2’s Role in mRNA Surveillance
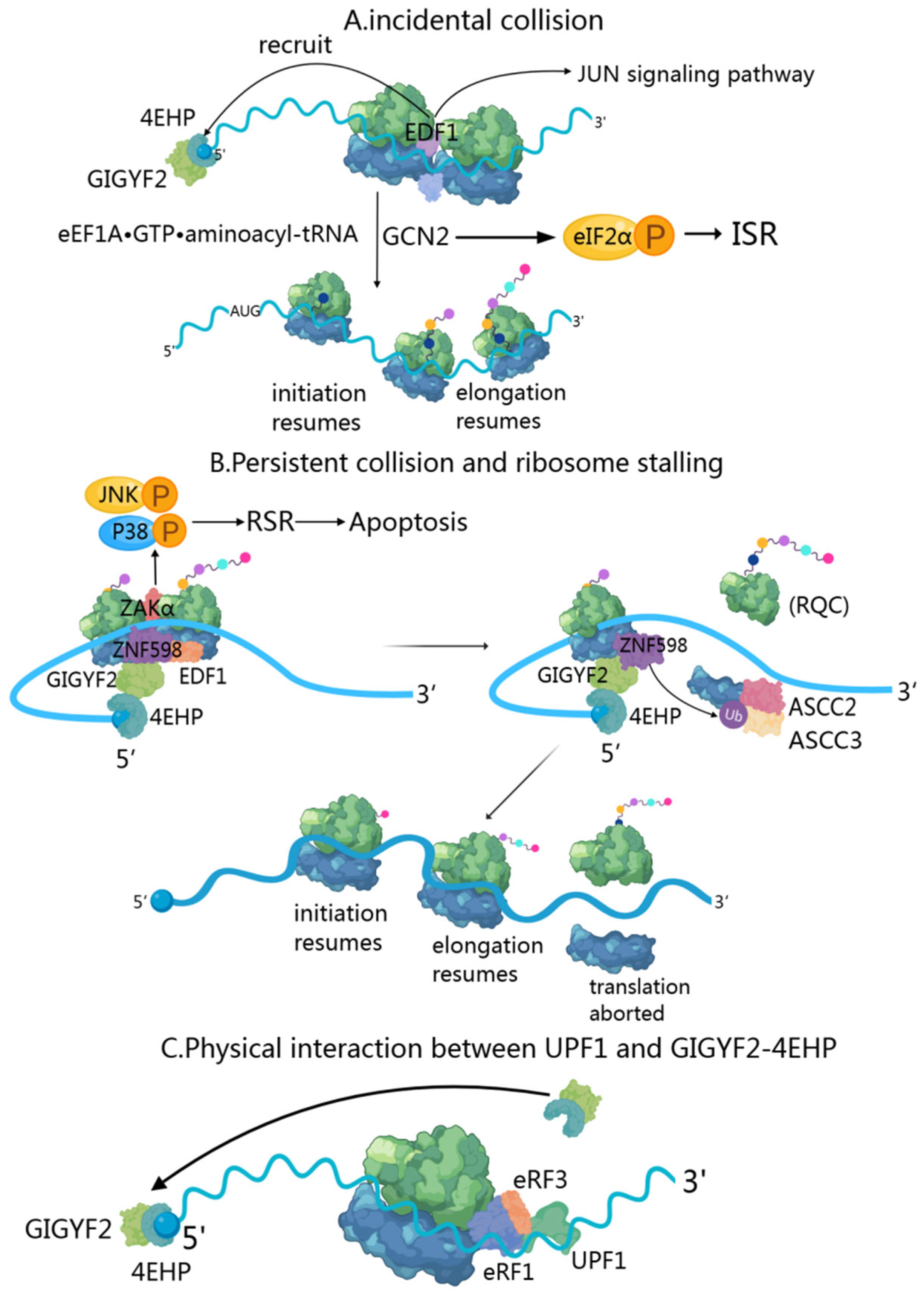
4.1. Transient Collision-Induced ISR Pathway: GIGYF2’s Early Response to Ribosomal Stalling
4.2. Persistent Collision-Activated RQC and RSR Pathways: GIGYF2’s Role in Sustained Translational Stress
4.3. Integration of NMD, NGD, and NSD Mechanisms: GIGYF2’s Comprehensive Approach to mRNA Surveillance
5. GIGYF2-Mediated mRNA Degradation Mechanisms
5.1. ZNF598-GIGYF2-4EHP Complex in Ribosome Collision-Mediated Decay
5.2. GIGYF2 as an RNA-Binding Protein in Direct mRNA Targeting
5.3. GIGYF2’s Recruitment to Target Transcripts via RNA-Binding Proteins (RBPs)
5.4. GIGYF2’s Role in miRNA-Mediated Silencing via TNRC6A Interaction
5.5. Convergence on mRNA Degradation Pathways and P-Body Association
5.6. Stress-Induced Regulation of GIGYF2 Activity
6. GIGYF2-Associated Pathological Conditions
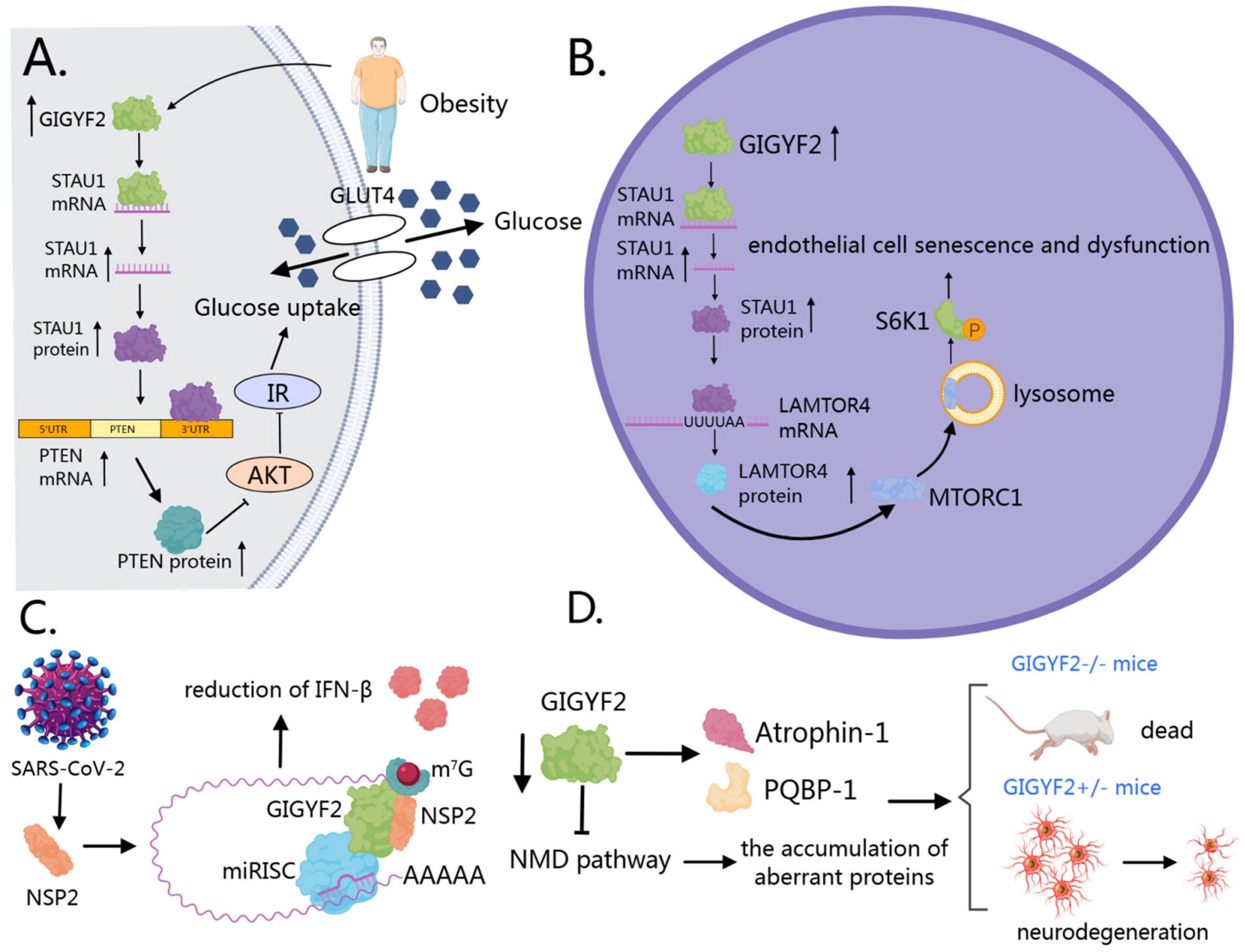
6.1. GIGYF2 in Metabolic Diseases
6.2. GIGYF2 in Vascular Aging
6.3. GIGYF2 in Viral Infections
6.4. GIGYF2 in Neurodegenerative Disorders
7. Discussion
7.1. Regulation of GIGYF2: An Underexplored Frontier
7.2. Nuclear Versus Cytoplasmic Functions: Complementary or Competitive Roles?
7.3. Disease-Associated Molecular Mechanisms: Current Limitations and Future Directions
7.4. Unresolved Mechanistic Questions and Future Perspectives
7.5. Conclusions and Therapeutic Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freund, C.; Dötsch, V.; Nishizawa, K.; Reinherz, E.L.; Wagner, G. The GYF domain is a novel structural fold that is involved in lymphoid signaling through proline-rich sequences. Nat. Struct. Biol. 1999, 6, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, K.; Freund, C.; Li, J.; Wagner, G.; Reinherz, E.L. Identification of a proline-binding motif regulating CD2-triggered T lymphocyte activation. Proc. Natl. Acad. Sci. USA 1998, 95, 14897–14902. [Google Scholar] [CrossRef]
- Kofler, M.M.; Freund, C. The GYF domain. FEBS J. 2006, 273, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Lillie, S.H.; Brown, S.S. Suppression of a myosin defect by a kinesin-related gene. Nature 1992, 356, 358–361. [Google Scholar] [CrossRef]
- Kofler, M.; Motzny, K.; Beyermann, M.; Freund, C. Novel interaction partners of the CD2BP2-GYF domain. J. Biol. Chem. 2005, 280, 33397–33402. [Google Scholar] [CrossRef] [PubMed]
- Kofler, M.; Motzny, K.; Freund, C. GYF domain proteomics reveals interaction sites in known and novel target proteins. Mol. Cell Proteom. 2005, 4, 1797–1811. [Google Scholar] [CrossRef]
- Ash, M.R.; Faelber, K.; Kosslick, D.; Albert, G.I.; Roske, Y.; Kofler, M.; Schuemann, M.; Krause, E.; Freund, C. Conserved beta-hairpin recognition by the GYF domains of Smy2 and GIGYF2 in mRNA surveillance and vesicular transport complexes. Structure 2010, 18, 944–954. [Google Scholar] [CrossRef]
- Bertazzon, M.; Hurtado-Pico, A.; Plaza-Sirvent, C.; Schuster, M.; Preußner, M.; Kuropka, B.; Liu, F.; Kirsten, A.Z.A.; Schmitt, X.J.; König, B.; et al. The nuclear GYF protein CD2BP2/U5-52K is required for T cell homeostasis. Front. Immunol. 2024, 15, 1415839. [Google Scholar] [CrossRef]
- Fu, R.; Olsen, M.T.; Webb, K.; Bennett, E.J.; Lykke-Andersen, J. Recruitment of the 4EHP-GYF2 cap-binding complex to tetraproline motifs of tristetraprolin promotes repression and degradation of mRNAs with AU-rich elements. RNA 2016, 22, 373–382. [Google Scholar] [CrossRef]
- Hale, V.A.; Guiney, E.L.; Goldberg, L.Y.; Haduong, J.H.; Kwartler, C.S.; Scangos, K.W.; Goutte, C. Notch signaling is antagonized by SAO-1, a novel GYF-domain protein that interacts with the E3 ubiquitin ligase SEL-10 in Caenorhabditis elegans. Genetics 2012, 190, 1043–1057. [Google Scholar] [CrossRef]
- Kofler, M.; Heuer, K.; Zech, T.; Freund, C. Recognition sequences for the GYF domain reveal a possible spliceosomal function of CD2BP2. J. Biol. Chem. 2004, 279, 28292–28297. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Ren, Y.; Li, Y.; Niu, F.; Li, Z.; Li, M.; Li, X.; Li, Q.; Huang, D.; Yu, Y.; et al. RNA-binding protein GIGYF2 orchestrates hepatic insulin resistance through STAU1/PTEN-mediated disruption of the PI3K/AKT signaling cascade. Mol. Med. 2024, 30, 124. [Google Scholar] [CrossRef] [PubMed]
- McCutchen-Maloney, S.L.; Matsuda, K.; Shimbara, N.; Binns, D.D.; Tanaka, K.; Slaughter, C.A.; DeMartino, G.N. cDNA cloning, expression, and functional characterization of PI31, a proline-rich inhibitor of the proteasome. J. Biol. Chem. 2000, 275, 18557–18565. [Google Scholar] [CrossRef]
- Schlundt, A.; Sticht, J.; Piotukh, K.; Kosslick, D.; Jahnke, N.; Keller, S.; Schuemann, M.; Krause, E.; Freund, C. Proline-rich sequence recognition: II. Proteomics analysis of Tsg101 ubiquitin-E2-like variant (UEV) interactions. Mol. Cell Proteom. 2009, 8, 2474–2486. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Chung, M.Y.; Keskeny, C.; Zinnall, U.; Landthaler, M.; Valkov, E.; Izaurralde, E.; Igreja, C. 4EHP and GIGYF1/2 Mediate Translation-Coupled Messenger RNA Decay. Cell Rep. 2020, 33, 108262. [Google Scholar] [CrossRef]
- Zaiss, D.M.; Standera, S.; Kloetzel, P.M.; Sijts, A.J. PI31 is a modulator of proteasome formation and antigen processing. Proc. Natl. Acad. Sci. USA 2002, 99, 14344–14349. [Google Scholar] [CrossRef]
- Choi, J.H.; Luo, J.; Hesketh, G.G.; Guo, S.; Pistofidis, A.; Ladak, R.J.; An, Y.; Naeli, P.; Alain, T.; Schmeing, T.M.; et al. Repression of mRNA translation initiation by GIGYF1 via disrupting the eIF3-eIF4G1 interaction. Sci. Adv. 2024, 10, eadl5638. [Google Scholar] [CrossRef]
- Giovannone, B.; Lee, E.; Laviola, L.; Giorgino, F.; Cleveland, K.A.; Smith, R.J. Two novel proteins that are linked to insulin-like growth factor (IGF-I) receptors by the Grb10 adapter and modulate IGF-I signaling. J. Biol. Chem. 2003, 278, 31564–31573. [Google Scholar] [CrossRef]
- Higashi, S.; Iseki, E.; Minegishi, M.; Togo, T.; Kabuta, T.; Wada, K. GIGYF2 is present in endosomal compartments in the mammalian brains and enhances IGF-1-induced ERK1/2 activation. J. Neurochem. 2010, 115, 423–437. [Google Scholar] [CrossRef]
- Morita, M.; Ler, L.W.; Fabian, M.R.; Siddiqui, N.; Mullin, M.; Henderson, V.C.; Alain, T.; Fonseca, B.D.; Karashchuk, G.; Bennett, C.F.; et al. A novel 4EHP-GIGYF2 translational repressor complex is essential for mammalian development. Mol. Cell Biol. 2012, 32, 3585–3593. [Google Scholar] [CrossRef]
- Saini, P.; Rudakou, U.; Yu, E.; Ruskey, J.A.; Asayesh, F.; Laurent, S.B.; Spiegelman, D.; Fahn, S.; Waters, C.; Monchi, O.; et al. Association study of DNAJC13, UCHL1, HTRA2, GIGYF2, and EIF4G1 with Parkinson’s disease. Neurobiol. Aging 2021, 100, e117–e119. [Google Scholar] [CrossRef]
- Wang, L.; Guo, J.F.; Zhang, W.W.; Xu, Q.; Zuo, X.; Shi, C.H.; Luo, L.Z.; Liu, J.; Hu, L.; Hu, Y.C.; et al. Novel GIGYF2 gene variants in patients with Parkinson’s disease in Chinese population. Neurosci. Lett. 2010, 473, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Peter, D.; Ruscica, V.; Bawankar, P.; Weber, R.; Helms, S.; Valkov, E.; Igreja, C.; Izaurralde, E. Molecular basis for GIGYF-Me31B complex assembly in 4EHP-mediated translational repression. Genes Dev. 2019, 33, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Kryszke, M.H.; Adjeriou, B.; Liang, F.; Chen, H.; Dautry, F. Post-transcriptional gene silencing activity of human GIGYF2. Biochem. Biophys. Res. Commun. 2016, 475, 289–294. [Google Scholar] [CrossRef]
- Amaya Ramirez, C.C.; Hubbe, P.; Mandel, N.; Béthune, J. 4EHP-independent repression of endogenous mRNAs by the RNA-binding protein GIGYF2. Nucleic Acids Res. 2018, 46, 5792–5808. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yuan, Q.; Zhang, S.; Zuo, M.; Li, T.; Li, J.; Zhou, X.; Li, M.; Feng, W.; Xia, X.; et al. Elevated GIGYF2 expression suppresses tumor migration and enhances sensitivity to temozolomide in malignant glioma. Cancer Gene Ther. 2022, 29, 750–757. [Google Scholar] [CrossRef]
- Kim, M.; Semple, I.; Kim, B.; Kiers, A.; Nam, S.; Park, H.W.; Park, H.; Ro, S.H.; Kim, J.S.; Juhász, G.; et al. Drosophila Gyf/GRB10 interacting GYF protein is an autophagy regulator that controls neuron and muscle homeostasis. Autophagy 2015, 11, 1358–1372. [Google Scholar] [CrossRef]
- Peter, D.; Weber, R.; Sandmeir, F.; Wohlbold, L.; Helms, S.; Bawankar, P.; Valkov, E.; Igreja, C.; Izaurralde, E. GIGYF1/2 proteins use auxiliary sequences to selectively bind to 4EHP and repress target mRNA expression. Genes Dev. 2017, 31, 1147–1161. [Google Scholar] [CrossRef]
- Naeli, P.; Zhang, X.; Snell, P.H.; Chatterjee, S.; Kamran, M.; Ladak, R.J.; Orr, N.; Duchaine, T.; Sonenberg, N.; Jafarnejad, S.M. The SARS-CoV-2 protein NSP2 enhances microRNA-mediated translational repression. J. Cell Sci. 2023, 136, jcs261286. [Google Scholar] [CrossRef]
- Xu, Z.; Choi, J.H.; Dai, D.L.; Luo, J.; Ladak, R.J.; Li, Q.; Wang, Y.; Zhang, C.; Wiebe, S.; Liu, A.C.H.; et al. SARS-CoV-2 impairs interferon production via NSP2-induced repression of mRNA translation. Proc. Natl. Acad. Sci. USA 2022, 119, e2204539119. [Google Scholar] [CrossRef]
- Zou, L.; Moch, C.; Graille, M.; Chapat, C. The SARS-CoV-2 protein NSP2 impairs the silencing capacity of the human 4EHP-GIGYF2 complex. iScience 2022, 25, 104646. [Google Scholar] [CrossRef]
- Joshi, B.; Cameron, A.; Jagus, R. Characterization of mammalian eIF4E-family members. Eur. J. Biochem. 2004, 271, 2189–2203. [Google Scholar] [CrossRef]
- Rom, E.; Kim, H.C.; Gingras, A.C.; Marcotrigiano, J.; Favre, D.; Olsen, H.; Burley, S.K.; Sonenberg, N. Cloning and characterization of 4EHP, a novel mammalian eIF4E-related cap-binding protein. J. Biol. Chem. 1998, 273, 13104–13109. [Google Scholar] [CrossRef] [PubMed]
- Zuberek, J.; Kubacka, D.; Jablonowska, A.; Jemielity, J.; Stepinski, J.; Sonenberg, N.; Darzynkiewicz, E. Weak binding affinity of human 4EHP for mRNA cap analogs. RNA 2007, 13, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, S.; Buchwald, G.; Falk, S.; Chakrabarti, S.; Prabu, J.R.; Conti, E. The conformational plasticity of eukaryotic RNA-dependent ATPases. FEBS J. 2015, 282, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Mathys, H.; Basquin, J.; Ozgur, S.; Czarnocki-Cieciura, M.; Bonneau, F.; Aartse, A.; Dziembowski, A.; Nowotny, M.; Conti, E.; Filipowicz, W. Structural and biochemical insights to the role of the CCR4-NOT complex and DDX6 ATPase in microRNA repression. Mol. Cell 2014, 54, 751–765. [Google Scholar] [CrossRef]
- Sobti, M.; Mead, B.J.; Stewart, A.G.; Igreja, C.; Christie, M. Molecular basis for GIGYF-TNRC6 complex assembly. RNA 2023, 29, 724–734. [Google Scholar] [CrossRef]
- Tollenaere, M.A.X.; Tiedje, C.; Rasmussen, S.; Nielsen, J.C.; Vind, A.C.; Blasius, M.; Batth, T.S.; Mailand, N.; Olsen, J.V.; Gaestel, M.; et al. GIGYF1/2-Driven Cooperation between ZNF598 and TTP in Posttranscriptional Regulation of Inflammatory Signaling. Cell Rep. 2019, 26, 3511–3521.e3514. [Google Scholar] [CrossRef]
- Schopp, I.M.; Amaya Ramirez, C.C.; Debeljak, J.; Kreibich, E.; Skribbe, M.; Wild, K.; Béthune, J. Split-BioID a conditional proteomics approach to monitor the composition of spatiotemporally defined protein complexes. Nat. Commun. 2017, 8, 15690. [Google Scholar] [CrossRef]
- Juszkiewicz, S.; Slodkowicz, G.; Lin, Z.; Freire-Pritchett, P.; Peak-Chew, S.Y.; Hegde, R.S. Ribosome collisions trigger cis-acting feedback inhibition of translation initiation. eLife 2020, 9, e60038. [Google Scholar] [CrossRef]
- Nordgaard, C.; Tollenaere, M.A.X.; Val, A.M.D.; Bekker-Jensen, D.B.; Blasius, M.; Olsen, J.V.; Bekker-Jensen, S. Regulation of the Golgi Apparatus by p38 and JNK Kinases during Cellular Stress Responses. Int. J. Mol. Sci. 2021, 22, 9595. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
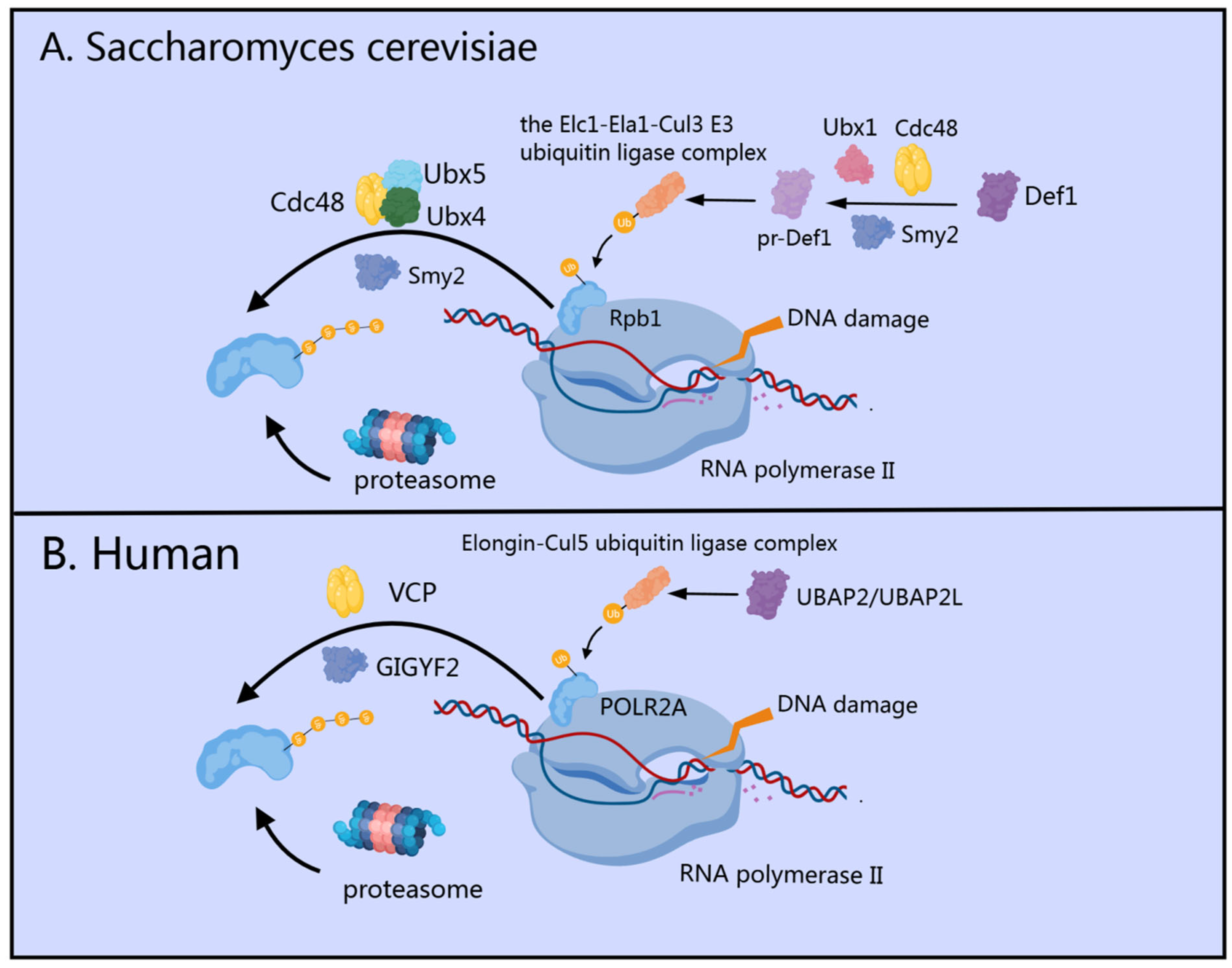
- Wilson, M.D.; Harreman, M.; Svejstrup, J.Q. Ubiquitylation and degradation of elongating RNA polymerase II: The last resort. Biochim. Biophys. Acta 2013, 1829, 151–157. [Google Scholar] [CrossRef]
- Noe Gonzalez, M.; Blears, D.; Svejstrup, J.Q. Causes and consequences of RNA polymerase II stalling during transcript elongation. Nat. Rev. Mol. Cell Biol. 2021, 22, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Oania, R.; Fang, R.; Smith, G.T.; Deshaies, R.J. Cdc48/p97 mediates UV-dependent turnover of RNA Pol II. Mol. Cell 2011, 41, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Iyama, T.; Wilson, D.M., 3rd. DNA repair mechanisms in dividing and non-dividing cells. DNA Repair 2013, 12, 620–636. [Google Scholar] [CrossRef]
- Herlihy, A.E.; Boeing, S.; Weems, J.C.; Walker, J.; Dirac-Svejstrup, A.B.; Lehner, M.H.; Conaway, R.C.; Conaway, J.W.; Svejstrup, J.Q. UBAP2/UBAP2L regulate UV-induced ubiquitylation of RNA polymerase II and are the human orthologues of yeast Def1. DNA Repair 2022, 115, 103343. [Google Scholar] [CrossRef]
- Huibregtse, J.M.; Yang, J.C.; Beaudenon, S.L. The large subunit of RNA polymerase II is a substrate of the Rsp5 ubiquitin-protein ligase. Proc. Natl. Acad. Sci. USA 1997, 94, 3656–3661. [Google Scholar] [CrossRef]
- Lehner, M.H.; Walker, J.; Temcinaite, K.; Herlihy, A.; Taschner, M.; Berger, A.C.; Corbett, A.H.; Dirac Svejstrup, A.B.; Svejstrup, J.Q. Yeast Smy2 and its human homologs GIGYF1 and -2 regulate Cdc48/VCP function during transcription stress. Cell Rep. 2022, 41, 111536. [Google Scholar] [CrossRef]
- Wilson, M.D.; Harreman, M.; Taschner, M.; Reid, J.; Walker, J.; Erdjument-Bromage, H.; Tempst, P.; Svejstrup, J.Q. Proteasome-mediated processing of Def1, a critical step in the cellular response to transcription stress. Cell 2013, 154, 983–995. [Google Scholar] [CrossRef]
- Olszewski, M.M.; Williams, C.; Dong, K.C.; Martin, A. The Cdc48 unfoldase prepares well-folded protein substrates for degradation by the 26S proteasome. Commun. Biol. 2019, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Forget, D.; Lacombe, A.A.; Cloutier, P.; Al-Khoury, R.; Bouchard, A.; Lavallée-Adam, M.; Faubert, D.; Jeronimo, C.; Blanchette, M.; Coulombe, B. The protein interaction network of the human transcription machinery reveals a role for the conserved GTPase RPAP4/GPN1 and microtubule assembly in nuclear import and biogenesis of RNA polymerase II. Mol. Cell Proteom. 2010, 9, 2827–2839. [Google Scholar] [CrossRef] [PubMed]
- Anindya, R.; Aygün, O.; Svejstrup, J.Q. Damage-induced ubiquitylation of human RNA polymerase II by the ubiquitin ligase Nedd4, but not Cockayne syndrome proteins or BRCA1. Mol. Cell 2007, 28, 386–397. [Google Scholar] [CrossRef]
- Varshavsky, A. The ubiquitin system. Trends Biochem. Sci. 1997, 22, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Pickart, C.M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001, 70, 503–533. [Google Scholar] [CrossRef]
- Harreman, M.; Taschner, M.; Sigurdsson, S.; Anindya, R.; Reid, J.; Somesh, B.; Kong, S.E.; Banks, C.A.; Conaway, R.C.; Conaway, J.W.; et al. Distinct ubiquitin ligases act sequentially for RNA polymerase II polyubiquitylation. Proc. Natl. Acad. Sci. USA 2009, 106, 20705–20710. [Google Scholar] [CrossRef]
- Bae, H.; Coller, J. Codon optimality-mediated mRNA degradation: Linking translational elongation to mRNA stability. Mol. Cell 2022, 82, 1467–1476. [Google Scholar] [CrossRef]
- Presnyak, V.; Alhusaini, N.; Chen, Y.H.; Martin, S.; Morris, N.; Kline, N.; Olson, S.; Weinberg, D.; Baker, K.E.; Graveley, B.R.; et al. Codon optimality is a major determinant of mRNA stability. Cell 2015, 160, 1111–1124. [Google Scholar] [CrossRef]
- Wu, Q.; Bazzini, A.A. Translation and mRNA Stability Control. Annu. Rev. Biochem. 2023, 92, 227–245. [Google Scholar] [CrossRef]
- Hanson, G.; Coller, J. Codon optimality, bias and usage in translation and mRNA decay. Nat. Rev. Mol. Cell Biol. 2018, 19, 20–30. [Google Scholar] [CrossRef]
- Yan, L.L.; Zaher, H.S. How do cells cope with RNA damage and its consequences? J. Biol. Chem. 2019, 294, 15158–15171. [Google Scholar] [CrossRef]
- Morris, C.; Cluet, D.; Ricci, E.P. Ribosome dynamics and mRNA turnover, a complex relationship under constant cellular scrutiny. Wiley Interdiscip. Rev. RNA 2021, 12, e1658. [Google Scholar] [CrossRef] [PubMed]
- Karousis, E.D.; Mühlemann, O. Nonsense-Mediated mRNA Decay Begins Where Translation Ends. Cold Spring Harb. Perspect. Biol. 2019, 11, a032862. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, K.; Tesina, P.; Matsuo, Y.; Sugiyama, T.; Cheng, J.; Saeki, Y.; Tanaka, K.; Becker, T.; Beckmann, R.; Inada, T. Collided ribosomes form a unique structural interface to induce Hel2-driven quality control pathways. EMBO J. 2019, 38, e100276. [Google Scholar] [CrossRef] [PubMed]
- Meydan, S.; Guydosh, N.R. A cellular handbook for collided ribosomes: Surveillance pathways and collision types. Curr. Genet. 2021, 67, 19–26. [Google Scholar] [CrossRef]
- Frischmeyer, P.A.; van Hoof, A.; O’Donnell, K.; Guerrerio, A.L.; Parker, R.; Dietz, H.C. An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science 2002, 295, 2258–2261. [Google Scholar] [CrossRef]
- van Hoof, A.; Frischmeyer, P.A.; Dietz, H.C.; Parker, R. Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science 2002, 295, 2262–2264. [Google Scholar] [CrossRef]
- Joazeiro, C.A.P. Mechanisms and functions of ribosome-associated protein quality control. Nat. Rev. Mol. Cell Biol. 2019, 20, 368–383. [Google Scholar] [CrossRef]
- Kulak, N.A.; Pichler, G.; Paron, I.; Nagaraj, N.; Mann, M. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat. Methods 2014, 11, 319–324. [Google Scholar] [CrossRef]
- Itzhak, D.N.; Tyanova, S.; Cox, J.; Borner, G.H. Global, quantitative and dynamic mapping of protein subcellular localization. eLife 2016, 5, e16950. [Google Scholar] [CrossRef]
- Sinha, N.K.; Ordureau, A.; Best, K.; Saba, J.A.; Zinshteyn, B.; Sundaramoorthy, E.; Fulzele, A.; Garshott, D.M.; Denk, T.; Thoms, M.; et al. EDF1 coordinates cellular responses to ribosome collisions. eLife 2020, 9, e58828. [Google Scholar] [CrossRef] [PubMed]
- Vind, A.C.; Genzor, A.V.; Bekker-Jensen, S. Ribosomal stress-surveillance: Three pathways is a magic number. Nucleic Acids Res. 2020, 48, 10648–10661. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, R.; Nagy, G.; Dotu, I.; Chuang, J.H.; Ackerman, S.L. Activation of GCN2 kinase by ribosome stalling links translation elongation with translation initiation. eLife 2016, 5, e14295. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Novoa, I.; Zhang, Y.; Zeng, H.; Wek, R.; Schapira, M.; Ron, D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 2000, 6, 1099–1108. [Google Scholar] [CrossRef]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M.; Sadri, N.; Yun, C.; Popko, B.; Paules, R.; et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 2003, 11, 619–633. [Google Scholar] [CrossRef]
- Matsuo, Y.; Inada, T. Co-Translational Quality Control Induced by Translational Arrest. Biomolecules 2023, 13, 317. [Google Scholar] [CrossRef]
- Yip, M.C.J.; Shao, S. Detecting and Rescuing Stalled Ribosomes. Trends Biochem. Sci. 2021, 46, 731–743. [Google Scholar] [CrossRef]
- Kim, K.Q.; Zaher, H.S. Canary in a coal mine: Collided ribosomes as sensors of cellular conditions. Trends Biochem. Sci. 2022, 47, 82–97. [Google Scholar] [CrossRef]
- Juszkiewicz, S.; Chandrasekaran, V.; Lin, Z.; Kraatz, S.; Ramakrishnan, V.; Hegde, R.S. ZNF598 Is a Quality Control Sensor of Collided Ribosomes. Mol. Cell 2018, 72, 469–481.e467. [Google Scholar] [CrossRef]
- Hickey, K.L.; Dickson, K.; Cogan, J.Z.; Replogle, J.M.; Schoof, M.; D’Orazio, K.N.; Sinha, N.K.; Hussmann, J.A.; Jost, M.; Frost, A.; et al. GIGYF2 and 4EHP Inhibit Translation Initiation of Defective Messenger RNAs to Assist Ribosome-Associated Quality Control. Mol. Cell 2020, 79, 950–962.e956. [Google Scholar] [CrossRef]
- Juszkiewicz, S.; Hegde, R.S. Initiation of Quality Control during Poly(A) Translation Requires Site-Specific Ribosome Ubiquitination. Mol. Cell 2017, 65, 743–750.e744. [Google Scholar] [CrossRef]
- D’Orazio, K.N.; Wu, C.C.; Sinha, N.; Loll-Krippleber, R.; Brown, G.W.; Green, R. The endonuclease Cue2 cleaves mRNAs at stalled ribosomes during No Go Decay. eLife 2019, 8, e49117. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Sugiyama, T.; Yamazaki, R.; Nobuta, R.; Inada, T. Identification of a novel trigger complex that facilitates ribosome-associated quality control in mammalian cells. Sci. Rep. 2020, 10, 3422. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Tesina, P.; Nakajima, S.; Mizuno, M.; Endo, A.; Buschauer, R.; Cheng, J.; Shounai, O.; Ikeuchi, K.; Saeki, Y.; et al. RQT complex dissociates ribosomes collided on endogenous RQC substrate SDD1. Nat. Struct. Mol. Biol. 2020, 27, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Juszkiewicz, S.; Speldewinde, S.H.; Wan, L.; Svejstrup, J.Q.; Hegde, R.S. The ASC-1 Complex Disassembles Collided Ribosomes. Mol. Cell 2020, 79, 603–614.e608. [Google Scholar] [CrossRef]
- Shao, S.; Brown, A.; Santhanam, B.; Hegde, R.S. Structure and assembly pathway of the ribosome quality control complex. Mol. Cell 2015, 57, 433–444. [Google Scholar] [CrossRef]
- Jandhyala, D.M.; Ahluwalia, A.; Obrig, T.; Thorpe, C.M. ZAK: A MAP3Kinase that transduces Shiga toxin- and ricin-induced proinflammatory cytokine expression. Cell Microbiol. 2008, 10, 1468–1477. [Google Scholar] [CrossRef]
- Wang, X.; Mader, M.M.; Toth, J.E.; Yu, X.; Jin, N.; Campbell, R.M.; Smallwood, J.K.; Christe, M.E.; Chatterjee, A.; Goodson, T., Jr.; et al. Complete inhibition of anisomycin and UV radiatsion but not cytokine induced JNK and p38 activation by an aryl-substituted dihydropyrrolopyrazole quinoline and mixed lineage kinase 7 small interfering RNA. J. Biol. Chem. 2005, 280, 19298–19305. [Google Scholar] [CrossRef]
- De, S.; Mühlemann, O. A comprehensive coverage insurance for cells: Revealing links between ribosome collisions, stress responses and mRNA surveillance. RNA Biol. 2022, 19, 609–621. [Google Scholar] [CrossRef]
- Zinshteyn, B.; Sinha, N.K.; Enam, S.U.; Koleske, B.; Green, R. Translational repression of NMD targets by GIGYF2 and EIF4E2. PLoS Genet. 2021, 17, e1009813. [Google Scholar] [CrossRef] [PubMed]
- Kashima, I.; Yamashita, A.; Izumi, N.; Kataoka, N.; Morishita, R.; Hoshino, S.; Ohno, M.; Dreyfuss, G.; Ohno, S. Binding of a novel SMG-1-Upf1-eRF1-eRF3 complex (SURF) to the exon junction complex triggers Upf1 phosphorylation and nonsense-mediated mRNA decay. Genes Dev. 2006, 20, 355–367. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Bonneau, F.; Schüssler, S.; Eppinger, E.; Conti, E. Phospho-dependent and phospho-independent interactions of the helicase UPF1 with the NMD factors SMG5-SMG7 and SMG6. Nucleic Acids Res. 2014, 42, 9447–9460. [Google Scholar] [CrossRef]
- Hurt, J.A.; Robertson, A.D.; Burge, C.B. Global analyses of UPF1 binding and function reveal expanded scope of nonsense-mediated mRNA decay. Genome Res. 2013, 23, 1636–1650. [Google Scholar] [CrossRef] [PubMed]
- Zünd, D.; Gruber, A.R.; Zavolan, M.; Mühlemann, O. Translation-dependent displacement of UPF1 from coding sequences causes its enrichment in 3′ UTRs. Nat. Struct. Mol. Biol. 2013, 20, 936–943. [Google Scholar] [CrossRef]
- Hogg, J.R.; Goff, S.P. Upf1 senses 3′UTR length to potentiate mRNA decay. Cell 2010, 143, 379–389. [Google Scholar] [CrossRef]
- Arribere, J.A.; Fire, A.Z. Nonsense mRNA suppression via nonstop decay. eLife 2018, 7, e33292. [Google Scholar] [CrossRef] [PubMed]
- Lareau, L.F.; Inada, M.; Green, R.E.; Wengrod, J.C.; Brenner, S.E. Unproductive splicing of SR genes associated with highly conserved and ultraconserved DNA elements. Nature 2007, 446, 926–929. [Google Scholar] [CrossRef]
- Lewis, B.P.; Green, R.E.; Brenner, S.E. Evidence for the widespread coupling of alternative splicing and nonsense-mediated mRNA decay in humans. Proc. Natl. Acad. Sci. USA 2003, 100, 189–192. [Google Scholar] [CrossRef]
- Morrison, M.; Harris, K.S.; Roth, M.B. smg mutants affect the expression of alternatively spliced SR protein mRNAs in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1997, 94, 9782–9785. [Google Scholar] [CrossRef]
- Alagar Boopathy, L.R.; Beadle, E.; Garcia-Bueno Rico, A.; Vera, M. Proteostasis regulation through ribosome quality control and no-go-decay. Wiley Interdiscip. Rev. RNA 2023, 14, e1809. [Google Scholar] [CrossRef] [PubMed]
- Nissan, T.; Rajyaguru, P.; She, M.; Song, H.; Parker, R. Decapping activators in Saccharomyces cerevisiae act by multiple mechanisms. Mol. Cell 2010, 39, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Ernoult-Lange, M.; Baconnais, S.; Harper, M.; Minshall, N.; Souquere, S.; Boudier, T.; Bénard, M.; Andrey, P.; Pierron, G.; Kress, M.; et al. Multiple binding of repressed mRNAs by the P-body protein Rck/p54. RNA 2012, 18, 1702–1715. [Google Scholar] [CrossRef]
- Coller, J.M.; Tucker, M.; Sheth, U.; Valencia-Sanchez, M.A.; Parker, R. The DEAD box helicase, Dhh1p, functions in mRNA decapping and interacts with both the decapping and deadenylase complexes. RNA 2001, 7, 1717–1727. [Google Scholar] [CrossRef]
- Ruscica, V.; Bawankar, P.; Peter, D.; Helms, S.; Igreja, C.; Izaurralde, E. Direct role for the Drosophila GIGYF protein in 4EHP-mediated mRNA repression. Nucleic Acids Res. 2019, 47, 7035–7048. [Google Scholar] [CrossRef]
- Niu, F.; Li, Z.; Ren, Y.; Li, Z.; Guan, H.; Li, Y.; Zhang, Y.; Li, Y.; Yang, J.; Qian, L.; et al. Aberrant hyper-expression of the RNA binding protein GIGYF2 in endothelial cells modulates vascular aging and function. Redox Biol. 2023, 65, 102824. [Google Scholar] [CrossRef]
- Kofler, M.; Schuemann, M.; Merz, C.; Kosslick, D.; Schlundt, A.; Tannert, A.; Schaefer, M.; Lührmann, R.; Krause, E.; Freund, C. Proline-rich sequence recognition: I. Marking GYF and WW domain assembly sites in early spliceosomal complexes. Mol. Cell Proteom. 2009, 8, 2461–2473. [Google Scholar] [CrossRef]
- Clark, A.R.; Dean, J.L. The control of inflammation via the phosphorylation and dephosphorylation of tristetraprolin: A tale of two phosphatases. Biochem. Soc. Trans. 2016, 44, 1321–1337. [Google Scholar] [CrossRef] [PubMed]
- Garzia, A.; Jafarnejad, S.M.; Meyer, C.; Chapat, C.; Gogakos, T.; Morozov, P.; Amiri, M.; Shapiro, M.; Molina, H.; Tuschl, T.; et al. The E3 ubiquitin ligase and RNA-binding protein ZNF598 orchestrates ribosome quality control of premature polyadenylated mRNAs. Nat. Commun. 2017, 8, 16056. [Google Scholar] [CrossRef]
- Naeli, P.; Winter, T.; Hackett, A.P.; Alboushi, L.; Jafarnejad, S.M. The intricate balance between microRNA-induced mRNA decay and translational repression. FEBS J. 2023, 290, 2508–2524. [Google Scholar] [CrossRef]
- Filipowicz, W.; Sonenberg, N. The long unfinished march towards understanding microRNA-mediated repression. RNA 2015, 21, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.E.; Huntzinger, E.; Fauser, M.; Izaurralde, E. GW182 proteins directly recruit cytoplasmic deadenylase complexes to miRNA targets. Mol. Cell 2011, 44, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Chekulaeva, M.; Mathys, H.; Zipprich, J.T.; Attig, J.; Colic, M.; Parker, R.; Filipowicz, W. miRNA repression involves GW182-mediated recruitment of CCR4-NOT through conserved W-containing motifs. Nat. Struct. Mol. Biol. 2011, 18, 1218–1226. [Google Scholar] [CrossRef]
- Fabian, M.R.; Cieplak, M.K.; Frank, F.; Morita, M.; Green, J.; Srikumar, T.; Nagar, B.; Yamamoto, T.; Raught, B.; Duchaine, T.F.; et al. miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat. Struct. Mol. Biol. 2011, 18, 1211–1217. [Google Scholar] [CrossRef]
- Rouya, C.; Siddiqui, N.; Morita, M.; Duchaine, T.F.; Fabian, M.R.; Sonenberg, N. Human DDX6 effects miRNA-mediated gene silencing via direct binding to CNOT1. RNA 2014, 20, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rivas, F.V.; Wohlschlegel, J.; Yates, J.R., 3rd; Parker, R.; Hannon, G.J. A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 2005, 7, 1261–1266. [Google Scholar] [CrossRef]
- Majerciak, V.; Zhou, T.; Kruhlak, M.J.; Zheng, Z.M. RNA helicase DDX6 and scaffold protein GW182 in P-bodies promote biogenesis of stress granules. Nucleic Acids Res. 2023, 51, 9337–9355. [Google Scholar] [CrossRef]
- Adjibade, P.; Mazroui, R. Control of mRNA turnover: Implication of cytoplasmic RNA granules. Semin. Cell Dev. Biol. 2014, 34, 15–23. [Google Scholar] [CrossRef]
- Eulalio, A.; Behm-Ansmant, I.; Izaurralde, E. P bodies: At the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 2007, 8, 9–22. [Google Scholar] [CrossRef]
- Parker, R.; Sheth, U. P bodies and the control of mRNA translation and degradation. Mol. Cell 2007, 25, 635–646. [Google Scholar] [CrossRef]
- Ingelfinger, D.; Arndt-Jovin, D.J.; Lührmann, R.; Achsel, T. The human LSm1-7 proteins colocalize with the mRNA-degrading enzymes Dcp1/2 and Xrnl in distinct cytoplasmic foci. RNA 2002, 8, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, E.; Cougot, N.; Meyer, S.; Babajko, S.; Wahle, E.; Séraphin, B. Human Dcp2: A catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. EMBO J. 2002, 21, 6915–6924. [Google Scholar] [CrossRef]
- Sheth, U.; Parker, R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 2003, 300, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Orban, T.I.; Izaurralde, E. Decay of mRNAs targeted by RISC requires XRN1, the Ski complex, and the exosome. RNA 2005, 11, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Almasi, S.; Crawford Parks, T.E.; Ravel-Chapuis, A.; MacKenzie, A.; Côté, J.; Cowan, K.N.; Jasmin, B.J. Differential regulation of autophagy by STAU1 in alveolar rhabdomyosarcoma and non-transformed skeletal muscle cells. Cell. Oncol. 2021, 44, 851–870. [Google Scholar] [CrossRef]
- Bu, L.; Wang, H.; Pan, J.A.; Chen, L.; Xing, F.; Wu, J.; Li, S.; Guo, D. PTEN suppresses tumorigenesis by directly dephosphorylating Akt. Signal Transduct. Target. Ther. 2021, 6, 262. [Google Scholar] [CrossRef]
- Driessen, G.J.; IJspeert, H.; Wentink, M.; Yntema, H.G.; van Hagen, P.M.; van Strien, A.; Bucciol, G.; Cogulu, O.; Trip, M.; Nillesen, W.; et al. Increased PI3K/Akt activity and deregulated humoral immune response in human PTEN deficiency. J. Allergy Clin. Immunol. 2016, 138, 1744–1747.e1745. [Google Scholar] [CrossRef]
- Yue, S.; Li, J.; Lee, S.Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; Ratliff, T.L.; et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef]
- Zoncu, R.; Bar-Peled, L.; Efeyan, A.; Wang, S.; Sancak, Y.; Sabatini, D.M. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 2011, 334, 678–683. [Google Scholar] [CrossRef]
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010, 141, 290–303. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, R.; Wang, Z.; Wang, X.; Wang, F.; Ding, J. Structural basis for Ragulator functioning as a scaffold in membrane-anchoring of Rag GTPases and mTORC1. Nat. Commun. 2017, 8, 1394. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, M.E.G.; Naschberger, A.; Fürnrohr, B.G.; Stasyk, T.; Dunzendorfer-Matt, T.; Lechner, S.; Welti, S.; Kremser, L.; Shivalingaiah, G.; Offterdinger, M.; et al. Crystal structure of the human lysosomal mTORC1 scaffold complex and its impact on signaling. Science 2017, 358, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Yepuri, G.; Velagapudi, S.; Xiong, Y.; Rajapakse, A.G.; Montani, J.P.; Ming, X.F.; Yang, Z. Positive crosstalk between arginase-II and S6K1 in vascular endothelial inflammation and aging. Aging Cell 2012, 11, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Huang, A.; Kaley, G.; Sun, D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1829–H1836. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Masood, K.I.; Yameen, M.; Ashraf, J.; Shahid, S.; Mahmood, S.F.; Nasir, A.; Nasir, N.; Jamil, B.; Ghanchi, N.K.; Khanum, I.; et al. Upregulated type I interferon responses in asymptomatic COVID-19 infection are associated with improved clinical outcome. Sci. Rep. 2021, 11, 22958. [Google Scholar] [CrossRef]
- Vinh, D.C.; Abel, L.; Bastard, P.; Cheng, M.P.; Condino-Neto, A.; Gregersen, P.K.; Haerynck, F.; Cicalese, M.P.; Hagin, D.; Soler-Palacín, P.; et al. Harnessing Type I IFN Immunity Against SARS-CoV-2 with Early Administration of IFN-β. J. Clin. Immunol. 2021, 41, 1425–1442. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Samaranch, L.; Lorenzo, E.; Pastor, M.A.; Riverol, M.; Luquin, M.R.; Rodríguez-Oroz, M.C.; Obeso, J.A.; Pastor, P. Analysis of the GIGYF2 gene in familial and sporadic Parkinson disease in the Spanish population. Eur. J. Neurol. 2010, 17, 321–325. [Google Scholar] [CrossRef]
- Tan, E.K.; Schapira, A.H. Summary of GIGYF2 studies in Parkinson’s disease: The burden of proof. Eur. J. Neurol. 2010, 17, 175–176. [Google Scholar] [CrossRef]
- Giovannone, B.; Tsiaras, W.G.; de la Monte, S.; Klysik, J.; Lautier, C.; Karashchuk, G.; Goldwurm, S.; Smith, R.J. GIGYF2 gene disruption in mice results in neurodegeneration and altered insulin-like growth factor signaling. Hum. Mol. Genet. 2009, 18, 4629–4639. [Google Scholar] [CrossRef] [PubMed]
- Bialkowska, A.; Kurlandzka, A. Proteins interacting with Lin 1p, a putative link between chromosome segregation, mRNA splicing and DNA replication in Saccharomyces cerevisiae. Yeast 2002, 19, 1323–1333. [Google Scholar] [CrossRef] [PubMed]

| Interacting Partner | Interaction Domain | References | Functional Consequence of the Interaction | References |
|---|---|---|---|---|
| 4EHP | residues 40–50 | [28] | Forms a complex that inhibits translation initiation by competing with eIF4E for 5’ cap binding | [20] |
| DDX6 | residues 280–310 | [23] | Essential for TTP-mediated translational repression of AU-rich mRNAs | [117] |
| ZNF598 | GYF domain (residues 529–597) | [38] | Recruits GIGYF2 to collided ribosomes during quality control of translation | [40] |
| TNRC6A | [37] | Connects GIGYF2-4EHP complex to miRNA-regulated transcripts | [37] | |
| TTP | [38] | Recruits GIGYF2-4EHP complex to ARE-containing mRNAs (inflammation-associated transcripts) | [38] | |
| NSP2 | residues 860–919 | [30] | Enhances the interaction between GIGYF2 and 4EHP | [31] |
| CCR4-NOT | multiple domains | [25] | Promotes deadenylation of target mRNAs | [25] |
| 14-3-3 proteins | phosphorylated serine residues S157 and S638 | [41] | Negatively modulates GIGYF2’s interactions with GYF domain-binding partners during stress conditions | [41] |
| UPF1 | unknown | Regulates ribosomal occupancy of mRNA containing premature termination codons | [91] | |
| VCP/p97 | unknown | Promotes extraction and proteasomal degradation of ubiquitylated Rpb1 from stalled transcription complexes during DNA damage response | [49] | |
| EDF1 | unknown | Recruits and stabilizes GIGYF2-4EHP complex to ribosome collision sites | [40] | |
| STAU1 mRNA | residues 1–495 | [12,106] | Enhances STAU1 mRNA stability | [12] |
| SVOPL mRNA | N-terminal domain (residues 1–532) and C-terminal domain (residues 606–1299) | [25] | Direct engagement with endogenous mRNAs for translational regulation and degradation | [25] |
| COL8A1 mRNA | ||||
| NPR3 mRNA | ||||
| CPM mRNA | ||||
| ECEL1 mRNA | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.-S.; Liu, S.-H.; Li, Z.-Y.; Chen, J.-Y.; Xiong, X.-Y. GIGYF2: A Multifunctional Regulator at the Crossroads of Gene Expression, mRNA Surveillance, and Human Disease. Cells 2025, 14, 1032. https://doi.org/10.3390/cells14131032
Zhao C-S, Liu S-H, Li Z-Y, Chen J-Y, Xiong X-Y. GIGYF2: A Multifunctional Regulator at the Crossroads of Gene Expression, mRNA Surveillance, and Human Disease. Cells. 2025; 14(13):1032. https://doi.org/10.3390/cells14131032
Chicago/Turabian StyleZhao, Chen-Shuo, Shu-Han Liu, Zheng-Yang Li, Jia-Yue Chen, and Xiang-Yang Xiong. 2025. "GIGYF2: A Multifunctional Regulator at the Crossroads of Gene Expression, mRNA Surveillance, and Human Disease" Cells 14, no. 13: 1032. https://doi.org/10.3390/cells14131032
APA StyleZhao, C.-S., Liu, S.-H., Li, Z.-Y., Chen, J.-Y., & Xiong, X.-Y. (2025). GIGYF2: A Multifunctional Regulator at the Crossroads of Gene Expression, mRNA Surveillance, and Human Disease. Cells, 14(13), 1032. https://doi.org/10.3390/cells14131032






