Novel Vitamin D3 Hydroxymetabolites Require Involvement of the Vitamin D Receptor or Retinoic Acid-Related Orphan Receptors for Their Antifibrogenic Activities in Human Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Western Blot Analyses Found That the Vitamin D Receptor Is Required for the Action of Vitamin D Hydroxyderivatives on Fibroblasts
2.3. Cell Proliferation Mediators Retinoic Acid-Related Orphan Receptor-α and Retinoic Acid-Related Orphan Receptor-γ Are Required for the Effects of Vitamin D Hydroxyderivatives on Fibroblasts
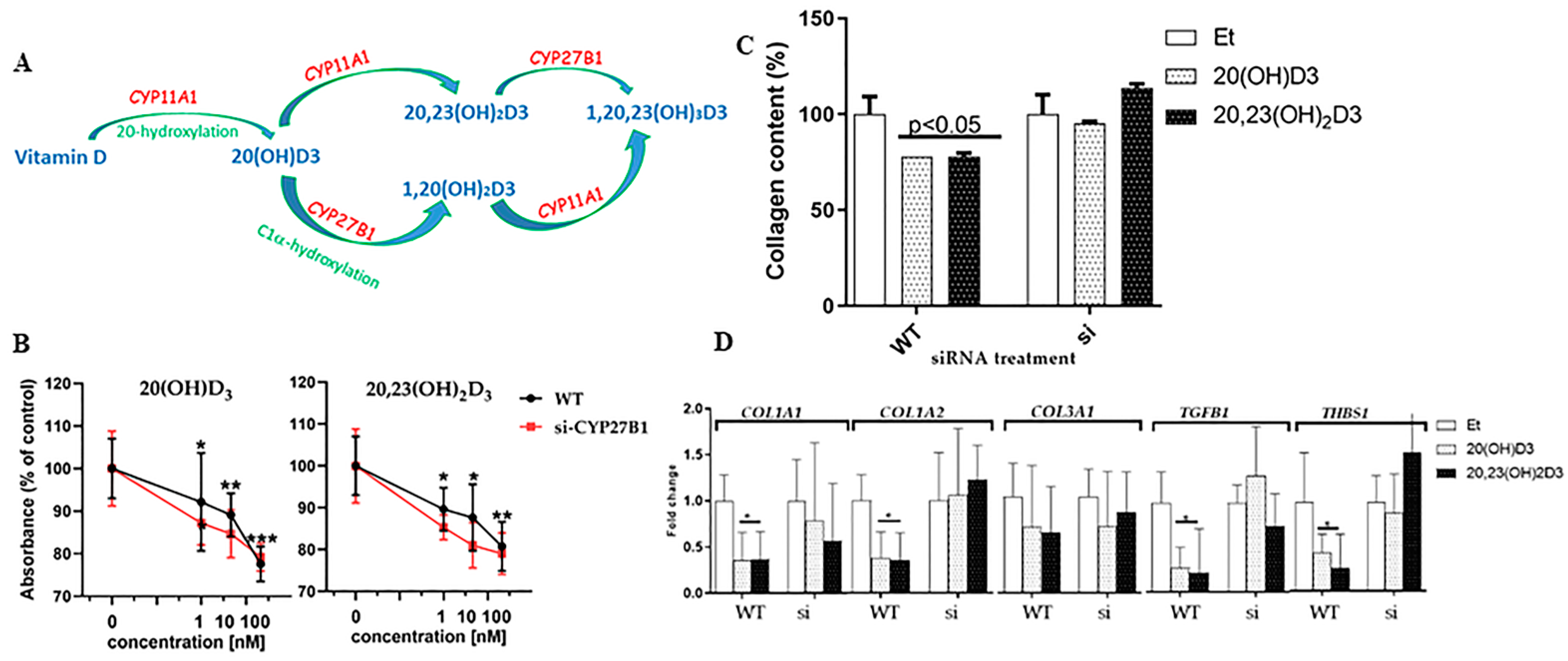
2.4. Ribonucleic Acid Isolation and Quantitative Reverse Transcription Polymerase Chain Reaction
2.5. Collagen Assay
3. Results
3.1. Silencing of the Vitamin D Receptor, Retinoic Acid-Related Orphan Receptors, or CYP27B1
3.2. The Vitamin D Receptor Is Required for the Action of Vitamin D Hydroxyderivatives on Fibroblasts
3.3. Retinoic Acid-Related Orphan Receptor-α and Retinoic Acid-Related Orphan Receptor-γ Are Required for Phenotypic Effects of Vitamin D Hydroxyderivatives on Fibroblasts
3.4. 1α-Hydroxylation Is Not Necessary for the Action of 20(OH)D3 or 20,23(OH)2D3 on Fibroblasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holick, M.F.; Clark, M.B. The photobiogenesis and metabolism of vitamin D. Fed. Proc. 1978, 37, 2567–2574. [Google Scholar]
- Holick, M.F. Vitamin D: A millenium perspective. J. Cell. Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M.; Bouillon, R.; Giovannucci, E.; Goltzman, D.; Meyer, B.M.; Welsh, J. Feldman and Pike’s Vitamin D, 5th ed.; Academic Press: Cambridge, MA, USA, 2024. [Google Scholar]
- McCollum, E.V. The paths to the discovery of vitamins A and D. J. Nutr. 1967, 91 (Suppl. S1), 32–38. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: An ancient hormone. Exp. Dermatol. 2011, 20, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef]
- Jenkinson, C. The vitamin D metabolome: An update on analysis and function. Cell Biochem. Funct. 2019, 37, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef]
- Zhu, J.G.; Ochalek, J.T.; Kaufmann, M.; Jones, G.; DeLuca, H.F. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 15650–15655. [Google Scholar] [CrossRef]
- Guryev, O.; Carvalho, R.A.; Usanov, S.; Gilep, A.; Estabrook, R.W. A pathway for the metabolism of vitamin D3: Unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1). Proc. Natl. Acad. Sci. USA 2003, 100, 14754–14759. [Google Scholar] [CrossRef]
- Tuckey, R.C.; Li, W.; Zjawiony, J.K.; Zmijewski, M.A.; Nguyen, M.N.; Sweatman, T.; Miller, D.; Slominski, A. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008, 275, 2585–2596. [Google Scholar] [CrossRef]
- Slominski, A.; Semak, I.; Zjawiony, J.; Wortsman, J.; Li, W.; Szczesniewski, A.; Tuckey, R.C. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005, 272, 4080–4090. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Shehabi, H.Z.; Semak, I.; Tang, E.K.; Nguyen, M.N.; Benson, H.A.; Korik, E.; Janjetovic, Z.; Chen, J.; et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012, 26, 3901–3915. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Li, W.; Tuckey, R.C. Classical and non-classical metabolic transformation of vitamin D in dermal fibroblasts. Exp. Dermatol. 2016, 25, 231–232. [Google Scholar] [CrossRef]
- Slominski, R.M.; Raman, C.; Elmets, C.; Jetten, A.M.; Slominski, A.T.; Tuckey, R.C. The significance of CYP11A1 expression in skin physiology and pathology. Mol. Cell. Endocrinol. 2021, 530, 111238. [Google Scholar] [CrossRef]
- Norlin, M.; Lundqvist, J.; Ellfolk, M.; Hellstrom Pigg, M.; Gustafsson, J.; Wikvall, K. Drug-Mediated Gene Regulation of Vitamin D(3) Metabolism in Primary Human Dermal Fibroblasts. Basic Clin. Pharmacol. Toxicol. 2017, 120, 59–63. [Google Scholar] [CrossRef]
- Slominski, A.; Janjetovic, Z.; Tuckey, R.C.; Nguyen, M.N.; Bhattacharya, K.G.; Wang, J.; Li, W.; Jiao, Y.; Gu, W.; Brown, M.; et al. 20S-hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J. Clin. Endocrinol. Metab. 2013, 98, E298–E303. [Google Scholar] [CrossRef]
- Janjetovic, Z.; Tuckey, R.C.; Nguyen, M.N.; Thorpe, E.M., Jr.; Slominski, A.T. 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J. Cell. Physiol. 2010, 223, 36–48. [Google Scholar] [CrossRef]
- Kim, T.K.; Wang, J.; Janjetovic, Z.; Chen, J.; Tuckey, R.C.; Nguyen, M.N.; Tang, E.K.; Miller, D.; Li, W.; Slominski, A.T. Correlation between secosteroid-induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Mol. Cell. Endocrinol. 2012, 361, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Brozyna, A.A.; Kim, T.K.; Elsayed, M.M.; Janjetovic, Z.; Qayyum, S.; Slominski, R.M.; Oak, A.S.W.; Li, C.; Podgorska, E.; et al. CYP11A1-derived vitamin D hydroxyderivatives as candidates for therapy of basal and squamous cell carcinomas. Int. J. Oncol. 2022, 61, 96. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Jarrett, S.G.; D’Orazio, J.A.; Holick, M.F.; Tang, E.K.Y.; Tuckey, R.C.; Panich, U.; Li, W.; et al. Protective effects of novel derivatives of vitamin D3 and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms. Redox Biol. 2019, 24, 101206. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Kim, T.K.; Zmijewski, M.A.; Janjetovic, Z.; Li, W.; Chen, J.; Kusniatsova, E.I.; Semak, I.; Postlethwaite, A.; Miller, D.D.; et al. Novel vitamin D photoproducts and their precursors in the skin. Dermato-Endocrinolog 2013, 5, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Janjetovic, Z.; Tuckey, R.C.; Bieniek, R.; Yue, J.; Li, W.; Chen, J.; Nguyen, M.N.; Tang, E.K.; et al. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am. J. Physiol.-Cell Physiol. 2011, 300, C526–C541. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Kim, T.K.; Tieu, E.W.; Tang, E.K.; Lin, Z.; Kovacic, D.; Miller, D.D.; Postlethwaite, A.; Tuckey, R.C.; et al. Novel vitamin D analogs as potential therapeutics: Metabolism, toxicity profiling, and antiproliferative activity. Anticancer Res. 2014, 34, 2153–2163. [Google Scholar]
- Slominski, A.T.; Janjetovic, Z.; Fuller, B.E.; Zmijewski, M.A.; Tuckey, R.C.; Nguyen, M.N.; Sweatman, T.; Li, W.; Zjawiony, J.; Miller, D.; et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS ONE 2010, 5, e9907. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Potter, J.J.; Liu, X.; Koteish, A.; Mezey, E. 1,25-dihydroxyvitamin D3 and its nuclear receptor repress human α1(I) collagen expression and type I collagen formation. Liver Int. 2013, 33, 677–686. [Google Scholar] [CrossRef]
- Perazzi, M.; Gallina, E.; Manfredi, G.F.; Patrucco, F.; Acquaviva, A.; Colangelo, D.; Pirisi, M.; Bellan, M. Vitamin D in Systemic Sclerosis: A Review. Nutrients 2022, 14, 3908. [Google Scholar] [CrossRef]
- Bikle, D.D. The vitamin D receptor: A tumor suppressor in skin. Discov. Med. 2011, 11, 7–17. [Google Scholar]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D in the Context of Evolution. Nutrients 2022, 14, 3018. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Hobrath, J.V.; Oak, A.S.W.; Tang, E.K.Y.; Tieu, E.W.; Li, W.; Tuckey, R.C.; Jetten, A.M. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as "biased" agonists on VDR and inverse agonists on RORα and RORγ. J. Steroid Biochem. Mol. Biol. 2017, 173, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Marepally, S.R.; Goh, E.S.Y.; Cheng, C.Y.S.; Janjetovic, Z.; Kim, T.K.; Miller, D.D.; Postlethwaite, A.E.; Slominski, A.T.; Tuckey, R.C.; et al. Investigation of 20S-hydroxyvitamin D3 analogs and their 1α-OH derivatives as potent vitamin D receptor agonists with anti-inflammatory activities. Sci. Rep. 2018, 8, 1478. [Google Scholar] [CrossRef] [PubMed]
- Mizwicki, M.T.; Norman, A.W. The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci. Signal. 2009, 2, re4. [Google Scholar] [CrossRef]
- Mizwicki, M.T.; Keidel, D.; Bula, C.M.; Bishop, J.E.; Zanello, L.P.; Wurtz, J.M.; Moras, D.; Norman, A.W. Identification of an alternative ligand-binding pocket in the nuclear vitamin D receptor and its functional importance in 1α,25(OH)2-vitamin D3 signaling. Proc. Natl. Acad. Sci. USA 2004, 101, 12876–12881. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Takeda, Y.; Janjetovic, Z.; Brozyna, A.A.; Skobowiat, C.; Wang, J.; Postlethwaite, A.; Li, W.; Tuckey, R.C.; et al. RORα and ROR γ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014, 28, 2775–2789. [Google Scholar] [CrossRef]
- Jetten, A.M. Retinoid-related orphan receptors (RORs): Critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal. 2009, 7, e003. [Google Scholar] [CrossRef]
- Jetten, A.M.; Kang, H.S.; Takeda, Y. Retinoic acid-related orphan receptors α and γ: Key regulators of lipid/glucose metabolism, inflammation, and insulin sensitivity. Front. Endocrinol. 2013, 4, 1. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Slominski, R.M.; Song, Y.; Janjetovic, Z.; Podgorska, E.; Reddy, S.B.; Song, Y.; Raman, C.; Tang, E.K.Y.; et al. Metabolic activation of tachysterol3 to biologically active hydroxyderivatives that act on VDR, AhR, LXRs, and PPARγ receptors. FASEB J. 2022, 36, e22451. [Google Scholar] [CrossRef]
- Huh, J.R.; Littman, D.R. Small molecule inhibitors of RORγt: Targeting Th17 cells and other applications. Eur. J. Immunol. 2012, 42, 2232–2237. [Google Scholar] [CrossRef]
- Smith, S.H.; Peredo, C.E.; Takeda, Y.; Bui, T.; Neil, J.; Rickard, D.; Millerman, E.; Therrien, J.P.; Nicodeme, E.; Brusq, J.M.; et al. Development of a Topical Treatment for Psoriasis Targeting RORγ: From Bench to Skin. PLoS ONE 2016, 11, e0147979. [Google Scholar] [CrossRef] [PubMed]
- Solt, L.A.; Griffin, P.R.; Burris, T.P. Ligand regulation of retinoic acid receptor-related orphan receptors: Implications for development of novel therapeutics. Curr. Opin. Lipidol. 2010, 21, 204–211. [Google Scholar] [CrossRef]
- Artaza, J.N.; Norris, K.C. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J. Endocrinol. 2009, 200, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P.; Chan, E.S. Molecular pathogenesis of skin fibrosis: Insight from animal models. Curr. Rheumatol. Rep. 2010, 12, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, D.; Brown, M.; Postlethwaite, B.C.; Postlethwaite, A.E. Pathogenesis of Systemic Sclerosis. Front. Immunol. 2015, 6, 272. [Google Scholar] [CrossRef]
- Gilbane, A.J.; Denton, C.P.; Holmes, A.M. Scleroderma pathogenesis: A pivotal role for fibroblasts as effector cells. Arthritis Res. Ther. 2013, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.M.; Wongtrakool, C.; Welch, T.; Steinmeyer, A.; Zugel, U.; Roman, J. Vitamin D inhibition of pro-fibrotic effects of transforming growth factor β1 in lung fibroblasts and epithelial cells. J. Steroid Biochem. Mol. Biol. 2010, 118, 142–150. [Google Scholar] [CrossRef]
- Ding, J.; Kwan, P.; Ma, Z.; Iwashina, T.; Wang, J.; Shankowsky, H.A.; Tredget, E.E. Synergistic effect of vitamin D and low concentration of transforming growth factor beta 1, a potential role in dermal wound healing. Burns 2016, 42, 1277–1286. [Google Scholar] [CrossRef]
- Tao, Q.; Wang, B.; Zheng, Y.; Jiang, X.; Pan, Z.; Ren, J. Vitamin D prevents the intestinal fibrosis via induction of vitamin D receptor and inhibition of transforming growth factor-beta1/Smad3 pathway. Dig. Dis. Sci. 2015, 60, 868–875. [Google Scholar] [CrossRef]
- Usategui, A.; Criado, G.; Del Rey, M.J.; Fare, R.; Pablos, J.L. Topical vitamin D analogue calcipotriol reduces skin fibrosis in experimental scleroderma. Arch. Dermatol. Res. 2014, 306, 757–761. [Google Scholar] [CrossRef]
- Greiling, D.; Thieroff-Ekerdt, R. 1α,25-dihydroxyvitamin D3 rapidly inhibits fibroblast-induced collagen gel contraction. J. Investig. Dermatol. 1996, 106, 1236–1241. [Google Scholar] [CrossRef][Green Version]
- Brown Lobbins, M.L.; Scott, I.O.; Slominski, A.T.; Hasty, K.A.; Zhang, S.; Miller, D.D.; Li, W.; Kim, T.K.; Janjetovic, Z.; Patel, T.S.; et al. 17,20S(OH)2pD Can Prevent the Development of Skin Fibrosis in the Bleomycin-Induced Scleroderma Mouse Model. Int. J. Mol. Sci. 2021, 22, 8926. [Google Scholar] [CrossRef]
- Slominski, A.T.; Li, W.; Bhattacharya, S.K.; Smith, R.A.; Johnson, P.L.; Chen, J.; Nelson, K.E.; Tuckey, R.C.; Miller, D.; Jiao, Y.; et al. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J. Investig. Dermatol. 2011, 131, 1167–1169. [Google Scholar] [CrossRef]
- Janjetovic, Z.; Postlethwaite, A.; Kang, H.S.; Kim, T.K.; Tuckey, R.C.; Crossman, D.K.; Qayyum, S.; Jetten, A.M.; Slominski, A.T. Antifibrogenic Activities of CYP11A1-derived Vitamin D3-hydroxyderivatives Are Dependent on RORγ. Endocrinology 2021, 162, bqaa198. [Google Scholar] [CrossRef] [PubMed]
- Dees, C.; Chakraborty, D.; Distler, J.H.W. Cellular and molecular mechanisms in fibrosis. Exp. Dermatol. 2021, 30, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.K.; Li, W.; Janjetovic, Z.; Nguyen, M.N.; Wang, Z.; Slominski, A.; Tuckey, R.C. Purified mouse CYP27B1 can hydroxylate 20,23-dihydroxyvitamin D3, producing 1α,20,23-trihydroxyvitamin D3, which has altered biological activity. Drug Metab. Dispos. 2010, 38, 1553–1559. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, H.; Belorusova, A.Y.; Bollinger, J.C.; Tang, E.K.Y.; Janjetovic, Z.; Kim, T.K.; Wu, Z.; Miller, D.D.; Slominski, A.T.; et al. 1α,20S-Dihydroxyvitamin D3 Interacts with Vitamin D Receptor: Crystal Structure and Route of Chemical Synthesis. Sci. Rep. 2017, 7, 10193. [Google Scholar] [CrossRef] [PubMed]
- Brzeminski, P.; Fabisiak, A.; Slominski, R.M.; Kim, T.K.; Janjetovic, Z.; Podgorska, E.; Song, Y.; Saleem, M.; Reddy, S.B.; Qayyum, S.; et al. Chemical synthesis, biological activities and action on nuclear receptors of 20S(OH)D3, 20S,25(OH)2D3, 20S,23S(OH)2D3 and 20S,23R(OH)2D3. Bioorg. Chem. 2022, 121, 105660. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Xu, Y.; Wang, L.; Widom, R.; Smith, B.D. Collagen α1(I) gene (COL1A1) is repressed by RFX family. J. Biol. Chem. 2005, 280, 21004–21014. [Google Scholar] [CrossRef]
- Wei, H.Y.; Liu, J.L.; Lv, B.J.; Xing, L.; Fu, S.Y. SPARC modulates expression of extracellular matrix genes in human trabecular meshwork cells. Acta Ophthalmol. 2012, 90, e138–e143. [Google Scholar] [CrossRef]
- Goldberg, M.T.; Han, Y.P.; Yan, C.; Shaw, M.C.; Garner, W.L. TNF-α suppresses α-smooth muscle actin expression in human dermal fibroblasts: An implication for abnormal wound healing. J. Investig. Dermatol. 2007, 127, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Carriere, V.; Roussel, L.; Ortega, N.; Lacorre, D.A.; Americh, L.; Aguilar, L.; Bouche, G.; Girard, J.P. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 282–287. [Google Scholar] [CrossRef]
- Sundararaj, K.P.; Samuvel, D.J.; Li, Y.; Sanders, J.J.; Lopes-Virella, M.F.; Huang, Y. Interleukin-6 released from fibroblasts is essential for up-regulation of matrix metalloproteinase-1 expression by U937 macrophages in coculture: Cross-talking between fibroblasts and U937 macrophages exposed to high glucose. J. Biol. Chem. 2009, 284, 13714–13724. [Google Scholar] [CrossRef]
- McLaughlin, J.N.; Mazzoni, M.R.; Cleator, J.H.; Earls, L.; Perdigoto, A.L.; Brooks, J.D.; Muldowney, J.A., 3rd; Vaughan, D.E.; Hamm, H.E. Thrombin modulates the expression of a set of genes including thrombospondin-1 in human microvascular endothelial cells. J. Biol. Chem. 2005, 280, 22172–22180. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Stefan, J.; Slominski, R.M.; Hanumanthu, V.S.; Raman, C.; Qayyum, S.; Song, Y.; et al. Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives. Cell Biochem. Biophys. 2020, 78, 165–180. [Google Scholar] [CrossRef]
- Zbytek, B.; Janjetovic, Z.; Tuckey, R.C.; Zmijewski, M.A.; Sweatman, T.W.; Jones, E.; Nguyen, M.N.; Slominski, A.T. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J. Investig. Dermatol. 2008, 128, 2271–2280. [Google Scholar] [CrossRef]
- Brown Lobbins, M.L.; Slominski, A.T.; Hasty, K.A.; Zhang, S.; Miller, D.D.; Li, W.; Kim, T.K.; Janjetovic, Z.; Tuckey, R.C.; Scott, I.O.; et al. Modulation by 17,20S(OH)2pD of Fibrosis-Related Mediators in Dermal Fibroblast Lines from Healthy Donors and from Patients with Systemic Sclerosis. Int. J. Mol. Sci. 2021, 23, 367. [Google Scholar] [CrossRef] [PubMed]
- Tuckey, R.C.; Cheng, C.Y.S.; Slominski, A.T. The serum vitamin D metabolome: What we know and what is still to discover. J. Steroid Biochem. Mol. Biol. 2019, 186, 4–21. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: Newer Concepts of Its Metabolism and Function at the Basic and Clinical Level. J. Endocr. Soc. 2020, 4, bvz038. [Google Scholar] [CrossRef]
- Tang, E.K.; Chen, J.; Janjetovic, Z.; Tieu, E.W.; Slominski, A.T.; Li, W.; Tuckey, R.C. Hydroxylation of CYP11A1-derived products of vitamin D3 metabolism by human and mouse CYP27B1. Drug Metab. Dispos. 2013, 41, 1112–1124. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Li, W.; Postlethwaite, A.; Tieu, E.W.; Tang, E.K.Y.; Tuckey, R.C. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci. Rep. 2015, 5, 14875. [Google Scholar] [CrossRef]
- Jenkinson, C.; Desai, R.; Slominski, A.T.; Tuckey, R.C.; Hewison, M.; Handelsman, D.J. Simultaneous measurement of 13 circulating vitamin D3 and D2 mono and dihydroxy metabolites using liquid chromatography mass spectrometry. Clin. Chem. Lab. Med. 2021, 59, 1642–1652. [Google Scholar] [CrossRef]
- Kim, T.K.; Atigadda, V.; Brzeminski, P.; Fabisiak, A.; Tang, E.K.Y.; Tuckey, R.C.; Slominski, A.T. Detection of 7-dehydrocholesterol and vitamin D3 derivatives in honey. Molecules 2020, 25, 2583. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Li, W.; Yi, A.K.; Postlethwaite, A.; Tuckey, R.C. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 28–39. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Bitterman, P.B.; Rennard, S.I.; Adelberg, S.; Crystal, R.G. Role of fibronectin as a growth factor for fibroblasts. J. Cell Biol. 1983, 97, 1925–1932. [Google Scholar] [CrossRef]
- Kim, K.K.; Sheppard, D.; Chapman, H.A. TGF-β1 Signaling and Tissue Fibrosis. Cold Spring Harb. Perspect. Biol. 2018, 10, a022293. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Li, F.; Singh, T.P.; Wolf, P.; Wang, X.J. The pro-inflammatory role of TGFβ1: A paradox? Int. J. Biol. Sci. 2012, 8, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.P.; Patterson, N.L.; Fields, G.B.; Lindsey, M.L. The history of matrix metalloproteinases: Milestones, myths, and misperceptions. Am. J. Physiol.-Heart Circ. Physiol. 2012, 303, H919–H930. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Janjetovic, Z.; Brozyna, A.A.; Zmijewski, M.A.; Xu, H.; Sutter, T.R.; Tuckey, R.C.; Jetten, A.M.; Crossman, D.K. Differential and Overlapping Effects of 20,23(OH)2D3 and 1,25(OH)2D3 on Gene Expression in Human Epidermal Keratinocytes: Identification of AhR as an Alternative Receptor for 20,23(OH)2D3. Int. J. Mol. Sci. 2018, 19, 3072. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qayyum, S.; Greer, R.A.; Slominski, R.M.; Raman, C.; Slominski, A.T.; Song, Y. Vitamin D3 and its hydroxyderivatives as promising drugs against COVID-19: A computational study. J. Biomol. Struct. Dyn. 2021, 40, 11594–11610. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Qayyum, S.; Song, Y.; Janjetovic, Z.; Oak, A.S.W.; Slominski, R.M.; Raman, C.; Stefan, J.; Mier-Aguilar, C.A.; et al. Vitamin D and lumisterol derivatives can act on liver X receptors (LXRs). Sci. Rep. 2021, 11, 8002. [Google Scholar] [CrossRef]
- Postlethwaite, A.E.; Tuckey, R.C.; Kim, T.K.; Li, W.; Bhattacharya, S.K.; Myers, L.K.; Brand, D.D.; Slominski, A.T. 20S-Hydroxyvitamin D3, a Secosteroid Produced in Humans, Is Anti-Inflammatory and Inhibits Murine Autoimmune Arthritis. Front. Immunol. 2021, 12, 678487. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Tuckey, R.C.; Jetten, A.M.; Holick, M.F. Recent Advances in Vitamin D Biology: Something New under the Sun. J. Investig. Dermatol. 2023, 143, 2340–2342. [Google Scholar] [CrossRef] [PubMed]



| Gene | Description | Sequence | Reference |
|---|---|---|---|
| Cyclophilin B | TGTGGTGTTTGGCAAAGTTC GTTTATCCCGGCTGTCTGTC | ||
| CYP27B1 | cytochrome P450 family 27 subfamily B member 1 | CTTGCGGACTGCTCACTG CGCAGACTACGTTGTTCAGG | |
| COL1A1 | collagen I, type alpha 1 | CAGGTCTCGGTCATGGTACCT TCGAGGGCCAAGACGAA | [60] |
| COL1A2 | collagen I, type alpha 2 | GCCCCCCAGGCAGAGA CCAACTCCTTTTCCATCATACTGA | [60] |
| COL3A1 | collagen III, type alpha 1 | CACTGGGGAATGGAGCAAAAC ATCAGGACCACCAATGTCATAGG | [61] |
| FN1 | fibronectin | GGAGAATTCAAGTGTGACCCT CA TGCCACTGTTCTCCTACGTGG | [62] |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase | AGCCACATCGCTCAGACAC GCCCAATACGACCAAATCCC | |
| IL-6 | cytokine of the IL-1 family | GAAGCTCTATCTCGCCTCCA AGCAGGCAACACCAGGAG | |
| IL-8 | cytokine of the IL-1 family | AGACAGCAGAGCACACAAGC ATGGTTCCTTCCGGTGGT | |
| IL-33 | cytokine of the IL-1 family | CACCCCTCAAATGAATCAGG GAGCTCCACAGAGTGTTCC | [63] |
| MMP1 | metallopeptidase 1 | CTGGGAAGCCATCACTTACCTTGC GTTTCTAGAGTCGCTGGGAAGCTG | [64] |
| PDGFA | platelet-derived growth factor, bone repair and regeneration | GCAGTCAGATCCACAGCATC TCCAAAGAATCCTCACTCCCTA | |
| RORA | RAR-related orphan receptor A | GTCAGCAGCTTCTACCTGGAC GTGTTGTTCTGAGAGTGAAAGGCACG | [37] |
| RORC | RAR-related orphan receptor C | CAGCGCTCCAACATCTTCT CCACATCTCCCACATGGACT | [37] |
| ACTA1 (α-SMA) | smooth muscle actin | CCGACCGAATGCAGAAG GA ACAGAGTATTTGCGCTCCGAA | [62] |
| VDR | vitamin D receptor | CTTACCTGCCCCCTGCTC AGGGTCAGGCAGGGAAGT | [23] |
| TGFB1 | transforming growth factor beta 1, cytokines family | GCAGCACGTGGAGCTGTA CAGCCGGTTGCTGAGGTA | |
| THBS1 | thrombospondin 1 | CTG ATC TGG GTT GTG GTT GTA CCT GTG ATG ATG ACG ATG A | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janjetovic, Z.; Qayyum, S.; Reddy, S.B.; Podgorska, E.; Scott, S.G.; Szpotan, J.; Mobley, A.A.; Li, W.; Boda, V.K.; Ravichandran, S.; et al. Novel Vitamin D3 Hydroxymetabolites Require Involvement of the Vitamin D Receptor or Retinoic Acid-Related Orphan Receptors for Their Antifibrogenic Activities in Human Fibroblasts. Cells 2024, 13, 239. https://doi.org/10.3390/cells13030239
Janjetovic Z, Qayyum S, Reddy SB, Podgorska E, Scott SG, Szpotan J, Mobley AA, Li W, Boda VK, Ravichandran S, et al. Novel Vitamin D3 Hydroxymetabolites Require Involvement of the Vitamin D Receptor or Retinoic Acid-Related Orphan Receptors for Their Antifibrogenic Activities in Human Fibroblasts. Cells. 2024; 13(3):239. https://doi.org/10.3390/cells13030239
Chicago/Turabian StyleJanjetovic, Zorica, Shariq Qayyum, Sivani B. Reddy, Ewa Podgorska, S. Gates Scott, Justyna Szpotan, Alisa A. Mobley, Wei Li, Vijay K. Boda, Senthilkumar Ravichandran, and et al. 2024. "Novel Vitamin D3 Hydroxymetabolites Require Involvement of the Vitamin D Receptor or Retinoic Acid-Related Orphan Receptors for Their Antifibrogenic Activities in Human Fibroblasts" Cells 13, no. 3: 239. https://doi.org/10.3390/cells13030239
APA StyleJanjetovic, Z., Qayyum, S., Reddy, S. B., Podgorska, E., Scott, S. G., Szpotan, J., Mobley, A. A., Li, W., Boda, V. K., Ravichandran, S., Tuckey, R. C., Jetten, A. M., & Slominski, A. T. (2024). Novel Vitamin D3 Hydroxymetabolites Require Involvement of the Vitamin D Receptor or Retinoic Acid-Related Orphan Receptors for Their Antifibrogenic Activities in Human Fibroblasts. Cells, 13(3), 239. https://doi.org/10.3390/cells13030239










