Day One Cell-Free DNA Levels as an Objective Prognostic Marker of Mortality in Major Burns Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohorts
2.1.1. Burns
2.1.2. Healthy Controls
2.2. Clinical Data Collection
2.3. Blood Sampling
2.4. Preparation of Platelet-Free Plasma (PFP) and Serum
2.5. Fluorometric Analysis of Plasma Cell-Free DNA Levels
2.6. Quantification of IL-6 and IL-10 Concentrations
2.7. Statistical Analyses
3. Results
3.1. Patient Demographics
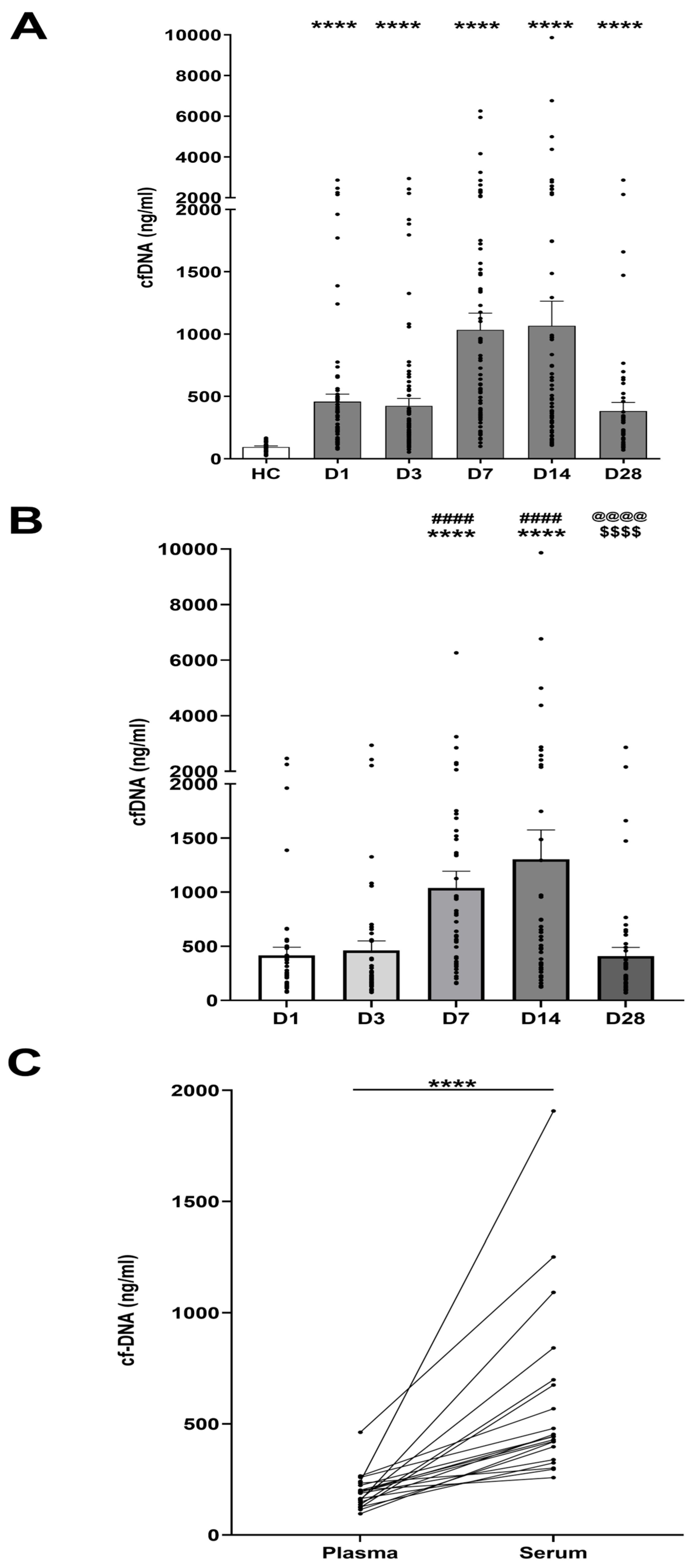
3.2. Thermal Injury Results in Elevated Concentrations of Circulating cfDNA
3.3. Comparison of Day One cfDNA Levels Measured in Serum Versus PFP
3.4. Correlation of Day One cfDNA Levels with Clinical Indices
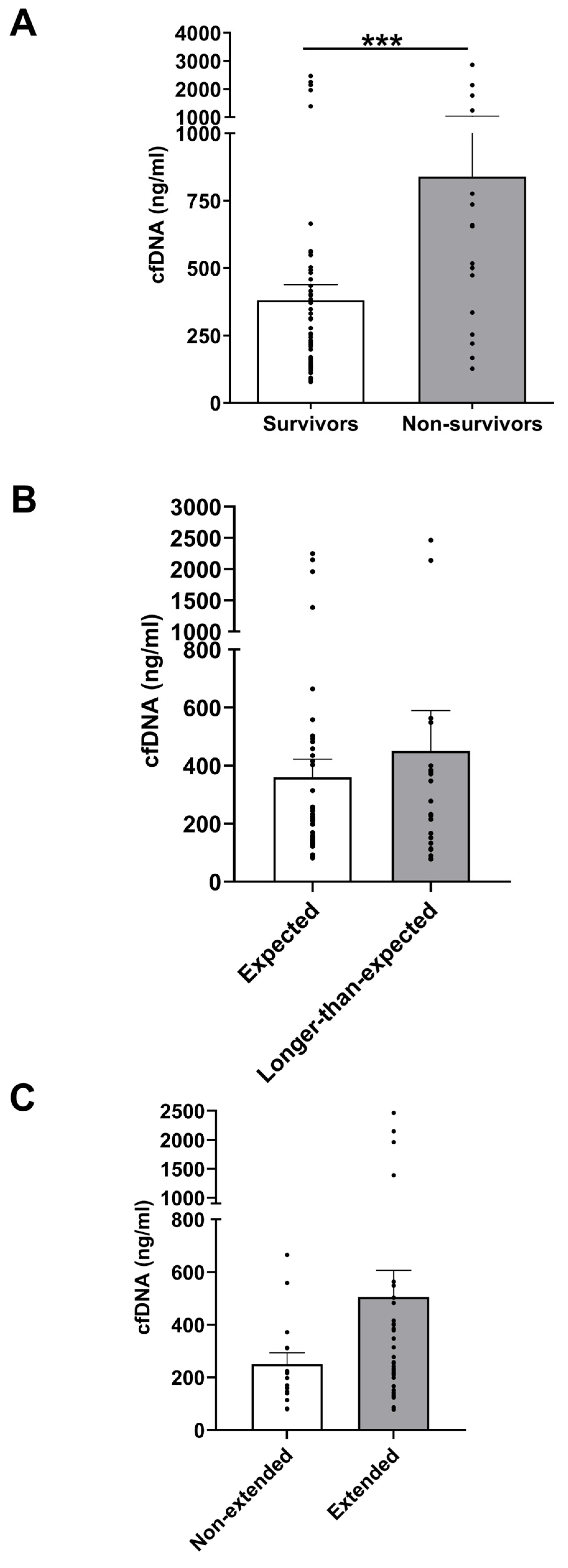
3.5. Day 1 cfDNA Level Comparisons Between Clinical Outcome Subgroups
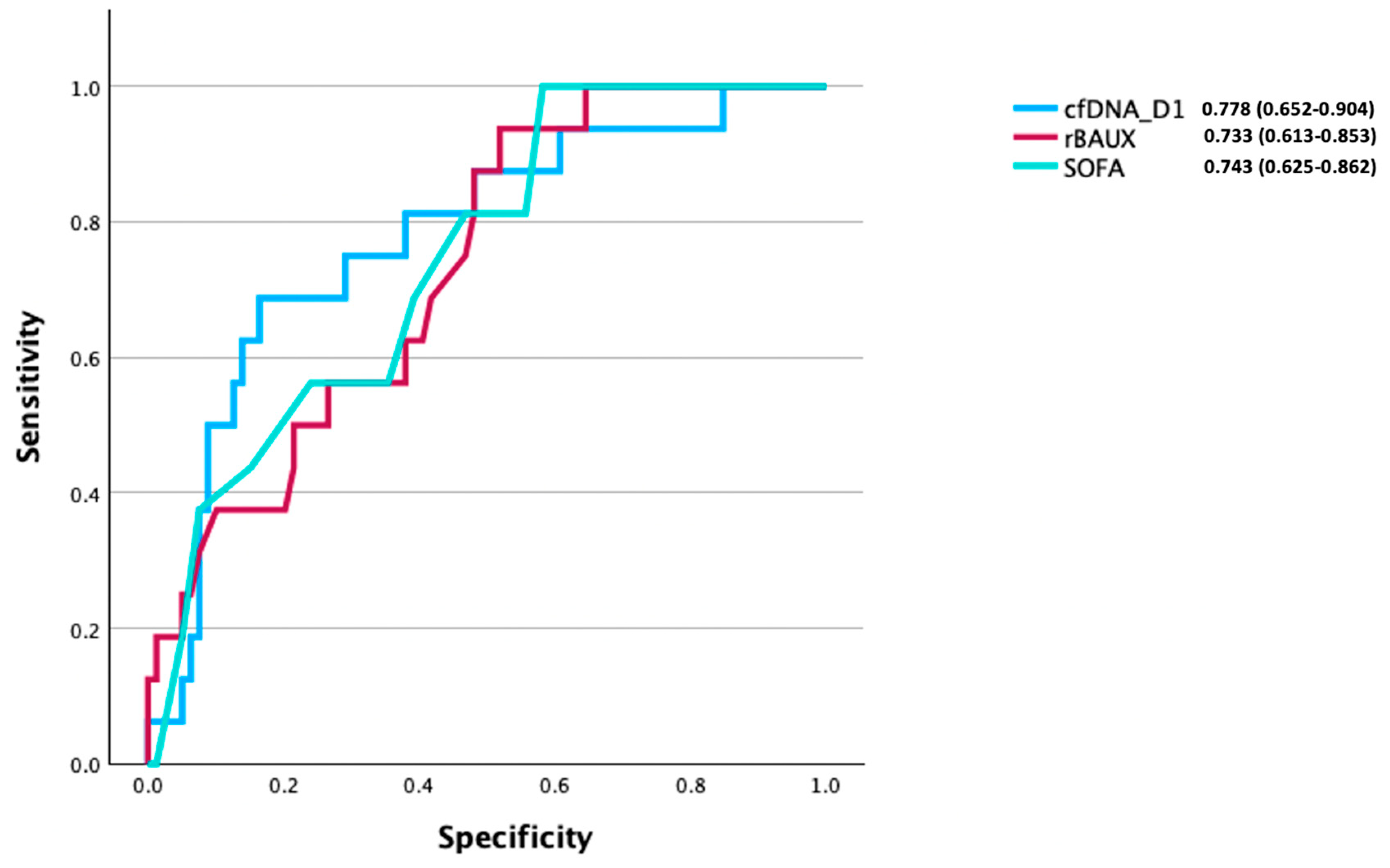
3.6. CfDNA as a Predictive Factor of Mortality Post-Burn
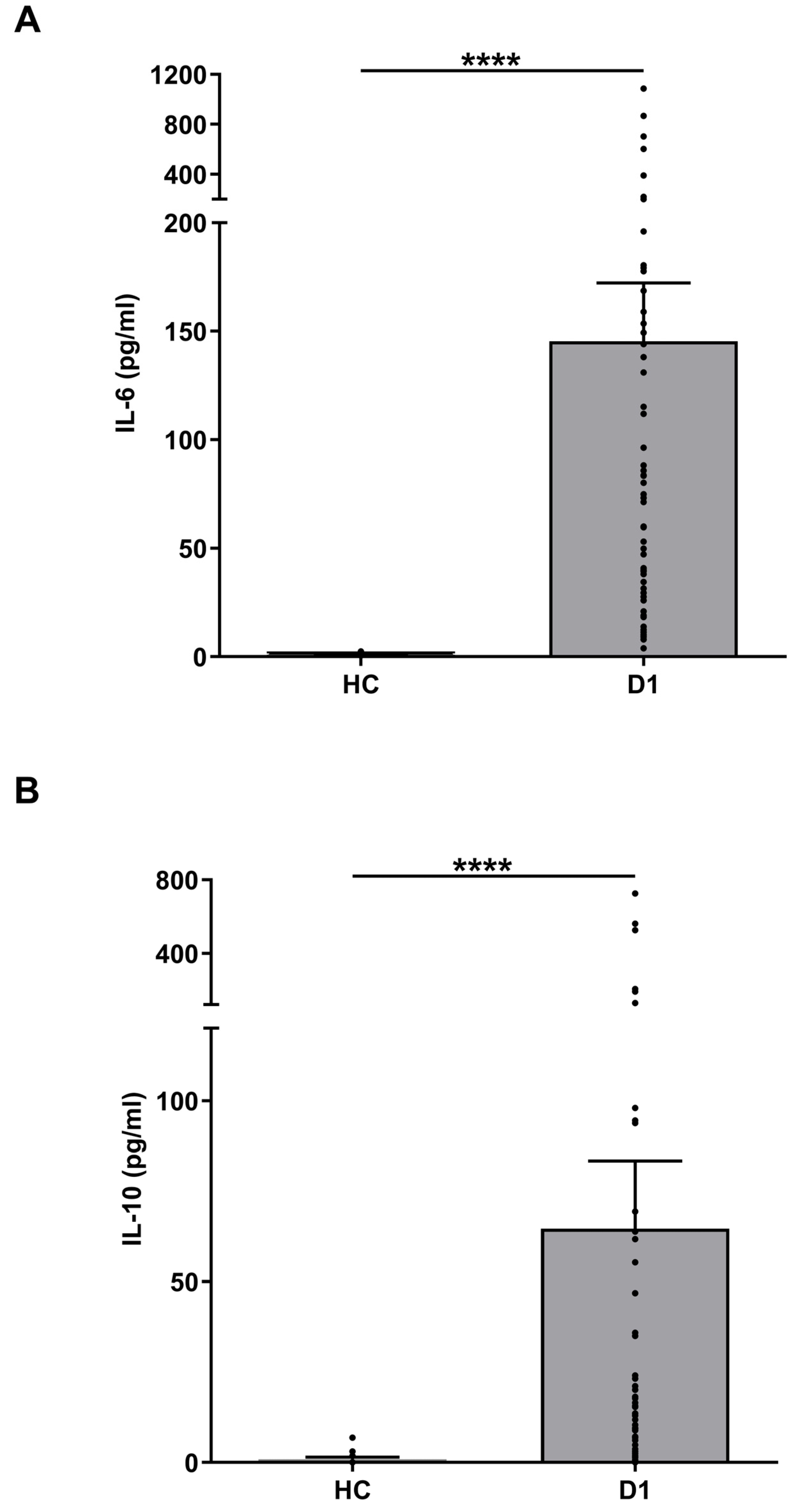
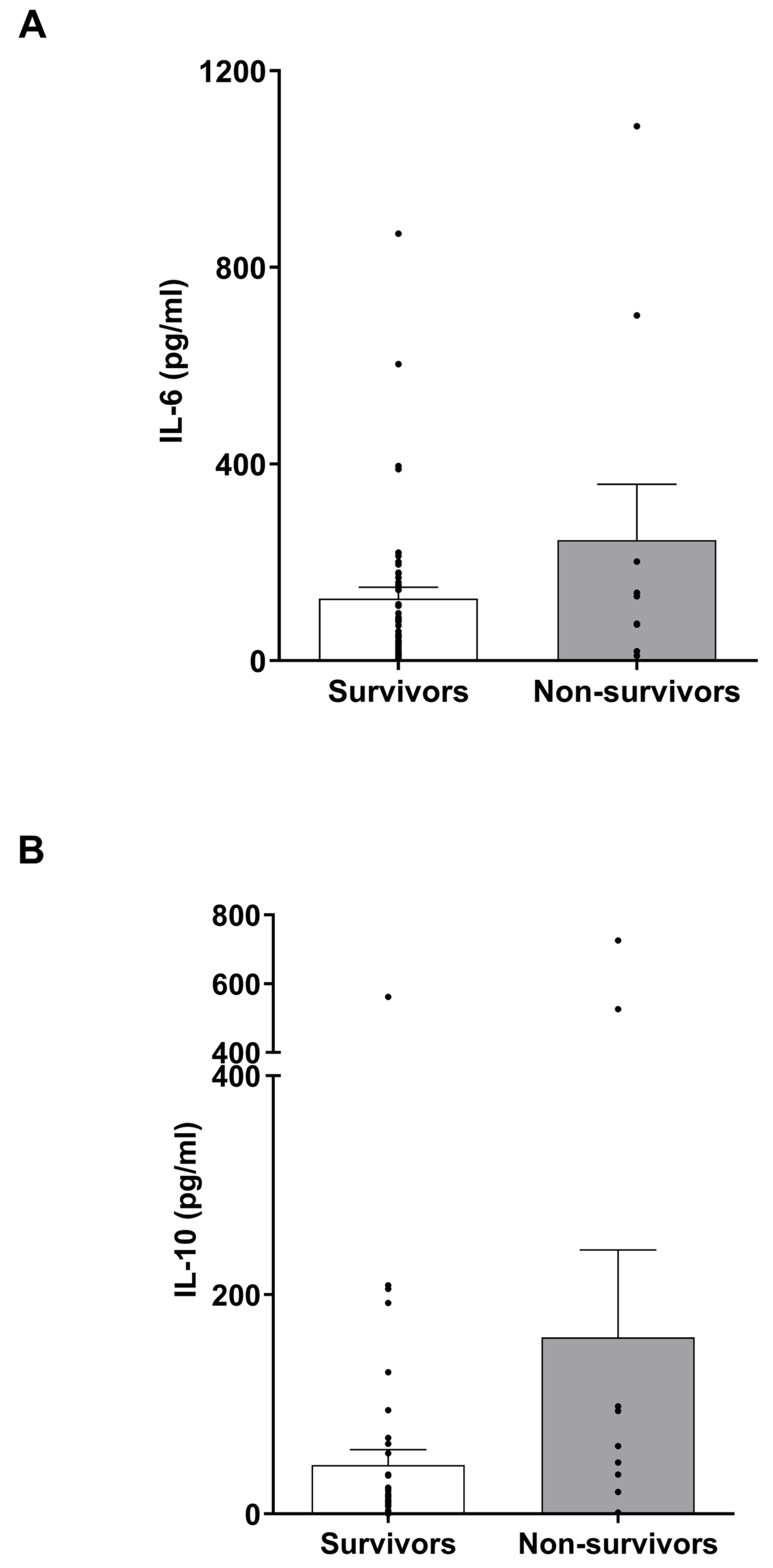
3.7. IL-6 and IL-10 Levels in Burns Patients
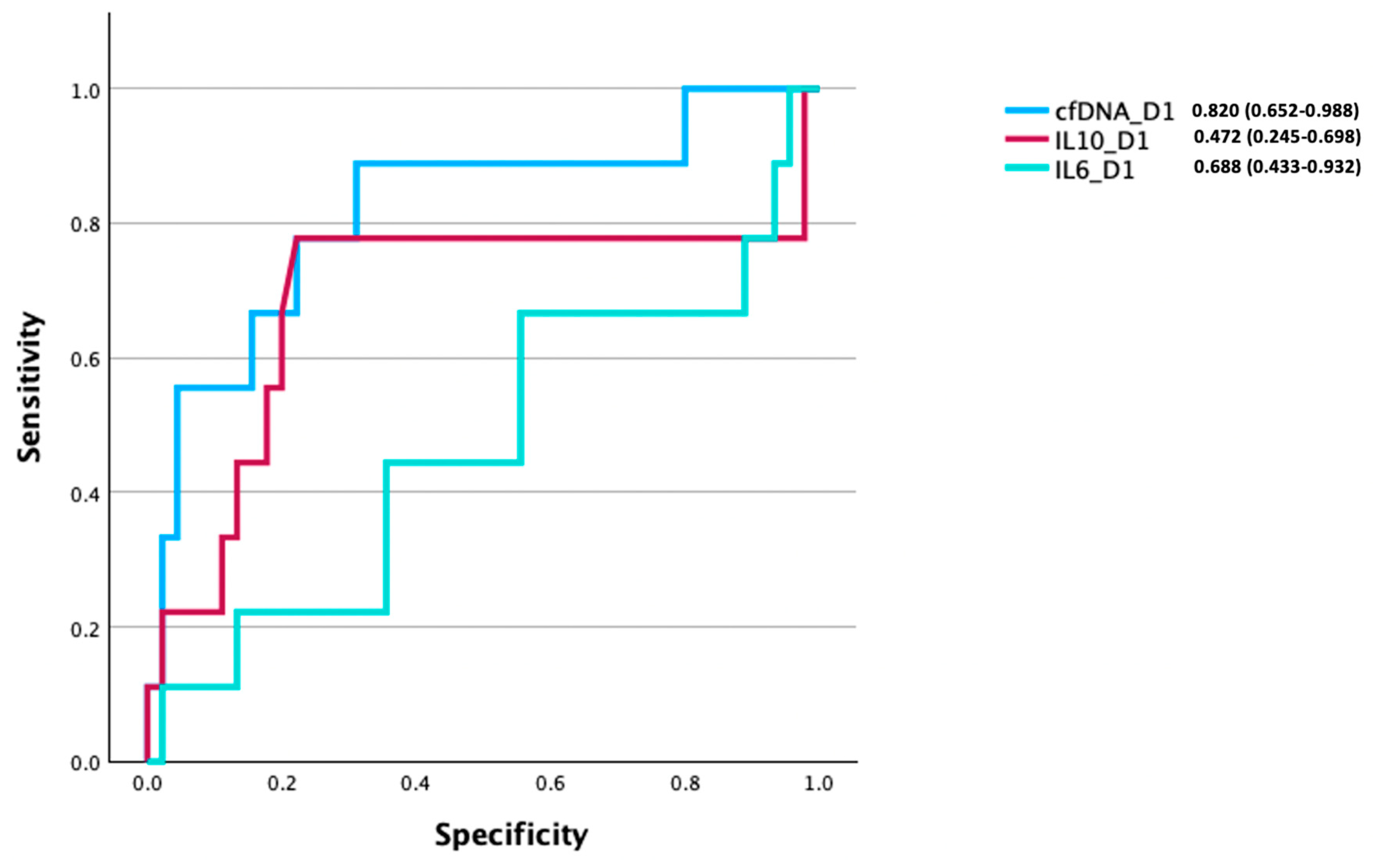
3.8. Comparison of the Prognostic Utility of cfDNA, IL-6, and IL-10 Levels for Discriminating Between Survivors and Non-Survivors of Burn Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHOIaV. Available online: https://www.who.int/news-room/fact-sheets/detail/injuries-and-violence (accessed on 30 October 2024).
- World Health Organization. Burns. Available online: https://www.who.int/en/news-room/fact-sheets/detail/burns (accessed on 30 October 2024).
- Smolle, C.; Cambiaso-Daniel, J.; Forbes, A.A.; Wurzer, P.; Hundeshagen, G.; Branski, L.K.; Huss, F.; Kamolz, L.-P. Recent trends in burn epidemiology worldwide: A systematic review. Burns 2017, 43, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Herndon, D.N.; Tompkins, R.G. Support of the metabolic response to burn injury. Lancet 2004, 363, 1895–1902. [Google Scholar] [CrossRef]
- Lord, J.M.; Midwinter, M.J.; Chen, Y.F.; Belli, A.; Brohi, K.; Kovacs, E.J.; Koenderman, L.; Kubes, P.; Lilford, R.J. The systemic immune response to trauma: An overview of pathophysiology and treatment. Lancet 2014, 384, 1455–1465. [Google Scholar] [CrossRef]
- Kraft, R.; Herndon, D.N.; Al-Mousawi, A.M.; Williams, F.N.; Finnerty, C.C.; Jeschke, M.G. Burn size and survival probability in paediatric patients in modern burn care: A prospective observational cohort study. Lancet 2012, 379, 1013–1021. [Google Scholar] [CrossRef]
- Williams, F.N.; Herndon, D.N.; Hawkins, H.K.; Lee, J.O.; Cox, R.A.; Kulp, G.A.; Finnerty, C.C.; Chinkes, D.L.; Jeschke, M.G. The leading causes of death after burn injury in a single pediatric burn center. Crit. Care 2009, 13, R183. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.; Gal, S.; Fisher, N.; Smythe, J.; Wainscoat, J.; Tyler, M.P.; Watt, S.M.; Harris, A.L. Quantification of circulating cell-free plasma DNA and endothelial gene RNA in patients with burns and relation to acute thermal injury. Burns 2008, 34, 809–816. [Google Scholar] [CrossRef]
- Hazeldine, J.; Naumann, D.N.; Toman, E.; Davies, D.; Bishop, J.R.B.; Su, Z.; Hampson, P.; Dinsdale, R.J.; Crombie, N.; Duggal, N.A.; et al. Prehospital immune responses and development of multiple organ dysfunction syndrome following traumatic injury: A prospective cohort study. PLoS Med. 2017, 14, e1002338. [Google Scholar] [CrossRef]
- Gögenur, M.; Burcharth, J.; Gögenur, I. The role of total cell-free DNA in predicting outcomes among trauma patients in the intensive care unit: A systematic review. Crit. Care 2017, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Margraf, S.; Lögters, T.; Reipen, J.; Altrichter, J.; Scholz, M.; Windolf, J. Neutrophil-derived circulating free DNA (cf-DNA/NETs): A potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock 2008, 30, 352–358. [Google Scholar] [CrossRef]
- Granger, V.; Faille, D.; Marani, V.; Noël, B.; Gallais, Y.; Szely, N.; Flament, H.; Pallardy, M.; Chollet-Martin, S.; de Chaisemartin, L. Human blood monocytes are able to form extracellular traps. J. Leukoc. Biol. 2017, 102, 775–781. [Google Scholar] [CrossRef]
- Lam, N.Y.; Rainer, T.H.; Chiu, R.W.; Joynt, G.M.; Lo, Y.M. Plasma mitochondrial DNA concentrations after trauma. Clin. Chem. 2004, 50, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Hampson, P.; Dinsdale, R.J.; Wearn, C.M.; Bamford, A.L.; Bishop, J.R.B.; Hazeldine, J.; Moiemen, N.S.; Harrison, P.; Lord, J.M. Neutrophil Dysfunction, Immature Granulocytes, and Cell-free DNA are Early Biomarkers of Sepsis in Burn-injured Patients: A Prospective Observational Cohort Study. Ann. Surg. 2017, 265, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.I.; Soliman, R.A.; Samir, S. Cell Free DNA and Procalcitonin as Early Markers of Complications in ICU Patients with Multiple Trauma and Major Surgery. Clin. Lab. 2016, 62, 2395–2404. [Google Scholar] [CrossRef]
- Rodrigues Filho, E.M.; Simon, D.; Ikuta, N.; Klovan, C.; Dannebrock, F.A.; Oliveira de Oliveira, C.; Regner, A. Elevated cell-free plasma DNA level as an independent predictor of mortality in patients with severe traumatic brain injury. J. Neurotrauma 2014, 31, 1639–1646. [Google Scholar] [CrossRef]
- Liaw, P.C.; Ito, T.; Iba, T.; Thachil, J.; Zeerleder, S. DAMP and DIC: The role of extracellular DNA and DNA-binding proteins in the pathogenesis of DIC. Blood Rev. 2016, 30, 257–261. [Google Scholar] [CrossRef]
- Naumann, D.N.; Hazeldine, J.; Dinsdale, R.J.; Bishop, J.R.; Midwinter, M.J.; Harrison, P.; Hutchings, S.D.; Lord, J.M. Endotheliopathy is associated with higher levels of cell-free DNA following major trauma: A prospective observational study. PLoS ONE 2017, 12, e0189870. [Google Scholar] [CrossRef]
- Chiu, T.W.; Young, R.; Chan, L.Y.; Burd, A.; Lo, D.Y. Plasma cell-free DNA as an indicator of severity of injury in burn patients. Clin. Chem. Lab. Med. 2006, 44, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Halpern, D.; Cohen, A.; Sharon, N.; Krieger, Y.; Silberstein, E.; Michael, T.; Douvdevani, A.; Shoham, Y. Admission Circulating Cell-Free DNA Levels as a Prognostic Factor in Pediatric Burns. Biomed. Res. Int. 2022, 2022, 5004282. [Google Scholar] [CrossRef]
- Hayun, Y.; Shoham, Y.; Krieger, Y.; Silberstein, E.; Douvdevani, A.; Ad-El, D. Circulating cell-free DNA as a potential marker in smoke inhalation injury. Medicine 2019, 98, e14863. [Google Scholar] [CrossRef]
- Altrichter, J.; Zedler, S.; Kraft, R.; Faist, E.; Mitzner, S.R.; Sauer, M.; Windolf, J.; Scholz, M.; Lögters, T. Neutrophil-derived circulating free DNA (cf-DNA/NETs), a potential prognostic marker for mortality in patients with severe burn injury. Eur. J. Trauma Emerg. Surg. 2010, 36, 551–557. [Google Scholar] [CrossRef]
- Dwivedi, D.J.; Toltl, L.J.; Swystun, L.L.; Pogue, J.; Liaw, K.L.; Weitz, J.I.; Cook, D.J.; Fox-Robichaud, A.E.; Liaw, P.C. Prognostic utility and characterization of cell-free DNA in patients with severe sepsis. Crit. Care 2012, 16, R151. [Google Scholar] [CrossRef] [PubMed]
- Saukkonen, K.; Lakkisto, P.; Pettilä, V.; Varpula, M.; Karlsson, S.; Ruokonen, E.; Pulkki, K. Cell-free plasma DNA as a predictor of outcome in severe sepsis and septic shock. Clin. Chem. 2008, 54, 1000–1007. [Google Scholar] [CrossRef]
- Rhodes, A.; Wort, S.J.; Thomas, H.; Collinson, P.; Bennett, E.D. Plasma DNA concentration as a predictor of mortality and sepsis in critically ill patients. Crit. Care 2006, 10, R60. [Google Scholar] [CrossRef]
- Lo, Y.M.; Rainer, T.H.; Chan, L.Y.; Hjelm, N.M.; Cocks, R.A. Plasma DNA as a prognostic marker in trauma patients. Clin. Chem. 2000, 46, 319–323. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Finnerty, C.C.; Herndon, D.N.; Mlcak, R.P.; Jeschke, M.G. Are serum cytokines early predictors for the outcome of burn patients with inhalation injuries who do not survive? Crit. Care 2008, 12, R81. [Google Scholar] [CrossRef]
- Bergquist, M.; Hästbacka, J.; Glaumann, C.; Freden, F.; Huss, F.; Lipcsey, M. The time-course of the inflammatory response to major burn injury and its relation to organ failure and outcome. Burns 2019, 45, 354–363. [Google Scholar] [CrossRef]
- Hazeldine, J.; McGee, K.C.; Al-Tarrah, K.; Hassouna, T.; Patel, K.; Imran, R.; Bishop, J.R.B.; Bamford, A.; Barnes, D.; Wilson, Y.; et al. Multicentre, longitudinal, observational cohort study to examine the relationship between neutrophil function and sepsis in adults and children with severe thermal injuries: A protocol for the Scientific Investigation of the Biological Pathways Following Thermal Injury-2 (SIFTI-2) study. BMJ Open 2021, 11, e052035. [Google Scholar] [PubMed]
- Friedman, A.B.; Delgado, M.K.; Auriemma, C.L.; Kilaru, A.S. Hospital-free days: A novel measure to study outcomes for emergency department care. Acad. Emerg. Med. 2024, 31, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Dolp, R.; Rehou, S.; McCann, M.R.; Jeschke, M.G. Contributors to the length-of-stay trajectory in burn-injured patients. Burns 2018, 44, 2011–2017. [Google Scholar] [CrossRef]
- Acharjee, A.; Hazeldine, J.; Bazarova, A.; Deenadayalu, L.; Zhang, J.; Bentley, C.; Russ, D.; Lord, J.M.; Gkoutos, G.V.; Young, S.P.; et al. Integration of Metabolomic and Clinical Data Improves the Prediction of Intensive Care Unit Length of Stay Following Major Traumatic Injury. Metabolites 2021, 12, 29. [Google Scholar] [CrossRef]
- Shoham, Y.; Krieger, Y.; Perry, Z.H.; Shaked, G.; Bogdanov-Berezovsky, A.; Silberstein, E.; Sagi, A.; Douvdevani, A. Admission cell free DNA as a prognostic factor in burns: Quantification by use of a direct rapid fluorometric technique. Biomed. Res. Int. 2014, 2014, 306580. [Google Scholar] [CrossRef]
- Nie, K.; Jia, Y.; Zhang, X. Cell-free circulating tumor DNA in plasma/serum of non-small cell lung cancer. Tumour Biol. 2015, 36, 7–19. [Google Scholar] [CrossRef]
- Zinkova, A.; Brynychova, I.; Svacina, A.; Jirkovska, M.; Korabecna, M. Cell-free DNA from human plasma and serum differs in content of telomeric sequences and its ability to promote immune response. Sci. Rep. 2017, 7, 2591. [Google Scholar] [CrossRef]
- Duke, J.M.; Rea, S.; Boyd, J.H.; Randall, S.M.; Wood, F.M. Mortality after burn injury in children: A 33-year population-based study. Pediatrics 2015, 135, e903–e910. [Google Scholar] [CrossRef]
- Duke, J.M.; Boyd, J.H.; Rea, S.; Randall, S.M.; Wood, F.M. Long-term mortality among older adults with burn injury: A population-based study in Australia. Bull. World Health Organ. 2015, 93, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Chinkes, D.L.; Finnerty, C.C.; Kulp, G.; Suman, O.E.; Norbury, W.B.; Branski, L.K.; Gauglitz, G.G.; Mlcak, R.P.; Herndon, D.N. Pathophysiologic response to severe burn injury. Ann. Surg. 2008, 248, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Nielson, C.B.; Duethman, N.C.; Howard, J.M.; Moncure, M.; Wood, J.G. Burns: Pathophysiology of Systemic Complications and Current Management. J. Burn. Care Res. 2017, 38, e469–e481. [Google Scholar] [CrossRef] [PubMed]
- Szczesny, B.; Brunyánszki, A.; Ahmad, A.; Oláh, G.; Porter, C.; Toliver-Kinsky, T.; Sidossis, L.; Herndon, D.N.; Szabo, C. Time-Dependent and Organ-Specific Changes in Mitochondrial Function, Mitochondrial DNA Integrity, Oxidative Stress and Mononuclear Cell Infiltration in a Mouse Model of Burn Injury. PLoS ONE 2015, 10, e0143730. [Google Scholar] [CrossRef]
- Korabecna, M.; Tesar, V. NETosis provides the link between activation of neutrophils on hemodialysis membrane and comorbidities in dialyzed patients. Inflamm. Res. 2017, 66, 369–378. [Google Scholar] [CrossRef]
- Herndon, D.N.; Barrow, R.E.; Rutan, R.L.; Rutan, T.C.; Desai, M.H.; Abston, S. A comparison of conservative versus early excision. Therapies in severely burned patients. Ann. Surg. 1989, 209, 547–552, discussion 552–543. [Google Scholar] [CrossRef]
- Henriksen, T.V.; Reinert, T.; Christensen, E.; Sethi, H.; Birkenkamp-Demtröder, K.; Gögenur, M.; Gögenur, I.; Zimmermann, B.G.; Dyrskjøt, L.; Andersen, C.L. The effect of surgical trauma on circulating free DNA levels in cancer patients-implications for studies of circulating tumor DNA. Mol. Oncol. 2020, 14, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Konishi, S.; Narita, T.; Hatakeyama, S.; Yoneyama, T.; Yoneyama, M.S.; Tobisawa, Y.; Noro, D.; Sato, T.; Togashi, K.; Okamoto, T.; et al. Utility of total cell-free DNA levels for surgical damage evaluation in patients with urological surgeries. Sci. Rep. 2021, 11, 22103. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Uchida, T.; Yamamoto, M.; Yamamoto, Y.; Kido, K.; Ito, H.; Ohno, N.; Asahara, M.; Yamada, Y.; Yamaguchi, O.; et al. Perioperative Elevation in Cell-Free DNA Levels in Patients Undergoing Cardiac Surgery: Possible Contribution of Neutrophil Extracellular Traps to Perioperative Renal Dysfunction. Anesthesiol. Res. Pract. 2016, 2016, 2794364. [Google Scholar] [CrossRef] [PubMed]
- Brodbeck, K.; Kern, S.; Schick, S.; Steinbruck, A.; Schwerer, M.; Bayer, B.; Anslinger, K.; Peldschus, S. Quantitative analysis of individual cell-free DNA concentration before and after penetrating trauma. Int. J. Legal Med. 2019, 133, 385–393. [Google Scholar] [CrossRef]
- Dinsdale, R.J.; Hazeldine, J.; Al Tarrah, K.; Hampson, P.; Devi, A.; Ermogenous, C.; Bamford, A.L.; Bishop, J.; Watts, S.; Kirkman, E.; et al. Dysregulation of the actin scavenging system and inhibition of DNase activity following severe thermal injury. Br. J. Surg. 2020, 107, 391–401. [Google Scholar] [CrossRef]
- Trulson, I.; Stahl, J.; Margraf, S.; Scholz, M.; Hoecherl, E.; Wolf, K.; Durner, J.; Klawonn, F.; Holdenrieder, S. Cell-Free DNA in Plasma and Serum Indicates Disease Severity and Prognosis in Blunt Trauma Patients. Diagnostics 2023, 13, 1150. [Google Scholar] [CrossRef]
- Otawara, M.; Roushan, M.; Wang, X.; Ellett, F.; Yu, Y.M.; Irimia, D. Microfluidic Assay Measures Increased Neutrophil Extracellular Traps Circulating in Blood after Burn Injuries. Sci. Rep. 2018, 8, 16983. [Google Scholar] [CrossRef]
- Boneschansker, L.; Inoue, Y.; Oklu, R.; Irimia, D. Capillary plexuses are vulnerable to neutrophil extracellular traps. Integr. Biol. 2016, 8, 149–155. [Google Scholar] [CrossRef]
- Roberts, G.; Lloyd, M.; Parker, M.; Martin, R.; Philp, B.; Shelley, O.; Dziewulski, P. The Baux score is dead. Long live the Baux score: A 27-year retrospective cohort study of mortality at a regional burns service. J. Trauma. Acute Care Surg. 2012, 72, 251–256. [Google Scholar] [CrossRef]
- Giretzlehner, M.; Dirnberger, J.; Owen, R.; Haller, H.L.; Lumenta, D.B.; Kamolz, L.P. The determination of total burn surface area: How much difference? Burns 2013, 39, 1107–1113. [Google Scholar] [CrossRef]
- Parvizi, D.; Kamolz, L.P.; Giretzlehner, M.; Haller, H.L.; Trop, M.; Selig, H.; Nagele, P.; Lumenta, D.B. The potential impact of wrong TBSA estimations on fluid resuscitation in patients suffering from burns: Things to keep in mind. Burns 2014, 40, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Tocco-Tussardi, I.; Presman, B.; Huss, F. Want Correct Percentage of TBSA Burned? Let a Layman Do the Assessment. J. Burn. Care Res. 2018, 39, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.J.; Malyon, A.D.; Scerri, G.V.; Burge, T.S. A comparison of serial halving and the rule of nines as a pre-hospital assessment tool in burns. Br. J. Plast. Surg. 2005, 58, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Hazeldine, J.; Dinsdale, R.J.; Naumann, D.N.; Acharjee, A.; Bishop, J.R.B.; Lord, J.M.; Harrison, P. Traumatic injury is associated with reduced deoxyribonuclease activity and dysregulation of the actin scavenging system. Burns Trauma 2021, 9, tkab001. [Google Scholar] [CrossRef] [PubMed]
- Shaked, G.; Douvdevani, A.; Yair, S.; Zlotnik, A.; Czeiger, D. The role of cell-free DNA measured by a fluorescent test in the management of isolated traumatic head injuries. Scand. J. Trauma Resusc. Emerg. Med. 2014, 22, 21. [Google Scholar] [CrossRef]
- Avriel, A.; Wiessman, M.P.; Almog, Y.; Perl, Y.; Novack, V.; Galante, O.; Klein, M.; Pencina, M.J.; Douvdevani, A. Admission cell free DNA levels predict 28-day mortality in patients with severe sepsis in intensive care. PLoS ONE 2014, 9, e100514. [Google Scholar] [CrossRef]
- Wang, X.; Sun, K.; Wang, H. Point-of-care nucleic acid testing with a one-step branched-DNA-based functional carbon biosensor. Cell Rep. Phys. Sci. 2024, 5, 101753. [Google Scholar] [CrossRef]
| Characteristic | Burns Patients (n = 98) |
|---|---|
| Age, years | 47 (16–84) |
| Gender (M:F) | 77:21 |
| % TBSA | 35 (15–85) |
| % FT TBSA | 19 (0–80) |
| Inhalation injury (Y:N) | 44:54 |
| Mechanism of injury: | |
| Flash, n (%) | 7 (7) |
| Flame, n (%) | 82 (84) |
| Flame and flash, n (%) | 5 (5) |
| Electrical, n (%) | 1 (1) |
| Scald, n (%) | 3 (3) |
| Co-morbidities: | |
| Respiratory, n (%) | 12 (12) |
| Cardiovascular, n (%) | 15 (15) |
| Endocrine/metabolic, n (%) | 12 (12) |
| Psychiatric, n (%) | 30 (31) |
| Neurological, n (%) | 10 (10) |
| Gastrointestinal, n (%) | 3 (3) |
| Musculoskeletal, n (%) | 4 (4) |
| ABSI | 7 (2–14) |
| Baux | 82 (34–143) |
| rBaux | 90 (39–160) |
| Day 1 SOFA | 7 (0–17) |
| Day 1 Denver | 2 (0–7) |
| ITU-free days | 15 (0–30) |
| Hospital-free days | 4 (0–20) |
| Mortality (Y:N) | 17:81 |
| Correlation (R) | Significance (p) | FDR Adjusted p-value | |
|---|---|---|---|
| TBSA % (n = 95) | 0.413 | <0.0001 | 0.0001 |
| TBSA FT (n = 95) | 0.241 | 0.019 | 0.021 |
| Baux (n = 95) | 0.347 | <0.001 | 0.0009 |
| rBaux (n = 95) | 0.365 | <0.0005 | 0.0005 |
| Denver (n = 95) | 0.454 | <0.0001 | <0.0001 |
| SOFA (n = 95) | 0.391 | <0.0001 | 0.0002 |
| ABSI (n = 95) | 0.308 | 0.002 | 0.003 |
| Hospital-free days (n = 95) | −0.387 | 0.0003 | 0.0002 |
| ITU-free days (n = 95) | −0.451 | <0.0001 | <0.0001 |
| Hospital LOS (n = 95) | 0.075 | 0.469 | 0.469 |
| ITU LOS (n = 95) | 0.299 | 0.003 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tullie, S.; Asiri, A.; Acharjee, A.; Moiemen, N.S.; Lord, J.M.; Harrison, P.; Hazeldine, J. Day One Cell-Free DNA Levels as an Objective Prognostic Marker of Mortality in Major Burns Patients. Cells 2025, 14, 821. https://doi.org/10.3390/cells14110821
Tullie S, Asiri A, Acharjee A, Moiemen NS, Lord JM, Harrison P, Hazeldine J. Day One Cell-Free DNA Levels as an Objective Prognostic Marker of Mortality in Major Burns Patients. Cells. 2025; 14(11):821. https://doi.org/10.3390/cells14110821
Chicago/Turabian StyleTullie, Sebastian, Ali Asiri, Animesh Acharjee, Naiem S. Moiemen, Janet M. Lord, Paul Harrison, and Jon Hazeldine. 2025. "Day One Cell-Free DNA Levels as an Objective Prognostic Marker of Mortality in Major Burns Patients" Cells 14, no. 11: 821. https://doi.org/10.3390/cells14110821
APA StyleTullie, S., Asiri, A., Acharjee, A., Moiemen, N. S., Lord, J. M., Harrison, P., & Hazeldine, J. (2025). Day One Cell-Free DNA Levels as an Objective Prognostic Marker of Mortality in Major Burns Patients. Cells, 14(11), 821. https://doi.org/10.3390/cells14110821








