Abstract
GIGYF2 (Grb10-interacting GYF protein 2) functions as a versatile adaptor protein that regulates gene expression at various levels. At the transcriptional level, GIGYF2 facilitates VCP/p97-mediated extraction of ubiquitylated Rpb1 from stalled RNA polymerase II complexes during DNA damage response. In mRNA surveillance, GIGYF2 participates in ribosome collision-induced quality control, nonsense-mediated decay, no-go decay, and non-stop decay pathways. Furthermore, GIGYF2 interacts with key factors including 4EHP, TTP, CCR4-NOT, DDX6, ZNF598, and TNRC6A to mediate translational repression and mRNA degradation. Additionally, dysregulation of GIGYF2 has been implicated in various pathological conditions, including metabolic diseases, vascular aging, viral infections, and neurodegenerative disorders. This review summarizes the structural and functional characteristics of GIGYF2, highlighting its importance in transcriptional regulation, mRNA surveillance, translational inhibition, and mRNA degradation, while also elucidating its potential as a therapeutic target for disease treatment.
1. Introduction
The GYF domain represents a highly conserved, versatile adaptor module that specifically recognizes proline-rich sequences (PRS) [1]. Initially identified in CD2BP2 as the structural element mediating interaction with PPPGHR amino acid repeats within the T-cell adhesion molecule CD2 [2], this domain derives its nomenclature from the signature glycine-tyrosine-phenylalanine tripeptide sequence [1]. Comprising approximately 60 amino acid residues, the GYF domain is characterized by a conserved signature motif GPF-X4-[M/V/I]-X2-W-X3-GYF that assembles into a distinctive bulge-helix-bulge tertiary structure [3]. The GYF domain family segregates into two distinct subfamilies: CD2BP2-type and SMY2-type [1,2,4]. The CD2BP2-type, first characterized in T-cell adhesion molecule CD2 binding protein 2, is distinguished by a tryptophan residue at position 22 and a longer β1-β2 loop region [5,6]. In contrast, the SMY2-type, named after the yeast suppressor of myosin 2 protein, exhibits an aspartic acid at the corresponding position 22, and the domain prefers ligands with the consensus motif PPGF (where f = hydrophobic amino acid, except for tryptophan) [7].
GYF domain-containing proteins participate in diverse cellular processes, including proteome-wide protein–protein interactions, translational regulation, mRNA splicing, surveillance mechanisms, ubiquitin conjugation, signal transduction pathways, and regulation of immunoproteasome [7,8,9,10,11,12,13,14,15,16].
The human genome encodes two principal SMY2-type GYF domain-containing proteins: GIGYF1 and GIGYF2. GIGYF2 (Grb10-interacting GYF protein 2) differs structurally from GIGYF1 by containing glutamic acid-rich and glutamine/proline-glutamine-rich C-terminal sequences, whereas GIGYF1 features a distinctive polyproline region [17]. However, given that the functional mechanisms and biological significance of GIGYF1 remain poorly characterized, this review focuses on elucidating the multifaceted roles of GIGYF2 in regulating various stages of gene expression.
GIGYF2 is encoded by the GIGYF2 gene, which belongs to the GIGYF protein family. GIGYF2 was initially discovered through yeast two-hybrid screening, where researchers used the N-terminal region of Grb10 (growth factor receptor-bound protein 10) as bait and identified novel interacting proteins, including GIGYF1 and GIGYF2, from a mouse fat cell cDNA library [18]. At the organ level, GIGYF2 is widely expressed throughout the body, with particularly high expression in the liver, pancreas, brain, lung, kidney, and spleen [19]. At the subcellular level, GIGYF2 exhibits dual localization patterns depending on cellular conditions. Under normal physiological conditions, GIGYF2 associates with the endoplasmic reticulum (ER) and Golgi apparatus, where it colocalizes with COPII vesicle proteins (particularly Sec31A) involved in protein transport [7]. However, under cellular stress conditions such as arsenite exposure, GIGYF2 redistributes to cytoplasmic stress granules, which contain translationally repressed mRNAs and RNA-binding proteins like TIA-1 [7].
Despite their structural similarities, GIGYF1 and GIGYF2 operate through distinct functional mechanisms. GIGYF1 primarily represses translation by strongly interacting with eIF3 complex subunits (particularly eIF3L, eIF3E, eIF3D, and eIF3G) through its C-terminal region, disrupting the critical eIF3-eIF4G1 interaction. In contrast, GIGYF2 relies approximately 50% on its interaction with 4EHP (eIF4E2) for translational repression [17,20].
The understanding of GIGYF2 function has undergone remarkable expansion in recent years. Initially identified as a binding partner of GRB10 and implicated in growth factor receptor signaling and insulin/IGF1 pathways [19], subsequent studies have revealed its multifaceted roles. Current research highlights GIGYF2’s involvement in neurodevelopment and neurodegenerative diseases [21,22], mRNA regulation through interactions with RNA-binding proteins (AGO2, DDX6) [23,24], direct participation in mRNA degradation as an RNA-binding protein [25], and potential tumor suppressor functions [26]. Additionally, GIGYF2 has been linked to autophagy regulation [12] and metabolic processes affecting glucose and lipid metabolism [27]. In this review, we summarized the role of GIGYF2 in various stages of gene expression regulation.
2. The Structure of GIGYF2
The human GIGYF2 gene is located on chromosome 2q37.1, spanning 163,275 base pairs and comprising 35 exons. The GIGYF2 protein consists of 1299 amino acids with a theoretical molecular weight of 150,070 Da. GIGYF2 contains three distinct domains that contribute to its repressive activity: an N-terminal domain (NTD, amino acids 1-532), a GYF domain (amino acids 533-596), and a C-terminal domain (CTD, amino acids 606-1299) [25]. The NTD includes binding domains for both 4EHP and DDX6 [23,28], while the CTD contains the NSP2 binding domain [29,30,31] (Figure 1A).
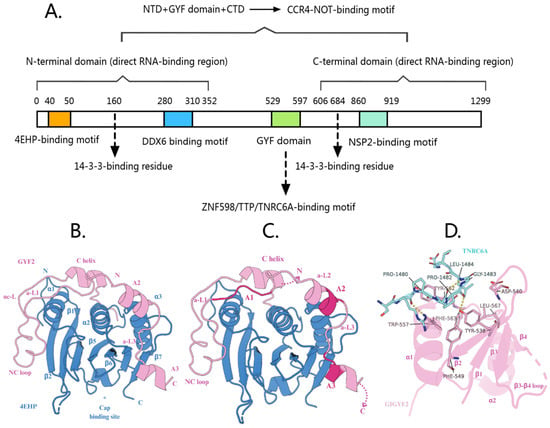
Figure 1.
(A) The functional domains of GIGYF2 protein are involved in gene expression regulation. (B) Crystal structure of the 4EHP-GIGYF2 complex. 4EHP is shown in blue and GIGYF2 in pink, illustrating the extensive binding interface and stable heterodimeric complex formation. Asterisks (*) indicate residues that are shown without their side chains for clarity. (C) Schematic representation of the tripartite binding mechanism between 4EHP (blue) and GIGYF2 (pink). The diagram shows three distinct binding regions: canonical motif (C) at the dorsal surface, non-canonical motif (NC) at the lateral surface, and auxiliary sequences (A1–A3) providing 4EHP-specific contacts. Auxiliary motifs are interconnected by linkers a-L1, a-L2, and a-L3, with the invariant PLAL motif in A1. This cooperative binding mechanism confers high affinity and specificity for 4EHP over eIF4E. (D) Crystal structure of GIGYF2 GYF domain (pink cartoon) in complex with TNRC6A PRS peptide (blue sticks). The peptide binds to a conserved hydrophobic groove formed by aromatic residues on the GYF domain surface. Yellow dashed lines indicate hydrogen bonds. This interaction facilitates recruitment of the 4EHP-GIGYF2 complex to miRISC for translational repression.
2.1. N-Terminal Domain
2.1.1. 4EHP-Binding Motif
GIGYF2 binds to 4EHP to form a complex that inhibits translation initiation by competing with eIF4E to bind to the 5’ cap structure of target mRNAs, thereby preventing the assembly of the translation initiation complex [20,32,33]. Although 4EHP exhibits lower affinity for the 5’ cap compared to eIF4E [33,34], this competition is likely enhanced by GIGYF2, which stabilizes 4EHP and may increase its cap-binding capability [28].
The 4EHP-binding region (4EHP-BR) of GIGYF2 employs a tripartite binding mechanism consisting of three distinct sequence elements that work cooperatively to achieve high-affinity and specific binding to 4EHP [28] (Table 1) (Figure 1B). First, the canonical motif (YXYX4LΦ consensus sequence, residues 41–49) forms the primary interaction by binding to the dorsal surface of 4EHP in a helical conformation, similar to how 4E-BPs bind to eIF4E. Second, the noncanonical motif (located ~12 residues C-terminal to the canonical motif) engages the lateral surface of 4EHP through hydrophobic interactions, providing additional binding stability. Third, and most importantly for 4EHP specificity, the auxiliary sequences (A1–A3) extend the binding interface and contact 4EHP-specific residues that are not conserved in eIF4E.
The auxiliary sequences can be subdivided into three functional elements: (1) Auxiliary motif 1 (A1), which contains the invariant PLAL motif and is connected to the noncanonical motif via auxiliary linker 1 (a-L1); (2) Auxiliary motif 2 (A2), which forms auxiliary helix α2; and (3) Auxiliary motif 3 (A3), which adopts auxiliary helix α3 and is positioned near the 4EHP cap-binding pocket (Figure 1C). These auxiliary motifs are interconnected by conserved linkers a-L2 and a-L3, with the a-L3 linker containing a critical VNS sequence that interacts with 4EHP-specific residues [28]. Importantly, the auxiliary sequences increase GIGYF2’s affinity for 4EHP by 30–40 fold compared to peptides lacking these sequences, and they interact specifically with 4EHP residues (R103, R140, E149) that differ from eIF4E, thereby conferring selectivity for 4EHP over eIF4E.
Within the asymmetric unit of 4EHP-GIGYF2 crystals, the GIGYF2 canonical and auxiliary motifs constitute part of a large interface (1008 Å2), which mediates the association of two adjacent 4EHP-GIGYF2 complexes. The putative dimer interface is stabilized by 4EHP-specific residues R202, M161, and Q159, and GIGYF2 residues E46 and E47 [28]. This complex dimerization potentially increases the local concentration of repressor complexes on target mRNAs, thereby amplifying their regulatory efficacy.
2.1.2. DDX6-Binding Motif
GIGYF2 interacts with the RNA-dependent ATPase DDX6/Me31B through a conserved Me31B/DDX6-binding motif (MBM) to mediate translational repression [23,35,36]. Structurally, the MBM employs a bipartite binding mechanism: the N-terminal PEW (Pro-Glu-Trp) sequence forms a short coil, with the tryptophan residue (W349 in Drosophila GIGYF, equivalent to W221 in 4E-T) inserting into the W pocket between helices α10 and α11 of Me31B (Table 1). The PEW region is connected via a flexible linker to a C-terminal β-hairpin structure containing a unique “split” FDx4F motif (F361, D362, and F367 in Drosophila GIGYF) that engages the FDF pocket of Me31B.

Table 1.
Key residues in GIGYF2 protein interactions with 4EHP (human), Me31B/DDX6 (Dm) and TNRC6A (Human).
Table 1.
Key residues in GIGYF2 protein interactions with 4EHP (human), Me31B/DDX6 (Dm) and TNRC6A (Human).
| Interaction Proteins | GIGYF2 Components | GIGYF2 Residues | Interaction Partners | References |
|---|---|---|---|---|
| 4EHP (Human) | Canonical Motif | Y41 | P55 | [28] |
| M48/L49/Y43 | 4EHP dorsal surface | |||
| F52 | W95/P78 | |||
| Noncanonical Loop | F67 | Y64/K83/I85 | ||
| I70 | Y64 | |||
| I70/Q72 | H100/V102 | |||
| Linker | I58/D55 | H100 | ||
| P59 | F97 | |||
| A1 | L79/A80 | E149 | ||
| L79/V82 | R146 | |||
| P77 | R103 | |||
| A3 | S98 | R138 | ||
| S98-OH | E177 | |||
| V101/L102 | R138/I211 | |||
| Me31B/DDX6 (Dm) | MBM Motif | P347/E348 /W349 | V283/L310/L311 /F370 | [23] |
| F361/F367 | A275/H284/C285 /L289/I421 | |||
| D362 | H368 | |||
| G365/F367 | F276 | |||
| F361 | K423 | |||
| TNRC6A (Human) | GYF Domain | Y538/F549 /W557/Y562 | P1481/P1482 | [37] |
This bipartite binding strategy distinguishes GIGYF from other DDX6-interacting proteins by combining structural elements reminiscent of the 4E-T PEW motif with its specialized “split” FDx4F configuration. Critically, the molecular basis for selective ternary complex formation lies in the differential binding partner requirements. NOT1, the scaffold protein of the CCR4-NOT deadenylase complex, binds to a distinct surface on the DDX6 RecA2 domain that is spatially adjacent to, but non-overlapping with, the FDF pocket utilized by other DDX6 partners [23].
The capacity for ternary complex assembly is determined by the electrostatic compatibility between binding partners and NOT1. Specifically, EDC3 and PatL1 contain negatively charged residues N-terminal to their FDF motifs, which generate unfavorable electrostatic repulsions with NOT1, precluding ternary complex formation. In contrast, both 4E-T and GIGYF feature polar rather than negatively charged residues preceding their respective binding motifs (IEL and FDx4F), thereby permitting the formation of stable ternary complexes: 4E-T-DDX6-NOT1 and GIGYF-DDX6-NOT1 [23]. This structural compatibility enables GIGYF to orchestrate translational repression of target mRNAs through coordinated recruitment of both the DDX6 helicase and the CCR4-NOT deadenylation machinery.
2.2. GYF Domain: Interactions with ZNF598/TTP/TNRC6A
The GYF domain of GIGYF2 (residues 529–597) is a highly conserved structural unit comprising a four-stranded antiparallel β-sheet with two α-helices packed onto one face [37]. Named after its characteristic glycine-tyrosine-phenylalanine motif within the larger GPF-X4-[M/V/I]-X2-W-X3-GYF signature sequence, this domain forms a bulge-helix-bulge structural element generating a hydrophobic ligand-binding surface [1]. Functionally, the GYF domain can be divided into two distinct regions: the N-terminal portion (β1, β2, α1, and α1-β3 loop) constitutes the essential ligand-binding interface for GIGYF2-mediated translational repression, while the C-terminal region shows greater variability across proteins [3]. Notably, the C-terminal phenylalanine residue (F592 in GIGYF2), termed the “Phe plug”, is highly conserved and inserts into a hydrophobic cavity between the α1 and α2 helices and the β-sheet’s inner surface, significantly contributing to domain stability and forming part of the hydrophobic core essential for the domain’s structural integrity [37].
The GYF domain of GIGYF2 mediates specific protein interactions by recognizing PPGΦ motifs, where P represents proline, G represents glycine, and Φ denotes a hydrophobic amino acid [6,7]. This recognition depends on a conserved binding groove formed by residues Y538, F549, W557, Y562, F563, and L567, which accommodates the PPII helical conformation adopted by proline residues in binding partners [37]. The conserved glycine in these motifs is critical as it prevents steric clashes with D540 and Q546 residues while enabling the hydrophobic Φ residue to insert into a specific cavity [7,37]. GIGYF2’s key binding partners—TNRC6A, TTP, and ZNF598—all utilize this interaction mechanism but display important molecular distinctions that confer functional specificity. TNRC6A contains PPGL motifs where leucine occupies the Φ position, precisely fitting into the Smy2 subclass-specific cavity of GIGYF2’s GYF domain [37,38] (Table 1) (Figure 1D). In contrast, both TTP and ZNF598 feature PPPPGF motifs with phenylalanine at the Φ position [7]. ZNF598 contains three polyproline stretches that are structurally similar to those in TTP [38]. Importantly, these interactions are predominantly transient and context-dependent rather than constitutive associations. TNRC6A-GIGYF2 interactions are specifically recruited during miRNA-mediated gene silencing when target mRNAs are bound by the miRISC complex [39]. TTP-GIGYF2 associations are primarily induced under inflammatory conditions when AU-rich element-containing mRNAs require translational repression [38]. ZNF598-GIGYF2 interactions are triggered during ribosome collision events and translational quality control responses [15,40]. Additionally, these GYF domain-dependent interactions can be negatively modulated by stress-induced GIGYF2 phosphorylation, which recruits 14-3-3 proteins and disrupts binding partner associations [41]. Notably, GIGYF2 targeting of mRNAs occurs through dual mechanisms: indirect recruitment via protein–protein interactions with RNA-binding partners (such as TTP recognizing AU-rich elements or TNRC6A in miRISC complexes) and direct RNA binding through its intrinsic RNA-binding regions located in both the N-terminal (residues 1–532) and C-terminal (residues 606–1299) domains [25]. In the context of ribosome collision events, GIGYF2 recruitment is primarily mediated through ZNF598 recognition of collided ribosome surfaces rather than through direct RNA binding or nascent peptide recognition [15,40]. This molecular architecture enables GIGYF proteins to participate in context-specific translational control through a common binding mechanism with partner-specific variations.
2.3. C-Terminal Domain Interactions: Cooperative Roles of Multiple Structural Elements
Studies have demonstrated that molecular interactions involving GIGYF2 frequently necessitate the coordinated participation of multiple structural domains. First, GIGYF2 is involved in the interaction with NSP2 through diverse regions [30,31]. The C-terminal region spanning residues 743–1085 of GIGYF2, along with 4EHP, functions as a critical contact interface for the N-terminal region of NSP2 (residues 1–350), which contains a highly conserved zinc finger domain. Additionally, GIGYF2’s N-terminal domain (residues 1–267), containing the 4EHP-binding motif, also participates in this interaction [31]. Second, GIGYF2 establishes multiple interfaces with the CCR4-NOT complex through RNA-independent mechanisms. The three major domains of GIGYF2 exhibit differential binding preferences with CCR4-NOT subunits—the GYF domain (when attached to the NTD) preferentially interacts with CNOT1 and CNOT7, while the CTD and NTD + GYF regions show stronger affinity for CNOT9 [25]. Third, GIGYF2 directly engages with endogenous mRNAs through two distinct RNA-binding regions: the N-terminal domain (amino acids 1–532) and the C-terminal domain (beyond amino acid 606) [25]. Fourth, GIGYF2 undergoes phosphorylation at two evolutionarily conserved serine residues, S157 and S638, which flank the GYF domain [41]. This phosphorylation is catalyzed by MAP kinase-activated protein kinase 2 (MK2), a downstream effector of the p38 MAPK signaling cascade that is activated during cellular stress responses [42]. The resultant phosphorylation generates binding sites for 14-3-3 proteins, which negatively affects GYF domain-dependent protein interactions [41].
3. Transcriptional Regulation by GIGYF2
When DNA sustains damage from agents such as UV radiation and conventional repair mechanisms are insufficient, the cellular “last resort” pathway is activated as a critical transcription stress response [43]. This pathway involves the selective extraction and degradation of the Rpb1 subunit from stalled RNA polymerase II (RNAPII) complexes in chromatin [43,44,45], which may contribute to the recruitment and activity of repair factors involved in the global genome nucleotide excision repair and transcription-coupled nucleotide excision repair [46,47].
In Saccharomyces cerevisiae, the GIGYF homolog Smy2 functions as a key regulatory factor in the evolutionarily conserved “last resort” pathway through a multi-step interaction mechanism (Figure 2A). The pathway begins with mono-ubiquitylation of Rpb1 (the largest subunit of RNA polymerase II) by the ubiquitin ligase Rsp5 [48]. Concurrently, Smy2 collaborates with Ubx1 and Cdc48 (cell division cycle protein 48) to facilitate efficient proteasome-mediated processing of Def1 into pr-Def1 [49]. The accumulation of pr-Def1 promotes the recruitment of the Elc1-Ela1-Cul3 ligase complex to mono-ubiquitylated Rpb1, catalyzing its subsequent polyubiquitylation [50]. Following this, Smy2 works in concert with Cdc48 and its co-factors Ubx4/5 to extract polyubiquitylated Rpb1 from damage-stalled RNAPII complexes within chromatin [45,49]. The extracted ubiquitylated Rpb1 is then targeted for degradation by the 26S proteasome [51]. Notably, immunoprecipitation experiments have revealed that significant co-precipitation of Cdc48 and Rpb1 occurs only in the presence of Smy2 expression, highlighting the essential bridging function of Smy2 in mediating this critical interaction [49].
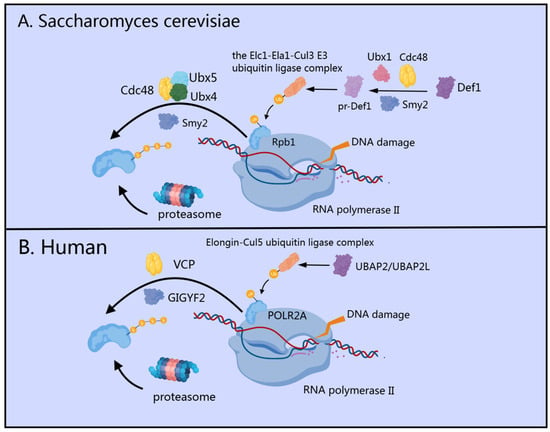
Figure 2.
Role of GIGYF2/Smy2 in the “Last Resort” Pathway During Transcription Stress Response. (A) In Saccharomyces cerevisiae, Smy2 collaborates with Ubx1 and Cdc48 to facilitate proteasome-mediated processing of Def1, recruiting the Elc1-Ela1-Cul3 ligase complex to monoubiquitylated Rpb1. Smy2 then works with Cdc48 and its co-factors Ubx4/5 to extract polyubiquitylated Rpb1 from damage-stalled RNAPII complexes for proteasomal degradation. (B) In human cells, GIGYF2 interacts with VCP (mammalian homolog of Cdc48) to facilitate extraction and proteasomal degradation of ubiquitylated POLR2A from stalled transcription complexes following UBAP2/UBAP2L-mediated recruitment of the Elongin-Cul5 ubiquitin ligase complex.
In human cells, GIGYF1/2 proteins serve as key regulatory factors that interact with VCP (the mammalian homolog of yeast Cdc48), specifically facilitating the extraction and proteasomal degradation of ubiquitylated POLR2A (the human homolog of yeast Rpb1) [47,52] from stalled transcription complexes [49] (Figure 2B). Sophisticated biochemical fractionation experiments have demonstrated that GIGYF2 maintains substantial chromatin association, which becomes markedly enhanced following UV-induced DNA damage, highlighting its direct involvement in the transcription stress response [49]. Recent research has identified UBAP2/UBAP2L as the human orthologs of yeast Def1, establishing their critical role in recruiting the Elongin-Cul5 ubiquitin ligase complex to sites of DNA damage [47]. This recruitment follows a highly coordinated two-step ubiquitylation mechanism: NEDD4 (the human ortholog of yeast Rsp5) acts as the initial E3 ubiquitin ligase [53], adding a single ubiquitin moiety to POLR2A, after which the Elongin-Cul5 ubiquitin ligase complex builds K48-linked polyubiquitin chains on this mono-ubiquitinated substrate [52,54,55,56]. These K48-linked chains effectively mark POLR2A for proteasomal degradation, representing a critical “last resort” pathway in the cellular response to transcription-blocking DNA damage [56].
These molecular mechanisms collectively establish GIGYF1/2 and Smy2 as regulators of the transcriptional stress response and DNA damage repair pathway. Their function as previously overlooked modulators of Cdc48/VCP activity extends beyond transcription stress to multiple cellular processes, representing an important advancement in our understanding of cellular quality control systems and stress response mechanisms.
4. GIGYF2’s Role in mRNA Surveillance
During the translation of aberrant transcripts, suboptimal codons with limited cognate tRNA availability significantly reduce ribosomal transit rates [57]. This programmed deceleration triggers ribosomal collisions along the mRNA transcript [58,59,60]. The collision events function as molecular signaling nodes, effectively coupling translation elongation kinetics to mRNA surveillance. The mRNA surveillance mechanism enables cells to identify and target aberrant mRNAs for degradation, preventing the translation of defective transcripts through three key pathways: nonsense-mediated decay (NMD), no-go decay (NGD), and non-stop decay (NSD) [61,62]. NMD targets transcripts with premature termination codons [63]. NGD recognizes and degrades transcripts on which ribosomes stall within the CDS due to the presence of inhibitory stem-loop structures or in stretches of rare codons [64,65]. NSD handles mRNAs lacking stop codons [66,67]. Working in concert with these systems, ribosome-associated quality control (RQC) resolves stalled translation complexes by rescuing ribosomes and degrading incomplete nascent polypeptides [68]. Recent studies have shown that GIGYF2 plays an important role in mRNA surveillance by forming a complex with 4EHP that binds to the 5’ cap of mRNAs, thus preventing ribosome loading to aberrant transcripts. This complex participates in multiple mRNA surveillance pathways and prevents the continued translation of defective mRNAs, particularly those encoding secretory proteins, membrane proteins, and tubulin subunits [15].
4.1. Transient Collision-Induced ISR Pathway: GIGYF2’s Early Response to Ribosomal Stalling
When two ribosomes collide, EDF1 (endothelial differentiation-related factor 1) is the first responder due to its high cellular abundance [40,69,70]. During transient collisions, EDF1’s engagement triggers a series of coordinated responses. Initially, EDF1 utilizes its conserved KKW motif and alpha-helical segment to clamp the mRNA in a headlock-like arrangement [71], stabilizing collided ribosomes and preventing frameshifting. Through RACK1-dependent and ZNF598-independent mechanisms, EDF1 recruits and stabilizes the GIGYF2-4EHP complex to collision sites [40] (Figure 3A). The GIGYF2-4EHP complex implements translational inhibition via 4EHP-mediated sequestration of the 5’ cap structure, effectively reducing ribosome density along the mRNA [20,71]. Notably, this regulatory mechanism requires both ribosome stalling and intact cap-binding functionality [15].
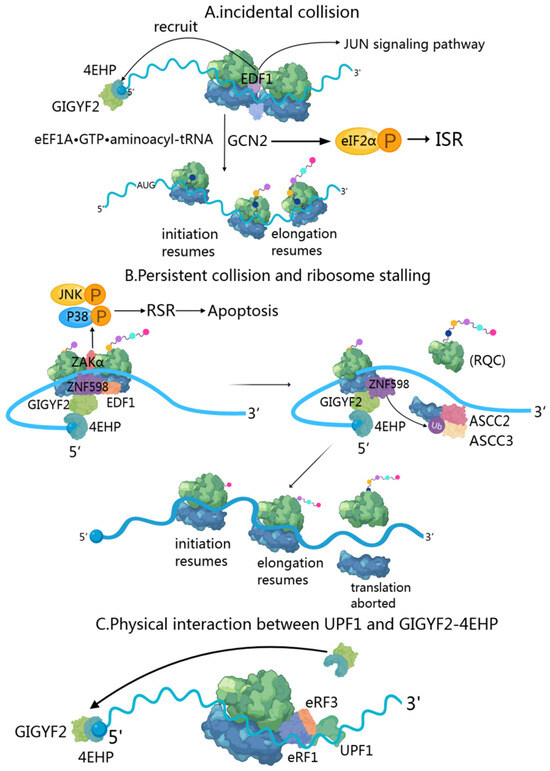
Figure 3.
GIGYF2’s role in mRNA surveillance. (A) Transient ribosome collision. EDF1 rapidly binds to collided ribosomes and recruits the GIGYF2-4EHP complex through RACK1-dependent mechanisms. GIGYF2-4EHP inhibits translation by competing with eIF4E for 5’ cap binding, reducing ribosome density. GCN2 senses collisions and activates the ISR pathway via eIF2α phosphorylation. (B) Persistent collision and ribosome stalling. ZNF598 recognizes collided di-ribosomes and ubiquitinates eS10, triggering ASCC-mediated ribosome dissociation. The GIGYF2-4EHP complex continues translation inhibition while RQC machinery processes incomplete peptides. ZAKα activates RSR pathways (p38/JNK), leading to stress responses. (C) Nonsense-mediated decay regulation. GIGYF2 interacts with UPF1 and EJC components at premature termination codons. The GIGYF2-4EHP complex prevents excessive ribosome loading on NMD targets, reducing collision likelihood. GIGYF2 also regulates NGD and NSD substrates, providing comprehensive quality control against aberrant mRNAs.
Resolution of the collision event proceeds through the activity of the eEF1A·GTP·aminoacyl-tRNA complex, which delivers the appropriate charged tRNA to the ribosome A-site, thereby facilitating the resumption of translational elongation [40]. Beyond its immediate role in collision resolution, EDF1 demonstrates additional regulatory functions, particularly in the JUN-mediated transcriptional response to ribosomal collisions [40]. Simultaneously with these events, GCN2 senses ribosome collision events, triggering phosphorylation of eIF2α and activation of the Integrated Stress Response (ISR) pathway [72,73]. The phosphorylated eIF2α inhibits eIF2B activity and consequently reduces global translation [74,75]. However, this translational reprogramming selectively preserves the expression of a small number of stress response mRNAs, including GCN4 and ATF4 [76].
4.2. Persistent Collision-Activated RQC and RSR Pathways: GIGYF2’s Role in Sustained Translational Stress
Ribosome collisions occur when trailing ribosomes encounter stalled leading ones during translation, primarily due to genetic mutations, mRNA processing errors, insufficient charged tRNAs, and poly(A) tracts created by premature polyadenylation [77,78,79]. This creates distinctive 40S-40S interfaces that serve as recognition sites for quality control mechanisms [64,80]. EDF1, a highly abundant protein, acts as the primary sensor of these collisions, binding to the collided ribosomes and stabilizing the recruitment of the ZNF598-GIGYF2-4EHP complex [40]. In persistent collisions, E3 ubiquitin-protein ligase ZNF598 recognizes collided di-ribosome structures on non-stop mRNA substrates [81] by specifically binding to target sites at the 40S-40S interface [64,80], particularly when a trailing ribosome catches up to a stalled one. The GIGYF2-4EHP complex inhibits translation initiation by competing with eIF4E for the mRNA 5’ cap, providing an initial response to reduce ribosome density on problematic transcripts (Figure 3B).
If collisions persist despite this initial response, ZNF598 ubiquitinates several 40S ribosomal proteins (primarily eS10) over approximately 2–5 min to accumulate to significant levels under near-physiological conditions [82]. This time delay ensures that ZNF598-dependent quality control is only initiated when persistent ribosome collisions cannot be resolved through conventional translation dynamics [40]. The ubiquitination of eS10 serves as a signal for the ASC-1 complex (ASCC), which contains the ASCC3 helicase, to recognize and act on the stalled ribosome. ASCC then directly dissociates the lead ribosome into 40S and 60S subunits, irreversibly aborting translation and triggering ribosome quality control (RQC) [62,80,83,84,85,86]. Following ribosome separation, the resultant 60S subunit-peptidyl-tRNA complex undergoes processing via the RQC machinery, comprising Listerin (an E3 ubiquitin ligase), NEMF, and additional factors that facilitate appropriate management of incomplete nascent peptides [87]. This mechanism prevents the accumulation of potentially cytotoxic protein fragments within the cellular environment. Upon resolution of the stalled lead ribosome, trailing ribosomes are liberated from their collision state and can resume translational elongation. The dissolution of the collision architecture induces the dissociation of both EDF1 and the ZNF598-GIGYF2-4EHP complex from the mRNA [40]. As 4EHP disengages from the 5’ cap, it no longer competes with eIF4E, thereby permitting the reinitiation of translation on the affected transcript. This hierarchical response system enables cellular machinery to dynamically modulate translation rates while maintaining translational fidelity, thus ensuring both qualitative and quantitative integrity of protein synthesis. Concurrently, ZAKα activates MAP kinase cascades of the ribotoxic stress response (RSR) [72], including p38 and JNK pathways [88,89], leading to cell cycle arrest or apoptotic responses depending on stress severity [90].
4.3. Integration of NMD, NGD, and NSD Mechanisms: GIGYF2’s Comprehensive Approach to mRNA Surveillance
When a translating ribosome encounters a premature termination codon, GIGYF2 becomes involved in the NMD through an RNA-dependent interaction with UPF1 and EJC components [91] (Figure 3C). UPF1, a key NMD factor with RNA helicase activity, initially associates with the terminating ribosome through interactions with release factors eRF1 and eRF3 [92]. While UPF1 phosphorylation by SMG1 is essential for NMD progression [93], the study shows this phosphorylation state does not affect the GIGYF2-UPF1 interaction [91]. The GIGYF2-4EHP complex functions primarily through translational repression of NMD targets with 3’ UTR introns [91,94,95,96]. These EJC-dependent NMD substrates characteristically display elevated ribosome densities. GIGYF2-EIF4E2 serves as a molecular brake that prevents excessive ribosome loading onto NMD targets, thereby reducing the likelihood of ribosome collisions and minimizing the number of ribosomes requiring rescue after SMG6-mediated transcript cleavage [97]. GIGYF2-4EHP regulates ribosomal occupancy of specific splicing isoforms like SRSF3, which contains an ultraconserved “poison cassette exon” with a PTC that triggers NMD. The SR splicing regulator encoded by SRSF3 autoregulates this exon’s inclusion, creating a feedback loop through alternative splicing and NMD to control its expression levels [98,99,100].
Additionally, GIGYF2 has been shown to inhibit the translation of non-stop decay (NSD) and no-go decay (NGD) substrates, with its inhibitory effect on NSD substrates being independent of ZNF598 [81]. The ribosome collisions activate the NGD pathway to degrade faulty mRNAs and initiate the RQC pathway to resolve stalled ribosomes, recycle ribosomal subunits, and degrade potentially harmful aberrant polypeptides via ZNF598-mediated ubiquitination of RPS10 [64,90]. Simultaneously, the EDF1-GIGYF2-4EHP complex inhibits translation initiation, preventing further ribosome loading onto problematic transcripts [71,101]. This provides a comprehensive cellular quality control mechanism that effectively suppresses the potential detrimental effects of aberrant polypeptides.
These surveillance mechanisms constitute a hierarchical quality control system that safeguards cellular proteostasis by preventing the accumulation of potentially toxic translation products. The dynamic interplay between transient and persistent ribosome collisions allows cells to calibrate their response according to translational stress severity, with GIGYF2 serving as a coordinator across multiple mRNA surveillance pathways. By operating at the critical nexus between translation and degradation, GIGYF2 ensures protein synthesis fidelity while linking translational stress to broader cellular responses.
5. GIGYF2-Mediated mRNA Degradation Mechanisms
GIGYF2 functions as a pivotal regulator of post-transcriptional gene expression by targeting specific mRNAs for translational repression and degradation. This multifunctional adaptor protein operates at the intersection of mRNA surveillance and decay pathways through diverse molecular mechanisms. Co-translational binding of GIGYF2 to mRNA marks transcripts with perturbed elongation for decay, a process that requires translation of the coding sequence (CDS) [15]. Through both cap-dependent inhibition and direct recruitment of degradation factors, GIGYF2 exerts precise control over mRNA fate in a context-dependent manner. We summarize four key mechanisms through which GIGYF2 mediates mRNA degradation, emphasizing its central role in RNA quality control and metabolism.
5.1. ZNF598-GIGYF2-4EHP Complex in Ribosome Collision-Mediated Decay
When ribosome collision occurs during translation of mRNAs encoding secreted and membrane-bound proteins or tubulin subunits, ZNF598 is recruited to the 40S-40S subunits of the collided ribosomes [64,80]. GIGYF2’s specific role in this pathway involves serving as a molecular scaffold that directly binds ZNF598 through its GYF domain via Pro-Pro-Gly-Φ motifs [15] (Figure 4A). The ribosome pausing and collision event serves as the essential initiating signal for GIGYF2-mediated translational repression and mRNA degradation [15].
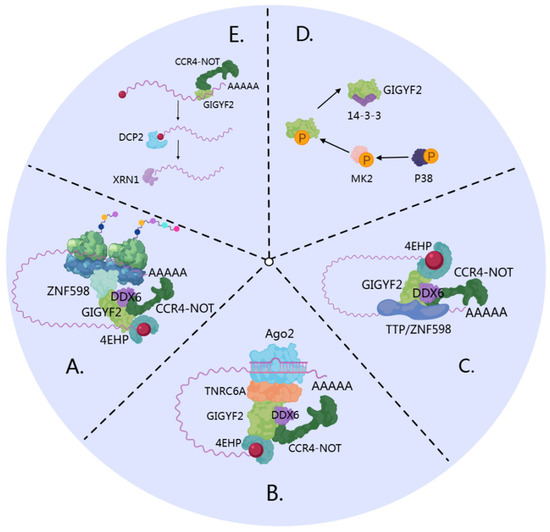
Figure 4.
GIGYF2-mediated mRNA decay pathways converging on P-bodies. (A) Ribosome collision-mediated decay: GIGYF2 serves as a molecular scaffold binding ZNF598 and orchestrating assembly of the CCR4-NOT4-4EHP-DDX6 degradation complex. GIGYF2-4EHP inhibits translation initiation by competing with eIF4E for 5’ cap binding. (B) miRNA-mediated silencing: GIGYF2 bridges miRNA targeting machinery (AGO2-miRNA-TNRC6A) and translational repression factors (4EHP) by binding TNRC6A through its GYF domain, facilitating target mRNA silencing. (C) TTP/ZNF598-mediated recruitment: GIGYF2 links sequence-specific RNA-binding proteins (TTP and ZNF598) to the translational repression machinery through GYF domain interactions, enabling 4EHP-mediated repression of inflammation-associated mRNAs. (D) Stress-induced regulation: p38 MAPK-activated MK2 phosphorylates GIGYF2 (S157, S638), creating 14-3-3 binding sites that negatively modulate GIGYF2’s interactions with GYF domain partners, inhibiting GIGYF2-mediated mRNA regulation. (E) Direct RNA-binding function: GIGYF2 directly binds target mRNAs (especially ER-destined transcripts) and recruits the CCR4-NOT complex for deadenylation, promoting mRNA degradation independent of 4EHP. All pathways converge through conventional decay involving DCP2 decapping and XRN1 exonucleolytic degradation.
Upon recruitment, GIGYF2 orchestrates the assembly of a degradation complex comprising CCR4-NOT4, 4EHP, and the DEAD-box RNA helicase DDX6 [15,25]. In this complex, GIGYF2 facilitates the functional coordination between different degradation factors: the CCR4-NOT4 complex mediates deadenylation of target mRNAs, while DDX6 promotes translational repression and facilitates decapping complex assembly [102,103,104]. This mechanism effectively integrates ribosome quality control with mRNA degradation through GIGYF2’s scaffolding function.
5.2. GIGYF2 as an RNA-Binding Protein in Direct mRNA Targeting
GIGYF2 functions as a sequence-specific RNA-binding protein (RBP) that directly recruits the CCR4-NOT complex to promote deadenylation of target mRNAs, accelerating their degradation through a mechanism independent of 4EHP [25,105] (Figure 4E). Five endogenous targets of GIGYF2-mediated silencing have been identified and validated: SVOPL, COL8A1, NPR3, CPM, and ECEL1 [25]. These targets were confirmed through RNA immunoprecipitation experiments demonstrating their direct association with GIGYF2, and their repression was shown to be independent of 4EHP binding [25]. GIGYF2 exhibits target selectivity with notable enrichment for mRNAs encoding proteins destined for the endoplasmic reticulum. Among the 23 endogenous RNAs identified as GIGYF2 targets, 15 out of 21 protein-coding transcripts encode transmembrane, GPI-anchored, or secreted proteins requiring ER-associated translation [25]. This spatial organization appears functionally relevant, as GIGYF2 localizes to the ER at steady-state [7], suggesting that GIGYF2-mediated repression may be spatially restricted to this cellular compartment. Notably, GIGYF2 can exert dual regulatory effects on mRNA stability as an RBP. The GIGYF2 fragment spanning amino acids 1-495 interacts with STAU1 mRNA and enhances its stability [12,106], demonstrating context-dependent regulatory functions.
5.3. GIGYF2’s Recruitment to Target Transcripts via RNA-Binding Proteins (RBPs)
GIGYF2 can be indirectly recruited to mRNAs through other RNA-binding proteins, particularly TTP and ZNF598, primarily facilitating 4EHP-dependent translational repression [25,38] (Figure 4C). GIGYF2’s role in this mechanism involves serving as a bridging factor that links sequence-specific RBPs to the translational repression machinery, particularly for inflammation-associated transcripts. The molecular basis of these interactions relies on GIGYF2’s GYF domains’ high affinity for polyproline stretches [107]. Both ZNF598 and TTP contain conserved polyproline stretches that bind to GIGYF2’s GYF domain, enabling integration into larger 4EHP-GIGYF2-RBP translation initiation inhibitory complexes [9,38]. ZNF598 and TTP exhibit partially overlapping mRNA-binding repertoires while maintaining distinct binding specificities. TTP recognizes adenylate-uridylate-rich elements (AREs) in 3’ UTRs through its two RNA-binding zinc fingers [108], whereas ZNF598 possesses C2H2-type zinc fingers with broader binding specificity for sequences in both coding and non-coding regions [109]. GIGYF2’s assembly function is critical for TTP-mediated regulation, as the formation of the 4EHP-GIGYF2-DDX6 complex is essential for TTP-mediated translational repression of AU-rich element-containing mRNAs [23]. GIGYF2’s recruitment of DDX6 serves as the critical molecular link between TTP’s ARE-binding activity and the translational repression machinery [23].
5.4. GIGYF2’s Role in miRNA-Mediated Silencing via TNRC6A Interaction
During miRNA-mediated silencing, GIGYF2 functions as a regulatory component that facilitates post-transcriptional gene regulation through direct interactions with TNRC6A in the miRNA-induced silencing complex (miRISC) [24,37] (Figure 4B). GIGYF2’s specific contribution involves facilitating the recruitment of 4EHP to Argonaute-miRNA complexes, thereby establishing a functional bridge between miRNA targeting machinery and translational repression factors [110]. Crystallographic analyses have elucidated the molecular basis of this interaction, revealing that GIGYF2’s GYF domain specifically recognizes and binds to a conserved PPGL motif within TNRC6A’s silencing domain [37,39]. TNRC6A serves as the primary scaffold protein within miRISC architecture [24,111], mediating CCR4-NOT complex recruitment via CNOT1 interactions [112,113,114]. CNOT1 directly binds DDX6, facilitating DDX6 localization to miRNA-targeted mRNAs [115].
5.5. Convergence on mRNA Degradation Pathways and P-Body Association
These four GIGYF2-mediated pathways converge on common mRNA degradation mechanisms involving deadenylation and decapping processes. While several GIGYF2-associated factors are known components of processing bodies (P-bodies), the extent of GIGYF2’s direct involvement in P-body dynamics requires further investigation [116,117,118,119]. Processing bodies represent discrete cytoplasmic foci that concentrate untranslating mRNAs with decay machinery, serving as sites where mRNAs undergo degradation, storage, or silencing [119,120]. The mRNA degradation process typically follows a sequential pathway: deadenylation through the CCR4-CAF1-NOT complex removes the poly(A) tail, followed by decapping via the DCP1/DCP2 complex and co-activators including Dhh1/RCK/p54, Pat1, and Lsm1-7 [121,122,123]. Subsequently, XRN1 mediates 5’-to-3’ exonucleolytic degradation [119,124]. Studies using Smy2 (GIGYF2 homolog) have provided insights into potential P-body associations, revealing binding to P-body components including Xrn1 exonuclease, Dcp2, seven Ccr4-NOT complex subunits, and Lsm proteins [7]. However, it should be noted that these binding partners are not exclusively localized to P-bodies and function in multiple cellular contexts.
5.6. Stress-Induced Regulation of GIGYF2 Activity
Under stress conditions, GIGYF2 undergoes regulatory phosphorylation at two evolutionarily conserved serine residues, S160 and S684, flanking the GYF domain [41] (Figure 4D). This phosphorylation is mediated by MAP kinase-activated protein kinase 2 (MK2), a downstream effector of the p38 MAPK pathway [42]. The resulting phosphorylation creates binding sites for 14-3-3 proteins, and this stress-induced 14-3-3 binding negatively modulates GIGYF2’s interactions with canonical binding partners, particularly those mediated by the GYF domain, thereby inhibiting GIGYF2-mediated transcriptional repression and mRNA degradation [41].
GIGYF2 orchestrates mRNA degradation through four distinct yet integrated pathways: coordinating degradation complexes at ribosome collision sites; directly binding target transcripts to recruit deadenylation factors; partnering with RNA-binding proteins to repress inflammation-associated mRNAs; and linking miRNA machinery to translational repression. These mechanisms converge on established mRNA degradation pathways involving deadenylation and decapping processes. Stress-induced phosphorylation enables 14-3-3 binding that modulates GIGYF2 activity, positioning it as a critical regulator that integrates diverse cellular signals to control transcript fate.
6. GIGYF2-Associated Pathological Conditions
The extensive involvement of GIGYF2 in multiple regulatory mechanisms spanning transcriptional control, translational repression, and mRNA degradation positions this protein as a significant coordinator of cellular homeostasis. The functional consequences of GIGYF2 activity extend beyond normal physiological processes to pathological states. The alterations in GIGYF2 expression, regulation, or protein–protein interactions have been documented across several disease contexts. The subsequent section examines the evidence linking GIGYF2 to specific pathological conditions, including metabolic diseases, vascular aging, viral infections, and neurodegenerative disorders, thereby elucidating the clinical implications of this molecular regulator.
6.1. GIGYF2 in Metabolic Diseases
In metabolic diseases, particularly hepatic insulin resistance and type 2 diabetes, GIGYF2 functions as an RNA-binding protein that enhances STAU1 mRNA stability [12,106] (Figure 5A). This upregulated STAU1 subsequently binds to and stabilizes PTEN transcripts [12,125], leading to increased PTEN protein expression that disrupts the PI3K/AKT signaling cascade. The consequent reduction in AKT phosphorylation at both Thr308 and Ser473 residues impairs downstream glucose metabolism and insulin sensitivity [126,127,128]. A pathological feedback loop is established where palmitic acid, abundant in obesity, induces GIGYF2 expression, triggering insulin resistance, which further exacerbates metabolic dysfunction [12].
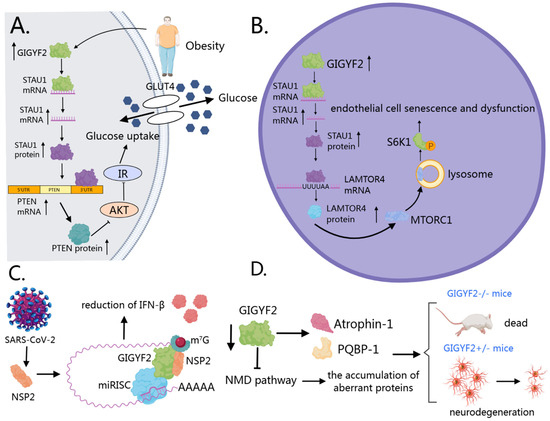
Figure 5.
GIGYF2’s roles in various pathological conditions. (A) Metabolic diseases: GIGYF2 stabilizes STAU1 mRNA, leading to increased STAU1 expression that enhances PTEN mRNA stability. Elevated PTEN disrupts PI3K/AKT signaling, reducing AKT phosphorylation and impairing glucose metabolism and insulin sensitivity. Palmitic acid induces GIGYF2 expression, creating a pathological feedback loop that exacerbates metabolic dysfunction. (B) Vascular aging: GIGYF2 promotes endothelial senescence by stabilizing STAU1 mRNA, which enhances LAMTOR4 mRNA stability. Elevated LAMTOR4 activates mTORC1-S6K1 signaling, triggering eNOS uncoupling and reducing nitric oxide production while increasing reactive oxygen species, leading to endothelial dysfunction and vascular aging. (C) Viral infections: SARS-CoV-2 NSP2 directly interacts with GIGYF2, enhancing GIGYF2-4EHP binding and increasing miRNA-mediated translation suppression. This interaction represses Ifnb1 mRNA translation, reducing IFN-β levels and compromising the host’s antiviral immune response. (D) Neurodegenerative disorders: GIGYF2 maintains neuronal proteostasis through direct interactions with disease-associated proteins (Atrophin-1, PQBP-1) and mRNA quality control via the GIGYF2-4EHP complex. GIGYF2 deficiency allows accumulation of aberrant proteins, contributing to neurodevelopmental and neuropsychiatric phenotypes.
6.2. GIGYF2 in Vascular Aging
In vascular aging, GIGYF2 promotes endothelial cell senescence and dysfunction through activation of the mTORC1-S6K1 signaling pathway [106] (Figure 5B). As an RNA-binding protein, GIGYF2 binds to and stabilizes STAU1 mRNA, leading to increased STAU1 expression. This upregulated STAU1 then binds to specific intron regions of LAMTOR4 mRNA, enhancing its stability and increasing LAMTOR4 expression [106]. Elevated LAMTOR4 facilitates the translocation of mTORC1 to lysosomes, activating the mTORC1-S6K1 signaling cascade [129,130,131,132]. This activation triggers eNOS uncoupling, resulting in decreased nitric oxide production and increased generation of reactive oxygen species, creating oxidative stress and endothelial dysfunction [133,134]. Consequently, senescent cells exhibit increased expression of senescence markers (p21, p16), decreased expression of anti-aging genes (SIRT1, SIRT6), and enhanced secretion of pro-inflammatory factors [106]. These cellular alterations contribute to vascular dysfunction through various mechanisms: overexpression of adhesion molecules (ICAM-1, VCAM-1), increased monocyte adhesion to endothelial cells, impaired endothelium-dependent vasodilation, and increased arterial stiffness [106]. Notably, GIGYF2 is hyper-expressed in senescent human endothelial cells and aged mouse aortas, while endothelial-specific GIGYF2 knockout provides protection against age-associated vascular dysfunction, highlighting its potential as a therapeutic target for vascular aging and age-related cardiovascular diseases.
6.3. GIGYF2 in Viral Infections
In viral infections, SARS-CoV-2 protein NSP2 directly interacts with human GIGYF2, enhancing the binding of GIGYF2 to 4EHP and partially increasing GIGYF2-mediated miRNA translation suppression [29,30,31] (Figure 5C). Notably, NSP2 displays selectivity, binding preferentially to the diffuse cytoplasmic form of GIGYF2 rather than its P-body-associated counterpart [31]. Studies have found that the NSP2-GIGYF2 interaction correlates with downstream effects on immune response, particularly the repression of Ifnb1 mRNA translation [30]. This results in reduced IFN-β levels, consequently diminishing the host’s antiviral immune defense mechanisms [135]. This interaction mechanism assumes greater significance in light of findings by Masood et al. demonstrating that upregulated type I interferon responses are associated with improved clinical outcomes in asymptomatic cases of COVID-19 [136,137]. Since SARS-CoV-2 NSP2 directly targets GIGYF2 to suppress IFN-β production, and production of type I interferons is vital to antiviral immunity as a host defense mechanism [135], disrupting the NSP2-GIGYF2 interaction could represent a promising therapeutic strategy. Zhou et al. (2020) established that SARS-CoV-2 shares 79.6% sequence identity with SARS-CoV and uses the same ACE2 receptor for cellular entry [138], providing further context for understanding viral pathogenesis. Given these findings, GIGYF2 warrants further investigation as a potential therapeutic target for SARS-CoV-2 infection, though additional studies are needed to validate this approach in appropriate experimental models and clinical settings.
6.4. GIGYF2 in Neurodegenerative Disorders
In neurodegenerative disorders, genetic studies have identified potential associations between GIGYF2 mutations and Parkinson’s disease, though the causative relationship remains debated due to inconsistent replication across different populations and the identification of mutations in both affected and unaffected individuals [21,38,139,140]. Animal model studies provide more compelling evidence for GIGYF2’s essential role in neurological function. Homozygous GIGYF2-null mice exhibit perinatal lethality due to feeding deficits, while heterozygous mice develop normally but progressively manifest age-related motor dysfunction, neurodegeneration, and disrupted IGF signaling [141], phenotypes reminiscent of human neurodegenerative conditions.
The molecular basis of GIGYF2’s neuroprotective function operates through two complementary mechanisms (Figure 5D). First, GIGYF2 directly interacts with neurological disease-associated proteins, including Atrophin-1 (implicated in dentatorubral pallidoluysian atrophy) and PQBP-1 (linked to intellectual disability) [7], potentially modulating their pathogenic activities. Second, GIGYF2 maintains protein homeostasis through its role in mRNA quality control. Specifically, the GIGYF2-4EHP complex prevents translation of nonsense-mediated decay (NMD) targets, thereby blocking production of potentially neurotoxic truncated proteins [91]. Consequently, GIGYF2 deficiency compromises this protective surveillance mechanism, allowing accumulation of aberrant proteins that may contribute to the neurodevelopmental and neuropsychiatric phenotypes observed in both mouse models and human patients [81].
The diverse pathologies associated with GIGYF2 dysregulation demonstrate its significance as a critical node in multiple disease networks. From metabolic disorders to vascular aging, viral infections, and neurodegenerative disorders, aberrant GIGYF2 activity contributes to disease progression through disruption of essential regulatory mechanisms. These findings collectively highlight GIGYF2 as a promising therapeutic target with broad clinical potential. Future research should focus on developing tissue-specific and context-dependent approaches to modulate GIGYF2 function, which may yield novel interventions for these challenging medical conditions while minimizing potential adverse effects on essential cellular processes.
7. Discussion
In various cellular contexts, GIGYF2 functions as an important regulator at the majority of stages of gene expression. At the transcriptional level, GIGYF2 is associated with transcriptional regulation through its interaction with VCP/p97 to extract ubiquitylated Rpb1 from stalled RNA polymerase II complexes during DNA damage response. In post-transcriptional processes, GIGYF2 is intricately involved in mRNA surveillance, translational repression, and mRNA degradation. Notably, under stress conditions, GIGYF2 also contributes to the subcellular localization of mRNAs to stress granules. As a translational repressor and regulator of RNA stability, GIGYF2 forms complexes through its multiple domains (N-terminal, GYF, and C-terminal) with multiple partners, including proteins and mRNAs (Table 2), to modulate gene expression in diverse cellular contexts. However, several questions regarding the precise molecular mechanisms of GIGYF2 function still remain unresolved.

Table 2.
GIGYF2’s protein and RNA interactions in gene expression regulation.
7.1. Regulation of GIGYF2: An Underexplored Frontier
Despite the extensive characterization of GIGYF2’s downstream effects, the upstream regulatory mechanisms governing GIGYF2 expression, activity, and subcellular localization remain largely unexplored. Current knowledge is limited to stress-induced phosphorylation by MK2 kinase at serine residues S157 and S638, which recruits 14-3-3 proteins and modulates GYF domain-dependent interactions [41]. However, this represents only a fraction of the potential regulatory landscape. Several critical questions remain unanswered: What transcriptional and post-transcriptional mechanisms control GIGYF2 expression levels across different cell types and developmental stages? Are there additional post-translational modifications beyond phosphorylation that fine-tune GIGYF2 activity? How do different cellular stresses specifically modulate GIGYF2 function, and what are the upstream signaling cascades involved?
The tissue-specific expression patterns of GIGYF2, with particularly high levels in the liver, pancreas, brain, lung, kidney, and spleen [19], suggest the existence of tissue-specific regulatory programs that remain to be elucidated. Furthermore, the dynamic redistribution of GIGYF2 from ER/Golgi to stress granules under cellular stress [7] implies the existence of sophisticated trafficking mechanisms that are currently poorly understood. The identification of these regulatory networks will be crucial for understanding how GIGYF2 activity is coordinated with broader cellular programs and how dysregulation contributes to pathological conditions.
7.2. Nuclear Versus Cytoplasmic Functions: Complementary or Competitive Roles?
An intriguing aspect of GIGYF2 biology is its dual subcellular localization and the potential interplay between its nuclear and cytoplasmic functions. While GIGYF2 exhibits predominantly cytoplasmic localization where it regulates translation and mRNA degradation, a subset of GIGYF2 localizes to the nucleus where it participates in transcriptional regulation through the VCP/p97-mediated extraction pathway [49]. This raises fundamental questions about whether these nuclear and cytoplasmic roles represent complementary or potentially competitive functions.
The nuclear function of GIGYF2 in facilitating POLR2A extraction during transcriptional stress may indirectly influence the cytoplasmic mRNA landscape by affecting transcript abundance and quality. Conversely, the cytoplasmic functions of GIGYF2 in mRNA surveillance and degradation may influence which transcripts are available for nuclear export and subsequent processing. This bidirectional relationship suggests a potential coordination between nuclear quality control and cytoplasmic RNA metabolism that warrants systematic investigation.
Additionally, the identification of spliceosomal proteins (Prp8, Lsm4, U2AF65, and U2AF35) as potential GIGYF2 interaction partners [7] raises the possibility of previously uncharacterized nuclear functions beyond transcriptional stress responses. Unlike other GYF domain proteins that are primarily nuclear and involved in pre-mRNA splicing [11,142], GIGYF2’s Smy2-type GYF domain and predominantly cytoplasmic localization suggest unique functional adaptations that may bridge nuclear and cytoplasmic RNA processing events.
7.3. Disease-Associated Molecular Mechanisms: Current Limitations and Future Directions
While GIGYF2 has been implicated in diverse pathological conditions, including metabolic diseases, vascular aging, viral infections, and neurodegenerative disorders, the molecular mechanisms identified in disease contexts remain limited and often correlative rather than mechanistically definitive. This limitation stems from several factors that constrain our current understanding. First, most disease-associated studies have focused on single pathway analyses, failing to capture the multifaceted nature of GIGYF2 function. For instance, while the GIGYF2-STAU1-PTEN axis has been well-characterized in metabolic diseases [12], how this pathway interacts with GIGYF2’s roles in mRNA surveillance and translational control remains unclear. The potential crosstalk between different GIGYF2-mediated pathways in disease progression requires systematic investigation. Second, the temporal dynamics of GIGYF2 dysfunction in disease development are poorly understood. It remains unclear whether GIGYF2 alterations represent primary causative events, secondary adaptive responses, or consequences of disease progression. This distinction is crucial for determining whether GIGYF2 represents a viable therapeutic target or primarily a disease biomarker. Third, the tissue-specific and context-dependent nature of GIGYF2 function complicates the translation of findings across different disease models and human pathology. The same molecular pathway may have distinct consequences in different cellular environments, necessitating more nuanced approaches to understanding GIGYF2’s role in human disease.
7.4. Unresolved Mechanistic Questions and Future Perspectives
Several specific mechanistic questions require resolution to advance our understanding of GIGYF2 function. The precise binding interface between GIGYF2 and VCP/p97 remains incompletely characterized [49], limiting our understanding of how this interaction is regulated and potentially targeted therapeutically. Similarly, the molecular determinants governing GIGYF2’s recruitment to stress granules and its potential role in regulating P-body/stress granule dynamics during cellular stress require further investigation. The relationship between GIGYF2’s RNA-binding capacity and its protein-mediated recruitment to target mRNAs represents another area requiring clarification. While GIGYF2 can directly bind RNA through its N-terminal and C-terminal domains [25], the relative contributions of direct RNA binding versus protein-mediated recruitment in different cellular contexts remain unclear.
7.5. Conclusions and Therapeutic Implications
GIGYF2 serves as a versatile regulator across multiple levels of gene expression, spanning transcription, translation, mRNA surveillance, and degradation through diverse protein–protein and protein-RNA interactions. Its involvement in metabolic diseases, vascular aging, viral infections, and neurodegenerative disorders highlights its clinical significance. However, the current limitations in understanding GIGYF2 regulation, the relationship between its nuclear and cytoplasmic functions, and the molecular mechanisms underlying disease associations represent significant opportunities for future research.
Addressing these knowledge gaps will be essential for developing targeted therapeutic strategies that can modulate GIGYF2 function in a tissue-specific and context-dependent manner. The multifaceted nature of GIGYF2 function suggests that successful therapeutic interventions will require nuanced approaches that consider the complex interplay between its various cellular roles. Future research should prioritize systematic approaches to understand GIGYF2 regulation, comprehensive analyses of its subcellular functional organization, and mechanistic studies of its role in human disease pathogenesis.
Author Contributions
Conceptualization, C.-S.Z. and X.-Y.X.; investigation, C.-S.Z., S.-H.L., Z.-Y.L. and J.-Y.C.; resources, Z.-Y.L., J.-Y.C. and S.-H.L.; writing—original draft preparation, C.-S.Z. and S.-H.L.; writing—review and editing, S.-H.L. and X.-Y.X.; supervision, S.-H.L., Z.-Y.L. and X.-Y.X.; funding acquisition, S.-H.L., J.-Y.C. and Z.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) (grant Nos. 82260531 and 81760509) and the Natural Science Foundation of Jiangxi Province of China (grant Nos. 20224BAB206057 and 20181BAB205043) to X.-Y.X.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors have declared that no competing interests exist.
References
- Freund, C.; Dötsch, V.; Nishizawa, K.; Reinherz, E.L.; Wagner, G. The GYF domain is a novel structural fold that is involved in lymphoid signaling through proline-rich sequences. Nat. Struct. Biol. 1999, 6, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, K.; Freund, C.; Li, J.; Wagner, G.; Reinherz, E.L. Identification of a proline-binding motif regulating CD2-triggered T lymphocyte activation. Proc. Natl. Acad. Sci. USA 1998, 95, 14897–14902. [Google Scholar] [CrossRef]
- Kofler, M.M.; Freund, C. The GYF domain. FEBS J. 2006, 273, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Lillie, S.H.; Brown, S.S. Suppression of a myosin defect by a kinesin-related gene. Nature 1992, 356, 358–361. [Google Scholar] [CrossRef]
- Kofler, M.; Motzny, K.; Beyermann, M.; Freund, C. Novel interaction partners of the CD2BP2-GYF domain. J. Biol. Chem. 2005, 280, 33397–33402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kofler, M.; Motzny, K.; Freund, C. GYF domain proteomics reveals interaction sites in known and novel target proteins. Mol. Cell Proteom. 2005, 4, 1797–1811. [Google Scholar] [CrossRef]
- Ash, M.R.; Faelber, K.; Kosslick, D.; Albert, G.I.; Roske, Y.; Kofler, M.; Schuemann, M.; Krause, E.; Freund, C. Conserved beta-hairpin recognition by the GYF domains of Smy2 and GIGYF2 in mRNA surveillance and vesicular transport complexes. Structure 2010, 18, 944–954. [Google Scholar] [CrossRef]
- Bertazzon, M.; Hurtado-Pico, A.; Plaza-Sirvent, C.; Schuster, M.; Preußner, M.; Kuropka, B.; Liu, F.; Kirsten, A.Z.A.; Schmitt, X.J.; König, B.; et al. The nuclear GYF protein CD2BP2/U5-52K is required for T cell homeostasis. Front. Immunol. 2024, 15, 1415839. [Google Scholar] [CrossRef]
- Fu, R.; Olsen, M.T.; Webb, K.; Bennett, E.J.; Lykke-Andersen, J. Recruitment of the 4EHP-GYF2 cap-binding complex to tetraproline motifs of tristetraprolin promotes repression and degradation of mRNAs with AU-rich elements. RNA 2016, 22, 373–382. [Google Scholar] [CrossRef]
- Hale, V.A.; Guiney, E.L.; Goldberg, L.Y.; Haduong, J.H.; Kwartler, C.S.; Scangos, K.W.; Goutte, C. Notch signaling is antagonized by SAO-1, a novel GYF-domain protein that interacts with the E3 ubiquitin ligase SEL-10 in Caenorhabditis elegans. Genetics 2012, 190, 1043–1057. [Google Scholar] [CrossRef]
- Kofler, M.; Heuer, K.; Zech, T.; Freund, C. Recognition sequences for the GYF domain reveal a possible spliceosomal function of CD2BP2. J. Biol. Chem. 2004, 279, 28292–28297. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Ren, Y.; Li, Y.; Niu, F.; Li, Z.; Li, M.; Li, X.; Li, Q.; Huang, D.; Yu, Y.; et al. RNA-binding protein GIGYF2 orchestrates hepatic insulin resistance through STAU1/PTEN-mediated disruption of the PI3K/AKT signaling cascade. Mol. Med. 2024, 30, 124. [Google Scholar] [CrossRef] [PubMed]
- McCutchen-Maloney, S.L.; Matsuda, K.; Shimbara, N.; Binns, D.D.; Tanaka, K.; Slaughter, C.A.; DeMartino, G.N. cDNA cloning, expression, and functional characterization of PI31, a proline-rich inhibitor of the proteasome. J. Biol. Chem. 2000, 275, 18557–18565. [Google Scholar] [CrossRef]
- Schlundt, A.; Sticht, J.; Piotukh, K.; Kosslick, D.; Jahnke, N.; Keller, S.; Schuemann, M.; Krause, E.; Freund, C. Proline-rich sequence recognition: II. Proteomics analysis of Tsg101 ubiquitin-E2-like variant (UEV) interactions. Mol. Cell Proteom. 2009, 8, 2474–2486. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Chung, M.Y.; Keskeny, C.; Zinnall, U.; Landthaler, M.; Valkov, E.; Izaurralde, E.; Igreja, C. 4EHP and GIGYF1/2 Mediate Translation-Coupled Messenger RNA Decay. Cell Rep. 2020, 33, 108262. [Google Scholar] [CrossRef]
- Zaiss, D.M.; Standera, S.; Kloetzel, P.M.; Sijts, A.J. PI31 is a modulator of proteasome formation and antigen processing. Proc. Natl. Acad. Sci. USA 2002, 99, 14344–14349. [Google Scholar] [CrossRef]
- Choi, J.H.; Luo, J.; Hesketh, G.G.; Guo, S.; Pistofidis, A.; Ladak, R.J.; An, Y.; Naeli, P.; Alain, T.; Schmeing, T.M.; et al. Repression of mRNA translation initiation by GIGYF1 via disrupting the eIF3-eIF4G1 interaction. Sci. Adv. 2024, 10, eadl5638. [Google Scholar] [CrossRef]
- Giovannone, B.; Lee, E.; Laviola, L.; Giorgino, F.; Cleveland, K.A.; Smith, R.J. Two novel proteins that are linked to insulin-like growth factor (IGF-I) receptors by the Grb10 adapter and modulate IGF-I signaling. J. Biol. Chem. 2003, 278, 31564–31573. [Google Scholar] [CrossRef]
- Higashi, S.; Iseki, E.; Minegishi, M.; Togo, T.; Kabuta, T.; Wada, K. GIGYF2 is present in endosomal compartments in the mammalian brains and enhances IGF-1-induced ERK1/2 activation. J. Neurochem. 2010, 115, 423–437. [Google Scholar] [CrossRef]
- Morita, M.; Ler, L.W.; Fabian, M.R.; Siddiqui, N.; Mullin, M.; Henderson, V.C.; Alain, T.; Fonseca, B.D.; Karashchuk, G.; Bennett, C.F.; et al. A novel 4EHP-GIGYF2 translational repressor complex is essential for mammalian development. Mol. Cell Biol. 2012, 32, 3585–3593. [Google Scholar] [CrossRef]
- Saini, P.; Rudakou, U.; Yu, E.; Ruskey, J.A.; Asayesh, F.; Laurent, S.B.; Spiegelman, D.; Fahn, S.; Waters, C.; Monchi, O.; et al. Association study of DNAJC13, UCHL1, HTRA2, GIGYF2, and EIF4G1 with Parkinson’s disease. Neurobiol. Aging 2021, 100, e117–e119. [Google Scholar] [CrossRef]
- Wang, L.; Guo, J.F.; Zhang, W.W.; Xu, Q.; Zuo, X.; Shi, C.H.; Luo, L.Z.; Liu, J.; Hu, L.; Hu, Y.C.; et al. Novel GIGYF2 gene variants in patients with Parkinson’s disease in Chinese population. Neurosci. Lett. 2010, 473, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Peter, D.; Ruscica, V.; Bawankar, P.; Weber, R.; Helms, S.; Valkov, E.; Igreja, C.; Izaurralde, E. Molecular basis for GIGYF-Me31B complex assembly in 4EHP-mediated translational repression. Genes Dev. 2019, 33, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Kryszke, M.H.; Adjeriou, B.; Liang, F.; Chen, H.; Dautry, F. Post-transcriptional gene silencing activity of human GIGYF2. Biochem. Biophys. Res. Commun. 2016, 475, 289–294. [Google Scholar] [CrossRef]
- Amaya Ramirez, C.C.; Hubbe, P.; Mandel, N.; Béthune, J. 4EHP-independent repression of endogenous mRNAs by the RNA-binding protein GIGYF2. Nucleic Acids Res. 2018, 46, 5792–5808. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yuan, Q.; Zhang, S.; Zuo, M.; Li, T.; Li, J.; Zhou, X.; Li, M.; Feng, W.; Xia, X.; et al. Elevated GIGYF2 expression suppresses tumor migration and enhances sensitivity to temozolomide in malignant glioma. Cancer Gene Ther. 2022, 29, 750–757. [Google Scholar] [CrossRef]
- Kim, M.; Semple, I.; Kim, B.; Kiers, A.; Nam, S.; Park, H.W.; Park, H.; Ro, S.H.; Kim, J.S.; Juhász, G.; et al. Drosophila Gyf/GRB10 interacting GYF protein is an autophagy regulator that controls neuron and muscle homeostasis. Autophagy 2015, 11, 1358–1372. [Google Scholar] [CrossRef]
- Peter, D.; Weber, R.; Sandmeir, F.; Wohlbold, L.; Helms, S.; Bawankar, P.; Valkov, E.; Igreja, C.; Izaurralde, E. GIGYF1/2 proteins use auxiliary sequences to selectively bind to 4EHP and repress target mRNA expression. Genes Dev. 2017, 31, 1147–1161. [Google Scholar] [CrossRef]
- Naeli, P.; Zhang, X.; Snell, P.H.; Chatterjee, S.; Kamran, M.; Ladak, R.J.; Orr, N.; Duchaine, T.; Sonenberg, N.; Jafarnejad, S.M. The SARS-CoV-2 protein NSP2 enhances microRNA-mediated translational repression. J. Cell Sci. 2023, 136, jcs261286. [Google Scholar] [CrossRef]
- Xu, Z.; Choi, J.H.; Dai, D.L.; Luo, J.; Ladak, R.J.; Li, Q.; Wang, Y.; Zhang, C.; Wiebe, S.; Liu, A.C.H.; et al. SARS-CoV-2 impairs interferon production via NSP2-induced repression of mRNA translation. Proc. Natl. Acad. Sci. USA 2022, 119, e2204539119. [Google Scholar] [CrossRef]
- Zou, L.; Moch, C.; Graille, M.; Chapat, C. The SARS-CoV-2 protein NSP2 impairs the silencing capacity of the human 4EHP-GIGYF2 complex. iScience 2022, 25, 104646. [Google Scholar] [CrossRef]
- Joshi, B.; Cameron, A.; Jagus, R. Characterization of mammalian eIF4E-family members. Eur. J. Biochem. 2004, 271, 2189–2203. [Google Scholar] [CrossRef]
- Rom, E.; Kim, H.C.; Gingras, A.C.; Marcotrigiano, J.; Favre, D.; Olsen, H.; Burley, S.K.; Sonenberg, N. Cloning and characterization of 4EHP, a novel mammalian eIF4E-related cap-binding protein. J. Biol. Chem. 1998, 273, 13104–13109. [Google Scholar] [CrossRef] [PubMed]
- Zuberek, J.; Kubacka, D.; Jablonowska, A.; Jemielity, J.; Stepinski, J.; Sonenberg, N.; Darzynkiewicz, E. Weak binding affinity of human 4EHP for mRNA cap analogs. RNA 2007, 13, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, S.; Buchwald, G.; Falk, S.; Chakrabarti, S.; Prabu, J.R.; Conti, E. The conformational plasticity of eukaryotic RNA-dependent ATPases. FEBS J. 2015, 282, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Mathys, H.; Basquin, J.; Ozgur, S.; Czarnocki-Cieciura, M.; Bonneau, F.; Aartse, A.; Dziembowski, A.; Nowotny, M.; Conti, E.; Filipowicz, W. Structural and biochemical insights to the role of the CCR4-NOT complex and DDX6 ATPase in microRNA repression. Mol. Cell 2014, 54, 751–765. [Google Scholar] [CrossRef]
- Sobti, M.; Mead, B.J.; Stewart, A.G.; Igreja, C.; Christie, M. Molecular basis for GIGYF-TNRC6 complex assembly. RNA 2023, 29, 724–734. [Google Scholar] [CrossRef]
- Tollenaere, M.A.X.; Tiedje, C.; Rasmussen, S.; Nielsen, J.C.; Vind, A.C.; Blasius, M.; Batth, T.S.; Mailand, N.; Olsen, J.V.; Gaestel, M.; et al. GIGYF1/2-Driven Cooperation between ZNF598 and TTP in Posttranscriptional Regulation of Inflammatory Signaling. Cell Rep. 2019, 26, 3511–3521.e3514. [Google Scholar] [CrossRef]
- Schopp, I.M.; Amaya Ramirez, C.C.; Debeljak, J.; Kreibich, E.; Skribbe, M.; Wild, K.; Béthune, J. Split-BioID a conditional proteomics approach to monitor the composition of spatiotemporally defined protein complexes. Nat. Commun. 2017, 8, 15690. [Google Scholar] [CrossRef]
- Juszkiewicz, S.; Slodkowicz, G.; Lin, Z.; Freire-Pritchett, P.; Peak-Chew, S.Y.; Hegde, R.S. Ribosome collisions trigger cis-acting feedback inhibition of translation initiation. eLife 2020, 9, e60038. [Google Scholar] [CrossRef]
- Nordgaard, C.; Tollenaere, M.A.X.; Val, A.M.D.; Bekker-Jensen, D.B.; Blasius, M.; Olsen, J.V.; Bekker-Jensen, S. Regulation of the Golgi Apparatus by p38 and JNK Kinases during Cellular Stress Responses. Int. J. Mol. Sci. 2021, 22, 9595. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.D.; Harreman, M.; Svejstrup, J.Q. Ubiquitylation and degradation of elongating RNA polymerase II: The last resort. Biochim. Biophys. Acta 2013, 1829, 151–157. [Google Scholar] [CrossRef]
- Noe Gonzalez, M.; Blears, D.; Svejstrup, J.Q. Causes and consequences of RNA polymerase II stalling during transcript elongation. Nat. Rev. Mol. Cell Biol. 2021, 22, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Oania, R.; Fang, R.; Smith, G.T.; Deshaies, R.J. Cdc48/p97 mediates UV-dependent turnover of RNA Pol II. Mol. Cell 2011, 41, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Iyama, T.; Wilson, D.M., 3rd. DNA repair mechanisms in dividing and non-dividing cells. DNA Repair 2013, 12, 620–636. [Google Scholar] [CrossRef]
- Herlihy, A.E.; Boeing, S.; Weems, J.C.; Walker, J.; Dirac-Svejstrup, A.B.; Lehner, M.H.; Conaway, R.C.; Conaway, J.W.; Svejstrup, J.Q. UBAP2/UBAP2L regulate UV-induced ubiquitylation of RNA polymerase II and are the human orthologues of yeast Def1. DNA Repair 2022, 115, 103343. [Google Scholar] [CrossRef]
- Huibregtse, J.M.; Yang, J.C.; Beaudenon, S.L. The large subunit of RNA polymerase II is a substrate of the Rsp5 ubiquitin-protein ligase. Proc. Natl. Acad. Sci. USA 1997, 94, 3656–3661. [Google Scholar] [CrossRef]
- Lehner, M.H.; Walker, J.; Temcinaite, K.; Herlihy, A.; Taschner, M.; Berger, A.C.; Corbett, A.H.; Dirac Svejstrup, A.B.; Svejstrup, J.Q. Yeast Smy2 and its human homologs GIGYF1 and -2 regulate Cdc48/VCP function during transcription stress. Cell Rep. 2022, 41, 111536. [Google Scholar] [CrossRef]
- Wilson, M.D.; Harreman, M.; Taschner, M.; Reid, J.; Walker, J.; Erdjument-Bromage, H.; Tempst, P.; Svejstrup, J.Q. Proteasome-mediated processing of Def1, a critical step in the cellular response to transcription stress. Cell 2013, 154, 983–995. [Google Scholar] [CrossRef]
- Olszewski, M.M.; Williams, C.; Dong, K.C.; Martin, A. The Cdc48 unfoldase prepares well-folded protein substrates for degradation by the 26S proteasome. Commun. Biol. 2019, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Forget, D.; Lacombe, A.A.; Cloutier, P.; Al-Khoury, R.; Bouchard, A.; Lavallée-Adam, M.; Faubert, D.; Jeronimo, C.; Blanchette, M.; Coulombe, B. The protein interaction network of the human transcription machinery reveals a role for the conserved GTPase RPAP4/GPN1 and microtubule assembly in nuclear import and biogenesis of RNA polymerase II. Mol. Cell Proteom. 2010, 9, 2827–2839. [Google Scholar] [CrossRef] [PubMed]
- Anindya, R.; Aygün, O.; Svejstrup, J.Q. Damage-induced ubiquitylation of human RNA polymerase II by the ubiquitin ligase Nedd4, but not Cockayne syndrome proteins or BRCA1. Mol. Cell 2007, 28, 386–397. [Google Scholar] [CrossRef]
- Varshavsky, A. The ubiquitin system. Trends Biochem. Sci. 1997, 22, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Pickart, C.M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001, 70, 503–533. [Google Scholar] [CrossRef]
- Harreman, M.; Taschner, M.; Sigurdsson, S.; Anindya, R.; Reid, J.; Somesh, B.; Kong, S.E.; Banks, C.A.; Conaway, R.C.; Conaway, J.W.; et al. Distinct ubiquitin ligases act sequentially for RNA polymerase II polyubiquitylation. Proc. Natl. Acad. Sci. USA 2009, 106, 20705–20710. [Google Scholar] [CrossRef]
- Bae, H.; Coller, J. Codon optimality-mediated mRNA degradation: Linking translational elongation to mRNA stability. Mol. Cell 2022, 82, 1467–1476. [Google Scholar] [CrossRef]
- Presnyak, V.; Alhusaini, N.; Chen, Y.H.; Martin, S.; Morris, N.; Kline, N.; Olson, S.; Weinberg, D.; Baker, K.E.; Graveley, B.R.; et al. Codon optimality is a major determinant of mRNA stability. Cell 2015, 160, 1111–1124. [Google Scholar] [CrossRef]
- Wu, Q.; Bazzini, A.A. Translation and mRNA Stability Control. Annu. Rev. Biochem. 2023, 92, 227–245. [Google Scholar] [CrossRef]
- Hanson, G.; Coller, J. Codon optimality, bias and usage in translation and mRNA decay. Nat. Rev. Mol. Cell Biol. 2018, 19, 20–30. [Google Scholar] [CrossRef]
- Yan, L.L.; Zaher, H.S. How do cells cope with RNA damage and its consequences? J. Biol. Chem. 2019, 294, 15158–15171. [Google Scholar] [CrossRef]
- Morris, C.; Cluet, D.; Ricci, E.P. Ribosome dynamics and mRNA turnover, a complex relationship under constant cellular scrutiny. Wiley Interdiscip. Rev. RNA 2021, 12, e1658. [Google Scholar] [CrossRef] [PubMed]
- Karousis, E.D.; Mühlemann, O. Nonsense-Mediated mRNA Decay Begins Where Translation Ends. Cold Spring Harb. Perspect. Biol. 2019, 11, a032862. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, K.; Tesina, P.; Matsuo, Y.; Sugiyama, T.; Cheng, J.; Saeki, Y.; Tanaka, K.; Becker, T.; Beckmann, R.; Inada, T. Collided ribosomes form a unique structural interface to induce Hel2-driven quality control pathways. EMBO J. 2019, 38, e100276. [Google Scholar] [CrossRef] [PubMed]
- Meydan, S.; Guydosh, N.R. A cellular handbook for collided ribosomes: Surveillance pathways and collision types. Curr. Genet. 2021, 67, 19–26. [Google Scholar] [CrossRef]
- Frischmeyer, P.A.; van Hoof, A.; O’Donnell, K.; Guerrerio, A.L.; Parker, R.; Dietz, H.C. An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science 2002, 295, 2258–2261. [Google Scholar] [CrossRef]
- van Hoof, A.; Frischmeyer, P.A.; Dietz, H.C.; Parker, R. Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science 2002, 295, 2262–2264. [Google Scholar] [CrossRef]
- Joazeiro, C.A.P. Mechanisms and functions of ribosome-associated protein quality control. Nat. Rev. Mol. Cell Biol. 2019, 20, 368–383. [Google Scholar] [CrossRef]
- Kulak, N.A.; Pichler, G.; Paron, I.; Nagaraj, N.; Mann, M. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat. Methods 2014, 11, 319–324. [Google Scholar] [CrossRef]
- Itzhak, D.N.; Tyanova, S.; Cox, J.; Borner, G.H. Global, quantitative and dynamic mapping of protein subcellular localization. eLife 2016, 5, e16950. [Google Scholar] [CrossRef]
- Sinha, N.K.; Ordureau, A.; Best, K.; Saba, J.A.; Zinshteyn, B.; Sundaramoorthy, E.; Fulzele, A.; Garshott, D.M.; Denk, T.; Thoms, M.; et al. EDF1 coordinates cellular responses to ribosome collisions. eLife 2020, 9, e58828. [Google Scholar] [CrossRef] [PubMed]
- Vind, A.C.; Genzor, A.V.; Bekker-Jensen, S. Ribosomal stress-surveillance: Three pathways is a magic number. Nucleic Acids Res. 2020, 48, 10648–10661. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, R.; Nagy, G.; Dotu, I.; Chuang, J.H.; Ackerman, S.L. Activation of GCN2 kinase by ribosome stalling links translation elongation with translation initiation. eLife 2016, 5, e14295. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Novoa, I.; Zhang, Y.; Zeng, H.; Wek, R.; Schapira, M.; Ron, D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 2000, 6, 1099–1108. [Google Scholar] [CrossRef]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M.; Sadri, N.; Yun, C.; Popko, B.; Paules, R.; et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 2003, 11, 619–633. [Google Scholar] [CrossRef]
- Matsuo, Y.; Inada, T. Co-Translational Quality Control Induced by Translational Arrest. Biomolecules 2023, 13, 317. [Google Scholar] [CrossRef]
- Yip, M.C.J.; Shao, S. Detecting and Rescuing Stalled Ribosomes. Trends Biochem. Sci. 2021, 46, 731–743. [Google Scholar] [CrossRef]
- Kim, K.Q.; Zaher, H.S. Canary in a coal mine: Collided ribosomes as sensors of cellular conditions. Trends Biochem. Sci. 2022, 47, 82–97. [Google Scholar] [CrossRef]
- Juszkiewicz, S.; Chandrasekaran, V.; Lin, Z.; Kraatz, S.; Ramakrishnan, V.; Hegde, R.S. ZNF598 Is a Quality Control Sensor of Collided Ribosomes. Mol. Cell 2018, 72, 469–481.e467. [Google Scholar] [CrossRef]
- Hickey, K.L.; Dickson, K.; Cogan, J.Z.; Replogle, J.M.; Schoof, M.; D’Orazio, K.N.; Sinha, N.K.; Hussmann, J.A.; Jost, M.; Frost, A.; et al. GIGYF2 and 4EHP Inhibit Translation Initiation of Defective Messenger RNAs to Assist Ribosome-Associated Quality Control. Mol. Cell 2020, 79, 950–962.e956. [Google Scholar] [CrossRef]
- Juszkiewicz, S.; Hegde, R.S. Initiation of Quality Control during Poly(A) Translation Requires Site-Specific Ribosome Ubiquitination. Mol. Cell 2017, 65, 743–750.e744. [Google Scholar] [CrossRef]
- D’Orazio, K.N.; Wu, C.C.; Sinha, N.; Loll-Krippleber, R.; Brown, G.W.; Green, R. The endonuclease Cue2 cleaves mRNAs at stalled ribosomes during No Go Decay. eLife 2019, 8, e49117. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Sugiyama, T.; Yamazaki, R.; Nobuta, R.; Inada, T. Identification of a novel trigger complex that facilitates ribosome-associated quality control in mammalian cells. Sci. Rep. 2020, 10, 3422. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Tesina, P.; Nakajima, S.; Mizuno, M.; Endo, A.; Buschauer, R.; Cheng, J.; Shounai, O.; Ikeuchi, K.; Saeki, Y.; et al. RQT complex dissociates ribosomes collided on endogenous RQC substrate SDD1. Nat. Struct. Mol. Biol. 2020, 27, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Juszkiewicz, S.; Speldewinde, S.H.; Wan, L.; Svejstrup, J.Q.; Hegde, R.S. The ASC-1 Complex Disassembles Collided Ribosomes. Mol. Cell 2020, 79, 603–614.e608. [Google Scholar] [CrossRef]
- Shao, S.; Brown, A.; Santhanam, B.; Hegde, R.S. Structure and assembly pathway of the ribosome quality control complex. Mol. Cell 2015, 57, 433–444. [Google Scholar] [CrossRef]
- Jandhyala, D.M.; Ahluwalia, A.; Obrig, T.; Thorpe, C.M. ZAK: A MAP3Kinase that transduces Shiga toxin- and ricin-induced proinflammatory cytokine expression. Cell Microbiol. 2008, 10, 1468–1477. [Google Scholar] [CrossRef]
- Wang, X.; Mader, M.M.; Toth, J.E.; Yu, X.; Jin, N.; Campbell, R.M.; Smallwood, J.K.; Christe, M.E.; Chatterjee, A.; Goodson, T., Jr.; et al. Complete inhibition of anisomycin and UV radiatsion but not cytokine induced JNK and p38 activation by an aryl-substituted dihydropyrrolopyrazole quinoline and mixed lineage kinase 7 small interfering RNA. J. Biol. Chem. 2005, 280, 19298–19305. [Google Scholar] [CrossRef]
- De, S.; Mühlemann, O. A comprehensive coverage insurance for cells: Revealing links between ribosome collisions, stress responses and mRNA surveillance. RNA Biol. 2022, 19, 609–621. [Google Scholar] [CrossRef]
- Zinshteyn, B.; Sinha, N.K.; Enam, S.U.; Koleske, B.; Green, R. Translational repression of NMD targets by GIGYF2 and EIF4E2. PLoS Genet. 2021, 17, e1009813. [Google Scholar] [CrossRef] [PubMed]
- Kashima, I.; Yamashita, A.; Izumi, N.; Kataoka, N.; Morishita, R.; Hoshino, S.; Ohno, M.; Dreyfuss, G.; Ohno, S. Binding of a novel SMG-1-Upf1-eRF1-eRF3 complex (SURF) to the exon junction complex triggers Upf1 phosphorylation and nonsense-mediated mRNA decay. Genes Dev. 2006, 20, 355–367. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Bonneau, F.; Schüssler, S.; Eppinger, E.; Conti, E. Phospho-dependent and phospho-independent interactions of the helicase UPF1 with the NMD factors SMG5-SMG7 and SMG6. Nucleic Acids Res. 2014, 42, 9447–9460. [Google Scholar] [CrossRef]
- Hurt, J.A.; Robertson, A.D.; Burge, C.B. Global analyses of UPF1 binding and function reveal expanded scope of nonsense-mediated mRNA decay. Genome Res. 2013, 23, 1636–1650. [Google Scholar] [CrossRef] [PubMed]
- Zünd, D.; Gruber, A.R.; Zavolan, M.; Mühlemann, O. Translation-dependent displacement of UPF1 from coding sequences causes its enrichment in 3′ UTRs. Nat. Struct. Mol. Biol. 2013, 20, 936–943. [Google Scholar] [CrossRef]
- Hogg, J.R.; Goff, S.P. Upf1 senses 3′UTR length to potentiate mRNA decay. Cell 2010, 143, 379–389. [Google Scholar] [CrossRef]
- Arribere, J.A.; Fire, A.Z. Nonsense mRNA suppression via nonstop decay. eLife 2018, 7, e33292. [Google Scholar] [CrossRef] [PubMed]
- Lareau, L.F.; Inada, M.; Green, R.E.; Wengrod, J.C.; Brenner, S.E. Unproductive splicing of SR genes associated with highly conserved and ultraconserved DNA elements. Nature 2007, 446, 926–929. [Google Scholar] [CrossRef]
- Lewis, B.P.; Green, R.E.; Brenner, S.E. Evidence for the widespread coupling of alternative splicing and nonsense-mediated mRNA decay in humans. Proc. Natl. Acad. Sci. USA 2003, 100, 189–192. [Google Scholar] [CrossRef]
- Morrison, M.; Harris, K.S.; Roth, M.B. smg mutants affect the expression of alternatively spliced SR protein mRNAs in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1997, 94, 9782–9785. [Google Scholar] [CrossRef]
- Alagar Boopathy, L.R.; Beadle, E.; Garcia-Bueno Rico, A.; Vera, M. Proteostasis regulation through ribosome quality control and no-go-decay. Wiley Interdiscip. Rev. RNA 2023, 14, e1809. [Google Scholar] [CrossRef] [PubMed]
- Nissan, T.; Rajyaguru, P.; She, M.; Song, H.; Parker, R. Decapping activators in Saccharomyces cerevisiae act by multiple mechanisms. Mol. Cell 2010, 39, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Ernoult-Lange, M.; Baconnais, S.; Harper, M.; Minshall, N.; Souquere, S.; Boudier, T.; Bénard, M.; Andrey, P.; Pierron, G.; Kress, M.; et al. Multiple binding of repressed mRNAs by the P-body protein Rck/p54. RNA 2012, 18, 1702–1715. [Google Scholar] [CrossRef]
- Coller, J.M.; Tucker, M.; Sheth, U.; Valencia-Sanchez, M.A.; Parker, R. The DEAD box helicase, Dhh1p, functions in mRNA decapping and interacts with both the decapping and deadenylase complexes. RNA 2001, 7, 1717–1727. [Google Scholar] [CrossRef]
- Ruscica, V.; Bawankar, P.; Peter, D.; Helms, S.; Igreja, C.; Izaurralde, E. Direct role for the Drosophila GIGYF protein in 4EHP-mediated mRNA repression. Nucleic Acids Res. 2019, 47, 7035–7048. [Google Scholar] [CrossRef]
- Niu, F.; Li, Z.; Ren, Y.; Li, Z.; Guan, H.; Li, Y.; Zhang, Y.; Li, Y.; Yang, J.; Qian, L.; et al. Aberrant hyper-expression of the RNA binding protein GIGYF2 in endothelial cells modulates vascular aging and function. Redox Biol. 2023, 65, 102824. [Google Scholar] [CrossRef]
- Kofler, M.; Schuemann, M.; Merz, C.; Kosslick, D.; Schlundt, A.; Tannert, A.; Schaefer, M.; Lührmann, R.; Krause, E.; Freund, C. Proline-rich sequence recognition: I. Marking GYF and WW domain assembly sites in early spliceosomal complexes. Mol. Cell Proteom. 2009, 8, 2461–2473. [Google Scholar] [CrossRef]
- Clark, A.R.; Dean, J.L. The control of inflammation via the phosphorylation and dephosphorylation of tristetraprolin: A tale of two phosphatases. Biochem. Soc. Trans. 2016, 44, 1321–1337. [Google Scholar] [CrossRef] [PubMed]
- Garzia, A.; Jafarnejad, S.M.; Meyer, C.; Chapat, C.; Gogakos, T.; Morozov, P.; Amiri, M.; Shapiro, M.; Molina, H.; Tuschl, T.; et al. The E3 ubiquitin ligase and RNA-binding protein ZNF598 orchestrates ribosome quality control of premature polyadenylated mRNAs. Nat. Commun. 2017, 8, 16056. [Google Scholar] [CrossRef]
- Naeli, P.; Winter, T.; Hackett, A.P.; Alboushi, L.; Jafarnejad, S.M. The intricate balance between microRNA-induced mRNA decay and translational repression. FEBS J. 2023, 290, 2508–2524. [Google Scholar] [CrossRef]
- Filipowicz, W.; Sonenberg, N. The long unfinished march towards understanding microRNA-mediated repression. RNA 2015, 21, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.E.; Huntzinger, E.; Fauser, M.; Izaurralde, E. GW182 proteins directly recruit cytoplasmic deadenylase complexes to miRNA targets. Mol. Cell 2011, 44, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Chekulaeva, M.; Mathys, H.; Zipprich, J.T.; Attig, J.; Colic, M.; Parker, R.; Filipowicz, W. miRNA repression involves GW182-mediated recruitment of CCR4-NOT through conserved W-containing motifs. Nat. Struct. Mol. Biol. 2011, 18, 1218–1226. [Google Scholar] [CrossRef]
- Fabian, M.R.; Cieplak, M.K.; Frank, F.; Morita, M.; Green, J.; Srikumar, T.; Nagar, B.; Yamamoto, T.; Raught, B.; Duchaine, T.F.; et al. miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat. Struct. Mol. Biol. 2011, 18, 1211–1217. [Google Scholar] [CrossRef]
- Rouya, C.; Siddiqui, N.; Morita, M.; Duchaine, T.F.; Fabian, M.R.; Sonenberg, N. Human DDX6 effects miRNA-mediated gene silencing via direct binding to CNOT1. RNA 2014, 20, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rivas, F.V.; Wohlschlegel, J.; Yates, J.R., 3rd; Parker, R.; Hannon, G.J. A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 2005, 7, 1261–1266. [Google Scholar] [CrossRef]
- Majerciak, V.; Zhou, T.; Kruhlak, M.J.; Zheng, Z.M. RNA helicase DDX6 and scaffold protein GW182 in P-bodies promote biogenesis of stress granules. Nucleic Acids Res. 2023, 51, 9337–9355. [Google Scholar] [CrossRef]
- Adjibade, P.; Mazroui, R. Control of mRNA turnover: Implication of cytoplasmic RNA granules. Semin. Cell Dev. Biol. 2014, 34, 15–23. [Google Scholar] [CrossRef]
- Eulalio, A.; Behm-Ansmant, I.; Izaurralde, E. P bodies: At the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 2007, 8, 9–22. [Google Scholar] [CrossRef]
- Parker, R.; Sheth, U. P bodies and the control of mRNA translation and degradation. Mol. Cell 2007, 25, 635–646. [Google Scholar] [CrossRef]
- Ingelfinger, D.; Arndt-Jovin, D.J.; Lührmann, R.; Achsel, T. The human LSm1-7 proteins colocalize with the mRNA-degrading enzymes Dcp1/2 and Xrnl in distinct cytoplasmic foci. RNA 2002, 8, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, E.; Cougot, N.; Meyer, S.; Babajko, S.; Wahle, E.; Séraphin, B. Human Dcp2: A catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. EMBO J. 2002, 21, 6915–6924. [Google Scholar] [CrossRef]
- Sheth, U.; Parker, R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 2003, 300, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Orban, T.I.; Izaurralde, E. Decay of mRNAs targeted by RISC requires XRN1, the Ski complex, and the exosome. RNA 2005, 11, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Almasi, S.; Crawford Parks, T.E.; Ravel-Chapuis, A.; MacKenzie, A.; Côté, J.; Cowan, K.N.; Jasmin, B.J. Differential regulation of autophagy by STAU1 in alveolar rhabdomyosarcoma and non-transformed skeletal muscle cells. Cell. Oncol. 2021, 44, 851–870. [Google Scholar] [CrossRef]
- Bu, L.; Wang, H.; Pan, J.A.; Chen, L.; Xing, F.; Wu, J.; Li, S.; Guo, D. PTEN suppresses tumorigenesis by directly dephosphorylating Akt. Signal Transduct. Target. Ther. 2021, 6, 262. [Google Scholar] [CrossRef]
- Driessen, G.J.; IJspeert, H.; Wentink, M.; Yntema, H.G.; van Hagen, P.M.; van Strien, A.; Bucciol, G.; Cogulu, O.; Trip, M.; Nillesen, W.; et al. Increased PI3K/Akt activity and deregulated humoral immune response in human PTEN deficiency. J. Allergy Clin. Immunol. 2016, 138, 1744–1747.e1745. [Google Scholar] [CrossRef]
- Yue, S.; Li, J.; Lee, S.Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; Ratliff, T.L.; et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef]
- Zoncu, R.; Bar-Peled, L.; Efeyan, A.; Wang, S.; Sancak, Y.; Sabatini, D.M. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 2011, 334, 678–683. [Google Scholar] [CrossRef]
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010, 141, 290–303. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, R.; Wang, Z.; Wang, X.; Wang, F.; Ding, J. Structural basis for Ragulator functioning as a scaffold in membrane-anchoring of Rag GTPases and mTORC1. Nat. Commun. 2017, 8, 1394. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, M.E.G.; Naschberger, A.; Fürnrohr, B.G.; Stasyk, T.; Dunzendorfer-Matt, T.; Lechner, S.; Welti, S.; Kremser, L.; Shivalingaiah, G.; Offterdinger, M.; et al. Crystal structure of the human lysosomal mTORC1 scaffold complex and its impact on signaling. Science 2017, 358, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Yepuri, G.; Velagapudi, S.; Xiong, Y.; Rajapakse, A.G.; Montani, J.P.; Ming, X.F.; Yang, Z. Positive crosstalk between arginase-II and S6K1 in vascular endothelial inflammation and aging. Aging Cell 2012, 11, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Huang, A.; Kaley, G.; Sun, D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1829–H1836. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Masood, K.I.; Yameen, M.; Ashraf, J.; Shahid, S.; Mahmood, S.F.; Nasir, A.; Nasir, N.; Jamil, B.; Ghanchi, N.K.; Khanum, I.; et al. Upregulated type I interferon responses in asymptomatic COVID-19 infection are associated with improved clinical outcome. Sci. Rep. 2021, 11, 22958. [Google Scholar] [CrossRef]
- Vinh, D.C.; Abel, L.; Bastard, P.; Cheng, M.P.; Condino-Neto, A.; Gregersen, P.K.; Haerynck, F.; Cicalese, M.P.; Hagin, D.; Soler-Palacín, P.; et al. Harnessing Type I IFN Immunity Against SARS-CoV-2 with Early Administration of IFN-β. J. Clin. Immunol. 2021, 41, 1425–1442. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Samaranch, L.; Lorenzo, E.; Pastor, M.A.; Riverol, M.; Luquin, M.R.; Rodríguez-Oroz, M.C.; Obeso, J.A.; Pastor, P. Analysis of the GIGYF2 gene in familial and sporadic Parkinson disease in the Spanish population. Eur. J. Neurol. 2010, 17, 321–325. [Google Scholar] [CrossRef]
- Tan, E.K.; Schapira, A.H. Summary of GIGYF2 studies in Parkinson’s disease: The burden of proof. Eur. J. Neurol. 2010, 17, 175–176. [Google Scholar] [CrossRef]
- Giovannone, B.; Tsiaras, W.G.; de la Monte, S.; Klysik, J.; Lautier, C.; Karashchuk, G.; Goldwurm, S.; Smith, R.J. GIGYF2 gene disruption in mice results in neurodegeneration and altered insulin-like growth factor signaling. Hum. Mol. Genet. 2009, 18, 4629–4639. [Google Scholar] [CrossRef] [PubMed]
- Bialkowska, A.; Kurlandzka, A. Proteins interacting with Lin 1p, a putative link between chromosome segregation, mRNA splicing and DNA replication in Saccharomyces cerevisiae. Yeast 2002, 19, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).