Interconnections between the Cation/Alkaline pH-Responsive Slt and the Ambient pH Response of PacC/Pal Pathways in Aspergillus nidulans
Abstract
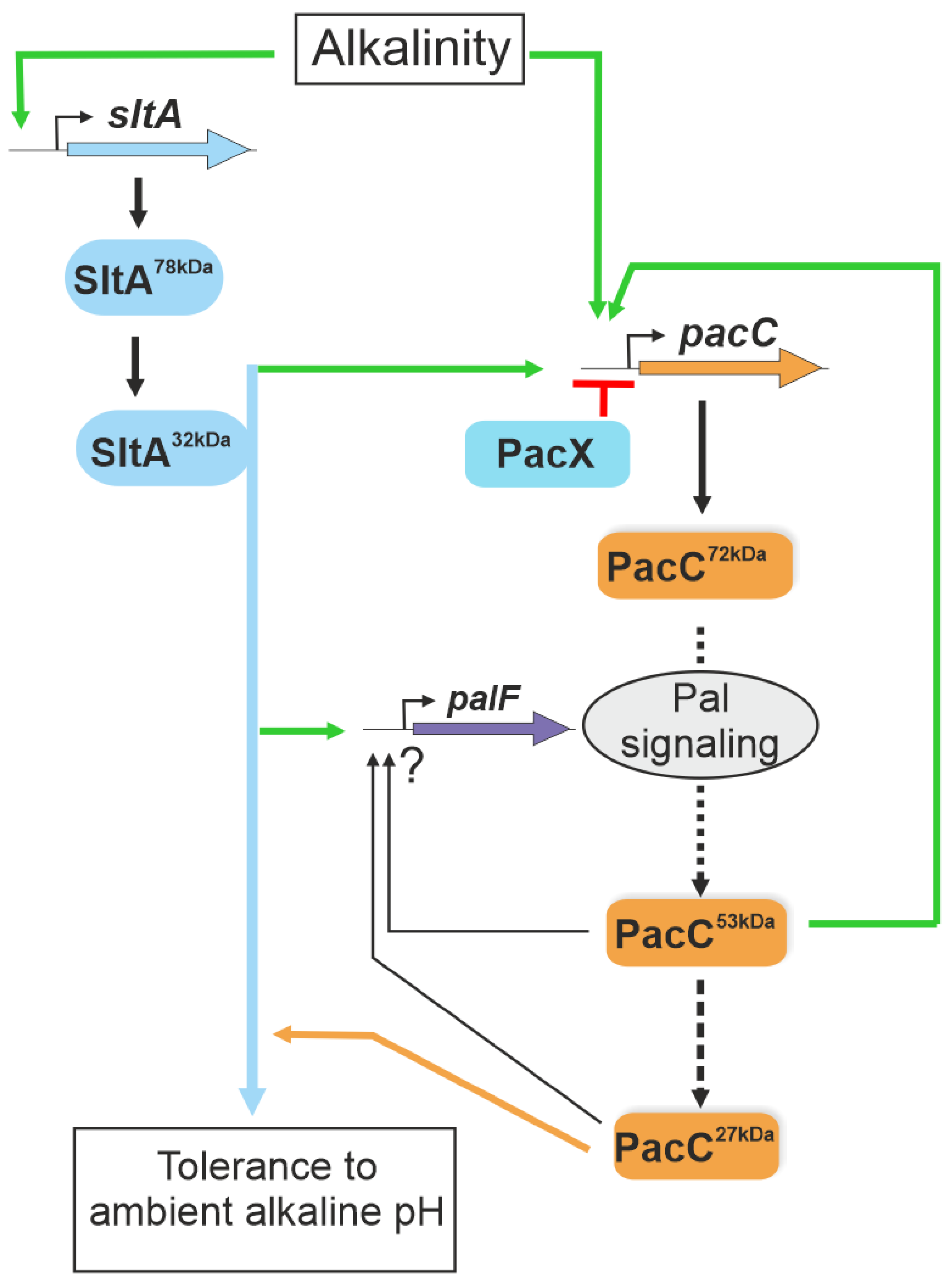
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. RNA Extraction and Quantitative PCR
2.3. Protein Extraction and Immunodetection
2.4. Fluorescence Microscopy
2.5. Statistical Analyses
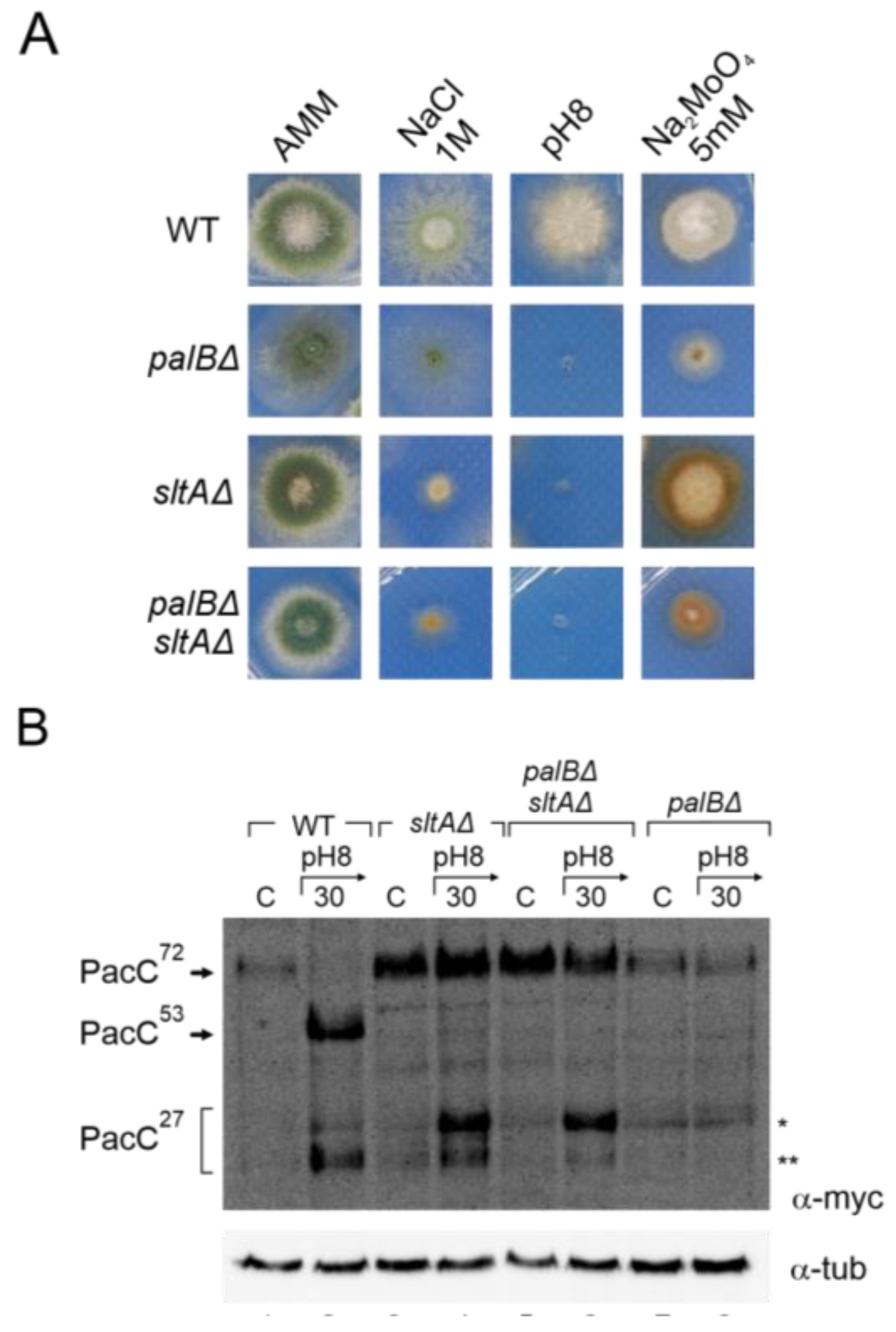
3. Results
3.1. Absence of SltA Function Altered pacC and palF Expression Levels in Response to Alkaline Ambient pH and High Sodium Concentration
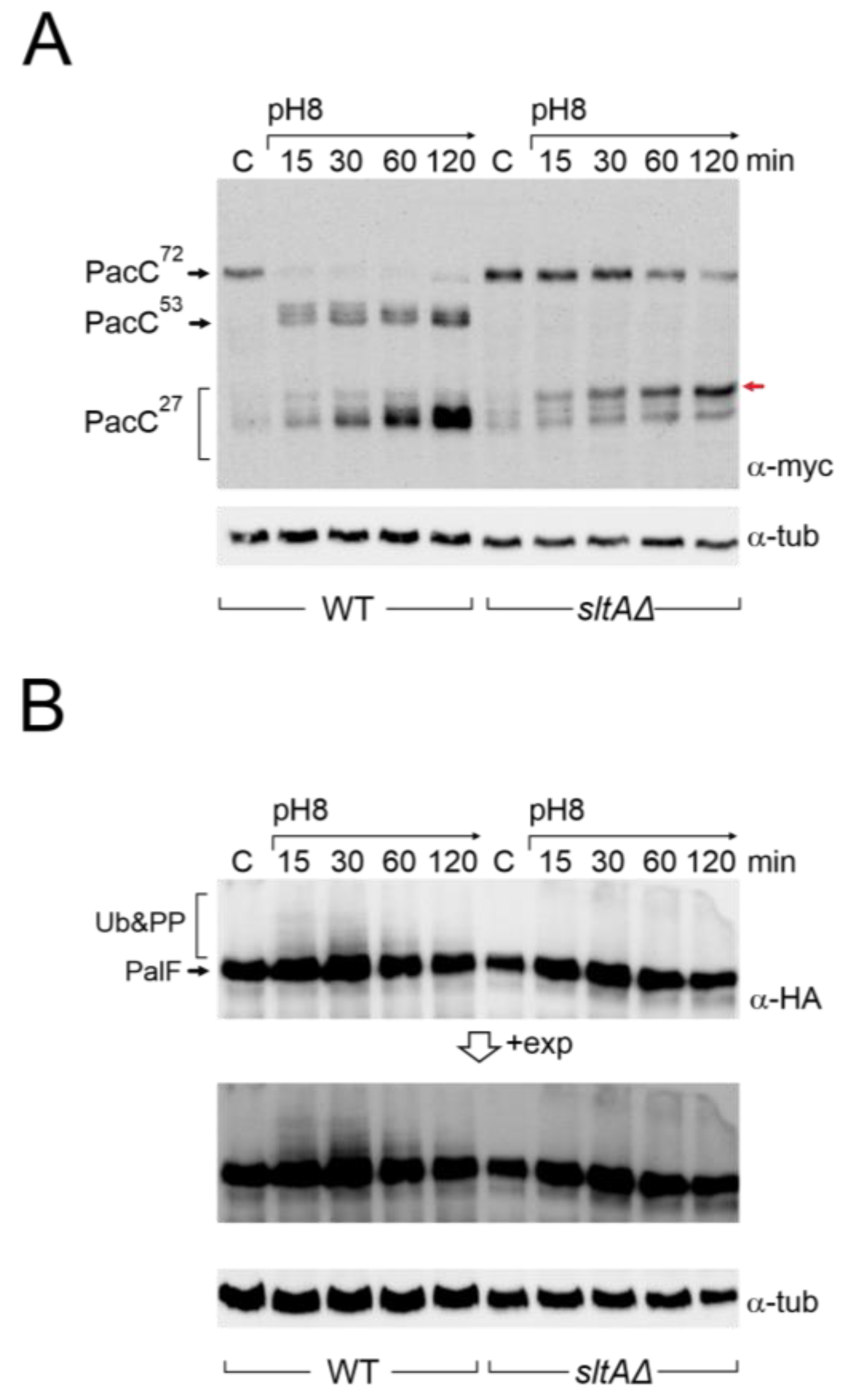
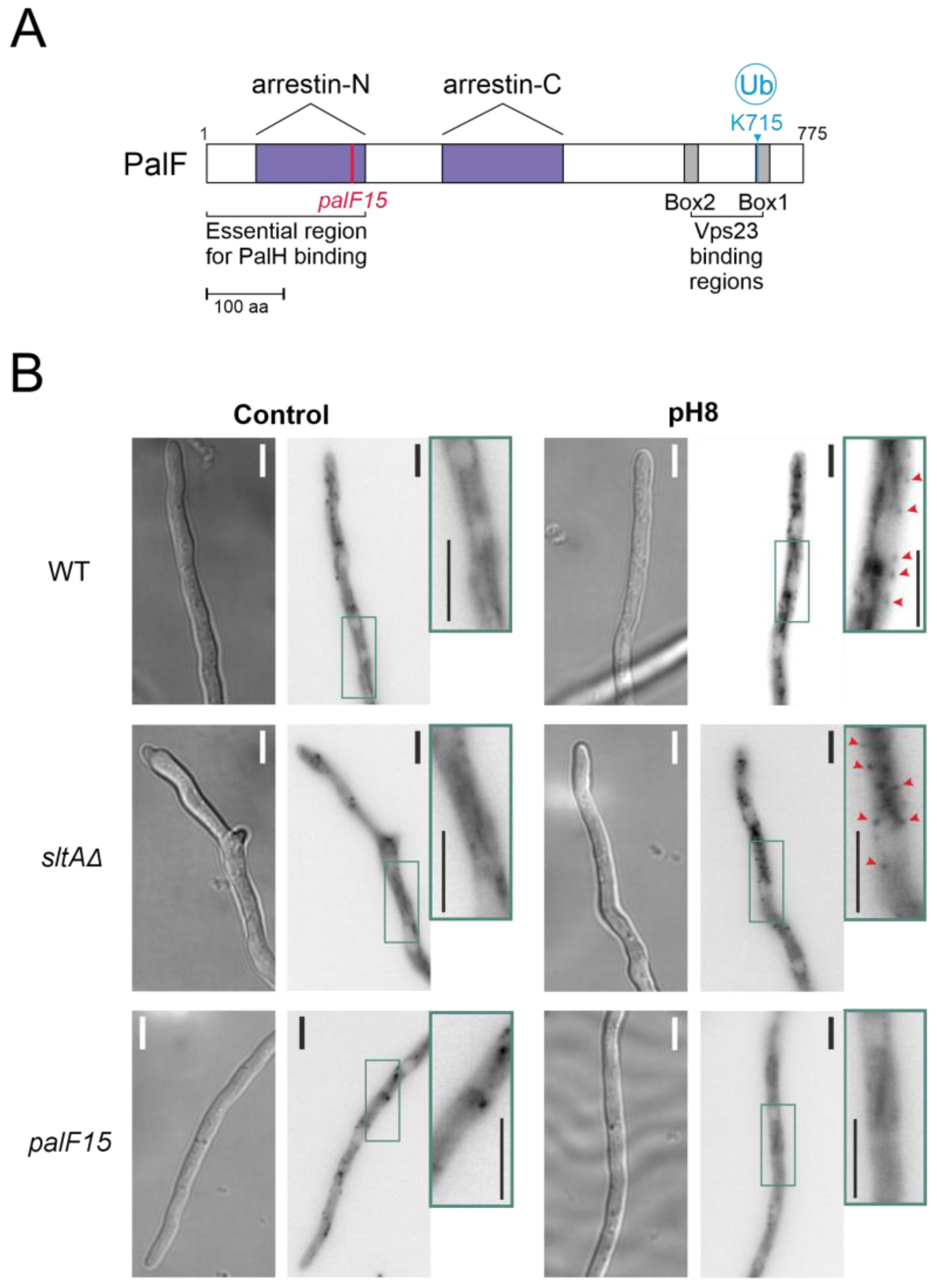
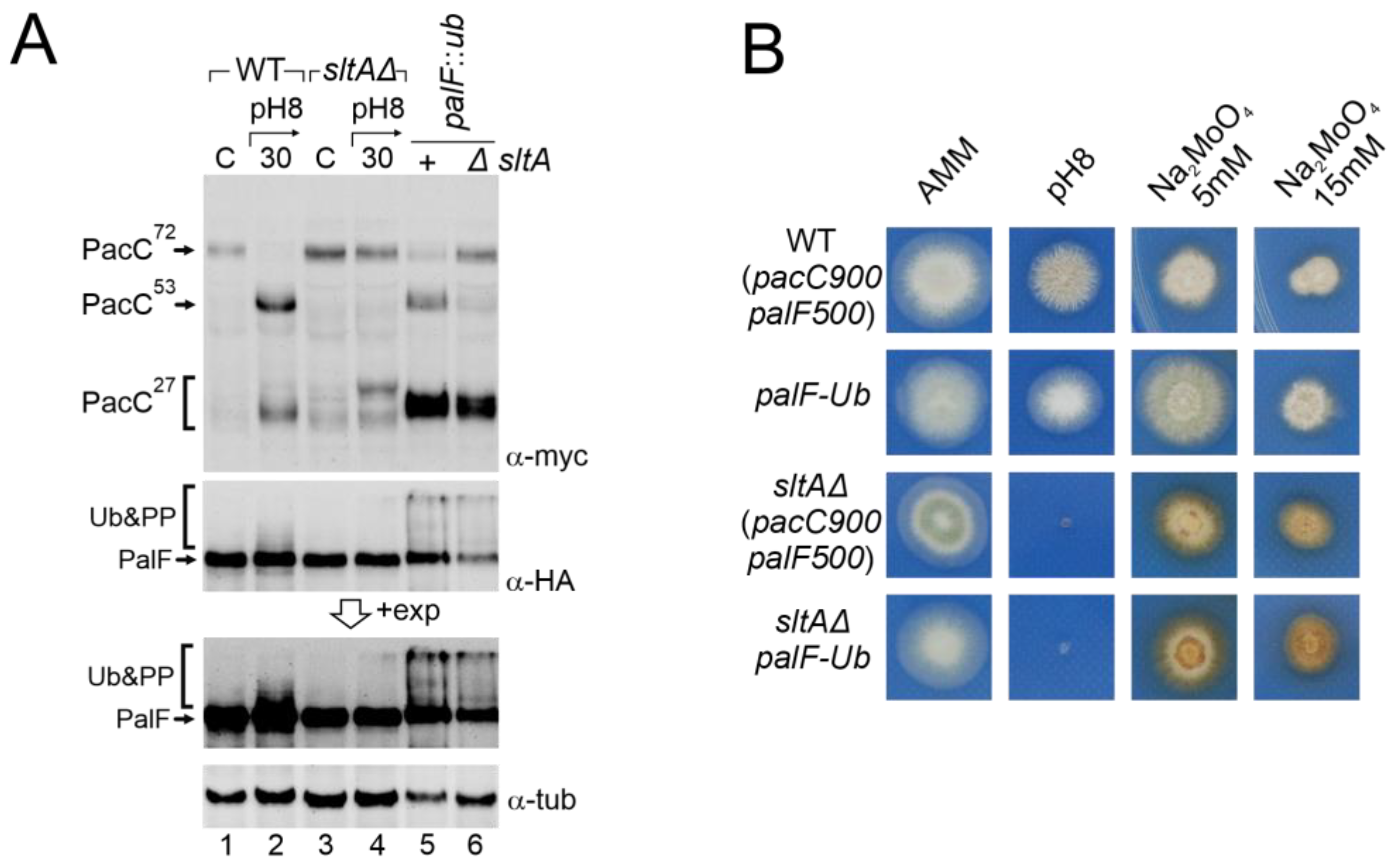
3.2. Absence of SltA Activity Prevents the Proper Signalling of PacC and PalF upon Exposure to Alkalinity
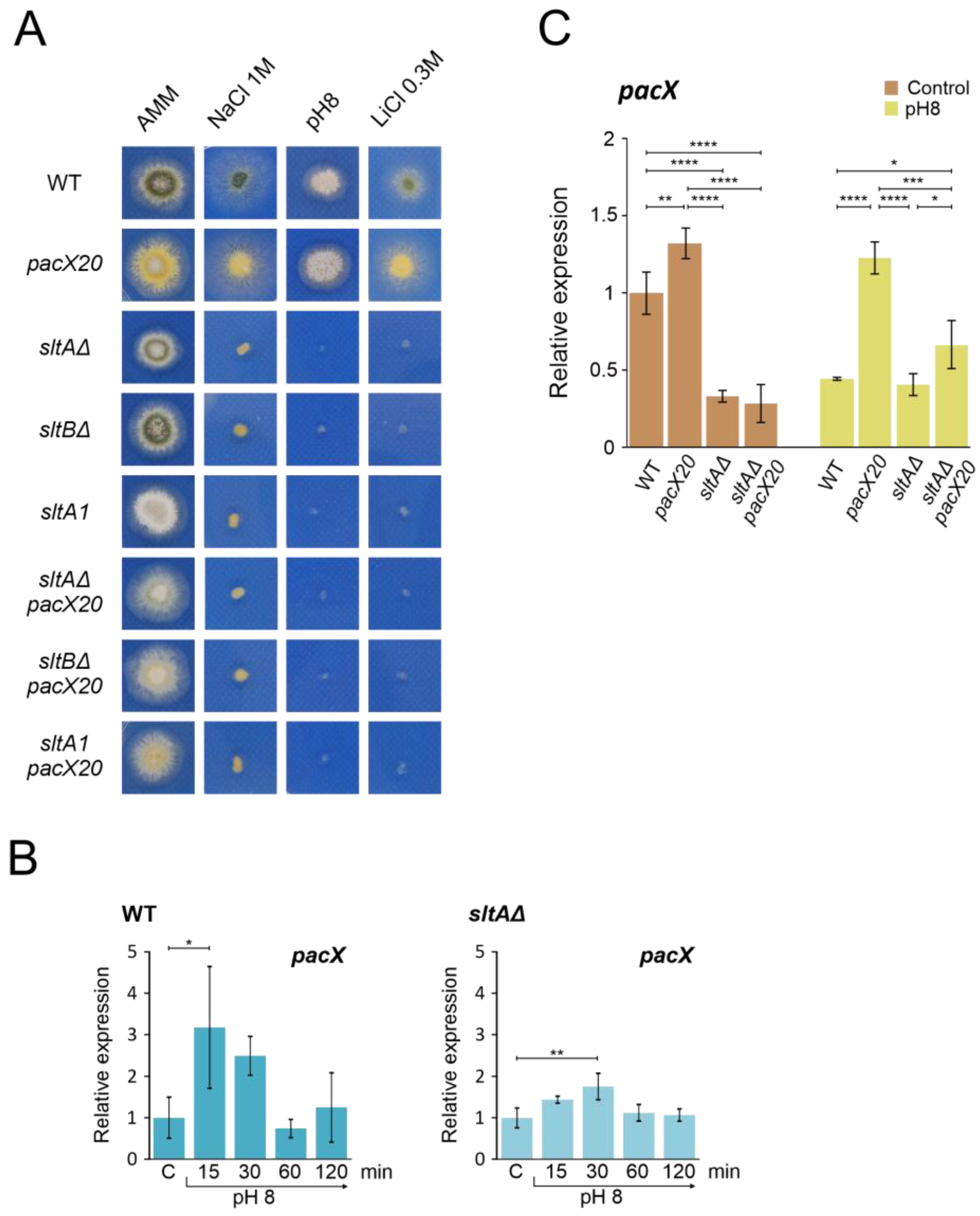
3.3. Absence of the Suppression of Null-sltA Phenotype by a pacX Null-like Mutation
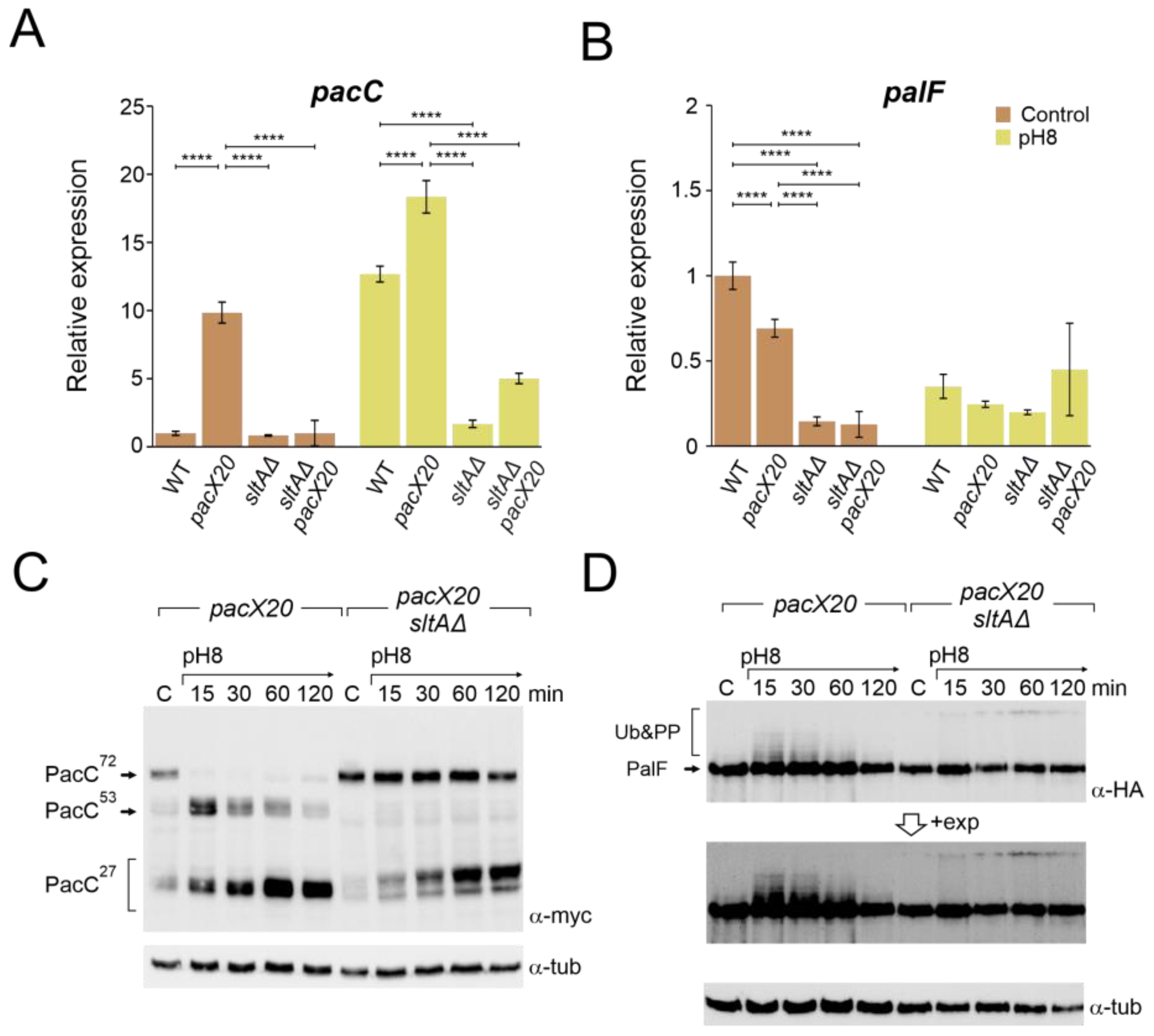
3.4. Absence of PacX Activity Did Not Rescue PacC and PalF Transcriptional and Translational Profiles in a sltAΔ Background
3.5. Altered Proteolytic Processing of PacC72kDa in a Null sltA Background Is Independent of the PalB Protease
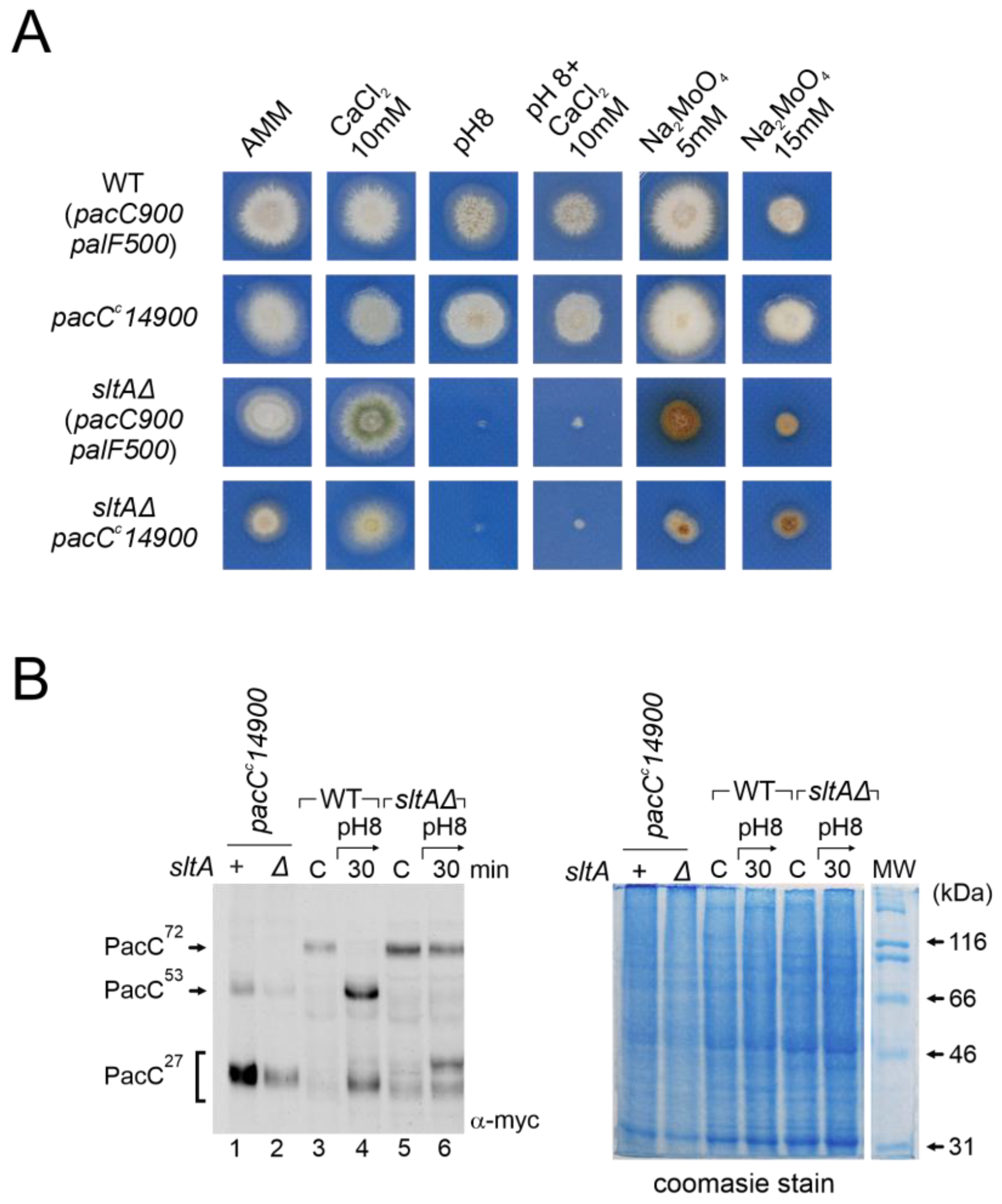
3.6. Constitutive Activation of the Pal Pathway Was Unable to Suppress sltAΔ Phenotypes
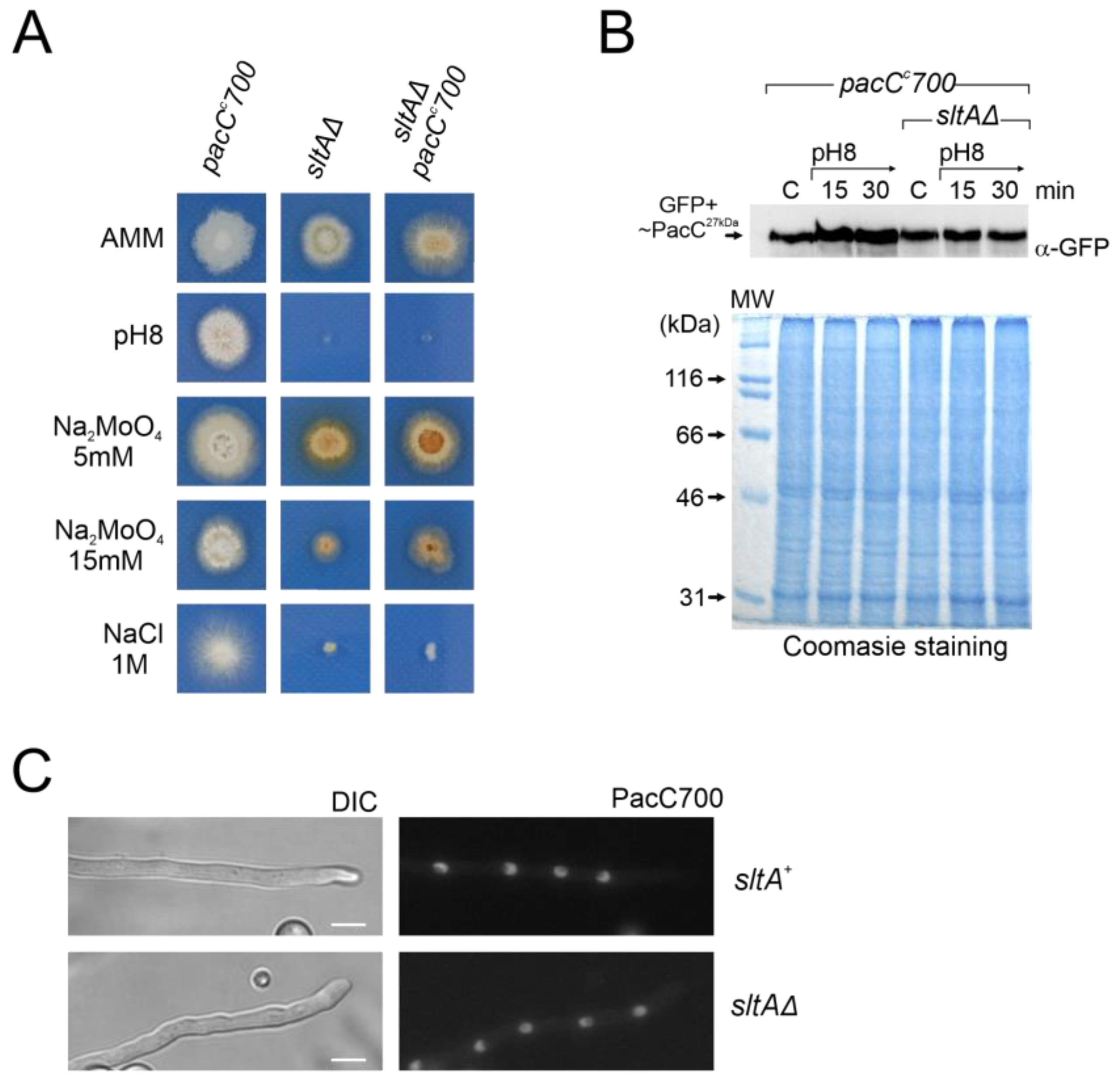
3.7. Epistasis of Null sltA Allele over pacC Constitutive Mutations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Etxebeste, O.; Espeso, E.A. Aspergillus nidulans in the post-genomic era: A top-model filamentous fungus for the study of signaling and homeostasis mechanisms. Int. Microbiol. 2020, 23, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.P.; Cowen, L.E.; di Pietro, A.; Quinn, J. Stress Adaptation. Microbiol. Spectr. 2017, 5, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Kelleher, N.L.; Keller, N.P. In the fungus where it happens: History and future propelling Aspergillus nidulans as the archetype of natural products research. Fungal Genet. Biol. 2020, 144, 103477. [Google Scholar] [CrossRef] [PubMed]
- Peñalva, M.A.; Tilburn, J.; Bignell, E.; Arst, H.N., Jr. Ambient pH gene regulation in fungi: Making connections. Trends Microbiol. 2008, 16, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.; Hansberg, W.; Navarro, R. Fungal responses to reactive oxygen species. Med. Mycol. 2006, 44, S101–S107. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, S.P.; Lima, A.M.; De Souza, C.R.B. Recent Molecular Advances on Downstream Plant Responses to Abiotic Stress. Int. J. Mol. Sci. 2012, 13, 8628–8647. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, D.; Sakai, K.; Suzuki, S.; Umemura, M.; Nogawa, T.; Kato, N.; Osada, H.; Watanabe, A.; Kawamoto, S.; Gonoi, T.; et al. Temperature during conidiation affects stress tolerance, pigmentation, and trypacidin accumulation in the conidia of the airborne pathogen Aspergillus fumigatus. PLoS ONE 2017, 12, e0177050. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Arst, H.N.; Peñalva, M.A. pH regulation in Aspergillus and parallels with higher eukaryotic regulatory systems. Trends Genet. 2003, 19, 224–231. [Google Scholar] [CrossRef]
- Razin, S.V.; Borunova, V.V.; Maksimenko, O.G.; Kantidze, O.L. Cys2His2 zinc finger protein family: Classification, functions, and major members. Biochemistry 2012, 77, 217–226. [Google Scholar] [CrossRef]
- Steyaert, J.M.; Weld, R.J.; Mendoza-Mendoza, A.; Stewart, A. Reproduction without sex: Conidiation in the filamentous fungus Trichoderma. Microbiol. 2010, 156, 2887–2900. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, M.G.; Batista-Garcia, R.A.; Arechiga-Carvajal, E.T. Alkaliphilic/Alkali-Tolerant Fungi: Molecular, Biochemical, and Biotechnological Aspects. J. Fungi 2023, 9, 652. [Google Scholar] [CrossRef]
- Rollins, J.A.; Dickman, M.B. pH signaling in Sclerotinia sclerotiorum: Identification of a pacC/RIM1 homolog. Appl. Environ. Microbiol. 2001, 67, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Cornet, M.; Gaillardin, C. pH signaling in human fungal pathogens: A new target for antifungal strategies. Eukaryot. Cell 2014, 13, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Davis, D. Adaptation to environmental pH in Candida albicans and its relation to pathogenesis. Curr. Genet. 2003, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mitchell, A.P. Proteolytic activation of Rim1p, a positive regulator of yeast sporulation and invasive growth. Genetics 1997, 145, 63–73. [Google Scholar] [CrossRef]
- Lamb, T.M.; Xu, W.; Diamond, A.; Mitchell, A.P. Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J. Biol. Chem. 2001, 276, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Peñalva, M.A.; Arst, H.N., Jr. Regulation of gene expression by ambient pH in filamentous fungi and yeasts. Microbiol. Mol. Biol. Rev. 2002, 66, 426–446. [Google Scholar] [CrossRef] [PubMed]
- Denison, S.H. pH regulation of gene expression in fungi. Fungal Genet. Biol. 2000, 29, 61–71. [Google Scholar] [CrossRef]
- Peñalva, M.A.; Lucena-Agell, D.; Arst, H.N., Jr. Liaison alcaline: Pals entice non-endosomal ESCRTs to the plasma membrane for pH signaling. Curr. Opin. Microbiol. 2014, 22, 49–59. [Google Scholar] [CrossRef]
- Herranz, S.; Rodriguez, J.M.; Bussink, H.J.; Sanchez-Ferrero, J.C.; Arst, H.N., Jr.; Peñalva, M.A.; Vincent, O. Arrestin-related proteins mediate pH signaling in fungi. Proc. Natl. Acad. Sci. USA 2005, 102, 12141–12146. [Google Scholar] [CrossRef]
- Galindo, A.; Calcagno-Pizarelli, A.M.; Arst, H.N., Jr.; Peñalva, M.A. An ordered pathway for the assembly of fungal ESCRT-containing ambient pH signalling complexes at the plasma membrane. J. Cell Sci. 2012, 125, 1784–1795. [Google Scholar] [CrossRef]
- Herrador, A.; Herranz, S.; Lara, D.; Vincent, O. Recruitment of the ESCRT machinery to a putative seven-transmembrane-domain receptor is mediated by an arrestin-related protein. Mol. Cell Biol. 2010, 30, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Vincent, O.; Rainbow, L.; Tilburn, J.; Arst, H.N., Jr.; Peñalva, M.A. YPXL/I is a protein interaction motif recognized by Aspergillus PalA and its human homologue, AIP1/Alix. Mol. Cell Biol. 2003, 23, 1647–1655. [Google Scholar] [CrossRef]
- Diez, E.; Alvaro, J.; Espeso, E.A.; Rainbow, L.; Suarez, T.; Tilburn, J.; Arst, H.N., Jr.; Peñalva, M.A. Activation of the Aspergillus PacC zinc finger transcription factor requires two proteolytic steps. EMBO J. 2002, 21, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Peñas, M.M.; Hervas-Aguilar, A.; Munera-Huertas, T.; Reoyo, E.; Peñalva, M.A.; Arst, H.N., Jr.; Tilburn, J. Further characterization of the signaling proteolysis step in the Aspergillus nidulans pH signal transduction pathway. Eukaryot. Cell 2007, 6, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Hervas-Aguilar, A.; Rodriguez, J.M.; Tilburn, J.; Arst, H.N., Jr.; Peñalva, M.A. Evidence for the direct involvement of the proteasome in the proteolytic processing of the Aspergillus nidulans zinc finger transcription factor PacC. J. Biol. Chem. 2007, 282, 34735–34747. [Google Scholar] [CrossRef] [PubMed]
- Caddick, M.X.; Brownlee, A.G.; Arst, H.N., Jr. Regulation of gene expression by pH of the growth medium in Aspergillus nidulans. Mol. Gen. Genet. 1986, 203, 346–353. [Google Scholar] [CrossRef]
- Peñalva, M.A.; Arst, H.N., Jr. Recent advances in the characterization of ambient pH regulation of gene expression in filamentous fungi and yeasts. Annu. Rev. Microbiol. 2004, 58, 425–451. [Google Scholar] [CrossRef]
- Markina-Iñarrairaegui, A.; Spielvogel, A.; Etxebeste, O.; Ugalde, U.; Espeso, E.A. Tolerance to alkaline ambient pH in Aspergillus nidulans depends on the activity of ENA proteins. Sci. Rep. 2020, 10, 14325. [Google Scholar] [CrossRef]
- Bussink, H.J.; Bignell, E.M.; Munera-Huertas, T.; Lucena-Agell, D.; Scazzocchio, C.; Espeso, E.A.; Bertuzzi, M.; Rudnicka, J.; Negrete-Urtasun, S.; Penas-Parilla, M.M.; et al. Refining the pH response in Aspergillus nidulans: A modulatory triad involving PacX, a novel zinc binuclear cluster protein. Mol. Microbiol. 2015, 98, 1051–1072. [Google Scholar] [CrossRef] [PubMed]
- Etxebeste, O. Transcription Factors in the Fungus Aspergillus nidulans: Markers of Genetic Innovation, Network Rewiring and Conflict between Genomics and Transcriptomics. J. Fungi 2021, 7, 600. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, S.; Larochelle, M.; Turcotte, B. A fungal family of transcriptional regulators: The zinc cluster proteins. Microbiol. Mol. Biol. Rev. 2006, 70, 583–604. [Google Scholar] [CrossRef]
- Clement, D.J.; Stanley, M.S.; Attwell, N.A.; Clipson, N.J.; Fincham, D.A.; Hooley, P. Evidence for sltA1 as a salt-sensitive allele of the arginase gene (agaA) in the ascomycete Aspergillus nidulans. Curr. Genet. 1996, 29, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Du, W.; Lu, L.; Zhang, S. Transcription factors SltA and CrzA reversely regulate calcium homeostasis under calcium-limited conditions. Appl. Environ. Microbiol. 2023, 89, e0117023. [Google Scholar] [CrossRef]
- O’Neil, J.D.; Bugno, M.; Stanley, M.S.; Barham-Morris, J.B.; Woodcock, N.A.; Clement, D.J.; Clipson, N.J.W.; Whitehead, M.P.; Fincham, D.A.; Hooley, P. Cloning of a novel gene encoding a C2H2 zinc finger protein that alleviates sensitivity to abiotic stresses in Aspergillus nidulans. Mycol. Res. 2002, 106, 491–498. [Google Scholar] [CrossRef]
- Spathas, D.H. A salt sensitive mutation on chromosome VI of Aspergillus Nidulans. Aspergillus Newsl. 1978, 46, 28. [Google Scholar]
- Spielvogel, A.; Findon, H.; Arst, H.N.; Araujo-Bazan, L.; Hernandez-Ortiz, P.; Stahl, U.; Meyer, V.; Espeso, E.A. Two zinc finger transcription factors, CrzA and SltA, are involved in cation homoeostasis and detoxification in Aspergillus nidulans. Biochem. J. 2008, 414, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, W.; Bruno, V.M.; Phan, Q.T.; Solis, N.V.; Woolford, C.A.; Ehrlich, R.L.; Shetty, A.C.; McCraken, C.; Lin, J.; et al. Determining Aspergillus fumigatus transcription factor expression and function during invasion of the mammalian lung. PLoS Pathog. 2021, 17, e1009235. [Google Scholar] [CrossRef]
- Baltussen, T.J.H.; van Rhijn, N.; Coolen, J.P.M.; Dijksterhuis, J.; Verweij, P.E.; Bromley, M.J.; Melchers, W.J.G. The C(2)H(2) transcription factor SltA is required for germination and hyphal development in Aspergillus fumigatus. Msphere 2023, 8, e0007623. [Google Scholar] [CrossRef]
- Aro, N.; Ilmen, M.; Saloheimo, A.; Penttila, M. ACEI of Trichoderma reesei is a repressor of cellulase and xylanase expression. Appl. Environ. Microbiol. 2003, 69, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Saloheimo, A.; Aro, N.; Ilmen, M.; Penttila, M. Isolation of the ace1 gene encoding a Cys(2)-His(2) transcription factor involved in regulation of activity of the cellulase promoter cbh1 of Trichoderma reesei. J. Biol. Chem. 2000, 275, 5817–5825. [Google Scholar] [CrossRef] [PubMed]
- Mellado, L.; Arst, H.N., Jr.; Espeso, E.A. Proteolytic activation of both components of the cation stress-responsive Slt pathway in Aspergillus nidulans. Mol. Biol. Cell 2016, 27, 2598–2612. [Google Scholar] [CrossRef] [PubMed]
- Picazo, I.; Etxebeste, O.; Requena, E.; Garzia, A.; Espeso, E.A. Defining the transcriptional responses of Aspergillus nidulans to cation/alkaline pH stress and the role of the transcription factor SltA. Microb. Genom. 2020, 6, e000415. [Google Scholar] [CrossRef]
- Todd, R.B.; Andrianopoulos, A. Evolution of a fungal regulatory gene family: The Zn(II)2Cys6 binuclear cluster DNA binding motif. Fungal Genet. Biol. 1997, 21, 388–405. [Google Scholar] [CrossRef]
- Cove, D.J. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim. Biophys. Acta 1966, 113, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Tilburn, J.; Scazzocchio, C.; Taylor, G.G.; Zabicky-Zissman, J.H.; Lockington, R.A.; Davies, R.W. Transformation by integration in Aspergillus nidulans. Gene 1983, 26, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Hervas-Aguilar, A.; Galindo, A.; Peñalva, M.A. Receptor-independent Ambient pH signaling by ubiquitin attachment to fungal arrestin-like PalF. J. Biol. Chem. 2010, 285, 18095–18102. [Google Scholar] [CrossRef] [PubMed]
- Nayak, T.; Szewczyk, E.; Oakley, C.E.; Osmani, A.; Ukil, L.; Murray, S.L.; Hynes, M.J.; Osmani, S.A.; Oakley, B.R. A versatile and efficient gene-targeting system for Aspergillus Nidulans. Genetics 2006, 172, 1557–1566. [Google Scholar] [CrossRef]
- Peñalva, M.A. Tracing the endocytic pathway of Aspergillus nidulans with FM4-64. Fungal Genet. Biol. 2005, 42, 963–975. [Google Scholar] [CrossRef]
- Calcagno-Pizarelli, A.M.; Hervas-Aguilar, A.; Galindo, A.; Abenza, J.F.; Peñalva, M.A.; Arst, H.N., Jr. Rescue of Aspergillus nidulans severely debilitating null mutations in ESCRT-0, I, II and III genes by inactivation of a salt-tolerance pathway allows examination of ESCRT gene roles in pH signalling. J. Cell Sci. 2011, 124, 4064–4076. [Google Scholar] [CrossRef] [PubMed]
- Denison, S.H.; Orejas, M.; Arst, H.N., Jr. Signaling of ambient pH in Aspergillus involves a cysteine protease. J. Biol. Chem. 1995, 270, 28519–28522. [Google Scholar] [CrossRef]
- Fernandez-Martinez, J.; Brown, C.V.; Diez, E.; Tilburn, J.; Arst, H.N., Jr.; Peñalva, M.A.; Espeso, E.A. Overlap of nuclear localisation signal and specific DNA-binding residues within the zinc finger domain of PacC. J. Mol. Biol. 2003, 334, 667–684. [Google Scholar] [CrossRef]
- Galindo, A.; Hervas-Aguilar, A.; Rodriguez-Galan, O.; Vincent, O.; Arst, H.N., Jr.; Tilburn, J.; Penalva, M.A. PalC, one of two Bro1 domain proteins in the fungal pH signalling pathway, localizes to cortical structures and binds Vps32. Traffic 2007, 8, 1346–1364. [Google Scholar] [CrossRef] [PubMed]
- Lucena-Agell, D.; Galindo, A.; Arst, H.N., Jr.; Penalva, M.A. Aspergillus nidulans Ambient pH Signaling Does Not Require Endocytosis. Eukaryot. Cell 2015, 14, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Galan, O.; Galindo, A.; Hervas-Aguilar, A.; Arst, H.N., Jr.; Peñalva, M.A. Physiological involvement in pH signaling of Vps24-mediated recruitment of Aspergillus PalB cysteine protease to ESCRT-III. J. Biol. Chem. 2009, 284, 4404–4412. [Google Scholar] [CrossRef]
- Lopez-Berges, M.S.; Arst, H.N.; Pinar, M.; Peñalva, M.A. Genetic studies on the physiological role of CORVET in Aspergillus nidulans. FEMS Microbiol. Lett. 2017, 364, fnx065. [Google Scholar] [CrossRef]
- Espeso, E.A.; Roncal, T.; Diez, E.; Rainbow, L.; Bignell, E.; Alvaro, J.; Suarez, T.; Denison, S.H.; Tilburn, J.; Arst, H.N., Jr.; et al. On how a transcription factor can avoid its proteolytic activation in the absence of signal transduction. EMBO J. 2000, 19, 719–728. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picazo, I.; Espeso, E.A. Interconnections between the Cation/Alkaline pH-Responsive Slt and the Ambient pH Response of PacC/Pal Pathways in Aspergillus nidulans. Cells 2024, 13, 651. https://doi.org/10.3390/cells13070651
Picazo I, Espeso EA. Interconnections between the Cation/Alkaline pH-Responsive Slt and the Ambient pH Response of PacC/Pal Pathways in Aspergillus nidulans. Cells. 2024; 13(7):651. https://doi.org/10.3390/cells13070651
Chicago/Turabian StylePicazo, Irene, and Eduardo A. Espeso. 2024. "Interconnections between the Cation/Alkaline pH-Responsive Slt and the Ambient pH Response of PacC/Pal Pathways in Aspergillus nidulans" Cells 13, no. 7: 651. https://doi.org/10.3390/cells13070651
APA StylePicazo, I., & Espeso, E. A. (2024). Interconnections between the Cation/Alkaline pH-Responsive Slt and the Ambient pH Response of PacC/Pal Pathways in Aspergillus nidulans. Cells, 13(7), 651. https://doi.org/10.3390/cells13070651






