Targeting Protein Tyrosine Phosphatases to Improve Cancer Immunotherapies
Abstract
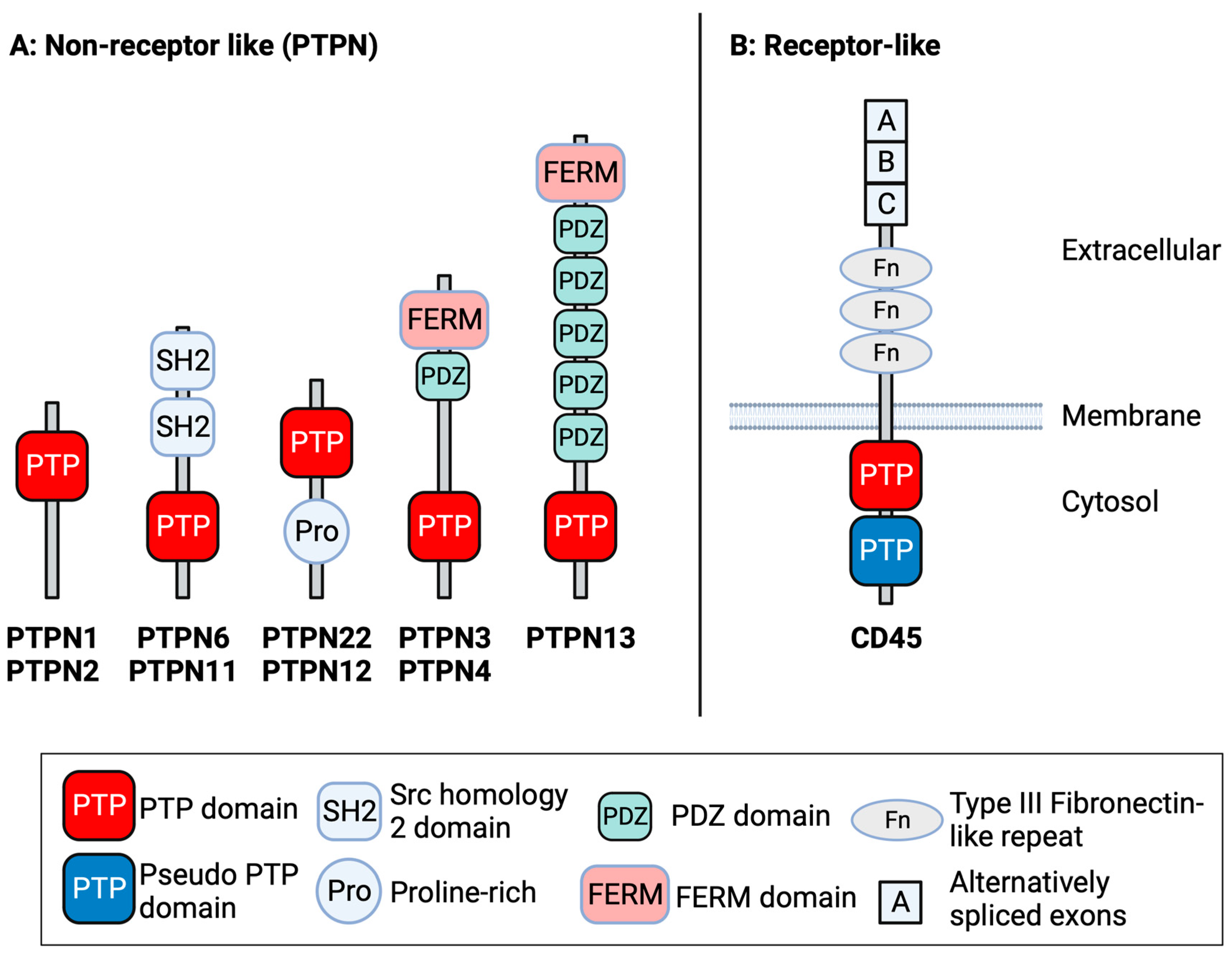
1. Introduction
2. PTPN1 and PTPN2
2.1. Roles of PTPN1 and PTPN2 in Cell Signalling
2.2. PTPN1 and PTPN2 as Regulators of Cancer Cell Biology
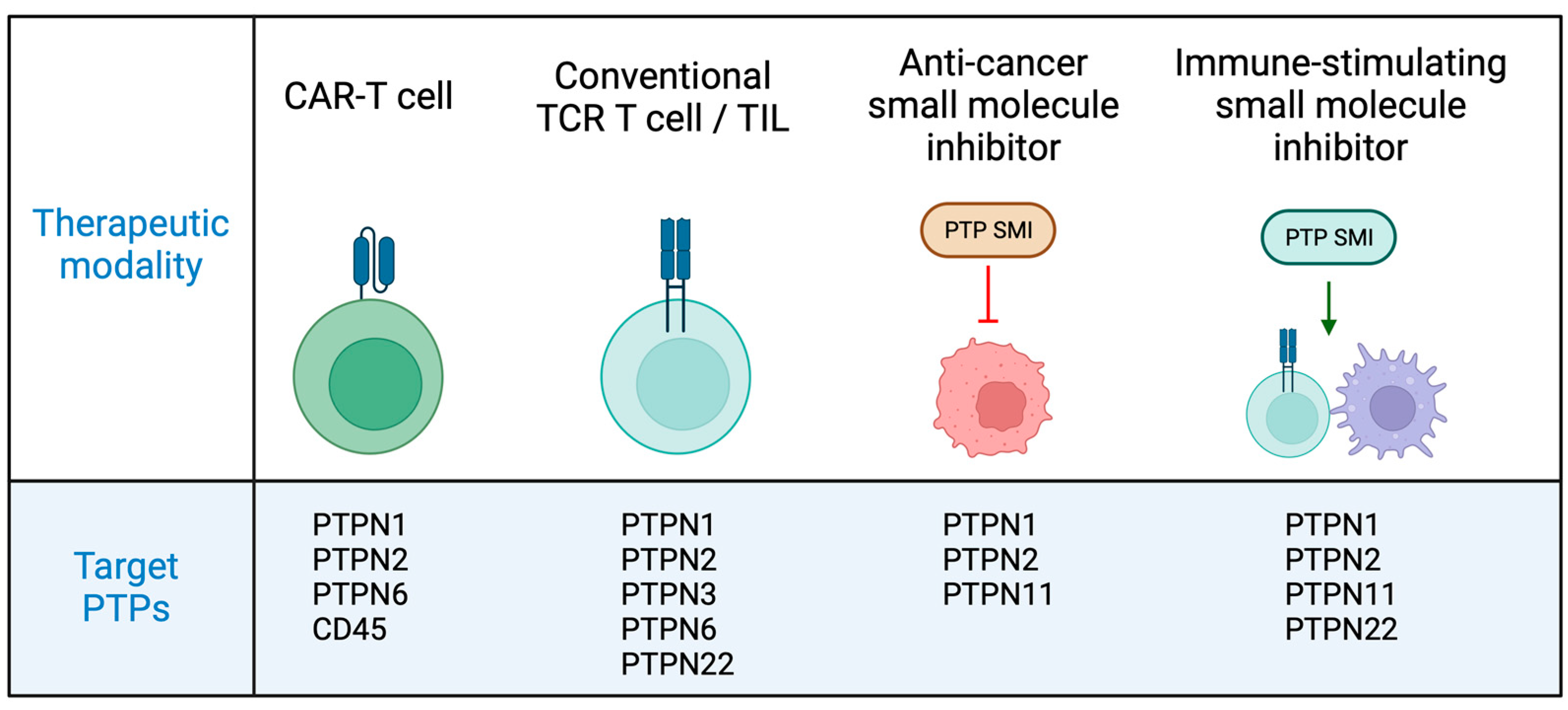
2.3. PTPN1 and PTPN2 as Targets in T Cell Cancer Immunotherapies
2.4. Dual PTPN1-PTPN2 Inhibitors as Cancer Therapeutics
3. PTPN6 and PTPN11
3.1. Roles of PTPN6 and PTPN11 in Cell Signalling
3.2. PTPN11 Is an Oncogenic Phosphatase
3.3. PTPN6 Acts as Tumour Suppressor
3.4. Role of PTPN6 and PTPN11 in PD-1 Signalling and Function
3.5. Targeting PTPN6 to Enhance T Cell Immune Responses to Cancer
4. PTPN22
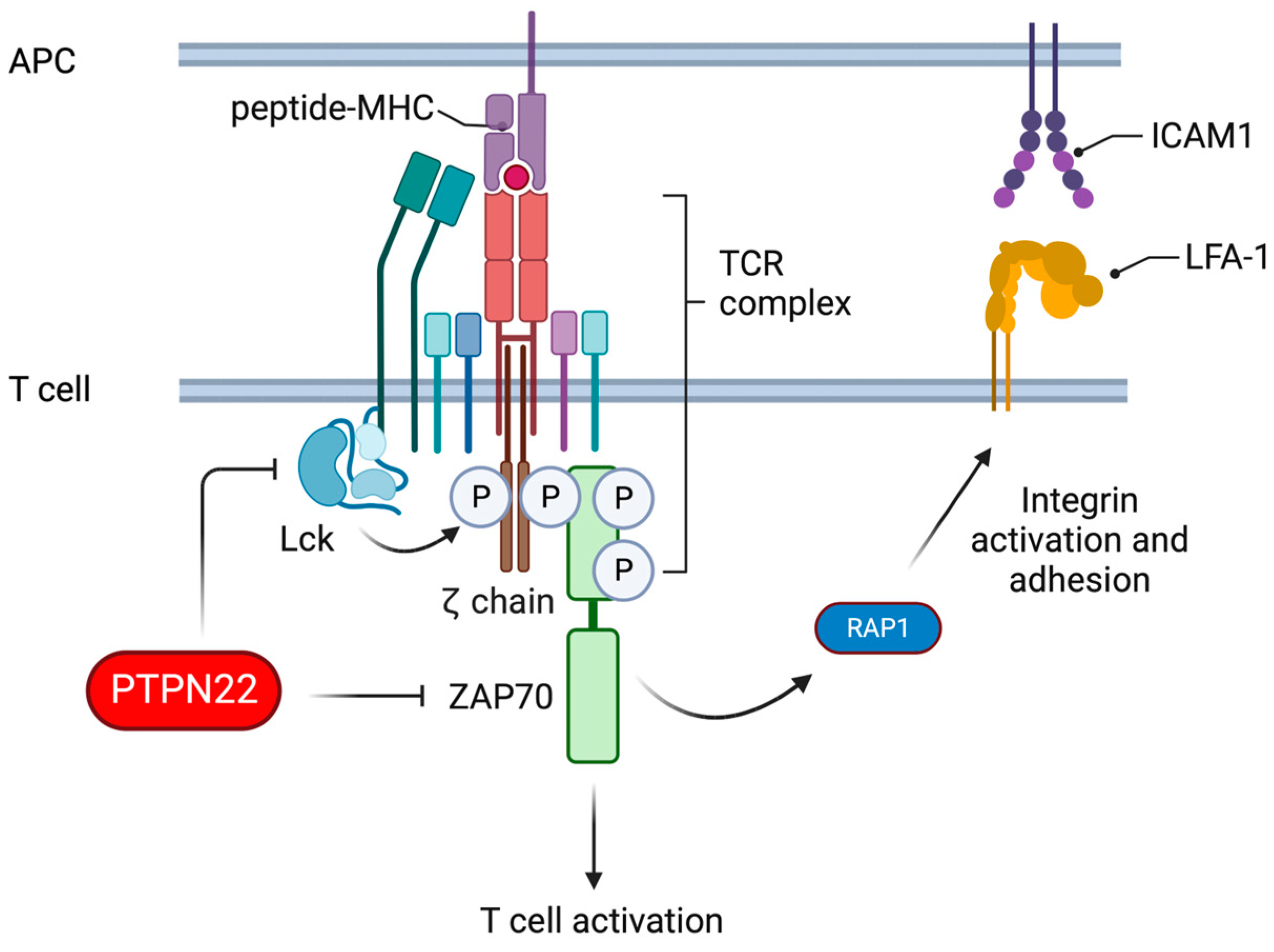
4.1. Roles of PTPN22 in T Cell Signalling
4.2. Deletion of PTPN22 Improves T Cell Responses to Cancer
4.3. PTPN22 Is a Druggable Target
5. Roles for Other Tyrosine Phosphatases in Cancer Biology and Tumour Immunity
5.1. PTPN3, PTPN4 and PTPN13
5.2. PTPN12
5.3. CD45
6. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jenkins, R.W.; Fisher, D.E. Treatment of Advanced Melanoma in 2020 and Beyond. J. Investig. Dermatol. 2021, 141, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.W.; Bell, R.B.; Bifulco, C.B.; Burtness, B.; Gillison, M.L.; Harrington, K.J.; Le, Q.T.; Lee, N.Y.; Leidner, R.; Lewis, R.L.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer 2019, 7, 184. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Perez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Atkins, M.B.; McDermott, D.F. Checkpoint inhibitor immunotherapy in kidney cancer. Nat. Rev. Urol. 2020, 17, 137–150. [Google Scholar] [CrossRef]
- Ghatalia, P.; Zibelman, M.; Geynisman, D.M.; Plimack, E. Approved checkpoint inhibitors in bladder cancer: Which drug should be used when? Ther. Adv. Med. Oncol. 2018, 10, 1758835918788310. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Tautz, L.; Critton, D.A.; Grotegut, S. Protein tyrosine phosphatases: Structure, function, and implication in human disease. Methods Mol. Biol. 2013, 1053, 179–221. [Google Scholar] [CrossRef]
- Tonks, N.K. Protein tyrosine phosphatases: From genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006, 7, 833–846. [Google Scholar] [CrossRef]
- Tonks, N.K. Protein tyrosine phosphatases—From housekeeping enzymes to master regulators of signal transduction. FEBS J. 2013, 280, 346–378. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yi, J.S.; Lawan, A.; Min, K.; Bennett, A.M. Mining the function of protein tyrosine phosphatases in health and disease. Semin. Cell Dev. Biol. 2015, 37, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Tiganis, T. PTP1B and TCPTP—Nonredundant phosphatases in insulin signaling and glucose homeostasis. FEBS J. 2013, 280, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Elchebly, M.; Payette, P.; Michaliszyn, E.; Cromlish, W.; Collins, S.; Loy, A.L.; Normandin, D.; Cheng, A.; Himms-Hagen, J.; Chan, C.C.; et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 1999, 283, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- You-Ten, K.E.; Muise, E.S.; Itie, A.; Michaliszyn, E.; Wagner, J.; Jothy, S.; Lapp, W.S.; Tremblay, M.L. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J. Exp. Med. 1997, 186, 683–693. [Google Scholar] [CrossRef]
- Heinonen, K.M.; Nestel, F.P.; Newell, E.W.; Charette, G.; Seemayer, T.A.; Tremblay, M.L.; Lapp, W.S. T-cell protein tyrosine phosphatase deletion results in progressive systemic inflammatory disease. Blood 2004, 103, 3457–3464. [Google Scholar] [CrossRef] [PubMed]
- Wiede, F.; Shields, B.J.; Chew, S.H.; Kyparissoudis, K.; van Vliet, C.; Galic, S.; Tremblay, M.L.; Russell, S.M.; Godfrey, D.I.; Tiganis, T. T cell protein tyrosine phosphatase attenuates T cell signaling to maintain tolerance in mice. J. Clin. Investig. 2011, 121, 4758–4774. [Google Scholar] [CrossRef]
- Wiede, F.; La Gruta, N.L.; Tiganis, T. PTPN2 attenuates T-cell lymphopenia-induced proliferation. Nat. Commun. 2014, 5, 3073. [Google Scholar] [CrossRef]
- Todd, J.A.; Walker, N.M.; Cooper, J.D.; Smyth, D.J.; Downes, K.; Plagnol, V.; Bailey, R.; Nejentsev, S.; Field, S.F.; Payne, F.; et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat. Genet. 2007, 39, 857–864. [Google Scholar] [CrossRef]
- Bentires-Alj, M.; Neel, B.G. Protein-tyrosine phosphatase 1B is required for HER2/Neu-induced breast cancer. Cancer Res. 2007, 67, 2420–2424. [Google Scholar] [CrossRef]
- Krishnan, N.; Koveal, D.; Miller, D.H.; Xue, B.; Akshinthala, S.D.; Kragelj, J.; Jensen, M.R.; Gauss, C.M.; Page, R.; Blackledge, M.; et al. Targeting the disordered C terminus of PTP1B with an allosteric inhibitor. Nat. Chem. Biol. 2014, 10, 558–566. [Google Scholar] [CrossRef]
- Le Sommer, S.; Morrice, N.; Pesaresi, M.; Thompson, D.; Vickers, M.A.; Murray, G.I.; Mody, N.; Neel, B.G.; Bence, K.K.; Wilson, H.M.; et al. Deficiency in Protein Tyrosine Phosphatase PTP1B Shortens Lifespan and Leads to Development of Acute Leukemia. Cancer Res. 2018, 78, 75–87. [Google Scholar] [CrossRef]
- Kleppe, M.; Lahortiga, I.; El Chaar, T.; De Keersmaecker, K.; Mentens, N.; Graux, C.; Van Roosbroeck, K.; Ferrando, A.A.; Langerak, A.W.; Meijerink, J.P.; et al. Deletion of the protein tyrosine phosphatase gene PTPN2 in T-cell acute lymphoblastic leukemia. Nat. Genet. 2010, 42, 530–535. [Google Scholar] [CrossRef]
- Kleppe, M.; Soulier, J.; Asnafi, V.; Mentens, N.; Hornakova, T.; Knoops, L.; Constantinescu, S.; Sigaux, F.; Meijerink, J.P.; Vandenberghe, P.; et al. PTPN2 negatively regulates oncogenic JAK1 in T-cell acute lymphoblastic leukemia. Blood 2011, 117, 7090–7098. [Google Scholar] [CrossRef] [PubMed]
- Shields, B.J.; Wiede, F.; Gurzov, E.N.; Wee, K.; Hauser, C.; Zhu, H.J.; Molloy, T.J.; O’Toole, S.A.; Daly, R.J.; Sutherland, R.L.; et al. TCPTP regulates SFK and STAT3 signaling and is lost in triple-negative breast cancers. Mol. Cell Biol. 2013, 33, 557–570. [Google Scholar] [CrossRef]
- Karaca Atabay, E.; Mecca, C.; Wang, Q.; Ambrogio, C.; Mota, I.; Prokoph, N.; Mura, G.; Martinengo, C.; Patrucco, E.; Leonardi, G.; et al. Tyrosine phosphatases regulate resistance to ALK inhibitors in ALK+ anaplastic large cell lymphoma. Blood 2022, 139, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Voena, C.; Conte, C.; Ambrogio, C.; Boeri Erba, E.; Boccalatte, F.; Mohammed, S.; Jensen, O.N.; Palestro, G.; Inghirami, G.; Chiarle, R. The tyrosine phosphatase Shp2 interacts with NPM-ALK and regulates anaplastic lymphoma cell growth and migration. Cancer Res. 2007, 67, 4278–4286. [Google Scholar] [CrossRef] [PubMed]
- Manguso, R.T.; Pope, H.W.; Zimmer, M.D.; Brown, F.D.; Yates, K.B.; Miller, B.C.; Collins, N.B.; Bi, K.; LaFleur, M.W.; Juneja, V.R.; et al. In Vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature 2017, 547, 413–418. [Google Scholar] [CrossRef]
- Wiede, F.; Lu, K.H.; Du, X.; Liang, S.; Hochheiser, K.; Dodd, G.T.; Goh, P.K.; Kearney, C.; Meyran, D.; Beavis, P.A.; et al. PTPN2 phosphatase deletion in T cells promotes anti-tumour immunity and CAR T-cell efficacy in solid tumours. EMBO J. 2020, 39, e103637. [Google Scholar] [CrossRef]
- Salmond, R.J. Unleashing T cell responses to cancer through removal of intracellular checkpoints. Immunol. Cell Biol. 2022, 100, 18–20. [Google Scholar] [CrossRef]
- Wiede, F.; Lu, K.H.; Du, X.; Zeissig, M.N.; Xu, R.; Goh, P.K.; Xirouchaki, C.E.; Hogarth, S.J.; Greatorex, S.; Sek, K.; et al. PTP1B Is an Intracellular Checkpoint that Limits T-cell and CAR T-cell Antitumor Immunity. Cancer Discov. 2022, 12, 752–773. [Google Scholar] [CrossRef]
- Zhu, Z.; Tang, R.; Huff, S.; Kummetha, I.R.; Wang, L.; Li, N.; Rana, T.M. Small-molecule PTPN2 Inhibitors Sensitize Resistant Melanoma to Anti-PD-1 Immunotherapy. Cancer Res. Commun. 2023, 3, 119–129. [Google Scholar] [CrossRef]
- Baumgartner, C.K.; Ebrahimi-Nik, H.; Iracheta-Vellve, A.; Hamel, K.M.; Olander, K.E.; Davis, T.G.R.; McGuire, K.A.; Halvorsen, G.T.; Avila, O.I.; Patel, C.H.; et al. The PTPN2/PTPN1 inhibitor ABBV-CLS-484 unleashes potent anti-tumour immunity. Nature 2023, 622, 850–862. [Google Scholar] [CrossRef]
- Liang, S.; Tran, E.; Du, X.; Dong, J.; Sudholz, H.; Chen, H.; Qu, Z.; Huntington, N.D.; Babon, J.J.; Kershaw, N.J.; et al. A small molecule inhibitor of PTP1B and PTPN2 enhances T cell anti-tumor immunity. Nat. Commun. 2023, 14, 4524. [Google Scholar] [CrossRef]
- Neel, B.G.; Gu, H.; Pao, L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 2003, 28, 284–293. [Google Scholar] [CrossRef]
- Salmond, R.J.; Alexander, D.R. SHP2 forecast for the immune system: Fog gradually clearing. Trends Immunol. 2006, 27, 154–160. [Google Scholar] [CrossRef]
- Abram, C.L.; Roberge, G.L.; Pao, L.I.; Neel, B.G.; Lowell, C.A. Distinct roles for neutrophils and dendritic cells in inflammation and autoimmunity in motheaten mice. Immunity 2013, 38, 489–501. [Google Scholar] [CrossRef]
- Johnson, D.J.; Pao, L.I.; Dhanji, S.; Murakami, K.; Ohashi, P.S.; Neel, B.G. Shp1 regulates T cell homeostasis by limiting IL-4 signals. J. Exp. Med. 2013, 210, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Pao, L.I.; Lam, K.P.; Henderson, J.M.; Kutok, J.L.; Alimzhanov, M.; Nitschke, L.; Thomas, M.L.; Neel, B.G.; Rajewsky, K. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity 2007, 27, 35–48. [Google Scholar] [CrossRef]
- Chiang, G.G.; Sefton, B.M. Specific dephosphorylation of the Lck tyrosine protein kinase at Tyr-394 by the SHP-1 protein-tyrosine phosphatase. J. Biol. Chem. 2001, 276, 23173–23178. [Google Scholar] [CrossRef]
- Saxton, T.M.; Henkemeyer, M.; Gasca, S.; Shen, R.; Rossi, D.J.; Shalaby, F.; Feng, G.S.; Pawson, T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997, 16, 2352–2364. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.K.; Nguyen, S.; Chen, J.; Feng, G.S. Requirement of Shp-2 tyrosine phosphatase in lymphoid and hematopoietic cell development. Blood 2001, 97, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, M.; Gelb, B.D. Noonan syndrome and related disorders: Genetics and pathogenesis. Annu. Rev. Genom. Hum. Genet. 2005, 6, 45–68. [Google Scholar] [CrossRef]
- Tartaglia, M.; Mehler, E.L.; Goldberg, R.; Zampino, G.; Brunner, H.G.; Kremer, H.; van der Burgt, I.; Crosby, A.H.; Ion, A.; Jeffery, S.; et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat. Genet. 2001, 29, 465–468. [Google Scholar] [CrossRef]
- Bentires-Alj, M.; Paez, J.G.; David, F.S.; Keilhack, H.; Halmos, B.; Naoki, K.; Maris, J.M.; Richardson, A.; Bardelli, A.; Sugarbaker, D.J.; et al. Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 2004, 64, 8816–8820. [Google Scholar] [CrossRef]
- Loh, M.L.; Reynolds, M.G.; Vattikuti, S.; Gerbing, R.B.; Alonzo, T.A.; Carlson, E.; Cheng, J.W.; Lee, C.M.; Lange, B.J.; Meshinchi, S.; et al. PTPN11 mutations in pediatric patients with acute myeloid leukemia: Results from the Children’s Cancer Group. Leukemia 2004, 18, 1831–1834. [Google Scholar] [CrossRef]
- Loh, M.L.; Vattikuti, S.; Schubbert, S.; Reynolds, M.G.; Carlson, E.; Lieuw, K.H.; Cheng, J.W.; Lee, C.M.; Stokoe, D.; Bonifas, J.M.; et al. Mutations in PTPN11 implicate the SHP-2 phosphatase in leukemogenesis. Blood 2004, 103, 2325–2331. [Google Scholar] [CrossRef]
- Tartaglia, M.; Niemeyer, C.M.; Fragale, A.; Song, X.; Buechner, J.; Jung, A.; Hahlen, K.; Hasle, H.; Licht, J.D.; Gelb, B.D. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat. Genet. 2003, 34, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Fedele, C.; Li, S.; Teng, K.W.; Foster, C.J.R.; Peng, D.; Ran, H.; Mita, P.; Geer, M.J.; Hattori, T.; Koide, A.; et al. SHP2 inhibition diminishes KRASG12C cycling and promotes tumor microenvironment remodeling. J. Exp. Med. 2021, 218, e20201414. [Google Scholar] [CrossRef] [PubMed]
- Ruess, D.A.; Heynen, G.J.; Ciecielski, K.J.; Ai, J.; Berninger, A.; Kabacaoglu, D.; Gorgulu, K.; Dantes, Z.; Wormann, S.M.; Diakopoulos, K.N.; et al. Mutant KRAS-driven cancers depend on PTPN11/SHP2 phosphatase. Nat. Med. 2018, 24, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Sharma, M.R.; Johnson, M.L.; Yap, T.A.; Gadgeel, S.; Nepert, D.; Feng, G.; Reddy, M.B.; Harney, A.S.; Elsayed, M.; et al. SHP2 Inhibition Sensitizes Diverse Oncogene-Addicted Solid Tumors to Re-treatment with Targeted Therapy. Cancer Discov. 2023, 13, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.T.; Bivona, T.G. Inhibition of SHP2 as an approach to block RAS-driven cancers. Adv. Cancer Res. 2022, 153, 205–236. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.L.; Haderk, F.; Bivona, T.G. Allosteric SHP2 inhibitors in cancer: Targeting the intersection of RAS, resistance, and the immune microenvironment. Curr. Opin. Chem. Biol. 2021, 62, 1–12. [Google Scholar] [CrossRef]
- Quintana, E.; Schulze, C.J.; Myers, D.R.; Choy, T.J.; Mordec, K.; Wildes, D.; Shifrin, N.T.; Belwafa, A.; Koltun, E.S.; Gill, A.L.; et al. Allosteric Inhibition of SHP2 Stimulates Antitumor Immunity by Transforming the Immunosuppressive Environment. Cancer Res. 2020, 80, 2889–2902. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.; Li, S.; Khodadadi-Jamayran, A.; Jen, J.; Han, H.; Guidry, K.; Chen, T.; Hao, Y.; Fedele, C.; Zebala, J.A.; et al. Combined Inhibition of SHP2 and CXCR1/2 Promotes Antitumor T-cell Response in NSCLC. Cancer Discov. 2022, 12, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Christofides, A.; Katopodi, X.L.; Cao, C.; Karagkouni, D.; Aliazis, K.; Yenyuwadee, S.; Aksoylar, H.I.; Pal, R.; Mahmoud, M.A.A.; Strauss, L.; et al. SHP-2 and PD-1-SHP-2 signaling regulate myeloid cell differentiation and antitumor responses. Nat. Immunol. 2023, 24, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Rota, G.; Niogret, C.; Dang, A.T.; Barros, C.R.; Fonta, N.P.; Alfei, F.; Morgado, L.; Zehn, D.; Birchmeier, W.; Vivier, E.; et al. Shp-2 Is Dispensable for Establishing T Cell Exhaustion and for PD-1 Signaling In Vivo. Cell Rep. 2018, 23, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Ouchida, M.; Koyama, M.; Ogama, Y.; Takada, S.; Nakatani, Y.; Tanaka, T.; Yoshino, T.; Hayashi, K.; Ohara, N.; et al. Gene silencing of the tyrosine phosphatase SHP1 gene by aberrant methylation in leukemias/lymphomas. Cancer Res. 2002, 62, 6390–6394. [Google Scholar] [PubMed]
- Chim, C.S.; Fung, T.K.; Cheung, W.C.; Liang, R.; Kwong, Y.L. SOCS1 and SHP1 hypermethylation in multiple myeloma: Implications for epigenetic activation of the Jak/STAT pathway. Blood 2004, 103, 4630–4635. [Google Scholar] [CrossRef] [PubMed]
- Chim, C.S.; Wong, K.Y.; Loong, F.; Srivastava, G. SOCS1 and SHP1 hypermethylation in mantle cell lymphoma and follicular lymphoma: Implications for epigenetic activation of the Jak/STAT pathway. Leukemia 2004, 18, 356–358. [Google Scholar] [CrossRef][Green Version]
- Varone, A.; Spano, D.; Corda, D. Shp1 in Solid Cancers and Their Therapy. Front. Oncol. 2020, 10, 935. [Google Scholar] [CrossRef]
- Wen, L.Z.; Ding, K.; Wang, Z.R.; Ding, C.H.; Lei, S.J.; Liu, J.P.; Yin, C.; Hu, P.F.; Ding, J.; Chen, W.S.; et al. SHP-1 Acts as a Tumor Suppressor in Hepatocarcinogenesis and HCC Progression. Cancer Res. 2018, 78, 4680–4691. [Google Scholar] [CrossRef]
- Fan, L.C.; Shiau, C.W.; Tai, W.T.; Hung, M.H.; Chu, P.Y.; Hsieh, F.S.; Lin, H.; Yu, H.C.; Chen, K.F. SHP-1 is a negative regulator of epithelial-mesenchymal transition in hepatocellular carcinoma. Oncogene 2015, 34, 5252–5263. [Google Scholar] [CrossRef] [PubMed]
- Chemnitz, J.M.; Parry, R.V.; Nichols, K.E.; June, C.H.; Riley, J.L. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J. Immunol. 2004, 173, 945–954. [Google Scholar] [CrossRef]
- Hui, E.; Cheung, J.; Zhu, J.; Su, X.; Taylor, M.J.; Wallweber, H.A.; Sasmal, D.K.; Huang, J.; Kim, J.M.; Mellman, I.; et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017, 355, 1428–1433. [Google Scholar] [CrossRef]
- Li, J.; Jie, H.B.; Lei, Y.; Gildener-Leapman, N.; Trivedi, S.; Green, T.; Kane, L.P.; Ferris, R.L. PD-1/SHP-2 inhibits Tc1/Th1 phenotypic responses and the activation of T cells in the tumor microenvironment. Cancer Res. 2015, 75, 508–518. [Google Scholar] [CrossRef]
- Fan, Z.; Tian, Y.; Chen, Z.; Liu, L.; Zhou, Q.; He, J.; Coleman, J.; Dong, C.; Li, N.; Huang, J.; et al. Blocking interaction between SHP2 and PD-1 denotes a novel opportunity for developing PD-1 inhibitors. EMBO Mol. Med. 2020, 12, e11571. [Google Scholar] [CrossRef]
- Niogret, C.; Birchmeier, W.; Guarda, G. SHP-2 in Lymphocytes’ Cytokine and Inhibitory Receptor Signaling. Front. Immunol. 2019, 10, 2468. [Google Scholar] [CrossRef] [PubMed]
- Ventura, P.M.O.; Gakovic, M.; Fischer, B.A.; Spinelli, L.; Rota, G.; Pathak, S.; Khameneh, H.J.; Zenobi, A.; Thomson, S.; Birchmeier, W.; et al. Concomitant deletion of Ptpn6 and Ptpn11 in T cells fails to improve anticancer responses. EMBO Rep. 2022, 23, e55399. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Hu, Y.; Zhu, Y.; Wang, X.; Li, W.; Tang, J.; Jia, X.; Wang, J.; Cong, Y.; Quan, M.; et al. SHP-1 Regulates CD8+ T Cell Effector Function but Plays a Subtle Role with SHP-2 in T Cell Exhaustion Due to a Stage-Specific Nonredundant Functional Relay. J. Immunol. 2023, 212, 397–409. [Google Scholar] [CrossRef]
- Mercadante, E.R.; Lorenz, U.M. T Cells Deficient in the Tyrosine Phosphatase SHP-1 Resist Suppression by Regulatory T Cells. J. Immunol. 2017, 199, 129–137. [Google Scholar] [CrossRef]
- Snook, J.P.; Soedel, A.J.; Ekiz, H.A.; O’Connell, R.M.; Williams, M.A. Inhibition of SHP-1 Expands the Repertoire of Antitumor T Cells Available to Respond to Immune Checkpoint Blockade. Cancer Immunol. Res. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Watson, H.A.; Dolton, G.; Ohme, J.; Ladell, K.; Vigar, M.; Wehenkel, S.; Hindley, J.; Mohammed, R.N.; Miners, K.; Luckwell, R.A.; et al. Purity of transferred CD8(+) T cells is crucial for safety and efficacy of combinatorial tumor immunotherapy in the absence of SHP-1. Immunol. Cell Biol. 2016, 94, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Zhong, M.; Long, Y.; Yang, W.; Liu, T.; Huang, X.; Ma, X. CRISPR/Cas9-mediated knockout of intracellular molecule SHP-1 enhances tumor-killing ability of CD133-targeted CAR T cells in vitro. Exp. Hematol. Oncol. 2023, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.K.; Wang, L.C.; Dolfi, D.V.; Wilson, C.B.; Ranganathan, R.; Sun, J.; Kapoor, V.; Scholler, J.; Pure, E.; Milone, M.C.; et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin. Cancer Res. 2014, 20, 4262–4273. [Google Scholar] [CrossRef]
- Velasco Cardenas, R.M.; Brandl, S.M.; Melendez, A.V.; Schlaak, A.E.; Buschky, A.; Peters, T.; Beier, F.; Serrels, B.; Taromi, S.; Raute, K.; et al. Harnessing CD3 diversity to optimize CAR T cells. Nat. Immunol. 2023, 24, 2135–2149. [Google Scholar] [CrossRef]
- Sun, C.; Shou, P.; Du, H.; Hirabayashi, K.; Chen, Y.; Herring, L.E.; Ahn, S.; Xu, Y.; Suzuki, K.; Li, G.; et al. THEMIS-SHP1 Recruitment by 4-1BB Tunes LCK-Mediated Priming of Chimeric Antigen Receptor-Redirected T Cells. Cancer Cell 2020, 37, 216–225.e216. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, J.F.; Veillette, A. Association of inhibitory tyrosine protein kinase p50csk with protein tyrosine phosphatase PEP in T cells and other hemopoietic cells. EMBO J. 1996, 15, 4909–4918. [Google Scholar] [CrossRef]
- Begovich, A.B.; Carlton, V.E.; Honigberg, L.A.; Schrodi, S.J.; Chokkalingam, A.P.; Alexander, H.C.; Ardlie, K.G.; Huang, Q.; Smith, A.M.; Spoerke, J.M.; et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am. J. Hum. Genet. 2004, 75, 330–337. [Google Scholar] [CrossRef]
- Bottini, N.; Musumeci, L.; Alonso, A.; Rahmouni, S.; Nika, K.; Rostamkhani, M.; MacMurray, J.; Meloni, G.F.; Lucarelli, P.; Pellecchia, M.; et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat. Genet. 2004, 36, 337–338. [Google Scholar] [CrossRef]
- Kyogoku, C.; Langefeld, C.D.; Ortmann, W.A.; Lee, A.; Selby, S.; Carlton, V.E.; Chang, M.; Ramos, P.; Baechler, E.C.; Batliwalla, F.M.; et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am. J. Hum. Genet. 2004, 75, 504–507. [Google Scholar] [CrossRef]
- Bottini, N.; Peterson, E.J. Tyrosine phosphatase PTPN22: Multifunctional regulator of immune signaling, development, and disease. Annu. Rev. Immunol. 2014, 32, 83–119. [Google Scholar] [CrossRef]
- Brownlie, R.J.; Zamoyska, R.; Salmond, R.J. Regulation of autoimmune and anti-tumour T-cell responses by PTPN22. Immunology 2018, 154, 377–382. [Google Scholar] [CrossRef]
- Salmond, R.J.; Brownlie, R.J.; Zamoyska, R. Multifunctional roles of the autoimmune disease-associated tyrosine phosphatase PTPN22 in regulating T cell homeostasis. Cell Cycle 2015, 14, 705–711. [Google Scholar] [CrossRef]
- Brownlie, R.J.; Miosge, L.A.; Vassilakos, D.; Svensson, L.M.; Cope, A.; Zamoyska, R. Lack of the phosphatase PTPN22 increases adhesion of murine regulatory T cells to improve their immunosuppressive function. Sci. Signal 2012, 5, ra87. [Google Scholar] [CrossRef]
- Hasegawa, K.; Martin, F.; Huang, G.; Tumas, D.; Diehl, L.; Chan, A.C. PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science 2004, 303, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Salmond, R.J.; Brownlie, R.J.; Morrison, V.L.; Zamoyska, R. The tyrosine phosphatase PTPN22 discriminates weak self peptides from strong agonist TCR signals. Nat. Immunol. 2014, 15, 875–883. [Google Scholar] [CrossRef]
- Anderson, W.; Barahmand-Pour-Whitman, F.; Linsley, P.S.; Cerosaletti, K.; Buckner, J.H.; Rawlings, D.J. PTPN22 R620W gene editing in T cells enhances low-avidity TCR responses. Elife 2023, 12, e81577. [Google Scholar] [CrossRef]
- Bray, C.; Wright, D.; Haupt, S.; Thomas, S.; Stauss, H.; Zamoyska, R. Crispr/Cas Mediated Deletion of PTPN22 in Jurkat T Cells Enhances TCR Signaling and Production of IL-2. Front. Immunol. 2018, 9, 2595. [Google Scholar] [CrossRef]
- Brownlie, R.J.; Garcia, C.; Ravasz, M.; Zehn, D.; Salmond, R.J.; Zamoyska, R. Resistance to TGFbeta suppression and improved anti-tumor responses in CD8(+) T cells lacking PTPN22. Nat. Commun. 2017, 8, 1343. [Google Scholar] [CrossRef] [PubMed]
- Cubas, R.; Khan, Z.; Gong, Q.; Moskalenko, M.; Xiong, H.; Ou, Q.; Pai, C.; Rodriguez, R.; Cheung, J.; Chan, A.C. Autoimmunity linked protein phosphatase PTPN22 as a target for cancer immunotherapy. J. Immunother. Cancer 2020, 8, e001439. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.J.; Croessmann, S.; Lin, J.; Phyo, Z.H.; Charmsaz, S.; Danilova, L.; Mohan, A.A.; Gross, N.E.; Chen, F.; Dong, J.; et al. Systemic inhibition of PTPN22 augments anticancer immunity. J. Clin. Investig. 2021, 131, e127847. [Google Scholar] [CrossRef] [PubMed]
- Orozco, R.C.; Marquardt, K.; Mowen, K.; Sherman, L.A. Proautoimmune Allele of Tyrosine Phosphatase, PTPN22, Enhances Tumor Immunity. J. Immunol. 2021, 207, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Darcy, P.K.; Wiede, F.; Tiganis, T. Targeting Protein Tyrosine Phosphatase 22 Does Not Enhance the Efficacy of Chimeric Antigen Receptor T Cells in Solid Tumors. Mol. Cell Biol. 2022, 42, e0044921. [Google Scholar] [CrossRef]
- Brownlie, R.J.; Wright, D.; Zamoyska, R.; Salmond, R.J. Deletion of PTPN22 improves effector and memory CD8+ T cell responses to tumors. JCI Insight 2019, 5, e127847. [Google Scholar] [CrossRef] [PubMed]
- Teagle, A.R.; Castro-Sanchez, P.; Brownlie, R.J.; Logan, N.; Kapoor, S.S.; Wright, D.; Salmond, R.J.; Zamoyska, R. Deletion of the protein tyrosine phosphatase PTPN22 for adoptive T cell therapy facilitates CTL effector function but promotes T cell exhaustion. J. Immunother. Cancer 2023, 11, e007614. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhao, H.; Du, J.; Li, X.; Li, K.; Zhao, Z.; Bi, W.; Zhang, X.; Yu, D.; Zhang, J.; et al. Discovery of benzofuran-2-carboxylic acid derivatives as lymphoid tyrosine phosphatase (LYP) inhibitors for cancer immunotherapy. Eur. J. Med. Chem. 2023, 258, 115599. [Google Scholar] [CrossRef] [PubMed]
- Bauler, T.J.; Hughes, E.D.; Arimura, Y.; Mustelin, T.; Saunders, T.L.; King, P.D. Normal TCR signal transduction in mice that lack catalytically active PTPN3 protein tyrosine phosphatase. J. Immunol. 2007, 178, 3680–3687. [Google Scholar] [CrossRef] [PubMed]
- Bauler, T.J.; Hendriks, W.J.; King, P.D. The FERM and PDZ domain-containing protein tyrosine phosphatases, PTPN4 and PTPN3, are both dispensable for T cell receptor signal transduction. PLoS ONE 2008, 3, e4014. [Google Scholar] [CrossRef]
- Young, J.A.; Becker, A.M.; Medeiros, J.J.; Shapiro, V.S.; Wang, A.; Farrar, J.D.; Quill, T.A.; Hooft van Huijsduijnen, R.; van Oers, N.S. The protein tyrosine phosphatase PTPN4/PTP-MEG1, an enzyme capable of dephosphorylating the TCR ITAMs and regulating NF-kappaB, is dispensable for T cell development and/or T cell effector functions. Mol. Immunol. 2008, 45, 3756–3766. [Google Scholar] [CrossRef]
- Fujimura, A.; Nakayama, K.; Imaizumi, A.; Kawamoto, M.; Oyama, Y.; Ichimiya, S.; Umebayashi, M.; Koya, N.; Morisaki, T.; Nakagawa, T.; et al. PTPN3 expressed in activated T lymphocytes is a candidate for a non-antibody-type immune checkpoint inhibitor. Cancer Immunol. Immunother. 2019, 68, 1649–1660. [Google Scholar] [CrossRef]
- Koga, S.; Onishi, H.; Masuda, S.; Fujimura, A.; Ichimiya, S.; Nakayama, K.; Imaizumi, A.; Nishiyama, K.; Kojima, M.; Miyoshi, K.; et al. PTPN3 is a potential target for a new cancer immunotherapy that has a dual effect of T cell activation and direct cancer inhibition in lung neuroendocrine tumor. Transl. Oncol. 2021, 14, 101152. [Google Scholar] [CrossRef]
- Iwamoto, N.; Onishi, H.; Masuda, S.; Imaizumi, A.; Sakanashi, K.; Morisaki, S.; Nagao, S.; Koga, S.; Ozono, K.; Umebayashi, M.; et al. PTPN3 inhibition contributes to the activation of the dendritic cell function to be a promising new immunotherapy target. J. Cancer Res. Clin. Oncol. 2023, 149, 14619–14630. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Liu, J.; Cao, J.; Yu, Y.; Zhang, H.; Wang, F.; Zhu, Y.; Xiao, M.; Liu, S.; Ye, Y.; et al. PTPN3 acts as a tumor suppressor and boosts TGF-beta signaling independent of its phosphatase activity. EMBO J. 2019, 38, e99945. [Google Scholar] [CrossRef] [PubMed]
- Kuchay, S.; Duan, S.; Schenkein, E.; Peschiaroli, A.; Saraf, A.; Florens, L.; Washburn, M.P.; Pagano, M. FBXL2- and PTPL1-mediated degradation of p110-free p85beta regulatory subunit controls the PI(3)K signalling cascade. Nat. Cell Biol. 2013, 15, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Carmona, A.; Skowronek, A.; Yu, F.; Collins, M.O.; Naik, S.; Murzeau, C.M.; Tseng, P.L.; Erdmann, K.S. Apoptotic signalling targets the post-endocytic sorting machinery of the death receptor Fas/CD95. Nat. Commun. 2019, 10, 3105. [Google Scholar] [CrossRef] [PubMed]
- McHeik, S.; Aptecar, L.; Coopman, P.; D’Hondt, V.; Freiss, G. Dual Role of the PTPN13 Tyrosine Phosphatase in Cancer. Biomolecules 2020, 10, 1659. [Google Scholar] [CrossRef] [PubMed]
- Glondu-Lassis, M.; Dromard, M.; Lacroix-Triki, M.; Nirde, P.; Puech, C.; Knani, D.; Chalbos, D.; Freiss, G. PTPL1/PTPN13 regulates breast cancer cell aggressiveness through direct inactivation of Src kinase. Cancer Res. 2010, 70, 5116–5126. [Google Scholar] [CrossRef]
- Scrima, M.; De Marco, C.; De Vita, F.; Fabiani, F.; Franco, R.; Pirozzi, G.; Rocco, G.; Malanga, D.; Viglietto, G. The nonreceptor-type tyrosine phosphatase PTPN13 is a tumor suppressor gene in non-small cell lung cancer. Am. J. Pathol. 2012, 180, 1202–1214. [Google Scholar] [CrossRef]
- Moshiri, H.; Cabrera Riofrio, D.A.; Lim, Y.J.; Lauhasurayotin, S.; Manisterski, M.; Elhasid, R.; Bonilla, F.A.; Dhanraj, S.; Armstrong, R.N.; Li, H.; et al. Germline PTPN13 mutations in patients with bone marrow failure and acute lymphoblastic leukemia. Leukemia 2022, 36, 2132–2135. [Google Scholar] [CrossRef]
- Nakahira, M.; Tanaka, T.; Robson, B.E.; Mizgerd, J.P.; Grusby, M.J. Regulation of signal transducer and activator of transcription signaling by the tyrosine phosphatase PTP-BL. Immunity 2007, 26, 163–176. [Google Scholar] [CrossRef]
- Li, J.; Davidson, D.; Martins Souza, C.; Zhong, M.C.; Wu, N.; Park, M.; Muller, W.J.; Veillette, A. Loss of PTPN12 Stimulates Progression of ErbB2-Dependent Breast Cancer by Enhancing Cell Survival, Migration, and Epithelial-to-Mesenchymal Transition. Mol. Cell Biol. 2015, 35, 4069–4082. [Google Scholar] [CrossRef]
- Nair, A.; Chung, H.C.; Sun, T.; Tyagi, S.; Dobrolecki, L.E.; Dominguez-Vidana, R.; Kurley, S.J.; Orellana, M.; Renwick, A.; Henke, D.M.; et al. Combinatorial inhibition of PTPN12-regulated receptors leads to a broadly effective therapeutic strategy in triple-negative breast cancer. Nat. Med. 2018, 24, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Aceto, N.; Meerbrey, K.L.; Kessler, J.D.; Zhou, C.; Migliaccio, I.; Nguyen, D.X.; Pavlova, N.N.; Botero, M.; Huang, J.; et al. Activation of multiple proto-oncogenic tyrosine kinases in breast cancer via loss of the PTPN12 phosphatase. Cell 2011, 144, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Taylor, P.; Andrade, J.; Ueberheide, B.; Shuch, B.; Glazer, P.M.; Bindra, R.S.; Moran, M.F.; Linehan, W.M.; Neel, B.G. Pathologic Oxidation of PTPN12 Underlies ABL1 Phosphorylation in Hereditary Leiomyomatosis and Renal Cell Carcinoma. Cancer Res. 2018, 78, 6539–6548. [Google Scholar] [CrossRef]
- Davidson, D.; Veillette, A. PTP-PEST, a scaffold protein tyrosine phosphatase, negatively regulates lymphocyte activation by targeting a unique set of substrates. EMBO J. 2001, 20, 3414–3426. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.; Shi, X.; Zhong, M.C.; Rhee, I.; Veillette, A. The phosphatase PTP-PEST promotes secondary T cell responses by dephosphorylating the protein tyrosine kinase Pyk2. Immunity 2010, 33, 167–180. [Google Scholar] [CrossRef]
- Rheinlander, A.; Schraven, B.; Bommhardt, U. CD45 in human physiology and clinical medicine. Immunol. Lett. 2018, 196, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.; Gamble, J.; Tooze, R.; Higgins, D.; Yang, F.T.; O’Brien, P.C.; Coleman, N.; Pingel, S.; Turner, M.; Alexander, D.R. Development of T-leukaemias in CD45 tyrosine phosphatase-deficient mutant lck mice. EMBO J. 2000, 19, 4644–4654. [Google Scholar] [CrossRef]
- Kresinsky, A.; Schnoder, T.M.; Jacobsen, I.D.; Rauner, M.; Hofbauer, L.C.; Ast, V.; Konig, R.; Hoffmann, B.; Svensson, C.M.; Figge, M.T.; et al. Lack of CD45 in FLT3-ITD mice results in a myeloproliferative phenotype, cortical porosity, and ectopic bone formation. Oncogene 2019, 38, 4773–4787. [Google Scholar] [CrossRef]
- Porcu, M.; Kleppe, M.; Gianfelici, V.; Geerdens, E.; De Keersmaecker, K.; Tartaglia, M.; Foa, R.; Soulier, J.; Cauwelier, B.; Uyttebroeck, A.; et al. Mutation of the receptor tyrosine phosphatase PTPRC (CD45) in T-cell acute lymphoblastic leukemia. Blood 2012, 119, 4476–4479. [Google Scholar] [CrossRef]
- Chang, V.T.; Fernandes, R.A.; Ganzinger, K.A.; Lee, S.F.; Siebold, C.; McColl, J.; Jonsson, P.; Palayret, M.; Harlos, K.; Coles, C.H.; et al. Initiation of T cell signaling by CD45 segregation at ‘close contacts’. Nat. Immunol. 2016, 17, 574–582. [Google Scholar] [CrossRef]
- Courtney, A.H.; Shvets, A.A.; Lu, W.; Griffante, G.; Mollenauer, M.; Horkova, V.; Lo, W.L.; Yu, S.; Stepanek, O.; Chakraborty, A.K.; et al. CD45 functions as a signaling gatekeeper in T cells. Sci. Signal 2019, 12, eaaw8151. [Google Scholar] [CrossRef]
- D’Oro, U.; Ashwell, J.D. Cutting edge: The CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. J. Immunol. 1999, 162, 1879–1883. [Google Scholar] [CrossRef] [PubMed]
- McNeill, L.; Salmond, R.J.; Cooper, J.C.; Carret, C.K.; Cassady-Cain, R.L.; Roche-Molina, M.; Tandon, P.; Holmes, N.; Alexander, D.R. The differential regulation of Lck kinase phosphorylation sites by CD45 is critical for T cell receptor signaling responses. Immunity 2007, 27, 425–437. [Google Scholar] [CrossRef]
- Zikherman, J.; Jenne, C.; Watson, S.; Doan, K.; Raschke, W.; Goodnow, C.C.; Weiss, A. CD45-Csk phosphatase-kinase titration uncouples basal and inducible T cell receptor signaling during thymic development. Immunity 2010, 32, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhang, X.; Tu, L.; Cao, J.; Hinrichs, C.S.; Su, X. Size-dependent activation of CAR-T cells. Sci. Immunol. 2022, 7, eabl3995. [Google Scholar] [CrossRef] [PubMed]
- Brana, I.; Shapiro, G.; Johnson, M.L.; Yu, H.A.; Robbrecht, D.; Tan, D.S.W.; Siu, L.L.; Minami, H.; Steeghs, N.; Hengelage, T.; et al. Initial results from a dose finding study of TNO155, a SHP2 inhibitor, in adults with advanced solid tumors. J. Clin. Oncol. 2021, 39, 3005. [Google Scholar] [CrossRef]
- Liu, R.; Mathieu, C.; Berthelet, J.; Zhang, W.; Dupret, J.M.; Rodrigues Lima, F. Human Protein Tyrosine Phosphatase 1B (PTP1B): From structure to clinical inhibitor perspectives. Int. J. Mol. Sci. 2022, 23, 7027. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, H.; Zhao, Z.; Huang, M.; Zhan, J. Status of research on natural protein tyrosine phosphatase 1B inhibitors as potential antidiabetic agents: Update. Biomed. Pharmacother. 2023, 157, 113990. [Google Scholar] [CrossRef]
| Inhibitor (Mechanism) | Target PTP(s) | Stage of Development | Cancers Targeted |
|---|---|---|---|
| MSI-1436 (allosteric) | PTPN1 | Phase I * | Metastatic breast cancer |
| Compound 8 (a.s.) | PTPN2 | Pre-clinical | Mouse models |
| PTP9 (a.s.) | PTPN2 | Pre-clinical | Mouse models |
| ABBV-CLS-484 (a.s.) | PTPN1/PTPN2 | Phase I | Locally advanced/metastatic solid tumours |
| Compound 182 (a.s.) | PTPN1/PTPN2 | Pre-clinical | Mouse models |
| TNO155 (allosteric) | PTPN11 | Phase I/II | Advanced solid tumours |
| PF-07284892 (allosteric) | PTPN11 | Phase I | Advanced solid tumours |
| RMC-4630 (allosteric) | PTPN11 | Phase I ** | Metastatic KRAS mutant tumours |
| BBP-398 (allosteric) | PTPN11 | Phase I | Advanced solid tumours with KRAS-G12C |
| JAB-3068 (allosteric) | PTPN11 | Phase I | Advanced solid tumours |
| JAB-3312 (allosteric) | PTPN11 | Phase I/IIa | Advanced solid tumours with KRAS-G12C |
| RLY-1971 (allosteric) | PTPN11 | Phase I *** | Advanced/metastatic solid tumours |
| HBI-2376 (allosteric) | PTPN11 | Phase I ** | Advanced solid tumours |
| ET0038 (allosteric) | PTPN11 | Phase I | Advanced solid tumours |
| ERAS-601 (allosteric) | PTPN11 | Phase I/Ib | Advanced solid tumours |
| BR790 (allosteric) | PTPN11 | Phase I/IIa | Advanced solid tumours |
| L-1 (a.s.) | PTPN22 | Pre-clinical | Mouse models |
| D14/D34 (a.s.) | PTPN22 | Pre-clinical | Mouse models |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmond, R.J. Targeting Protein Tyrosine Phosphatases to Improve Cancer Immunotherapies. Cells 2024, 13, 231. https://doi.org/10.3390/cells13030231
Salmond RJ. Targeting Protein Tyrosine Phosphatases to Improve Cancer Immunotherapies. Cells. 2024; 13(3):231. https://doi.org/10.3390/cells13030231
Chicago/Turabian StyleSalmond, Robert J. 2024. "Targeting Protein Tyrosine Phosphatases to Improve Cancer Immunotherapies" Cells 13, no. 3: 231. https://doi.org/10.3390/cells13030231
APA StyleSalmond, R. J. (2024). Targeting Protein Tyrosine Phosphatases to Improve Cancer Immunotherapies. Cells, 13(3), 231. https://doi.org/10.3390/cells13030231




