Nuclear Factor-κB Signaling Regulates the Nociceptin Receptor but Not Nociceptin Itself
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.1.1. Dose–Response Experiments
2.1.2. Co-Stimulation of THP-1 with PMA and Cytokines
2.1.3. Inhibition Experiments
2.2. RNA Isolation, cDNA Synthesis, and Relative Quantification
2.3. Flow Cytometry
2.3.1. Measurement of Cell Membrane NOP
2.3.2. Measurement of Intracellular Nociceptin
2.3.3. Measurement of Phosphorylated NFκB/p65
2.4. Statistical Analysis
3. Results
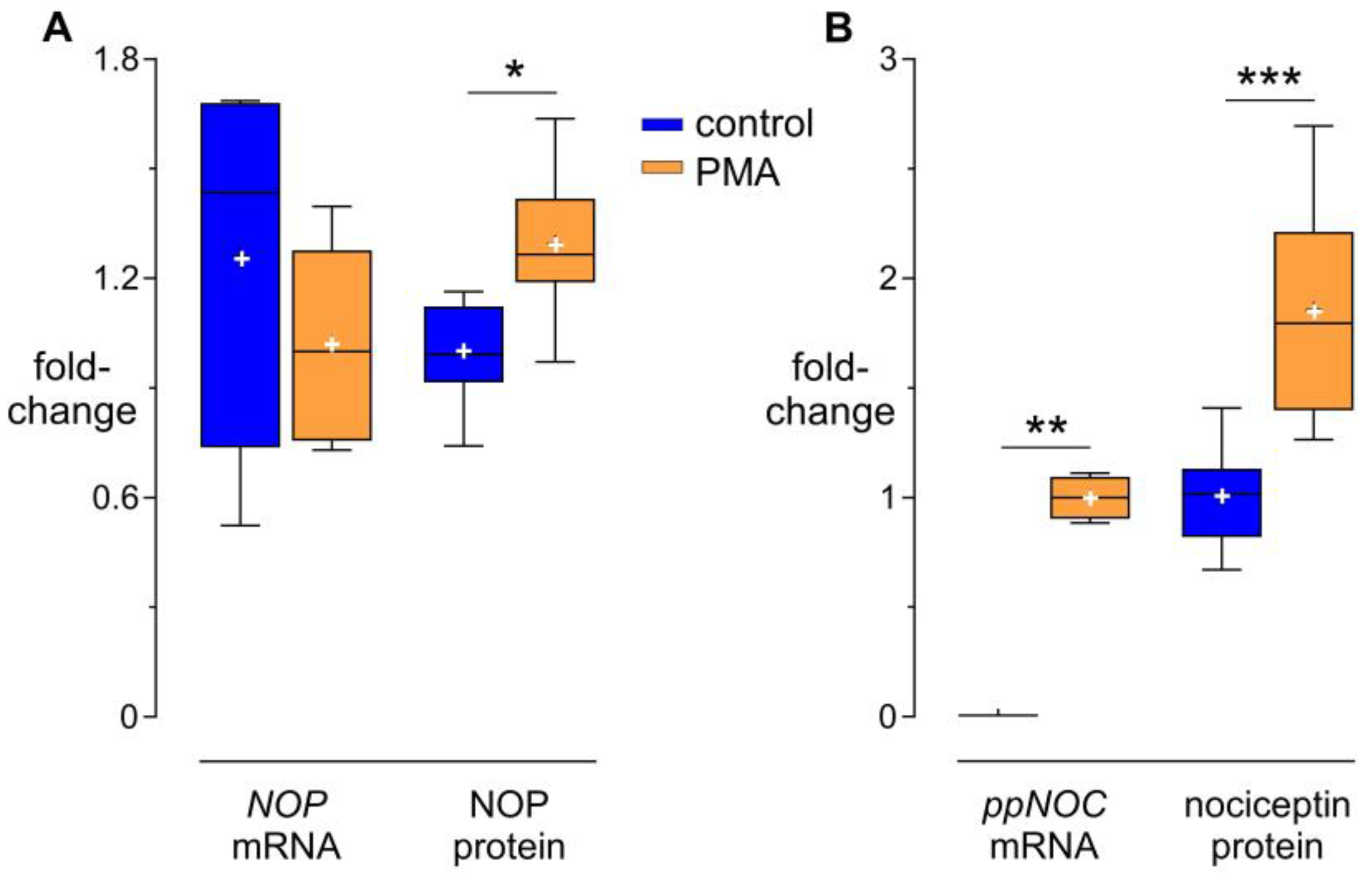
3.1. Modulation of NOP and Nociceptin by PMA
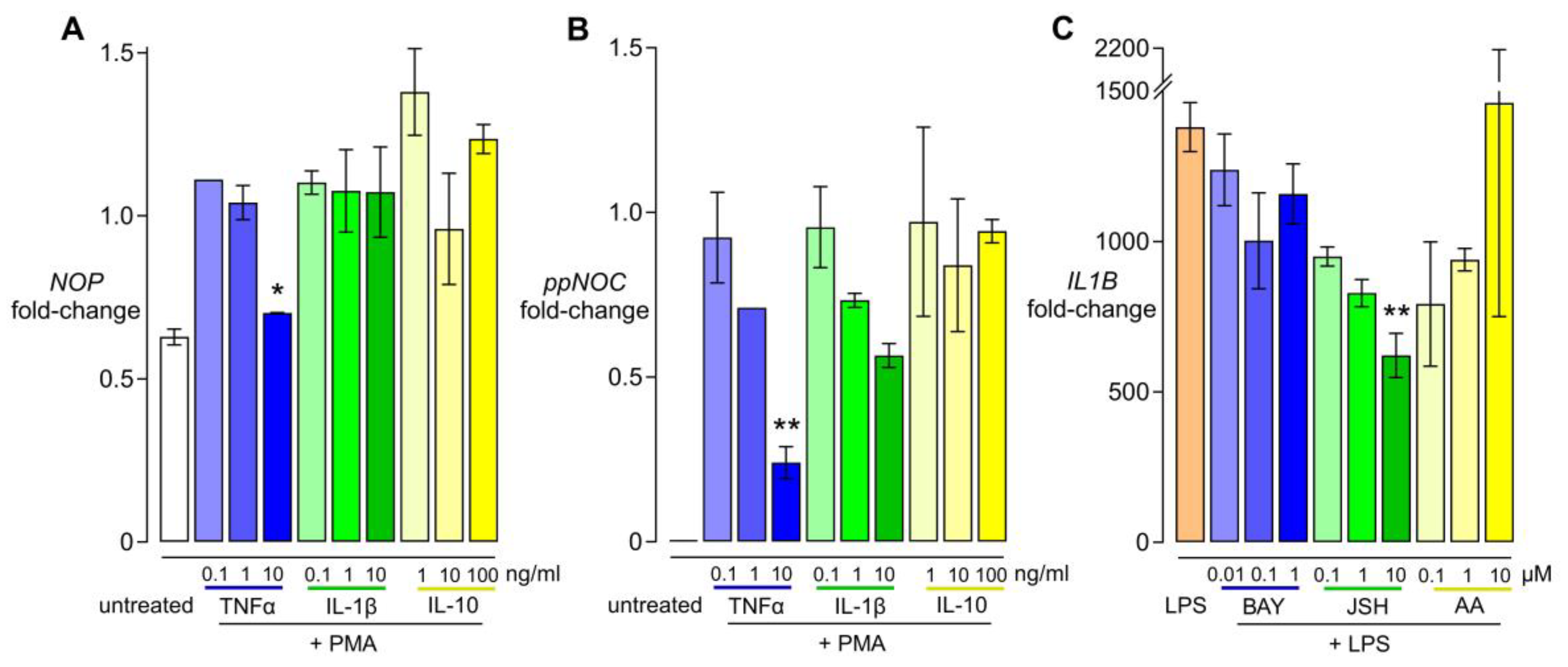
3.2. Dose-Dependent Effects of Cytokines and NFκB Inhibitors
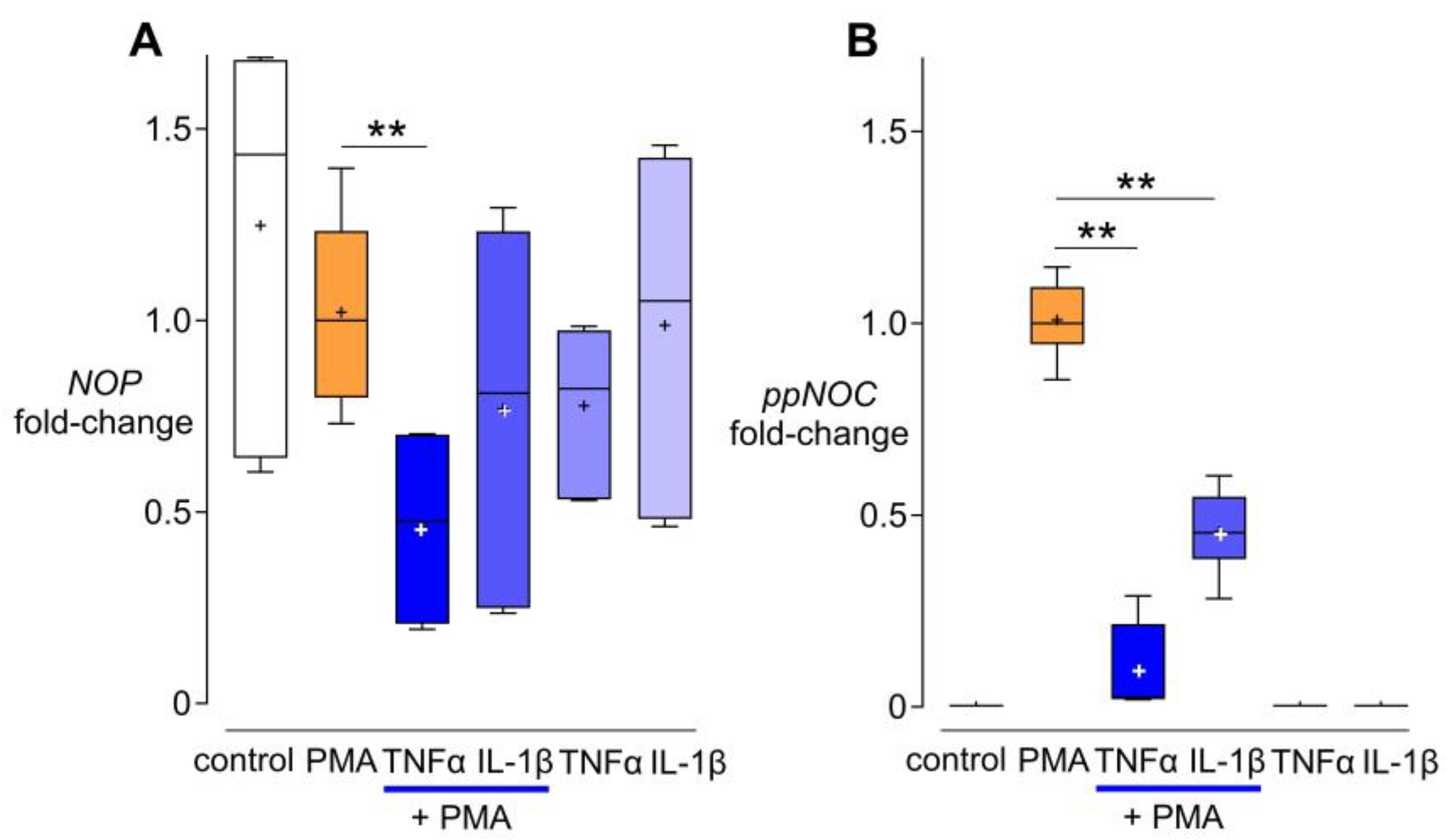
3.3. Effects of TNF-α and IL-1β on NOP and ppNOC mRNA
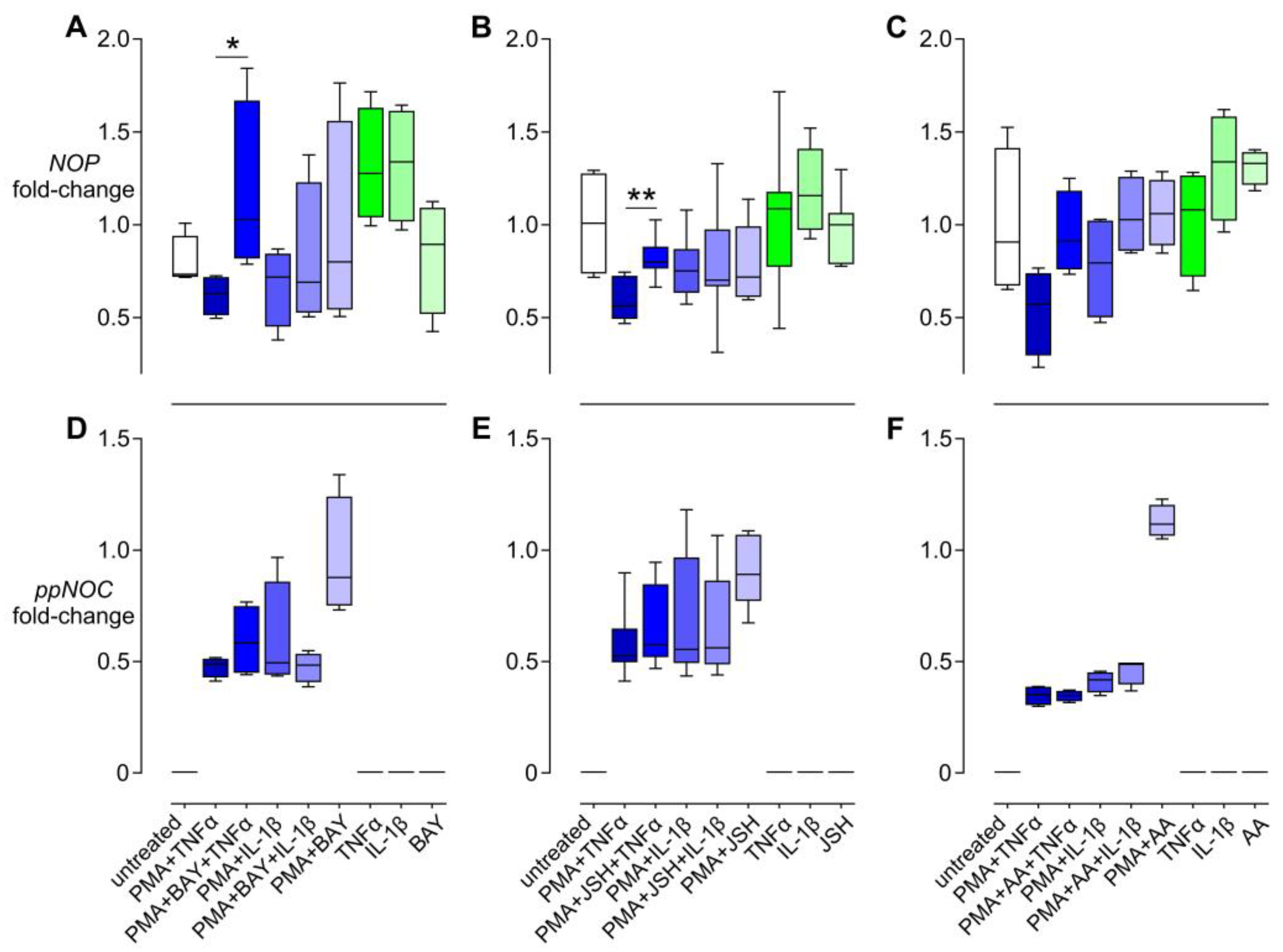
3.4. Effects of NFκB Inhibitors on the Regulation of NOP and ppNOC mRNA
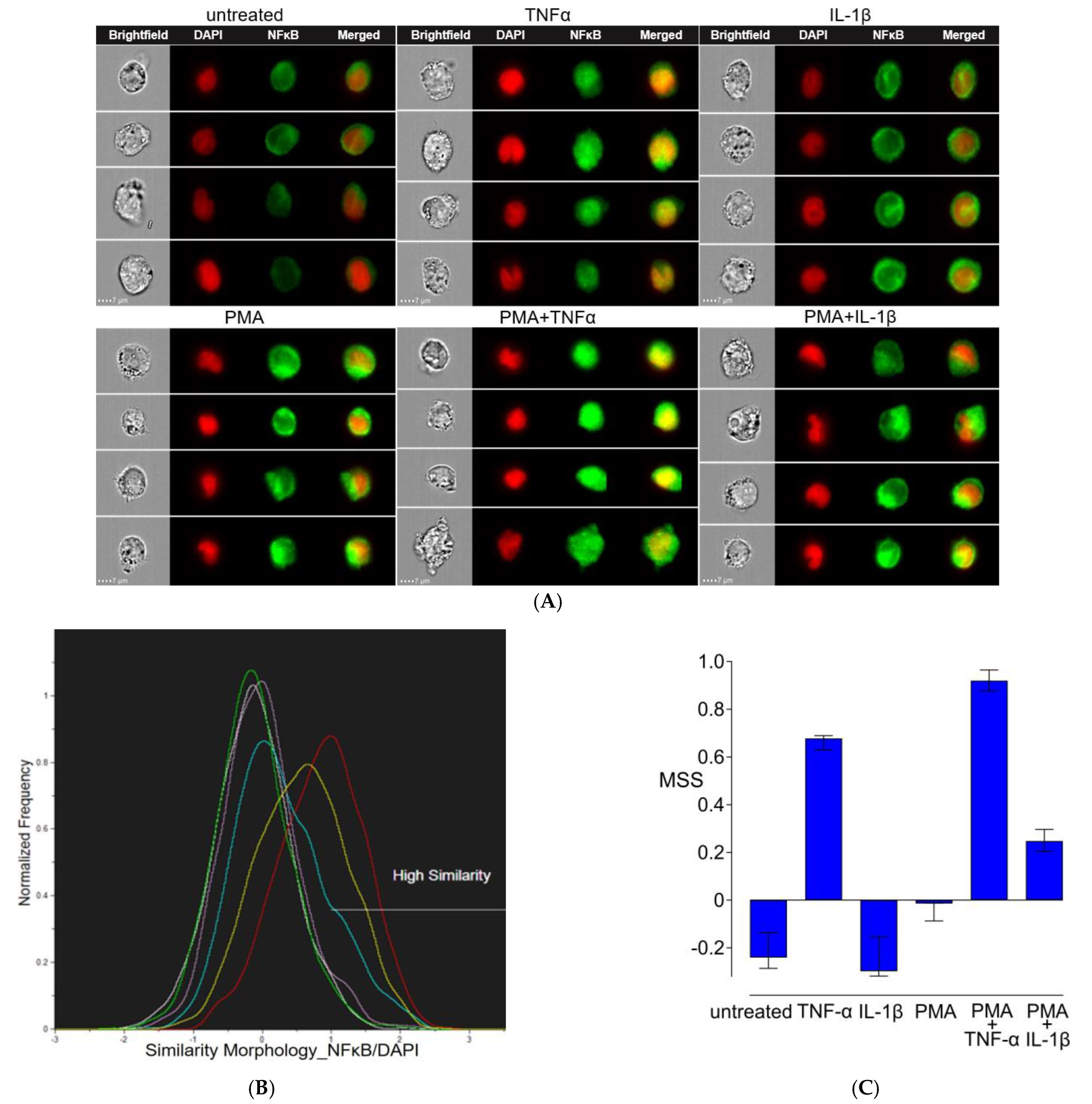
3.5. Nuclear Translocation of NFκB/p65
4. Discussion
4.1. ppNOC, NOP mRNA, and Cytokines
4.2. NFκB Regulates NOP mRNA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bird, M.F.; Hebbes, C.P.; Scott, S.W.M.; Willets, J.; Thompson, J.P.; Lambert, D.G. A novel bioassay to detect Nociceptin/Orphanin FQ release from single human polymorphonuclear cells. PLoS ONE 2022, 17, e0268868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Stuber, F.; Lippuner, C.; Schiff, M.; UM, S. ERK and p38 contribute to the regulation of nociceptin and the nociceptin receptor in human peripheral blood leukocytes. Mol. Pain. 2019, 15, 1744806919828921. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.G. Mixed mu-nociceptin/orphanin FQ opioid receptor agonists and the search for the analgesic holy grail. Br. J. Anaesth. 2019, 122, e95–e97. [Google Scholar] [CrossRef] [PubMed]
- Calo, G.; Lambert, D.G. Nociceptin/orphanin FQ receptor ligands and translational challenges: Focus on cebranopadol as an innovative analgesic. Br. J. Anaesth. 2018, 121, 1105–1114. [Google Scholar] [CrossRef]
- El Daibani, A.; Che, T. Spotlight on Nociceptin/Orphanin FQ Receptor in the Treatment of Pain. Molecules 2022, 27, 595. [Google Scholar] [CrossRef]
- Guan, Q.; Velho, R.V.; Jordan, A.; Pommer, S.; Radde, I.; Sehouli, J.; Mechsner, S. Nociceptin/Orphanin FQ Opioid Peptide-Receptor Expression in the Endometriosis-Associated Nerve Fibers-Possible Treatment Option? Cells 2023, 12, 1395. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, N.T. Nociceptin Opioid Receptor (NOP) as a Therapeutic Target: Progress in Translation from Preclinical Research to Clinical Utility. J. Med. Chem. 2016, 59, 7011–7028. [Google Scholar] [CrossRef]
- Zhang, L.; Stamer, U.M.; Huang, M.Y.; Stüber, F. Interactions between the Nociceptin and Toll-like Receptor Systems. Cells 2022, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Stuber, F.; Lippuner, C.; Schiff, M.; Stamer, U.M. Phorbol-12-myristate-13-acetate induces nociceptin in human Mono Mac 6 cells via multiple transduction signalling pathways. Br. J. Anaesth. 2016, 117, 250–257. [Google Scholar] [CrossRef]
- Gomes, A.; Capela, J.P.; Ribeiro, D.; Freitas, M.; Silva, A.M.; Pinto, D.C.; Santos, C.M.; Cavaleiro, J.A.; Lima, J.L.; Fernandes, E. Inhibition of NF-kB activation and cytokines production in THP-1 monocytes by 2-styrylchromones. Med. Chem. 2015, 11, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yin, S.; Chen, Y.; Wu, Y.; Zheng, W.; Dong, H.; Bai, Y.; Qin, Y.; Li, J.; Feng, S.; et al. LPS-induced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NF-kappaB, STAT3 or AP-1 activation. Mol. Med. Rep. 2018, 17, 5484–5491. [Google Scholar]
- Kunrade, L.; Rembergs, R.; Jekabsons, K.; Klavins, L.; Klavins, M.; Muceniece, R.; Riekstin, U. Inhibition of NF-κB pathway in LPS-stimulated THP-1 monocytes and COX-2 activity in vitro by berry pomace extracts from five Vaccinium species. J. Berry Res. 2020, 10, 381–396. [Google Scholar] [CrossRef]
- Sharif, O.; Bolshakov, V.N.; Raines, S.; Newham, P.; Perkins, N.D. Transcriptional profiling of the LPS induced NF-kappaB response in macrophages. BMC Immunol. 2007, 8, 1. [Google Scholar] [CrossRef]
- Takashiba, S.; Van Dyke, T.E.; Amar, S.; Murayama, Y.; Soskolne, A.W.; Shapira, L. Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor kappaB. Infect. Immun. 1999, 67, 5573–5578. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Hwang, J.H.; Lee, H.T. Differential susceptibility to lipopolysaccharide affects the activation of toll-like-receptor 4 signaling in THP-1 cells and PMA-differentiated THP-1 cells. Innate Immun. 2022, 28, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.G.; Son, M.; Kenna, E.; Thom, N.; Tay, S. NF-κB memory coordinates transcriptional responses to dynamic inflammatory stimuli. Cell Rep. 2022, 40, 111159. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Jimi, E.; Zeiss, C.; Hayden, M.S.; Ghosh, S. Constitutively active NF-kappaB triggers systemic TNFalpha-dependent inflammation and localized TNFalpha-independent inflammatory disease. Genes Dev. 2010, 24, 1709–1717. [Google Scholar] [CrossRef]
- Zhang, L.; Stuber, F.; Stamer, U.M. Inflammatory mediators influence the expression of nociceptin and its receptor in human whole blood cultures. PLoS ONE 2013, 8, e74138. [Google Scholar] [CrossRef][Green Version]
- Medepalli, K.; Alphenaar, B.W.; Keynton, R.S.; Sethu, P. A new technique for reversible permeabilization of live cells for intracellular delivery of quantum dots. Nanotechnology 2013, 24, 205101. [Google Scholar] [CrossRef] [PubMed]
- Fiset, M.E.; Gilbert, C.; Poubelle, P.E.; Pouliot, M. Human neutrophils as a source of nociceptin: A novel link between pain and inflammation. Biochemistry 2003, 42, 10498–10505. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.G. The nociceptin/orphanin FQ receptor: A target with broad therapeutic potential. Nat. Rev. Drug Discov. 2008, 7, 694–710. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.G.; Bird, M.F.; Rowbotham, D.J. Cebranopadol: A first in-class example of a nociceptin/orphanin FQ receptor and opioid receptor agonist. Br. J. Anaesth. 2015, 114, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Gargiulo, S.; Gamba, P.; Testa, G.; Rossin, D.; Biasi, F.; Poli, G.; Leonarduzzi, G. Relation between TLR4/NF-κB signaling pathway activation by 27-hydroxycholesterol and 4-hydroxynonenal, and atherosclerotic plaque instability. Aging Cell 2015, 14, 569–581. [Google Scholar] [CrossRef]
- Rex, J.; Lutz, A.; Faletti, L.E.; Albrecht, U.; Thomas, M.; Bode, J.G.; Borner, C.; Sawodny, O.; Merfort, I. IL-1β and TNFα Differentially Influence NF-κB Activity and FasL-Induced Apoptosis in Primary Murine Hepatocytes During LPS-Induced Inflammation. Front. Physiol. 2019, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Holden, N.S.; Squires, P.E.; Kaur, M.; Bland, R.; Jones, C.E.; Newton, R. Phorbol ester-stimulated NF-kappaB-dependent transcription: Roles for isoforms of novel protein kinase C. Cell Signal 2008, 20, 1338–1348. [Google Scholar] [CrossRef]
- Wang, L.; Du, F.; Wang, X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell 2008, 133, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Isaacs, J.S.; Lee, S.; Trepel, J.; Neckers, L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003, 17, 2115–2117. [Google Scholar] [CrossRef]
- Donica, C.L.; Ramirez, V.I.; Awwad, H.O.; Zaveri, N.T.; Toll, L.; Standifer, K.M. Orphanin FQ/nociceptin activates nuclear factor kappa B. J. Neuroimmune Pharmacol. 2011, 6, 617–625. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wołyniak, M.; Małecka-Wojciesko, E.; Zielińska, M.; Fabisiak, A. A Crosstalk between the Cannabinoid Receptors and Nociceptin Receptors in Colitis-Clinical Implications. J. Clin. Med. 2022, 11, 6675. [Google Scholar] [CrossRef]
- Bedini, A.; Baiula, M.; Vincelli, G.; Formaggio, F.; Lombardi, S.; Caprini, M.; Spampinato, S. Nociceptin/orphanin FQ antagonizes lipopolysaccharide-stimulated proliferation, migration and inflammatory signaling in human glioblastoma U87 cells. Biochem. Pharmacol. 2017, 140, 89–104. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Stamer, U.M.; Moolan-Vadackumchery, R.; Stüber, F. Nuclear Factor-κB Signaling Regulates the Nociceptin Receptor but Not Nociceptin Itself. Cells 2024, 13, 2111. https://doi.org/10.3390/cells13242111
Zhang L, Stamer UM, Moolan-Vadackumchery R, Stüber F. Nuclear Factor-κB Signaling Regulates the Nociceptin Receptor but Not Nociceptin Itself. Cells. 2024; 13(24):2111. https://doi.org/10.3390/cells13242111
Chicago/Turabian StyleZhang, Lan, Ulrike M. Stamer, Robin Moolan-Vadackumchery, and Frank Stüber. 2024. "Nuclear Factor-κB Signaling Regulates the Nociceptin Receptor but Not Nociceptin Itself" Cells 13, no. 24: 2111. https://doi.org/10.3390/cells13242111
APA StyleZhang, L., Stamer, U. M., Moolan-Vadackumchery, R., & Stüber, F. (2024). Nuclear Factor-κB Signaling Regulates the Nociceptin Receptor but Not Nociceptin Itself. Cells, 13(24), 2111. https://doi.org/10.3390/cells13242111





