Gestational Intermittent Hypoxia Enhances Mammary Stem Cells and Alters Tumor Phenotype in Adult Female Offspring
Abstract
1. Introduction
2. Materials and Methods
2.1. Intermittent Hypoxia Model
2.2. Mammary Whole Mounts
2.3. Histology
2.4. Collagen Quantification
2.5. Isolation of Mammary Epithelial and Stromal Cells
2.6. Progenitor Assays
2.7. Differentiation Assays
2.8. Gene Expression Analyses
2.9. Statistical Analyses
3. Results
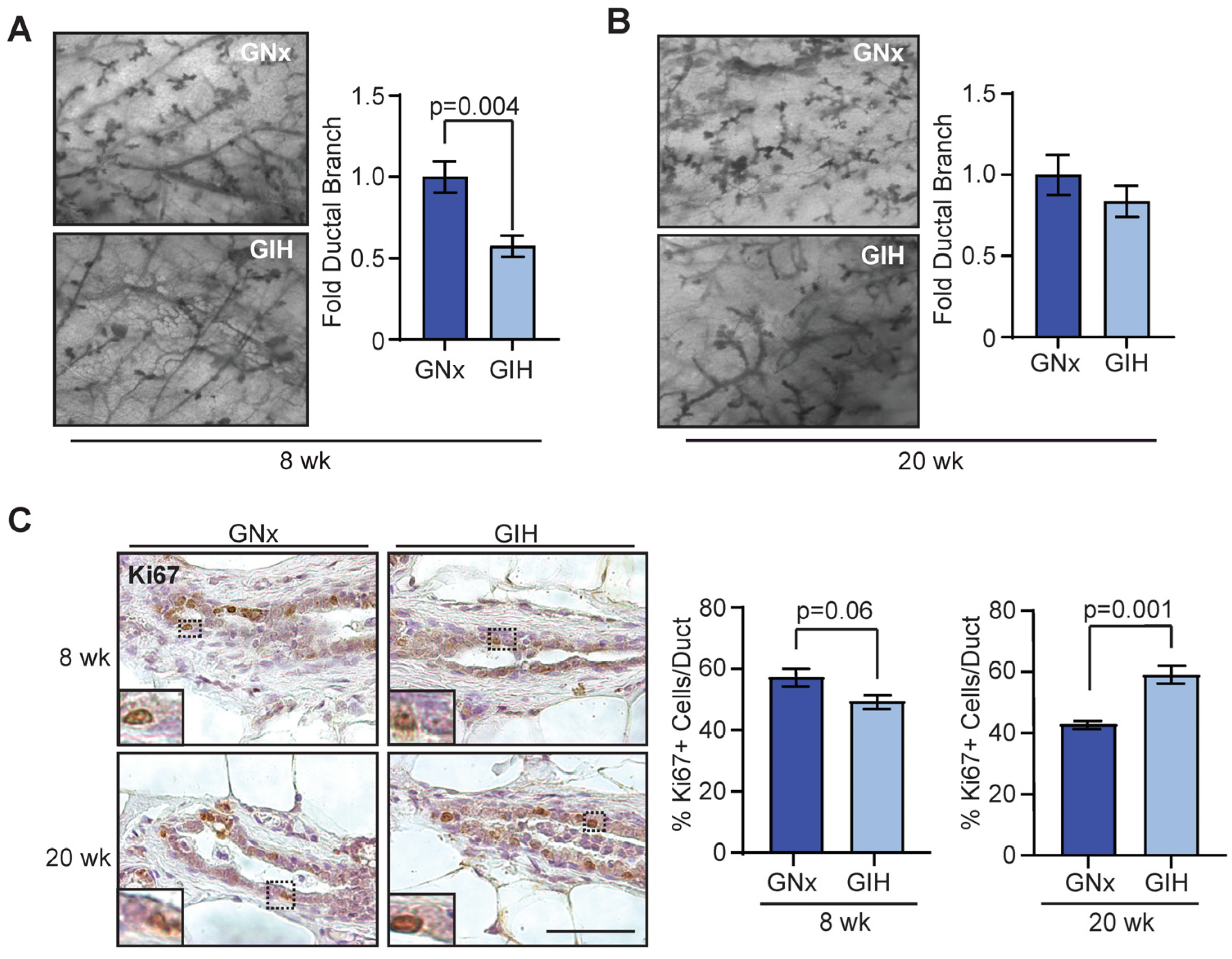
3.1. Delayed Development of Mammary Glands in GIH Female Offspring
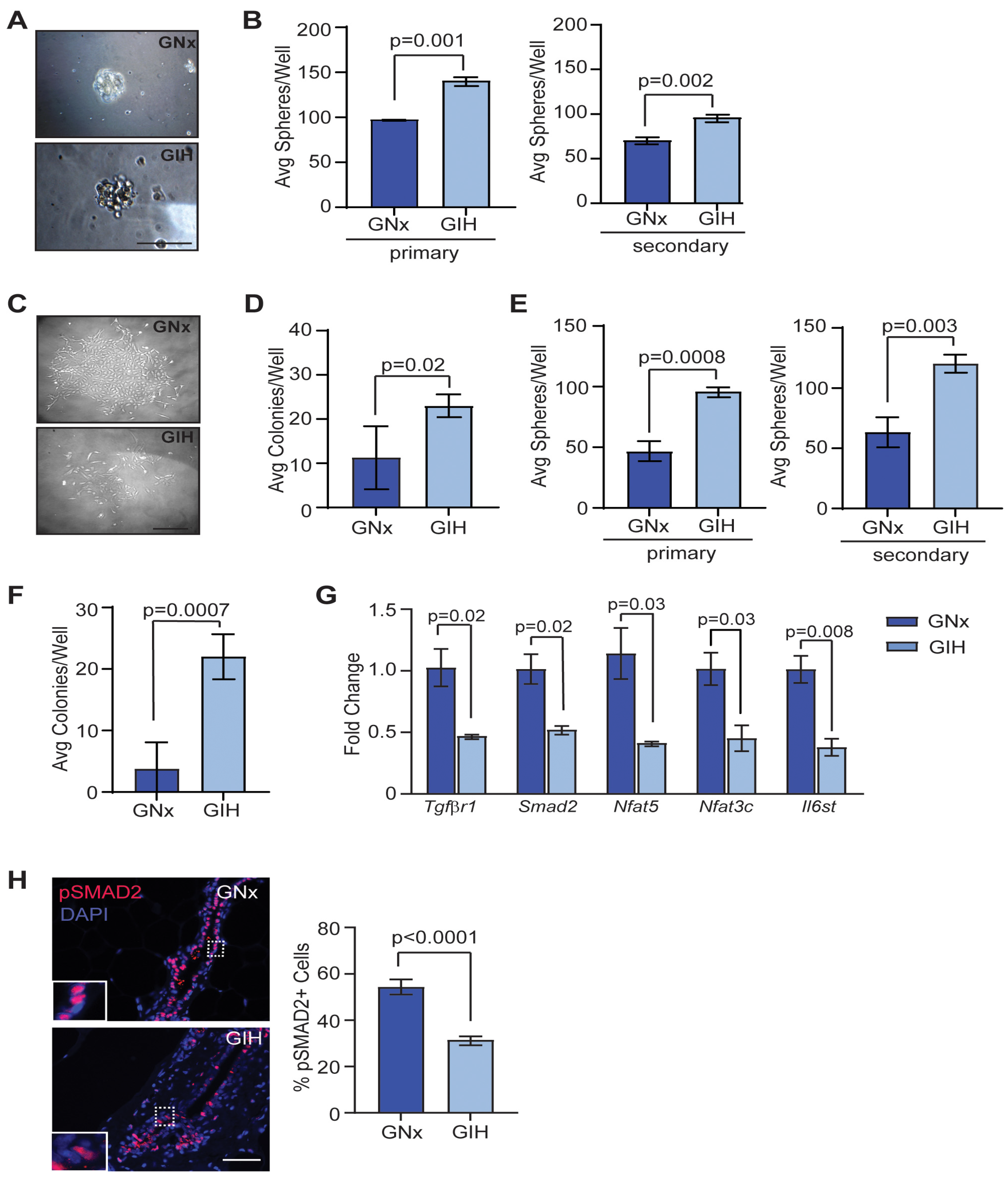
3.2. Enhanced Mammary Stem/Progenitor Activity in GIH Offspring
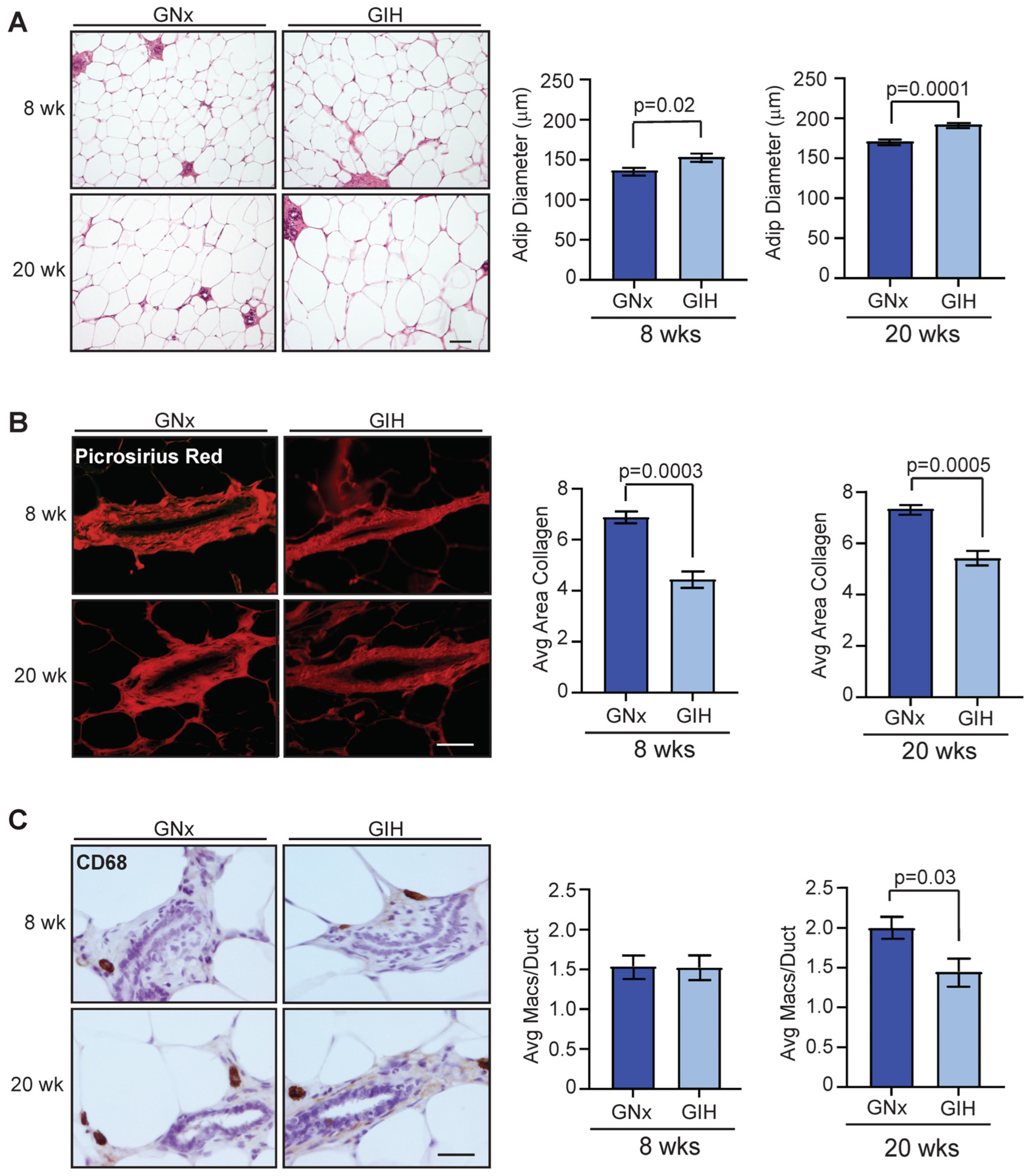
3.3. Microenvironmental Changes Observed in Mammary Glands of GIH Offspring
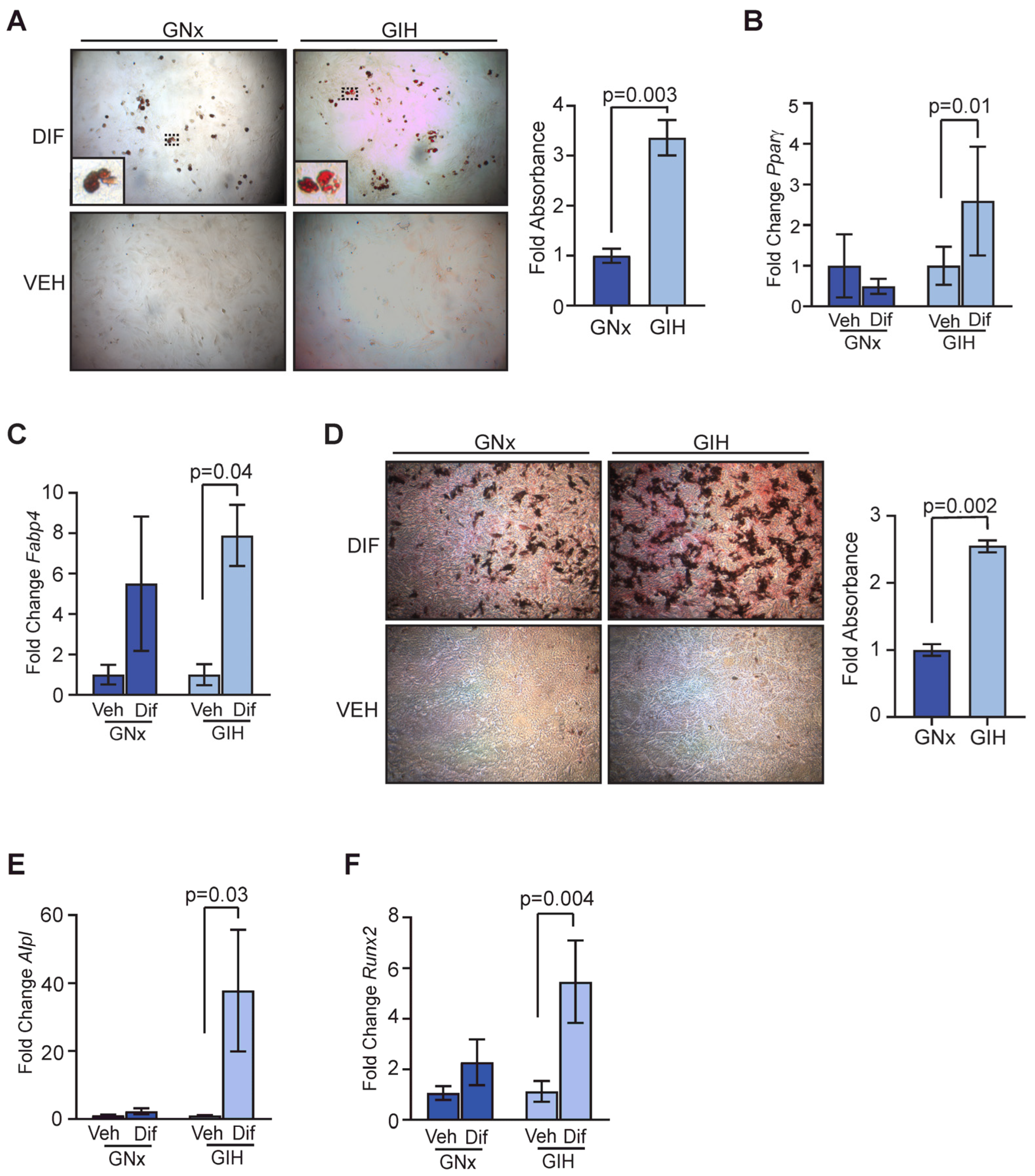
3.4. Increased Differentiation Potential in Adipose-Derived Stromal Cells from GIH Offspring
3.5. Fibrous Tumors Enhanced in Mammary Glands of GIH Offspring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Cancer Facts and Figures 2023; American Cancer Society: Atlanta, GA, USA, 2023; Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html (accessed on 20 December 2023).
- World Health Organization. Obesity and Overweight. 2021. Available online: https://www.who.int-news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 December 2023).
- Johns, E.C.; Denison, F.C.; Reynolds, R.M. Sleep disordered breathing in pregnancy: A review of the pathophysiology of adverse pregnancy outcomes. Acta Physiol. 2020, 229, e13458. [Google Scholar] [CrossRef]
- Young, T.; Peppard, P.E.; Gottlieb, D.J. Epidemiology of obstructive sleep apnea: A population health perspective. Am. J. Respir. Crit. Care Med. 2002, 165, 1217–1239. [Google Scholar] [CrossRef]
- Antony, K.M.; Jacobson, N.M.; Rice, L.; Wiedmer, A.M.; Mourey, H.; Bazalakova, M.H. Obstructive sleep apnea in pregnancy: Early lessons from our sleep pregnancy clinic. WMJ 2021, 120, 34–40. [Google Scholar]
- Chang, W.P.; Liu, M.E.; Chang, W.C.; Yang, A.C.; Ku, Y.C.; Pai, J.T.; Lin, Y.W.; Tsai, S.J. Sleep apnea and the subsequent risk of breast cancer in women: A nationwide population-based cohort study. Sleep Med. 2014, 15, 1016–1020. [Google Scholar] [CrossRef]
- Yap, D.W.T.; Tan, N.K.W.; Tan, B.K.J.; Teo, Y.H.; Tan, V.K.M.; See, A.; Toh, S.T. The association of obstructive sleep apnea with breast cancer incidence and mortality: A systematic review and meta-analysis. J. Breast Cancer 2022, 25, 149–163. [Google Scholar] [CrossRef]
- Warland, J.; Dorrian, J.; Morrison, J.L.; O’Brien, L.M. Maternal sleep during pregnancy and poor fetal outcomes: A scoping review of the literature with meta-analysis. Sleep Med. Rev. 2018, 41, 197–219. [Google Scholar] [CrossRef]
- Song, R.; Mishra, J.S.; Dangudubiyyam, S.V.; Antony, K.M.; Baker, T.L.; Watters, J.J.; Kumar, S. Gestational intermittent hypoxia induces sex-specific impairment in endothelial mechanisms and sex steroid hormone levels in male rat offspring. Reprod. Sci. 2022, 29, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Randhawa, K.S.; Epstein, J.J.; Gustafson, E.; Hocker, A.D.; Huxtable, A.G.; Baker, T.L.; Watters, J.J. Gestational intermittent hypoxia increases susceptibility to neuroinflammation and alters respiratory motor control in neonatal rats. Respir. Physiol. Neurobiol. 2018, 256, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Vanderplow, A.M.; Kermath, B.A.; Bernhardt, C.R.; Gums, K.T.; Seablom, E.N.; Radcliff, A.B.; Ewald, A.C.; Jones, M.V.; Baker, T.L.; Watters, J.J.; et al. A feature of maternal sleep apnea during gestation causes autism-relevant neuronal and behavioral phenotypes in offspring. PLoS Biol. 2022, 20, e3001502. [Google Scholar] [CrossRef]
- Chamberlin, T.; Clack, M.; Silvers, C.; Kuziel, G.; Thompson, V.; Johnson, H.; Arendt, L.M. Targeting obesity-induced macrophages during preneoplastic growth promotes mammary epithelial stem/progenitor activity, DNA damage, and tumor formation. Cancer Res. 2020, 80, 4465–4475. [Google Scholar] [CrossRef] [PubMed]
- Kuziel, G.; Moore, B.N.; Haugstad, G.P.; Arendt, L.M. Fibrocytes enhance mammary gland fibrosis in obesity. FASEB J. 2023, 37, e23049. [Google Scholar] [CrossRef] [PubMed]
- Tovar, E.A.; Sheridan, R.; Essenburg, C.J.; Dischinger, P.S.; Arumugam, M.; Callaghan, M.E.; Graveel, C.R.; Steensma, M.R. Dissecting the rat mammary gland: Isolation, characterization, and culture of purified mammary epithelial cells and fibroblasts. Bio Protoc. 2020, 10, e3818. [Google Scholar] [CrossRef] [PubMed]
- El Mouedden, M.; Laurent, G.; Mingeot-Leclercq, M.P.; Tulkens, P.M. Gentamicin-induced apoptosis in renal cell lines and embryonic rat fibroblasts. Toxicol. Sci. 2000, 56, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Chamberlin, T.; D’Amato, J.V.; Arendt, L.M. Obesity reversibly depletes the basal cell population and enhances mammary epithelial cell estrogen receptor alpha expression and progenitor activity. Breast Cancer Res. 2017, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.C.; Brady, D.C.; Po, P.; Chuang, L.P.; Marcondes, L.; Kim, E.Y.; Keenan, B.T.; Guo, X.; Maislin, G.; Galante, R.J.; et al. Simulating obstructive sleep apnea patients’ oxygenation characteristics into a mouse model of cyclical intermittent hypoxia. J. Appl. Physiol. 2015, 118, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Filgo, A.J.; Foley, J.F.; Puvanesarajah, S.; Borde, A.R.; Midkiff, B.R.; Reed, C.E.; Chappell, V.A.; Alexander, L.B.; Borde, P.R.; Troester, M.A.; et al. Mammary gland evaluation in juvenile toxicity studies: Temporal developmental patterns in the male and female Harlan Sprague-Dawley rat. Toxicol. Pathol. 2016, 44, 1034–1058. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Wicha, M.S. Mammary stem cell number as a determinate of breast cancer risk. Breast Cancer Res. 2007, 9, 109. [Google Scholar] [CrossRef]
- Terry, M.B.; Michels, K.B.; Brody, J.G.; Byrne, C.; Chen, S.; Jerry, D.J.; Malecki, K.M.C.; Martin, M.B.; Miller, R.L.; Neuhausen, S.L.; et al. Environmental exposures during windows of susceptibility for breast cancer: A framework for prevention research. Breast Cancer Res. 2019, 21, 96. [Google Scholar] [CrossRef]
- Kordon, E.C.; McKnight, R.A.; Jhappan, C.; Hennighausen, L.; Merlino, G.; Smith, G.H. Ectopic TGF beta 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev. Biol. 1995, 168, 47–61. [Google Scholar] [CrossRef]
- Boulanger, C.A.; Smith, G.H. Reducing mammary cancer risk through premature stem cell senescence. Oncogene 2001, 20, 2264–2272. [Google Scholar] [CrossRef]
- Feng, X.-H.; Derynck, R. Specificity and versatility in TGF-β signaling through SMADS. Ann. Rev. Cell Dev. Biol. 2005, 21, 659–693. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Cortese, R.; Qiao, Z.; Ye, H.; Bao, R.; Andrade, J.; Gozal, D. Late gestational intermittent hypoxia induces metabolic and epigenetic changes in male adult offspring mice. J. Physiol. 2017, 595, 2551–2568. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chaffee, T.S.; LaRue, R.S.; Huggins, D.N.; Witschen, P.M.; Ibrahim, A.M.; Nelson, A.C.; Machado, H.L.; Schwertfeger, K.L. Tissue-resident macrophages promote extracellular matrix homeostasis in the mammary gland stroma of nulliparous mice. eLife 2020, 9, e57438. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019, 114, 108765. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.K.; Stevenson, G.T.; Busch, K.A. Tumor incidence in normal Sprague-Dawley female rats. Cancer Res. 1956, 16, 194–197. [Google Scholar] [PubMed]
- Meites, J. Changes in neuroendocrine control of anterior pituitary function during aging. Neuroendocrinology 1982, 34, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Facco, F.L.; Chan, M.; Patel, S.R. Common sleep disorders in pregnancy. Obstet. Gynecol. 2022, 140, 321–339. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Y.; Rychahou, P.; Liu, C.; Weiss, H.L.; Evers, B.M. NFAT5 represses canonical Wnt signaling via inhibition of β-catenin acetylation and participates in regulating intestinal cell differentiation. Cell Death Dis. 2013, 4, e671. [Google Scholar] [CrossRef]
- Lee, H.; Chouinard, L.; Bonin, M.; Michel, R.N. NFATc3 deficiency may contribute to the development of mammary gland adenocarcinoma in aging female mice. Mol. Carcinog. 2005, 44, 219–222. [Google Scholar] [CrossRef]
- Jia, R.; Weng, Y.; Li, Z.; Liang, W.; Ji, Y.; Liang, Y.; Ning, P. Bioinformatics analysis identifies IL6ST as a potential tumor suppressor gene for triple-negative breast cancer. Reprod. Sci. 2021, 28, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.; Yassin, B.A.; Lin, D.T.S.; Kobor, M.S.; Ayas, N.; Laher, I. Gestational intermittent hypoxia induces endothelial dysfunction, reduces perivascular adiponectin and causes epigenetic changes in adult male offspring. J. Physiol. 2019, 597, 5349–5364. [Google Scholar] [CrossRef]
- Jotzu, C.; Alt, E.; Welte, G.; Li, J.; Hennessy, B.T.; Devarajan, E.; Krishnappa, S.; Pinilla, S.; Droll, L.; Song, Y.H. Adipose tissue derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor derived factors. Cell. Oncol. 2011, 34, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Chan, M.K.; Li, J.S.; Chan, A.S.; Tang, P.C.; Leung, K.T.; To, K.F.; Lan, H.Y.; Tang, P.M. TGF-β signaling: From tissue fibrosis to tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 7575. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Y.; Tang, W.; Luo, J.; Wang, M.; Lin, H.; Guo, H.; Ma, Y.; Zhang, J.; Li, Q. Association between the degree of fibrosis in fibrotic focus and the unfavorable clinicopathological prognostic features of breast cancer. PeerJ 2019, 7, e8067. [Google Scholar] [CrossRef]
- Piersma, B.; Hayward, M.K.; Weaver, V.M. Fibrosis and cancer: A strained relationship. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef] [PubMed]
- Olivarez, S.A.; Maheshwari, B.; McCarthy, M.; Zacharias, N.; van den Veyver, I.; Casturi, L.; Sangi-Haghpeykar, H.; Aagaard-Tillery, K. Prospective trial on obstructive sleep apnea in pregnancy and fetal heart rate monitoring. Am. J. Obstet. Gynecol. 2010, 202, 552.e1–552.e7. [Google Scholar] [CrossRef] [PubMed]
- May, A.M.; Mehra, R. Obstructive sleep apnea: Role of intermittent hypoxia and inflammation. Semin. Respir. Crit. Care Med. 2014, 35, 531–544. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, T.W.; Su, M.C.; Chen, C.J.; Chen, K.D.; Liou, C.W.; Tang, P.; Wang, T.Y.; Chang, J.C.; Wang, C.C.; et al. Whole genome DNA methylation analysis of obstructive sleep apnea: IL1R2, NPR2, AR, SP140 methylation and clinical phenotype. Sleep 2016, 39, 743–755. [Google Scholar] [CrossRef]
- Nanduri, J.; Semenza, G.L.; Prabhakar, N.R. Epigenetic changes by DNA methylation in chronic and intermittent hypoxia. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L1096–L1100. [Google Scholar] [CrossRef]
- Zhu, Z.; Cao, F.; Li, X. Epigenetic programming and fetal metabolic programming. Front. Endocrinol. 2019, 10, 764. [Google Scholar] [CrossRef]
- Elshenawy, S.; Simmons, R. Maternal obesity and prenatal programming. Mol. Cell Endocrinol. 2016, 435, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Hilakivi-Clarke, L.; de Assis, S. Fetal origins of breast cancer. Trends Endocrinol. Metab. 2006, 17, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Pathiraja, T.N.; Nayak, S.R.; Xi, Y.; Jiang, S.; Garee, J.P.; Edwards, D.P.; Lee, A.V.; Chen, J.; Shea, M.J.; Santen, R.J.; et al. Epigenetic reprogramming of HOXC10 in endocrine-resistant breast cancer. Sci. Transl. Med. 2014, 6, 229ra241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Widschwendter, M.; Siegmund, K.D.; Müller, H.M.; Fiegl, H.; Marth, C.; Müller-Holzner, E.; Jones, P.A.; Laird, P.W. Association of breast cancer DNA methylation profiles with hormone receptor status and response to tamoxifen. Cancer Res. 2004, 64, 3807–3813. [Google Scholar] [CrossRef] [PubMed]
- Mabry, S.; Wilson, E.N.; Bradshaw, J.L.; Gardner, J.J.; Fadeyibi, O.; Vera, E., Jr.; Osikoya, O.; Cushen, S.C.; Karamichos, D.; Goulopoulou, S.; et al. Sex and age differences in social and cognitive function in offspring exposed to late gestational hypoxia. Biol. Sex. Differ. 2023, 14, 81. [Google Scholar] [CrossRef]
- Bin, Y.S.; Cistulli, P.A.; Roberts, C.L.; Ford, J.B. Childhood health and educational outcomes associated with maternal sleep apnea: A population record-linkage study. Sleep 2017, 40, zsx158. [Google Scholar] [CrossRef]
- Tauman, R.; Zuk, L.; Uliel-Sibony, S.; Ascher-Landsberg, J.; Katsav, S.; Farber, M.; Sivan, Y.; Bassan, H. The effect of maternal sleep-disordered breathing on the infant’s neurodevelopment. Am. J. Obstet. Gynecol. 2015, 212, 656.e1–656.e7. [Google Scholar] [CrossRef]
- Casale, M.; Pappacena, M.; Rinaldi, V.; Bressi, F.; Baptista, P.; Salvinelli, F. Obstructive sleep apnea syndrome: From phenotype to genetic basis. Curr. Genom. 2009, 10, 119–126. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, J.; Xiong, Y.; Kuhn, M.; Radcliff, A.B.; Baker, T.L.; Watters, J.J.; Arendt, L.M. Gestational Intermittent Hypoxia Enhances Mammary Stem Cells and Alters Tumor Phenotype in Adult Female Offspring. Cells 2024, 13, 249. https://doi.org/10.3390/cells13030249
Joshi J, Xiong Y, Kuhn M, Radcliff AB, Baker TL, Watters JJ, Arendt LM. Gestational Intermittent Hypoxia Enhances Mammary Stem Cells and Alters Tumor Phenotype in Adult Female Offspring. Cells. 2024; 13(3):249. https://doi.org/10.3390/cells13030249
Chicago/Turabian StyleJoshi, Jaitri, Yue Xiong, Molly Kuhn, Abigail B. Radcliff, Tracy L. Baker, Jyoti J. Watters, and Lisa M. Arendt. 2024. "Gestational Intermittent Hypoxia Enhances Mammary Stem Cells and Alters Tumor Phenotype in Adult Female Offspring" Cells 13, no. 3: 249. https://doi.org/10.3390/cells13030249
APA StyleJoshi, J., Xiong, Y., Kuhn, M., Radcliff, A. B., Baker, T. L., Watters, J. J., & Arendt, L. M. (2024). Gestational Intermittent Hypoxia Enhances Mammary Stem Cells and Alters Tumor Phenotype in Adult Female Offspring. Cells, 13(3), 249. https://doi.org/10.3390/cells13030249






