Brain-Type Glycogen Phosphorylase (PYGB) in the Pathologies of Diseases: A Systematic Review
Abstract
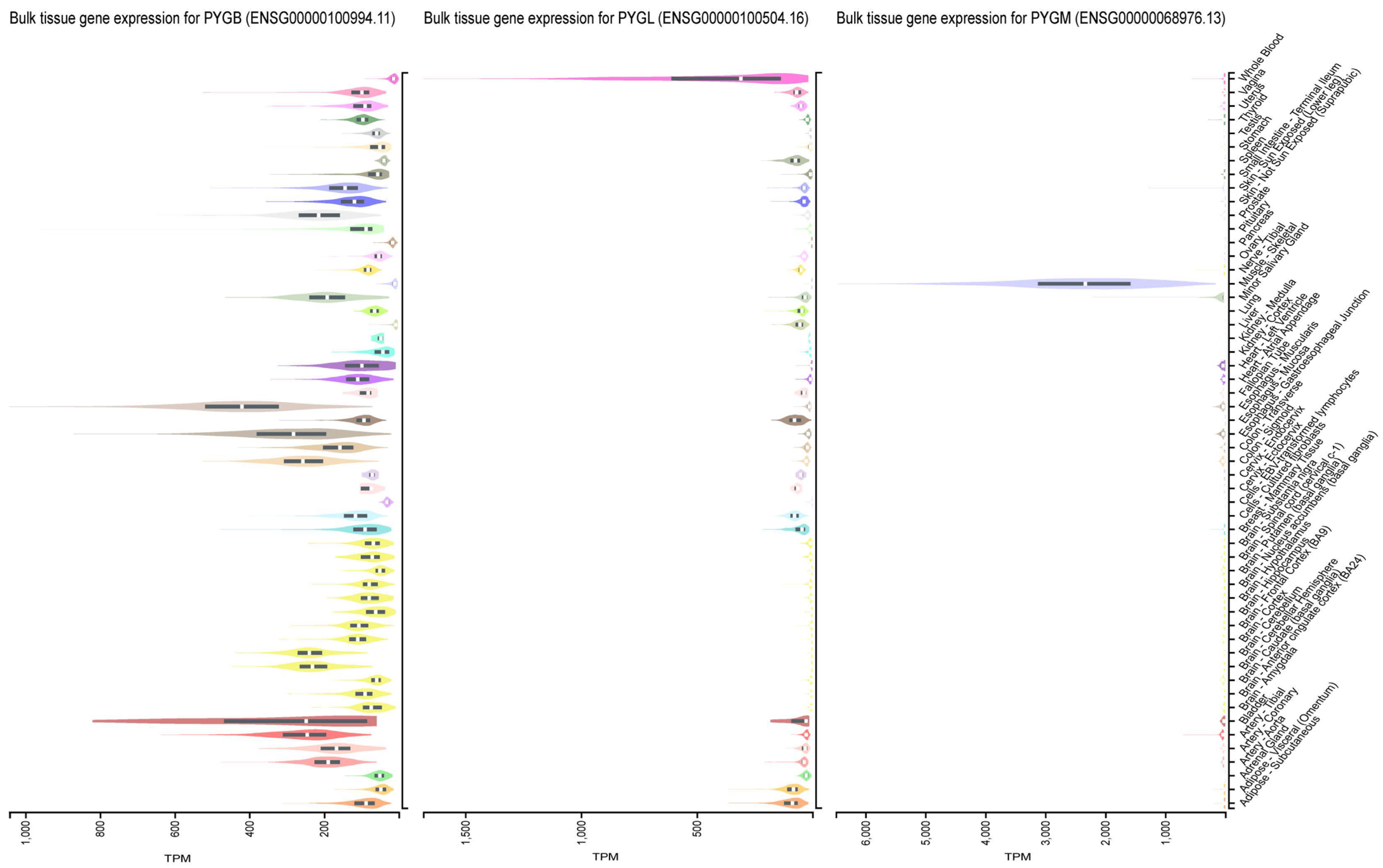
1. Introduction
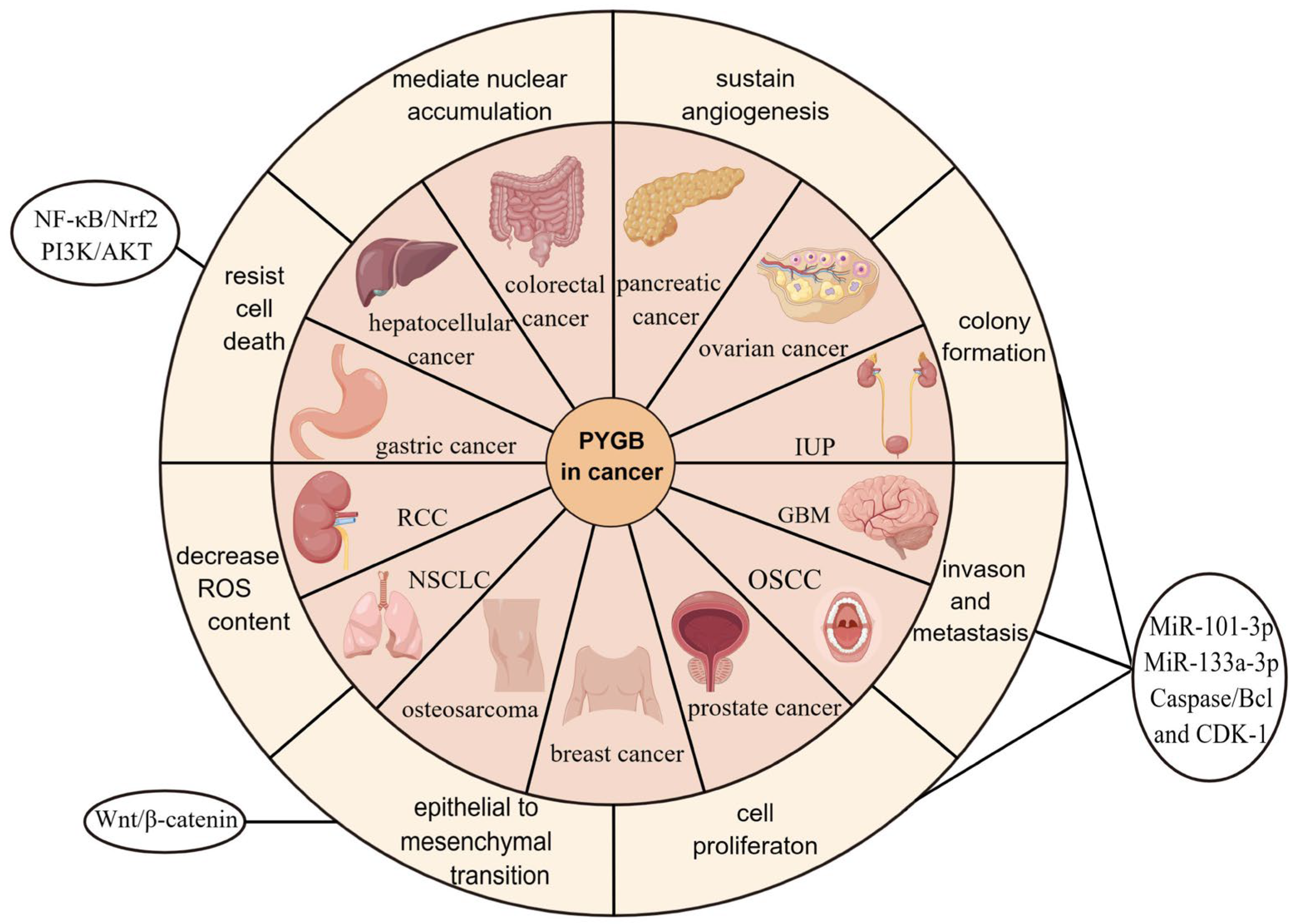
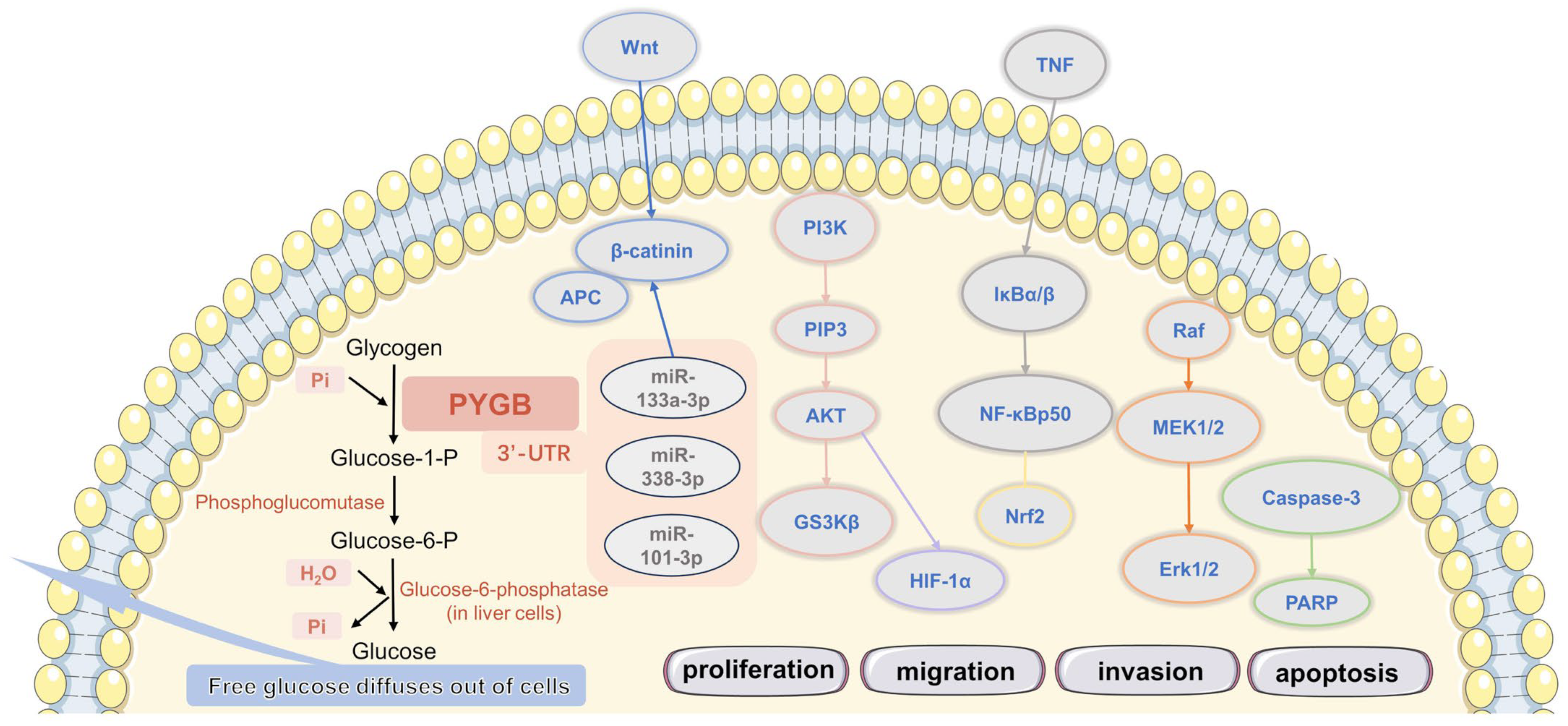
2. PYGB in Cancer
2.1. PYGB in Ovarian Cancer
2.2. PYGB in Gastric Cancer
2.3. PYGB in Hepatocellular Carcinoma
2.4. PYGB in Pancreatic Cancer
2.5. PYGB in Colorectal Cancer
2.6. PYGB in Prostate Cancer
2.7. PYGB in Osteosarcoma
2.8. PYGB in Non-Small-Cell Lung Cancer (NSCLC)
2.9. PYGB in Breast Cancer
2.10. PYGB in Renal-Cell Carcinoma (RCC)
2.11. PYGB in Inverted Urothelial Papilloma (IUP)
2.12. PYGB in Oral Squamous-Cell Carcinoma (OSCC)
2.13. PYGB in Glioblastoma Multiforme (GBM)
3. PYGB in Cardiovascular Disease
3.1. PYGB in Ischemic Myocardial Injury
3.1.1. Acute Myocardial Infarction (AMI)
3.1.2. Unstable Angina Pectoris (UA)
3.1.3. Coronary Artery Bypass Grafting (CABG)
3.2. PYGB in Acute Ischemic Stroke (AIS)
3.3. PYGB in Brain Ischemia
3.4. PYGB in Preeclampsia (PE)
4. PYGB in Metabolic Diseases
4.1. PYGB in McArdle Disease
4.2. PYGB in Type 2 Diabetes (T2D)
4.3. PYGB in Late-Onset Pompe Disease (LOPD)
5. PYGB in Nervous System Diseases
5.1. PYGB in Alzheimer’s Disease (AD)
5.2. PYGB in Amyotrophic Lateral Sclerosis (ALS)
5.3. PYGB in Depression
5.4. PYGB in Spinal Muscular Atrophy (SMA)
6. PYGB in Other Diseases
6.1. PYGB in Liver Regenerative Medicine
6.2. PYGB In Vitro Fertilization (IVF)
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GP | Glycogen phosphorylase |
| Glc-1-P | glucose-1-phosphate |
| PYGB | brain type glycogen phosphorylase |
| PYGL | liver type glycogen phosphorylase |
| PYGM | muscle type glycogen phosphorylase |
| ROS | reactive oxygen species |
| GC | Gastric cancer |
| IM | intestinal metaplasia |
| LNI | lymph node involvement |
| TNM stage | tumor, node, metastasis stage |
| EMT | epithelial-mesenchymal transformation |
| HCC | hepatocellular carcinoma |
| 2-DG | 2-deoxy-D-glucose |
| ACS | adenoma-carcinoma sequence |
| NSCLC | non-small cell lung cancer |
| RCC | renal cell carcinoma |
| IUP | inverted urothelial papilloma |
| PUC | papillary urothelial carcinoma |
| OSCC | oral squamous cell carcinoma |
| GBM | glioblastoma multiforme |
| CHD | coronary atherosclerotic heart disease |
| ACS | acute coronary syndrome |
| AMI | acute myocardial infarction |
| UA | unstable angina |
| MB | myoglobin |
| cTn | cardiac troponin |
| AIS | acute ischaemic stroke |
| PE | pre-eclampsia |
| SGA | small for gestational age |
| T2D | type 2 diabetes |
| IR | insulin resistance |
| AD | Alzheimer’s disease |
| ALS | amyotrophic lateral sclerosis |
| SMA | spinal muscular atrophy |
| IVF | in vitro fertilization |
References
- Gaboriaud-Kolar, N.; Skaltsounis, A.L. Glycogen phosphorylase inhibitors: A patent review (2008–2012). Expert Opin. Ther. Pat. 2013, 23, 1017–1032. [Google Scholar] [CrossRef]
- Newgard, C.B.; Hwang, P.K.; Fletterick, R.J. The family of glycogen phosphorylases: Structure and function. Crit. Rev. Biochem. Mol. Biol. 1989, 24, 69–99. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Dupret, J.M.; Rodrigues-Lima, F. The Structure and the Regulation of Glycogen Phosphorylases in Brain. Adv. Neurobiol. 2019, 23, 125–145. [Google Scholar] [PubMed]
- Newgard, C.B.; Littman, D.R.; van Genderen, C.; Smith, M.; Fletterick, R.J. Human brain glycogen phosphorylase. Cloning, sequence analysis, chromosomal mapping, tissue expression, and comparison with the human liver and muscle isozymes. J. Biol. Chem. 1988, 263, 3850–3857. [Google Scholar] [CrossRef] [PubMed]
- Ørtenblad, N.; Westerblad, H.; Nielsen, J. Muscle glycogen stores and fatigue. J. Physiol. 2013, 591, 4405–4413. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.S.; Pedersen, S.E.; Walls, A.B.; Waagepetersen, H.S.; Bak, L.K. Isoform-selective regulation of glycogen phosphorylase by energy deprivation and phosphorylation in astrocytes. Glia 2015, 63, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Agius, L. Role of glycogen phosphorylase in liver glycogen metabolism. Mol. Asp. Med. 2015, 46, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Proux, D.; Dreyfus, J.C. Phosphorylase isoenzymes in tissues: Prevalence of the liver type in man. Clin. Chim. Acta Int. J. Clin. Chem. 1973, 48, 167–172. [Google Scholar] [CrossRef]
- Hudson, J.W.; Golding, G.B.; Crerar, M.M. Evolution of allosteric control in glycogen phosphorylase. J. Mol. Biol. 1993, 234, 700–721. [Google Scholar] [CrossRef]
- Chasiotis, D. The regulation of glycogen phosphorylase and glycogen breakdown in human skeletal muscle. Acta Physiol. Scand. Suppl. 1983, 518, 1–68. [Google Scholar]
- Hue, L.; Bontemps, F.; Hers, H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem. J. 1975, 152, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.M.; Goodwin, G.W.; Guthrie, P.H.; Taegtmeyer, H. Effects of insulin on glucose uptake by rat hearts during and after coronary flow reduction. Am. J. Physiol. 1997, 273, H2170–H2177. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A.; Carlson, G.M. Major Advances in Brain Glycogen Research: Understanding of the Roles of Glycogen Have Evolved from Emergency Fuel Reserve to Dynamic, Regulated Participant in Diverse Brain Functions. In Brain Glycogen Metabolism; Advances in Neurobiology; Springer: Cham, Switzerland, 2019; Volume 23, pp. 1–16. [Google Scholar]
- Llavero, F.; Arrazola Sastre, A.; Luque Montoro, M.; Gálvez, P.; Lacerda, H.M.; Parada, L.A.; Zugaza, J.L. McArdle Disease: New Insights into Its Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2019, 20, 5919. [Google Scholar] [CrossRef] [PubMed]
- Lillpopp, L.; Tzikas, S.; Ojeda, F.; Zeller, T.; Baldus, S.; Bickel, C.; Sinning, C.R.; Wild, P.S.; Genth-Zotz, S.; Warnholtz, A.; et al. Prognostic information of glycogen phosphorylase isoenzyme BB in patients with suspected acute coronary syndrome. Am. J. Cardiol. 2012, 110, 1225–1230. [Google Scholar] [CrossRef]
- Pudil, R.; Vasatová, M.; Lenco, J.; Tichý, M.; Rehácek, V.; Fucíková, A.; Horácek, J.M.; Vojácek, J.; Pleskot, M.; Stulík, J.; et al. Plasma glycogen phosphorylase BB is associated with pulmonary artery wedge pressure and left ventricle mass index in patients with hypertrophic cardiomyopathy. Clin. Chem. Lab. Med. 2010, 48, 1193–1195. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Y.; Zha, Y.; Yang, Y.; Wang, L.; Li, J.; Jin, W. PYGB siRNA inhibits the cell proliferation of human osteosarcoma cell lines. Mol. Med. Rep. 2018, 18, 715–722. [Google Scholar] [CrossRef]
- Mathieu, C.; Duval, R.; Cocaign, A.; Petit, E.; Bui, L.C.; Haddad, I.; Vinh, J.; Etchebest, C.; Dupret, J.M.; Rodrigues-Lima, F. An Isozyme-specific Redox Switch in Human Brain Glycogen Phosphorylase Modulates Its Allosteric Activation by AMP. J. Biol. Chem. 2016, 291, 23842–23853. [Google Scholar] [CrossRef] [PubMed]
- Gelinas, R.P.; Froman, B.E.; McElroy, F.; Tait, R.C.; Gorin, F.A. Human brain glycogen phosphorylase: Characterization of fetal cDNA and genomic sequences. Brain Res. Mol. Brain Res. 1989, 6, 177–185. [Google Scholar] [CrossRef]
- Hashimoto, K.; Tamura, K.; Otani, H.; Tanaka, O. Histocytochemical and immunohistochemical studies related to the role of glycogen in human developing digestive organs. Anat. Embryol. 1995, 192, 497–505. [Google Scholar] [CrossRef]
- Sato, K.; Morris, H.P.; Weinhouse, S. Phosphorylase: A new isozyme in rat hepatic tumors and fetal liver. Science 1972, 178, 879–881. [Google Scholar] [CrossRef]
- Duran, J.; Guinovart, J.J. Brain glycogen in health and disease. Mol. Asp. Med. 2015, 46, 70–77. [Google Scholar] [CrossRef]
- Saez, I.; Duran, J.; Sinadinos, C.; Beltran, A.; Yanes, O.; Tevy, M.F.; Martínez-Pons, C.; Milán, M.; Guinovart, J.J. Neurons have an active glycogen metabolism that contributes to tolerance to hypoxia. J. Cereb. Blood Flow. Metab. Off. J. Int. Soc. Cereb. Blood Flow. Metab. 2014, 34, 945–955. [Google Scholar] [CrossRef]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef]
- Obel, L.F.; Müller, M.S.; Walls, A.B.; Sickmann, H.M.; Bak, L.K.; Waagepetersen, H.S.; Schousboe, A. Brain glycogen-new perspectives on its metabolic function and regulation at the subcellular level. Front. Neuroenerg. 2012, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Altemus, M.A.; Goo, L.E.; Little, A.C.; Yates, J.A.; Cheriyan, H.G.; Wu, Z.F.; Merajver, S.D. Breast cancers utilize hypoxic glycogen stores via PYGB, the brain isoform of glycogen phosphorylase, to promote metastatic phenotypes. PLoS ONE 2019, 14, e0220973. [Google Scholar] [CrossRef] [PubMed]
- Azkargorta, M.; Escobes, I.; Iloro, I.; Osinalde, N.; Corral, B.; Ibañez-Perez, J.; Exposito, A.; Prieto, B.; Elortza, F.; Matorras, R. Differential proteomic analysis of endometrial fluid suggests increased inflammation and impaired glucose metabolism in non-implantative IVF cycles and pinpoints PYGB as a putative implantation marker. Hum. Reprod. 2018, 33, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Philips, K.B.; Kurtoglu, M.; Leung, H.J.; Liu, H.; Gao, N.; Lehrman, M.A.; Murray, T.G.; Lampidis, T.J. Increased sensitivity to glucose starvation correlates with downregulation of glycogen phosphorylase isoform PYGB in tumor cell lines resistant to 2-deoxy-D-glucose. Cancer Chemother. Pharmacol. 2014, 73, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, G.; Liu, Q.; Zhang, W.; Wang, J. Silencing of PYGB suppresses growth and promotes the apoptosis of prostate cancer cells via the NF-κB/Nrf2 signaling pathway. Mol. Med. Rep. 2018, 18, 3800–3808. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Chiu, C.C.; Liang, J.D.; Chiou, L.L.; Huang, G.T.; Yu, M.J.; Lee, H.S. Brain isoform glycogen phosphorylase as a novel hepatic progenitor cell marker. PLoS ONE 2015, 10, e0122528. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Shiomori, K.; Tashima, S.; Tsuruta, J.; Ogawa, M. Frequent p53 mutation in brain (fetal)-type glycogen phosphorylase positive foci adjacent to human ‘de novo’ colorectal carcinomas. Br. J. Cancer 2001, 84, 1497–1504. [Google Scholar] [CrossRef]
- Shimada, S.; Matsuzaki, H.; Marutsuka, T.; Shiomori, K.; Ogawa, M. Gastric and intestinal phenotypes of gastric carcinoma with reference to expression of brain (fetal)-type glycogen phosphorylase. J. Gastroenterol. 2001, 36, 457–464. [Google Scholar] [CrossRef]
- Tashima, S.; Shimada, S.; Yamaguchi, K.; Tsuruta, J.; Ogawa, M. Expression of brain-type glycogen phosphorylase is a potentially novel early biomarker in the carcinogenesis of human colorectal carcinomas. Am. J. Gastroenterol. 2000, 95, 255–263. [Google Scholar] [CrossRef]
- Shimada, S.; Tashima, S.; Yamaguchi, K.; Matsuzaki, H.; Ogawa, M. Carcinogenesis of intestinal-type gastric cancer and colorectal cancer is commonly accompanied by expression of brain (fetal)-type glycogen phosphorylase. J. Exp. Clin. Cancer Res. CR 1999, 18, 111–118. [Google Scholar]
- Yoshida, G.J. Metabolic reprogramming: The emerging concept and associated therapeutic strategies. J. Exp. Clin. Cancer Res. 2015, 34, 111. [Google Scholar] [CrossRef] [PubMed]
- Zois, C.E.; Harris, A.L. Glycogen metabolism has a key role in the cancer microenvironment and provides new targets for cancer therapy. J. Mol. Med. 2016, 94, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Favaro, E.; Harris, A.L. Targeting glycogen metabolism: A novel strategy to inhibit cancer cell growth? Oncotarget 2013, 4, 3–4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uno, K.; Shimada, S.; Tsuruta, J.; Matsuzaki, H.; Tashima, S.; Ogawa, M. Nuclear localization of brain-type glycogen phosphorylase in some gastrointestinal carcinoma. Histochem. J. 1998, 30, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Zhang, K.; Liu, C. PYGB Promoted Tumor Progression by Regulating Wnt/β-Catenin Pathway in Gastric Cancer. Technol. Cancer Res. Treat. 2020, 19, 1533033820926592. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jin, Z.; Wang, C. Glycogen phosphorylase B promotes ovarian cancer progression via Wnt/β-catenin signaling and is regulated by miR-133a-3p. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 120, 109449. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kim, J.H.; Lee, C.H.; Kim, J.M.; Kang, C.D.; Kim, Y.D.; Choi, K.U.; Kim, H.W.; Kim, J.Y.; Park, D.Y.; et al. Clinicopathological significance of BGP expression in non-small-cell lung carcinoma: Relationship with histological type, microvessel density and patients’ survival. Pathology 2006, 38, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.X.; Zheng, L.Y.; Qu, H.X.; Yu, F.H.; Zou, S.S.; Hong, Y.; Tan, Y.X.; Wang, H.; Hu, H.P.; Wang, H.Y. Identification and differential expression of human carcinoma-associated antigens in hepatocellular carcinoma tissues. Exp. Biol. Med. 2013, 238, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Maeno, M.; Misumi, A.; Takano, S.; Akagi, M. Antigen reversion of glycogen phosphorylase isoenzyme in carcinoma and proliferative zone of intestinal metaplasia of the human stomach: An immunohistochemical study. Gastroenterology 1987, 93, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, H.; Shimada, S.; Uno, K.; Tsuruta, J.; Ogawa, M. Novel subtyping of intestinal metaplasia in the human stomach: Brain-type glycogen phosphorylase expression in the proliferative zone and its relationship with carcinogenesis. Am. J. Clin. Pathol. 1998, 109, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Shiomori, K.; Honmyo, U.; Maeno, M.; Yagi, Y.; Ogawa, M. BGP expression in gastric biopsies may predict the development of new lesions after local treatment for early gastric cancer. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2002, 5, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Terashima, M.; Fujita, Y.; Togashi, Y.; Sakai, K.; De Velasco, M.A.; Tomida, S.; Nishio, K. KIAA1199 interacts with glycogen phosphorylase kinase β-subunit (PHKB) to promote glycogen breakdown and cancer cell survival. Oncotarget 2014, 5, 7040–7050. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, S.; Deng, T.; Yang, Y.; Zhang, Y. Analysis of the expression, function and signaling of glycogen phosphorylase isoforms in hepatocellular carcinoma. Oncol. Lett. 2022, 24, 244. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Wang, H.; Liu, W.; Xing, J.; Song, W.; Zeng, Z.; Liu, L.; Wang, H.; Wang, X.; Luo, H.; et al. Glycogen Phosphorylase B Is Regulated by miR101-3p and Promotes Hepatocellular Carcinoma Tumorigenesis. Front. Cell Dev. Biol. 2020, 8, 566494. [Google Scholar] [CrossRef]
- Wang, G.; Ni, X.; Wang, J.; Dai, M. METTL3-mediated m(6)A methylation of PYGB facilitates pancreatic ductal adenocarcinoma progression through the activation of NF-κB signaling. Pathol. Res. Pract. 2023, 248, 154645. [Google Scholar] [CrossRef]
- Chipps, H.D.; Duff, G.L. Glycogen Infiltration of the Liver Cell Nuclei. Am. J. Pathol. 1942, 18, 645–659. [Google Scholar]
- Schnier, J.B.; Nishi, K.; Monks, A.; Gorin, F.A.; Bradbury, E.M. Inhibition of glycogen phosphorylase (GP) by CP-91,149 induces growth inhibition correlating with brain GP expression. Biochem. Biophys. Res. Commun. 2003, 309, 126–134. [Google Scholar] [CrossRef]
- Sun, R.C.; Dukhande, V.V.; Zhou, Z.; Young, L.E.A.; Emanuelle, S.; Brainson, C.F.; Gentry, M.S. Nuclear Glycogenolysis Modulates Histone Acetylation in Human Non-Small Cell Lung Cancers. Cell Metab. 2019, 30, 903–916.e7. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, W.; Huangfu, Q.; Tao, H.; Zhang, J. PYGB facilitates cell proliferation and invasiveness in non-small cell lung cancer by activating the Wnt-β-catenin signaling pathway. Biochem. Cell Biol. 2020, 98, 565–574. [Google Scholar] [CrossRef]
- Zhan, Y.; Chen, R.; Wang, T.; Shan, S.; Zhu, H. Glycogen phosphorylase B promotes cell proliferation and migration through PI3K/AKT pathway in non-small cell lung cancer. Exp. Lung Res. 2021, 47, 111–120. [Google Scholar] [PubMed]
- Lei, K.; Tan, B.; Liang, R.; Lyu, Y.; Wang, K.; Wang, W.; Wang, K.; Hu, X.; Wu, D.; Lin, H.; et al. Development and clinical validation of a necroptosis-related gene signature for prediction of prognosis and tumor immunity in lung adenocarcinoma. Am. J. Cancer Res. 2022, 12, 5160–5182. [Google Scholar] [PubMed]
- Li, J.; Li, H.; Zhang, C.; Zhang, C.; Wang, H. Integrative analysis of genomic alteration, immune cells infiltration and prognosis of lung squamous cell carcinoma (LUSC) to identify smoking-related biomarkers. Int. Immunopharmacol. 2020, 89, 107053. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, S.; Yan, H.; Chen, H.; Wang, Y.; Wang, Y. Development and validation of a combined glycolysis and immune prognostic signature for lung squamous cell carcinoma. Front. Genet. 2022, 13, 907058. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.S.; Roberts, H.; Leek, R.; Harris, A.L.; Geradts, J. Differential gene expression patterns in HER2/neu-positive and -negative breast cancer cell lines and tissues. Am. J. Pathol. 2002, 161, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Takashi, M.; Koshikawa, T.; Kurobe, N.; Kato, K. Elevated concentrations of brain-type glycogen phosphorylase in renal cell carcinoma. Jpn. J. Cancer Res. Gann 1989, 80, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Song, J.; Godfrey, J.; Riscal, R.; Skuli, N.; Nissim, I.; Simon, M.C. Glycogen metabolism is dispensable for tumour progression in clear cell renal cell carcinoma. Nat. Metab. 2021, 3, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Lee, C.; Han, D.; Kim, K.; Yang, S.; Nikas, I.P.; Moon, K.C.; Kim, H.; Song, M.J.; Kim, B.; et al. Proteomic-Based Machine Learning Analysis Reveals PYGB as a Novel Immunohistochemical Biomarker to Distinguish Inverted Urothelial Papilloma From Low-Grade Papillary Urothelial Carcinoma With Inverted Growth. Front. Oncol. 2022, 12, 841398. [Google Scholar] [CrossRef]
- Busso-Lopes, A.F.; Carnielli, C.M.; Winck, F.V.; Patroni, F.M.S.; Oliveira, A.K.; Granato, D.C.; RAP, E.C.; Domingues, R.R.; Pauletti, B.A.; Riaño-Pachón, D.M.; et al. A Reductionist Approach Using Primary and Metastatic Cell-Derived Extracellular Vesicles Reveals Hub Proteins Associated with Oral Cancer Prognosis. Mol. Cell. Proteom. 2021, 20, 100118. [Google Scholar] [CrossRef]
- Ferraro, G.; Mozzicafreddo, M.; Ettari, R.; Corsi, L.; Monti, M.C. A Proteomic Platform Unveils the Brain Glycogen Phosphorylase as a Potential Therapeutic Target for Glioblastoma Multiforme. Int. J. Mol. Sci. 2022, 23, 8200. [Google Scholar] [CrossRef]
- Institute of Medicine Committee on Social Security Cardiovascular Disability Commission. Cardiovascular Disability: Updating the Social Security Listings; National Academies Press: Washington, DC, USA, 2010; ISBN 10.17226/12940. [Google Scholar]
- Entman, M.L.; Bornet, E.P.; Van Winkle, W.B.; Goldstein, M.A.; Schwartz, A. Association of glycogenolysis with cardiac sarcoplasmic reticulum: II. Effect of glycogen depletion, deoxycholate solubilization and cardiac ischemia: Evidence for a phorphorylase kinase membrane complex. J. Mol. Cell. Cardiol. 1977, 9, 515–528. [Google Scholar] [CrossRef]
- Krause, E.G.; Rabitzsch, G.; Noll, F.; Mair, J.; Puschendorf, B. Glycogen phosphorylase isoenzyme BB in diagnosis of myocardial ischaemic injury and infarction. Mol. Cell. Biochem. 1996, 160–161, 289–295. [Google Scholar] [CrossRef]
- Mair, J. Glycogen phosphorylase isoenzyme BB to diagnose ischaemic myocardial damage. Clin. Chim. Acta Int. J. Clin. Chem. 1998, 272, 79–86. [Google Scholar] [CrossRef]
- Rabitzsch, G.; Mair, J.; Lechleitner, P.; Noll, F.; Hofmann, V.; Krause, E.G.; Dienstl, F.; Puschendorf, B. Isoenzyme BB of glycogen phosphorylase b and myocardial infarction. Lancet 1993, 341, 1032–1033. [Google Scholar] [CrossRef]
- Rabitzsch, G.; Mair, J.; Lechleitner, P.; Noll, F.; Hofmann, U.; Krause, E.G.; Dienstl, F.; Puschendorf, B. Immunoenzymometric assay of human glycogen phosphorylase isoenzyme BB in diagnosis of ischemic myocardial injury. Clin. Chem. 1995, 41, 966–978. [Google Scholar] [CrossRef]
- Rabitzsch, G.; Schulz, H.; Onnen, K.; Kössler, A.; Krause, E.G. Immunoinhibition assay of the serum activity of human glycogen isophosphorylase BB in the diagnosis of the acute myocardial ischaemia. Biomed. Biochim. Acta 1987, 46, S584–S588. [Google Scholar] [PubMed]
- Mair, J.; Puschendorf, B.; Smidt, J.; Lechleitner, P.; Dienstl, F.; Noll, F.; Krause, E.G.; Rabitzsch, G. Early release of glycogen phosphorylase in patients with unstable angina and transient ST-T alterations. Br. Heart J. 1994, 72, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhang, J.; Zhou, Z.; Zhu, X.; Fang, W. Changes of serum glycogen phosphorylase isoenzyme BB in patients with unstable angina pectoris and its clinical significance. Chin. J. Cardiol. 2001, 29, 309. [Google Scholar]
- Peetz, D.; Post, F.; Schinzel, H.; Schweigert, R.; Schollmayer, C.; Steinbach, K.; Dati, F.; Noll, F.; Lackner, K.J. Glycogen phosphorylase BB in acute coronary syndromes. Clin. Chem. Lab. Med. 2005, 43, 1351–1358. [Google Scholar] [CrossRef]
- Cubranic, Z.; Madzar, Z.; Matijevic, S.; Dvornik, S.; Fisic, E.; Tomulic, V.; Kunisek, J.; Laskarin, G.; Kardum, I.; Zaputovic, L. Diagnostic accuracy of heart fatty acid binding protein (H-FABP) and glycogen phosphorylase isoenzyme BB (GPBB) in diagnosis of acute myocardial infarction in patients with acute coronary syndrome. Biochem. Med. 2012, 22, 225–236. [Google Scholar] [CrossRef]
- Mair, P.; Mair, J.; Krause, E.G.; Balogh, D.; Puschendorf, B.; Rabitzsch, G. Glycogen phosphorylase isoenzyme BB mass release after coronary artery bypass grafting. Eur. J. Clin. Chem. Clin. Biochem. J. Forum Eur. Clin. Chem. Soc. 1994, 32, 543–547. [Google Scholar] [CrossRef][Green Version]
- Mion, M.M.; Novello, E.; Altinier, S.; Rocco, S.; Zaninotto, M.; Plebani, M. Analytical and clinical performance of a fully automated cardiac multi-markers strategy based on protein biochip microarray technology. Clin. Biochem. 2007, 40, 1245–1251. [Google Scholar] [CrossRef]
- Meune, C.; Wahbi, K.; Weber, S.; Zuily, S.; Cynober, L.; Chenevier-Gobeaux, C. Performance of glycogen phosphorylase isoenzyme BB is weak in the detection of patients with non-ST-elevation acute coronary syndrome. Clin. Biochem. 2011, 44, 1343–1345. [Google Scholar] [CrossRef]
- Lippi, G.; Mattiuzzi, C.; Comelli, I.; Cervellin, G. Glycogen phosphorylase isoenzyme BB in the diagnosis of acute myocardial infarction: A meta-analysis. Biochem. Med. 2013, 23, 78–82. [Google Scholar] [CrossRef]
- McCann, C.J.; Glover, B.M.; Menown, I.B.; Moore, M.J.; McEneny, J.; Owens, C.G.; Smith, B.; Sharpe, P.C.; Young, I.S.; Adgey, J.A. Prognostic value of a multimarker approach for patients presenting to hospital with acute chest pain. Am. J. Cardiol. 2009, 103, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Dobric, M.; Giga, V.; Beleslin, B.; Ignjatovic, S.; Paunovic, I.; Stepanovic, J.; Djordjevic-Dikic, A.; Kostic, J.; Nedeljkovic, I.; Nedeljkovic, M.; et al. Glycogen phosphorylase isoenzyme BB plasma kinetics is not related to myocardial ischemia induced by exercise stress echo test. Clin. Chem. Lab. Med. 2013, 51, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Dobric, M.; Ostojic, M.; Giga, V.; Djordjevic-Dikic, A.; Stepanovic, J.; Radovanovic, N.; Beleslin, B. Glycogen phosphorylase BB in myocardial infarction. Clin. Chim. Acta Int. J. Clin. Chem. 2015, 438, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gius, D. Glycogen Phosphorylase: A Novel Biomarker in Doxorubicin-Induced Cardiac Injury. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 1516–1517. [Google Scholar] [CrossRef] [PubMed]
- Yarana, C.; Carroll, D.; Chen, J.; Chaiswing, L.; Zhao, Y.; Noel, T.; Alstott, M.; Bae, Y.; Dressler, E.V.; Moscow, J.A.; et al. Extracellular Vesicles Released by Cardiomyocytes in a Doxorubicin-Induced Cardiac Injury Mouse Model Contain Protein Biomarkers of Early Cardiac Injury. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 1644–1653. [Google Scholar] [CrossRef]
- Ghimire, A.; Giri, S.; Khanal, N.; Rayamajhi, S.; Thapa, A.; Bist, A.; Devkota, S. Diagnostic accuracy of glycogen phosphorylase BB for myocardial infarction: A systematic review and meta-analysis. J. Clin. Lab. Anal. 2022, 36, e24368. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Shimizu, A.; Kurobe, N.; Takashi, M.; Koshikawa, T. Human brain-type glycogen phosphorylase: Quantitative localization in human tissues determined with an immunoassay system. J. Neurochem. 1989, 52, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Ignacio, P.C.; Baldwin, B.A.; Vijayan, V.K.; Tait, R.C.; Gorin, F.A. Brain isozyme of glycogen phosphorylase: Immunohistological localization within the central nervous system. Brain Res. 1990, 529, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Ay, I.; Avery, R.; Caceres, J.A.; Siket, M.S.; Pontes-Neto, O.M.; Zheng, H.; Rost, N.S.; Furie, K.L.; Sorensen, A.G.; et al. New biomarker for acute ischaemic stroke: Plasma glycogen phosphorylase isoenzyme BB. J. Neurol. Neurosurg. Psychiatry 2018, 89, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Guo, H.; Fan, Z.; Zhang, X.; Wu, D.; Tang, W.; Gu, T.; Wang, S.; Yin, A.; Tao, L.; et al. Glycogenolysis Is Crucial for Astrocytic Glycogen Accumulation and Brain Damage after Reperfusion in Ischemic Stroke. iScience 2020, 23, 101136. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, S.; Wang, Y.; Yan, Z.; Guo, Y.; Zhang, L. A Novel 5-Chloro-N-phenyl-1H-indole-2-carboxamide Derivative as Brain-Type Glycogen Phosphorylase Inhibitor: Validation of Target PYGB. Molecules 2023, 28, 1697. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Li, S.; Wang, Y.; Li, J.; Ma, C.; Guo, Y.; Zhang, L. Discovery of novel heterocyclic derivatives as potential glycogen phosphorylase inhibitors with a cardioprotective effect. Bioorg. Chem. 2022, 129, 106120. [Google Scholar] [CrossRef]
- Kanasaki, K.; Kalluri, R. The biology of preeclampsia. Kidney Int. 2009, 76, 831–837. [Google Scholar] [CrossRef]
- Uzan, J.; Carbonnel, M.; Piconne, O.; Asmar, R.; Ayoubi, J.M. Pre-eclampsia: Pathophysiology, diagnosis, and management. Vasc. Health Risk Manag. 2011, 7, 467–474. [Google Scholar]
- Craici, I.; Wagner, S.; Garovic, V.D. Preeclampsia and future cardiovascular risk: Formal risk factor or failed stress test? Ther. Adv. Cardiovasc. Dis. 2008, 2, 249–259. [Google Scholar] [CrossRef]
- Lee, J.; Romero, R.; Dong, Z.; Lee, D.C.; Dong, Y.; Mittal, P.; Chaiworapongsa, T.; Hassan, S.S.; Kim, C.J. Glycogen phosphorylase isoenzyme BB plasma concentration is elevated in pregnancy and preterm preeclampsia. Hypertension 2012, 59, 274–282. [Google Scholar] [CrossRef]
- Kenny, L.; Doyle, A.; Khashan, A. PP054. Elevated maternal plasma glycogen phosphorylase isoenzyme BB as time of disease biomarker of pre-eclampsia and small-for-gestational-age. Pregnancy Hypertens. 2013, 3, 86. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, F.; Doyle, A.; Khashan, A.; Kenny, L. Assessment of glycogen phosphorylase isoenzyme BB as a biomarker for pre-eclampsia and small for gestational age. Lancet 2015, 385 (Suppl. S1), S67. [Google Scholar] [CrossRef]
- McCarthy, F.P.; Doyle, A.; Khashan, A.S.; Kenny, L.C. Altered Maternal Plasma Glycogen Phosphorylase Isoenzyme BB as a Biomarker for Preeclampsia and Small for Gestational Age. Reprod. Sci. 2016, 23, 738–747. [Google Scholar] [CrossRef]
- Conti-Ramsden, F.; Gill, C.; Seed, P.T.; Bramham, K.; Chappell, L.C.; McCarthy, F.P. Markers of maternal cardiac dysfunction in pre-eclampsia and superimposed pre-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 237, 151–156. [Google Scholar] [CrossRef] [PubMed]
- De Luna, N.; Brull, A.; Guiu, J.M.; Lucia, A.; Martin, M.A.; Arenas, J.; Martí, R.; Andreu, A.L.; Pinós, T. Sodium valproate increases the brain isoform of glycogen phosphorylase: Looking for a compensation mechanism in McArdle disease using a mouse primary skeletal-muscle culture in vitro. Dis. Models Mech. 2015, 8, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Durrant, D.; Koka, S.; Salloum, F.N.; Xi, L.; Kukreja, R.C. Mammalian target of rapamycin (mTOR) inhibition with rapamycin improves cardiac function in type 2 diabetic mice: Potential role of attenuated oxidative stress and altered contractile protein expression. J. Biol. Chem. 2014, 289, 4145–4160. [Google Scholar] [CrossRef] [PubMed]
- Kotnik, P.; Knapič, E.; Kokošar, J.; Kovač, J.; Jerala, R.; Battelino, T.; Horvat, S. Identification of novel alleles associated with insulin resistance in childhood obesity using pooled-DNA genome-wide association study approach. Int. J. Obes. 2018, 42, 686–695. [Google Scholar] [CrossRef]
- De Filippi, P.; Errichiello, E.; Toscano, A.; Mongini, T.; Moggio, M.; Ravaglia, S.; Filosto, M.; Servidei, S.; Musumeci, O.; Giannini, F.; et al. Distribution of Exonic Variants in Glycogen Synthesis and Catabolism Genes in Late Onset Pompe Disease (LOPD). Curr. Issues Mol. Biol. 2023, 45, 2847–2860. [Google Scholar] [CrossRef]
- San Segundo-Acosta, P.; Montero-Calle, A.; Fuentes, M.; Rábano, A.; Villalba, M.; Barderas, R. Identification of Alzheimer’s Disease Autoantibodies and Their Target Biomarkers by Phage Microarrays. J. Proteome Res. 2019, 18, 2940–2953. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wei, Q.; Gu, X.; Chen, Y.; Chen, X.; Cao, B.; Ou, R.; Shang, H. Decreased Glycogenolysis by miR-338-3p Promotes Regional Glycogen Accumulation Within the Spinal Cord of Amyotrophic Lateral Sclerosis Mice. Front. Mol. Neurosci. 2019, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fan, Z.; Zhao, Q.; Li, J.; Cai, G.; Wang, R.; Liang, Y.; Lu, N.; Kang, J.; Luo, D.; et al. Brain-Type Glycogen Phosphorylase Is Crucial for Astrocytic Glycogen Accumulation in Chronic Social Defeat Stress-Induced Depression in Mice. Front. Mol. Neurosci. 2021, 14, 819440. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Kline, R.A.; Synowsky, S.A.; Shirran, S.L.; Holt, I.; Sillence, K.A.; Claus, P.; Wirth, B.; Wishart, T.M.; Fuller, H.R. The Proteome Signatures of Fibroblasts from Patients with Severe, Intermediate and Mild Spinal Muscular Atrophy Show Limited Overlap. Cells 2022, 11, 2624. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Wang, H.; Shao, M.; Chu, F.; He, Y.; Chen, X.; Fan, J.; Chen, J.; Cai, Q.; Wu, C. Brain-Type Glycogen Phosphorylase (PYGB) in the Pathologies of Diseases: A Systematic Review. Cells 2024, 13, 289. https://doi.org/10.3390/cells13030289
Yang C, Wang H, Shao M, Chu F, He Y, Chen X, Fan J, Chen J, Cai Q, Wu C. Brain-Type Glycogen Phosphorylase (PYGB) in the Pathologies of Diseases: A Systematic Review. Cells. 2024; 13(3):289. https://doi.org/10.3390/cells13030289
Chicago/Turabian StyleYang, Caiting, Haojun Wang, Miaomiao Shao, Fengyu Chu, Yuyu He, Xiaoli Chen, Jiahui Fan, Jingwen Chen, Qianqian Cai, and Changxin Wu. 2024. "Brain-Type Glycogen Phosphorylase (PYGB) in the Pathologies of Diseases: A Systematic Review" Cells 13, no. 3: 289. https://doi.org/10.3390/cells13030289
APA StyleYang, C., Wang, H., Shao, M., Chu, F., He, Y., Chen, X., Fan, J., Chen, J., Cai, Q., & Wu, C. (2024). Brain-Type Glycogen Phosphorylase (PYGB) in the Pathologies of Diseases: A Systematic Review. Cells, 13(3), 289. https://doi.org/10.3390/cells13030289




