Stem Cell-Associated Proteins and Extracellular Matrix Composition of the Human Atrioventricular Junction
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Human Cardiac Biopsies
2.3. Histology
2.4. Proteomic Sample Preparation
2.5. Nano-Liquid Chromatography and Mass Spectrometry Analysis
2.6. Database Matching and Protein Quantification
2.7. Processing of Quantitative Proteomics Data
2.8. RNA Extraction
2.9. RNA Sequencing Analysis
2.10. Statistics and Bioinformatics for Gene Analysis
2.11. Immunohistochemistry
2.12. Bioimage Analysis
3. Results
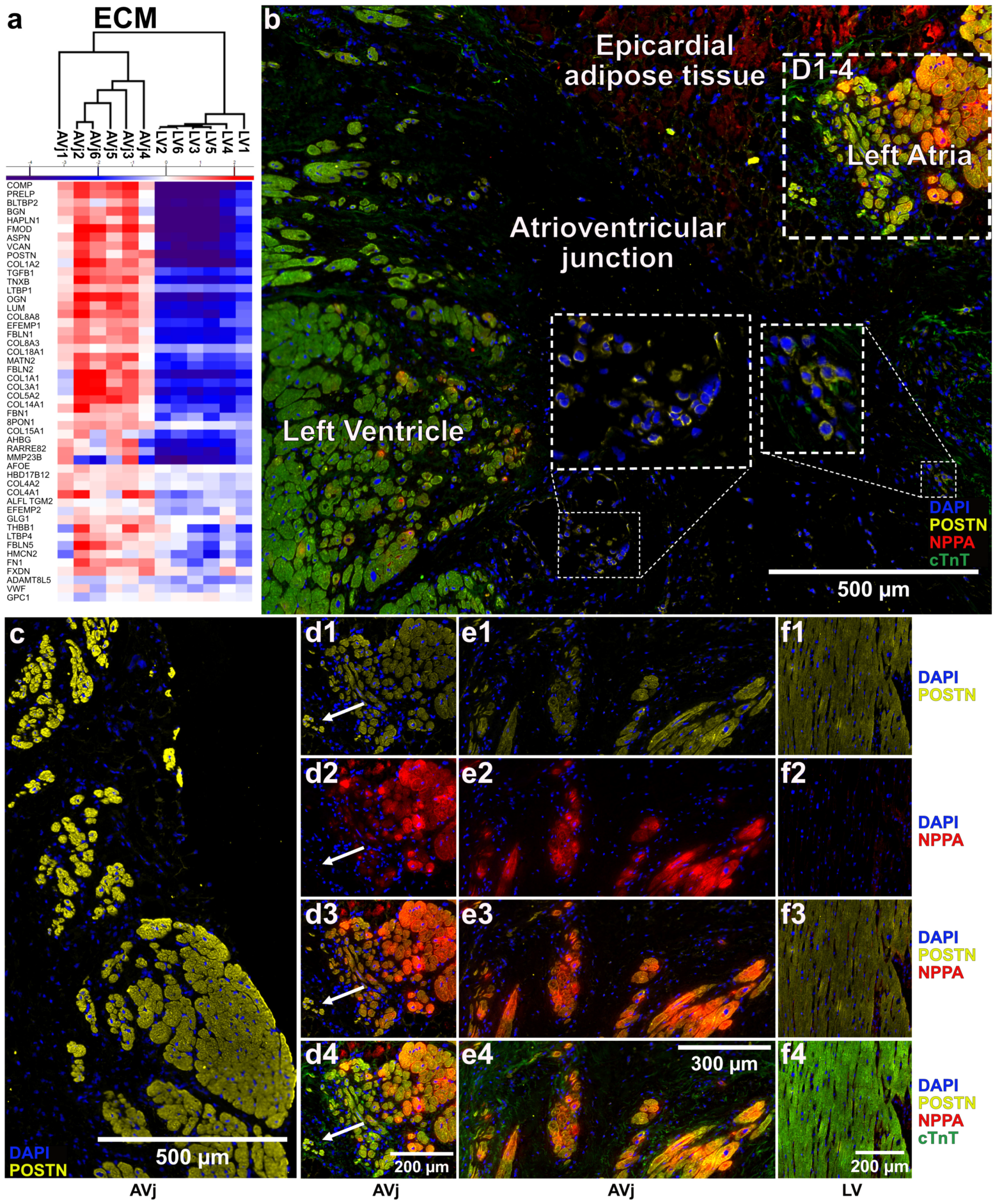
3.1. Histology of the AVj
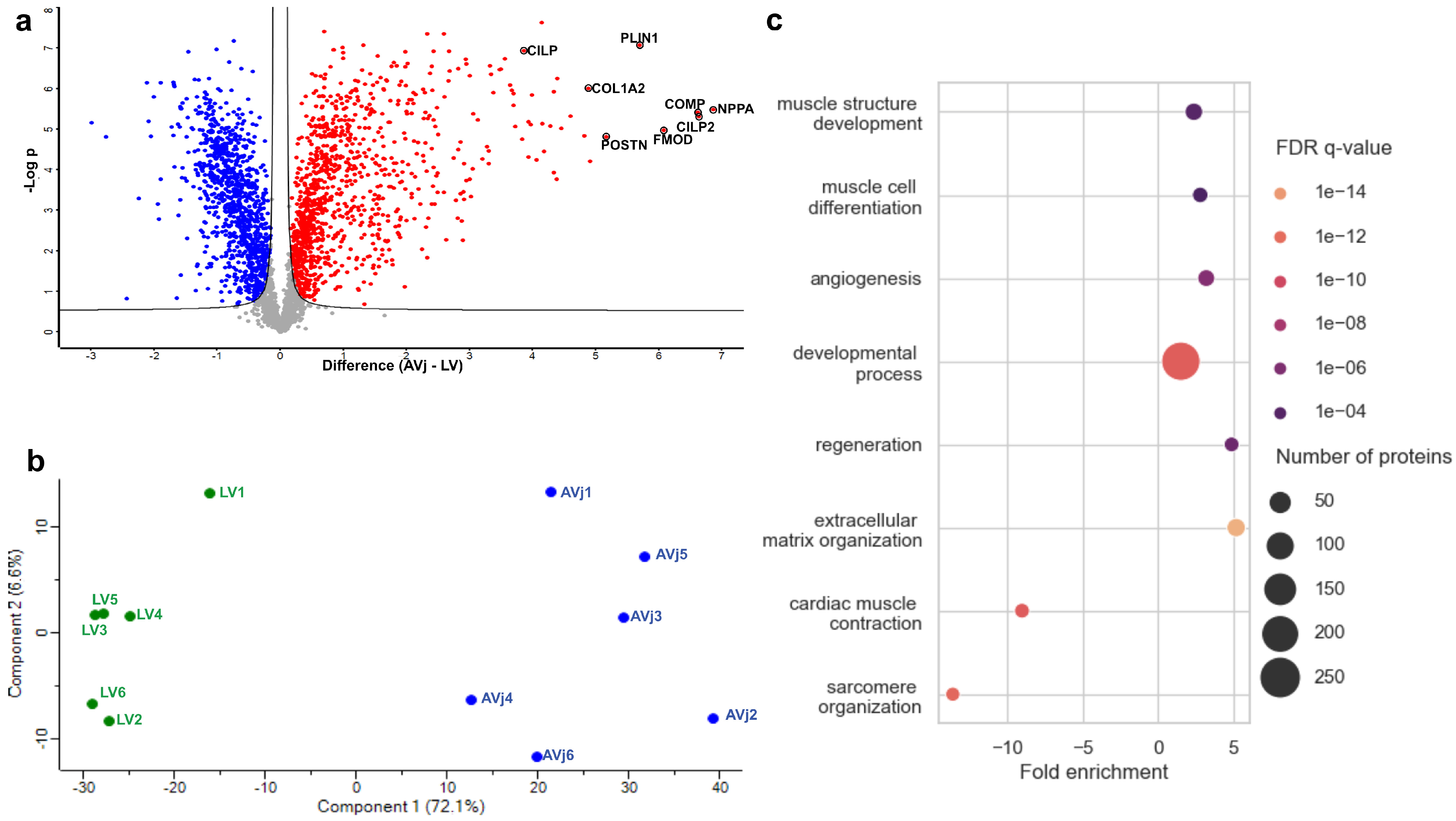
3.2. Differences Between the AVj and LV with Global Quantitative Proteomics
3.3. Gene Expression in the AVj Compared to the LV
3.4. Comparison of Proteomics and Gene Expression Data
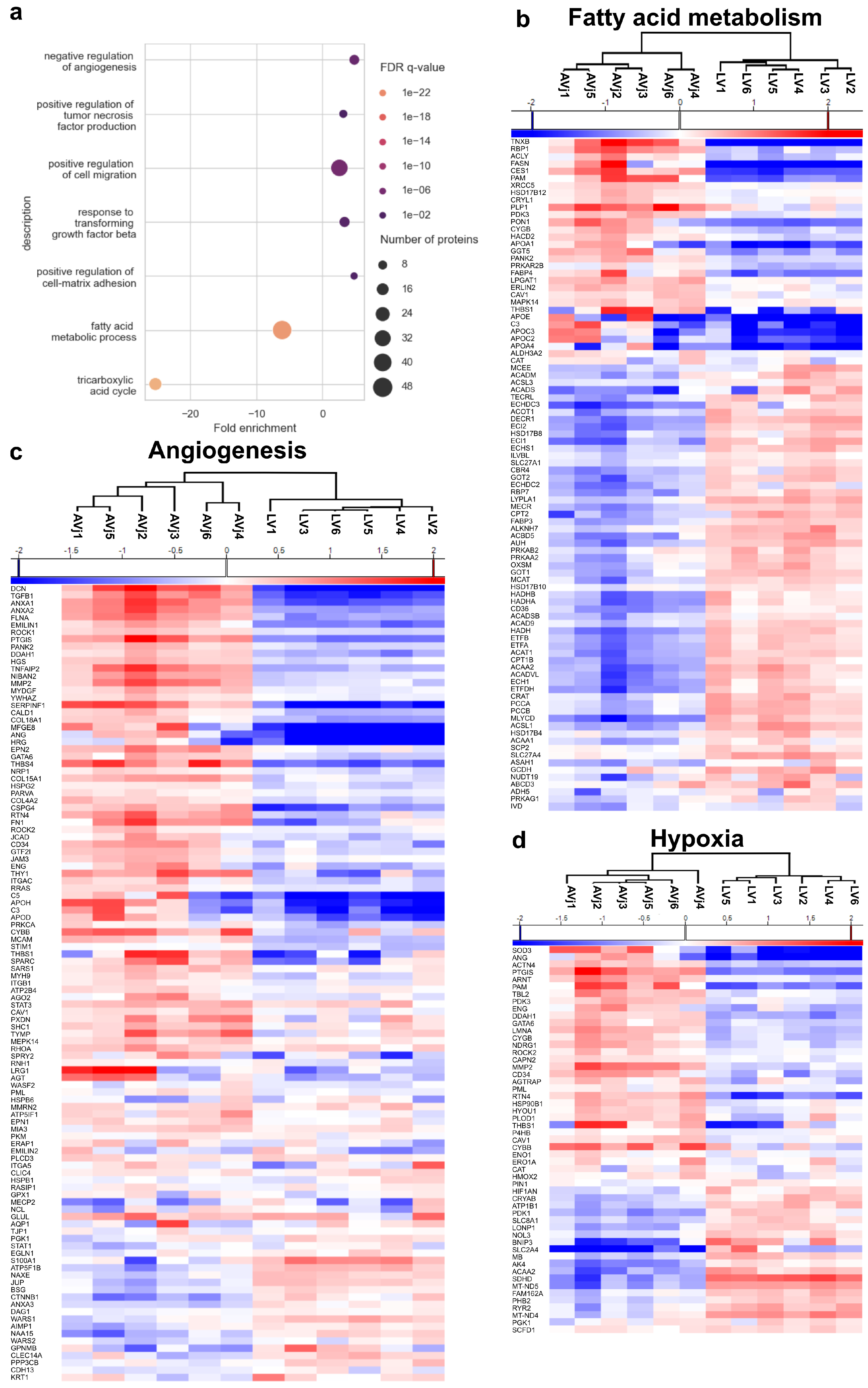
3.5. Stem Cell Niche-Related Proteome of the AVj
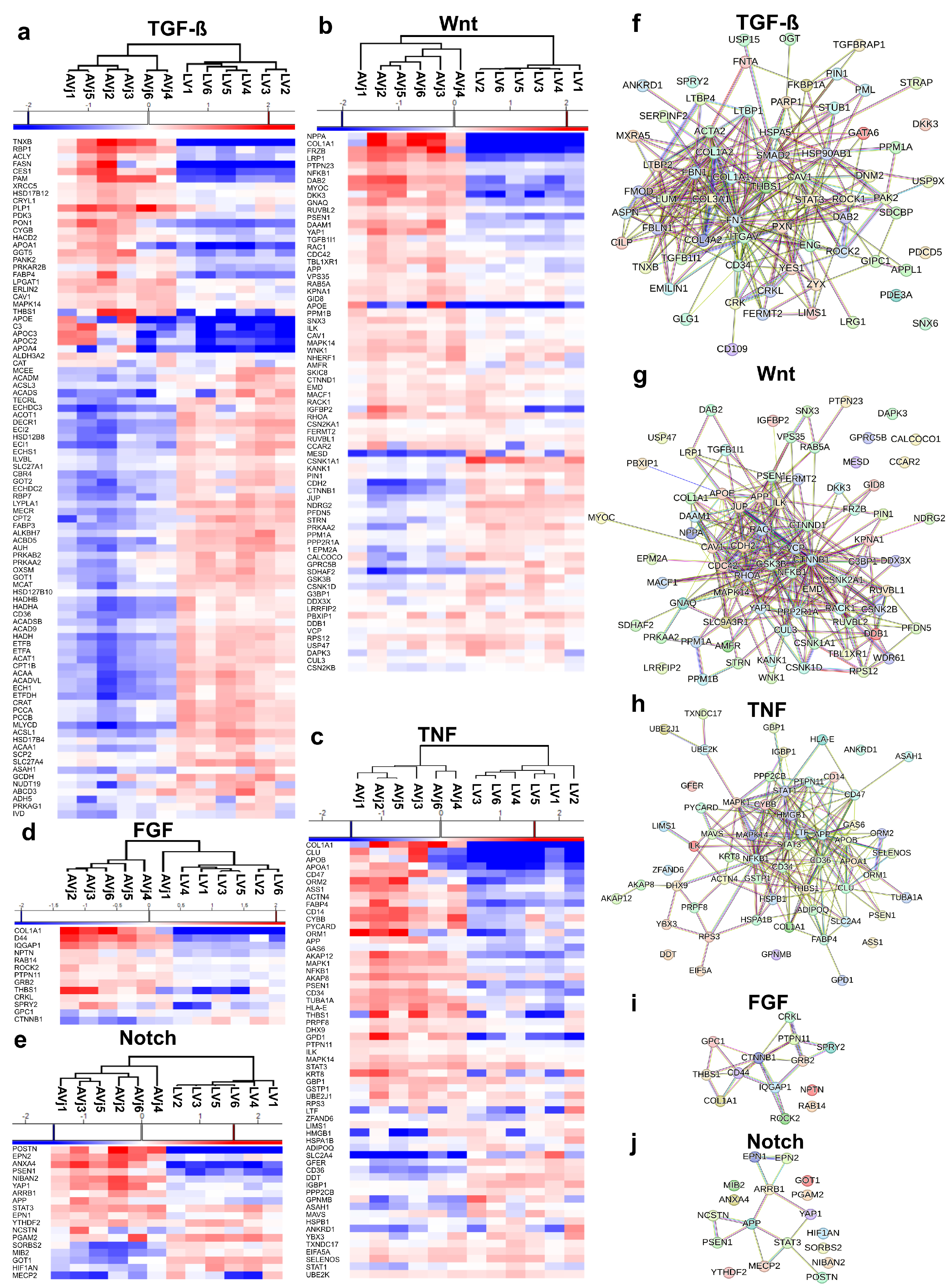
3.6. Enrichment of Developmental Signalling Pathways in the AVj
3.7. Extra Cellular Matrix Composition and Immunohistology of the AVj
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabe-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; Dos Remedios, C.; et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart Regeneration in Zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef] [PubMed]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.D.; Guerquin-Kern, J.L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Vukusic, K.; Asp, J.; Henriksson, H.B.; Brisby, H.; Lindahl, A.; Sandstedt, J. Physical exercise affects slow cycling cells in the rat heart and reveals a new potential niche area in the atrioventricular junction. J. Mol. Histol. 2015, 46, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Vukusic, K.; Sandstedt, M.; Jonsson, M.; Jansson, M.; Oldfors, A.; Jeppsson, A.; Dellgren, G.; Lindahl, A.; Sandstedt, J. The Atrioventricular Junction: A Potential Niche Region for Progenitor Cells in the Adult Human Heart. Stem Cells Dev. 2019, 28, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Sjölin, L.; Jonsson, M.; Orback, C.; Oldfors, A.; Jeppsson, A.; Synnergren, J.; Rotter Sopasakis, V.; Vukusic, K. Expression of Stem Cell Niche-Related Biomarkers at the Base of the Human Tricuspid Valve. Stem Cells Dev. 2022, 32, 140–151. [Google Scholar] [CrossRef]
- Tumbar, T.; Guasch, G.; Greco, V.; Blanpain, C.; Lowry, W.E.; Rendl, M.; Fuchs, E. Defining the epithelial stem cell niche in skin. Science 2004, 303, 359–363. [Google Scholar] [CrossRef]
- Fuchs, E.; Chen, T. A matter of life and death: Self-renewal in stem cells. EMBO Rep. 2013, 14, 39–48. [Google Scholar] [CrossRef]
- Lymperi, S.; Ferraro, F.; Scadden, D.T. The HSC niche concept has turned 31. Has our knowledge matured? Ann. N. Y. Acad. Sci. 2010, 1192, 12–18. [Google Scholar] [CrossRef]
- Simsek, T.; Kocabas, F.; Zheng, J.; Deberardinis, R.J.; Mahmoud, A.I.; Olson, E.N.; Schneider, J.W.; Zhang, C.C.; Sadek, H.A. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 2010, 7, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Takubo, K.; Semenza, G.L. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 2011, 9, 298–310. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhao, Z.; Cheng, M.; Li, M.; Si, J.; Lin, K.; Yu, H. HIF-1α Regulates Osteogenesis of Periosteum-Derived Stem Cells Under Hypoxia Conditions via Modulating POSTN Expression. Front. Cell Dev. Biol. 2022, 10, 836285. [Google Scholar] [CrossRef]
- Rathman-Josserand, M.; Genty, G.; Lecardonnel, J.; Chabane, S.; Cousson, A.; François Michelet, J.; Bernard, B.A. Human Hair Follicle Stem/Progenitor Cells Express Hypoxia Markers. J. Investig. Dermatol. 2013, 133, 2094–2097. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gong, S.H.; He, Y.L.; Cao, Y.; Chen, Y.; Huang, G.H.; Wang, Y.F.; Zhao, M.; Cheng, X.; Zhou, Y.Z.; et al. Autophagy Is Essential for Neural Stem Cell Proliferation Promoted by Hypoxia. Stem Cells 2023, 41, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, J.; O’Brien, W.T.; Johnson, R.S.; LaManna, J.C.; Chavez, J.C.; Klein, P.S.; Simon, M.C. O2 regulates stem cells through Wnt/β-catenin signalling. Nat. Cell Biol. 2010, 12, 1007–1013. [Google Scholar] [CrossRef]
- Chen, X.D. Extracellular matrix provides an optimal niche for the maintenance and propagation of mesenchymal stem cells. Birth Defects Res. C Embryo Today 2010, 90, 45–54. [Google Scholar] [CrossRef]
- Henriksson, H.B.; Papadimitriou, N.; Tschernitz, S.; Svala, E.; Skioldebrand, E.; Windahl, S.; Junevik, K.; Brisby, H. Indications of that migration of stem cells is influenced by the extra cellular matrix architecture in the mammalian intervertebral disk region. Tissue Cell 2015, 47, 439–455. [Google Scholar] [CrossRef]
- Sugimura, R.; He, X.C.; Venkatraman, A.; Arai, F.; Box, A.; Semerad, C.; Haug, J.S.; Peng, L.; Zhong, X.B.; Suda, T.; et al. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell 2012, 150, 351–365. [Google Scholar] [CrossRef]
- Fuchs, E.; Tumbar, T.; Guasch, G. Socializing with the Neighbors: Stem Cells and Their Niche. Cell 2004, 116, 769–778. [Google Scholar] [CrossRef]
- Bjornson, C.R.; Cheung, T.H.; Liu, L.; Tripathi, P.V.; Steeper, K.M.; Rando, T.A. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells 2012, 30, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Q.; Shi, J.; Xu, X.; Xu, J. The role of TNF-α in the fate regulation and functional reprogramming of mesenchymal stem cells in an inflammatory microenvironment. Front. Immunol. 2023, 14, 1074863. [Google Scholar] [CrossRef]
- Otsuka, T.; Mengsteab, P.Y.; Laurencin, C.T. Control of mesenchymal cell fate via application of FGF-8b in vitro. Stem Cell Res. 2021, 51, 102155. [Google Scholar] [CrossRef] [PubMed]
- Kimura, W.; Sadek, H.A. The cardiac hypoxic niche: Emerging role of hypoxic microenvironment in cardiac progenitors. Cardiovasc. Diagn. Ther. 2012, 2, 278–289. [Google Scholar] [CrossRef]
- Aguilar-Sanchez, C.; Michael, M.; Pennings, S. Cardiac Stem Cells in the Postnatal Heart: Lessons from Development. Stem Cells Int. 2018, 2018, 1247857. [Google Scholar] [CrossRef]
- Moretti, A.; Caron, L.; Nakano, A.; Lam, J.T.; Bernshausen, A.; Chen, Y.; Qyang, Y.; Bu, L.; Sasaki, M.; Martin-Puig, S.; et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 2006, 127, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.C.; Matthiesen, T.S.; Brechtken, J.; Grindle, S.; Goh, S.K.; Nelson, W.; Taylor, D.A. The adult human heart as a source for stem cells: Repair strategies with embryonic-like progenitor cells. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4 (Suppl. S1), S27–S39. [Google Scholar] [CrossRef]
- Duim, S.N.; Goumans, M.J.; Kruithof, B.P.T. WT1 in Cardiac Development and Disease. In Wilms Tumor [Internet]; van den Heuvel-Eibrink, M.M., Ed.; Codon Publications: Brisbane, Australia, 2016; Chapter 13. [Google Scholar] [PubMed]
- Oh, H.; Bradfute, S.B.; Gallardo, T.D.; Nakamura, T.; Gaussin, V.; Mishina, Y.; Pocius, J.; Michael, L.H.; Behringer, R.R.; Garry, D.J.; et al. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. USA 2003, 100, 12313–12318. [Google Scholar] [CrossRef] [PubMed]
- Sandstedt, J.; Jonsson, M.; Kajic, K.; Sandstedt, M.; Lindahl, A.; Dellgren, G.; Jeppsson, A.; Asp, J. Left atrium of the human adult heart contains a population of side population cells. Basic Res. Cardiol. 2012, 107, 255. [Google Scholar] [CrossRef]
- Pfister, O.; Mouquet, F.; Jain, M.; Summer, R.; Helmes, M.; Fine, A.; Colucci, W.S.; Liao, R. CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ. Res. 2005, 97, 52–61. [Google Scholar] [CrossRef]
- Santini, M.P.; Forte, E.; Harvey, R.P.; Kovacic, J.C. Developmental origin and lineage plasticity of endogenous cardiac stem cells. Development 2016, 143, 1242–1258. [Google Scholar] [CrossRef] [PubMed]
- Sandstedt, J.; Jonsson, M.; Vukusic, K.; Dellgren, G.; Lindahl, A.; Jeppsson, A.; Asp, J. SSEA-4+ CD34- cells in the adult human heart show the molecular characteristics of a novel cardiomyocyte progenitor population. Cells Tissues Organs 2014, 199, 103–116. [Google Scholar] [CrossRef]
- Taylor, G.; Lehrer, M.S.; Jensen, P.J.; Sun, T.T.; Lavker, R.M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 2000, 102, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, A.; Spicher, A.; Roehrich, M.E.; Glauche, I.; Vogt, P.; Vassalli, G. Immunohistochemical and flow cytometric analysis of long-term label-retaining cells in the adult heart. Stem Cells Dev. 2011, 20, 211–222. [Google Scholar] [CrossRef]
- Godwin, J.W.; Debuque, R.; Salimova, E.; Rosenthal, N.A. Heart regeneration in the salamander relies on macrophage-mediated control of fibroblast activation and the extracellular landscape. NPJ Regen. Med. 2017, 2, 22. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Hackam, D.G.; Redelmeier, D.A. Translation of research evidence from animals to humans. JAMA 2006, 296, 1731–1732. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T. Look back in anger—What clinical studies tell us about preclinical work. Altex 2013, 30, 275–291. [Google Scholar] [CrossRef]
- Huang, F.K.; Zhang, G.; Lawlor, K.; Nazarian, A.; Philip, J.; Tempst, P.; Dephoure, N.; Neubert, T.A. Deep Coverage of Global Protein Expression and Phosphorylation in Breast Tumor Cell Lines Using TMT 10-plex Isobaric Labeling. J. Proteome Res. 2017, 16, 1121–1132. [Google Scholar] [CrossRef]
- O’Connell, J.D.; Paulo, J.A.; O’Brien, J.J.; Gygi, S.P. Proteome-Wide Evaluation of Two Common Protein Quantification Methods. J. Proteome Res. 2018, 17, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- McAlister, G.C.; Nusinow, D.P.; Jedrychowski, M.P.; Wühr, M.; Huttlin, E.L.; Erickson, B.K.; Rad, R.; Haas, W.; Gygi, S.P. MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal. Chem. 2014, 86, 7150–7158. [Google Scholar] [CrossRef]
- Ow, S.Y.; Salim, M.; Noirel, J.; Evans, C.; Rehman, I.; Wright, P.C. iTRAQ underestimation in simple and complex mixtures: “the good, the bad and the ugly”. J. Proteome Res. 2009, 8, 5347–5355. [Google Scholar] [CrossRef]
- Gallien, S.; Duriez, E.; Crone, C.; Kellmann, M.; Moehring, T.; Domon, B. Targeted proteomic quantification on quadrupole-orbitrap mass spectrometer. Mol. Cell. Proteom. 2012, 11, 1709–1723. [Google Scholar] [CrossRef] [PubMed]
- Houweling, A.C.; van Borren, M.M.; Moorman, A.F.M.; Christoffels, V.M. Expression and regulation of the atrial natriuretic factor encoding gene Nppa during development and disease. Cardiovasc. Res. 2005, 67, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Person, A.D.; Garriock, R.J.; Krieg, P.A.; Runyan, R.B.; Klewer, S.E. Frzb modulates Wnt-9a-mediated beta-catenin signaling during avian atrioventricular cardiac cushion development. Dev. Biol. 2005, 278, 35–48. [Google Scholar] [CrossRef][Green Version]
- Duchamp de Lageneste, O.; Julien, A.; Abou-Khalil, R.; Frangi, G.; Carvalho, C.; Cagnard, N.; Cordier, C.; Conway, S.J.; Colnot, C. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat. Commun. 2018, 9, 773. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Qing, Q.; Zhang, Y.J.; Ning, L.J.; Cui, J.; Yao, X.; Luo, J.C.; Ding, W.; Qin, T.W. Enhancement of tenogenic differentiation of rat tendon-derived stem cells by biglycan. J. Cell. Physiol. 2019, 234, 15898–15910. [Google Scholar] [CrossRef]
- Westermann, D.; Mersmann, J.; Melchior, A.; Freudenberger, T.; Petrik, C.; Schaefer, L.; Lüllmann-Rauch, R.; Lettau, O.; Jacoby, C.; Schrader, J.; et al. Biglycan Is Required for Adaptive Remodeling After Myocardial Infarction. Circulation 2008, 117, 1269–1276. [Google Scholar] [CrossRef]
- Zuo, C.; Li, X.; Huang, J.; Chen, D.; Ji, K.; Yang, Y.; Xu, T.; Zhu, D.; Yan, C.; Gao, P. Osteoglycin attenuates cardiac fibrosis by suppressing cardiac myofibroblast proliferation and migration through antagonizing lysophosphatidic acid 3/matrix metalloproteinase 2/epidermal growth factor receptor signalling. Cardiovasc. Res. 2018, 114, 703–712. [Google Scholar] [CrossRef]
- Jinton, H.; Sopasakis, V.R.; Sjölin, L.; Oldfors, A.; Jeppsson, A.; Oras, J.; Wernbom, M.; Vukusic, K. Global ischemia induces stemness and dedifferentiation in human adult cardiomyocytes after cardiac arrest. Sci. Rep. 2024, 14, 14256. [Google Scholar] [CrossRef]
- Li, X.; Wu, F.; Günther, S.; Looso, M.; Kuenne, C.; Zhang, T.; Wiesnet, M.; Klatt, S.; Zukunft, S.; Fleming, I.; et al. Inhibition of fatty acid oxidation enables heart regeneration in adult mice. Nature 2023, 622, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Long, Q.; Wu, H.; Zhou, Y.; Duan, L.; Yuan, H.; Ding, Y.; Huang, Y.; Wu, Y.; Huang, J.; et al. Nuclear localization of mitochondrial TCA cycle enzymes modulates pluripotency via histone acetylation. Nat. Commun. 2022, 13, 7414. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, M.; Couillard-Despres, S.; Raber, K.A.; Stephan, M.; Lehner, B.; Winner, B.; Kohl, Z.; Rivera, F.J.; Nguyen, H.P.; Riess, O.; et al. Stem cell quiescence in the hippocampal neurogenic niche is associated with elevated transforming growth factor-beta signaling in an animal model of Huntington disease. J. Neuropathol. Exp. Neurol. 2010, 69, 717–728. [Google Scholar] [CrossRef]
- Lim, X.; Tan, S.H.; Koh, W.L.; Chau, R.M.; Yan, K.S.; Kuo, C.J.; van Amerongen, R.; Klein, A.M.; Nusse, R. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science 2013, 342, 1226–1230. [Google Scholar] [CrossRef]
- Yatsenko, A.S.; Shcherbata, H.R. Distant activation of Notch signaling induces stem cell niche assembly. PLoS Genet. 2021, 17, e1009489. [Google Scholar] [CrossRef]
- Pei, F.; Ma, L.; Jing, J.; Feng, J.; Yuan, Y.; Guo, T.; Han, X.; Ho, T.V.; Lei, J.; He, J.; et al. Sensory nerve niche regulates mesenchymal stem cell homeostasis via FGF/mTOR/autophagy axis. Nat. Commun. 2023, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Belenguer, G.; Duart-Abadia, P.; Jordán-Pla, A.; Domingo-Muelas, A.; Blasco-Chamarro, L.; Ferrón, S.R.; Morante-Redolat, J.M.; Fariñas, I. Adult Neural Stem Cells Are Alerted by Systemic Inflammation through TNF-α Receptor Signaling. Cell Stem Cell 2021, 28, 285–299.e289. [Google Scholar] [CrossRef]
- Salm, S.; Burger, P.E.; Wilson, E.L. TGF-β and stem cell factor regulate cell proliferation in the proximal stem cell niche. Prostate 2012, 72, 998–1005. [Google Scholar] [CrossRef]
- Yamazaki, S.; Iwama, A.; Takayanagi, S.; Eto, K.; Ema, H.; Nakauchi, H. TGF-beta as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood 2009, 113, 1250–1256. [Google Scholar] [CrossRef]
- Altshuler, A.; Amitai-Lange, A.; Tarazi, N.; Dey, S.; Strinkovsky, L.; Hadad-Porat, S.; Bhattacharya, S.; Nasser, W.; Imeri, J.; Ben-David, G.; et al. Discrete limbal epithelial stem cell populations mediate corneal homeostasis and wound healing. Cell Stem Cell 2021, 28, 1248–1261.e1248. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, S.R.; Ryan, C.J. Experimental reproducibility limits the correlation between mRNA and protein abundances in tumor proteomic profiles. Cell Rep. Methods 2022, 2, 100288. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cui, Y.; Luan, J.; Zhang, X.; Li, C.; Zhou, X.; Shi, L.; Wang, H.; Han, J. PRELP (proline/arginine-rich end leucine-rich repeat protein) promotes osteoblastic differentiation of preosteoblastic MC3T3-E1 cells by regulating the β-catenin pathway. Biochem. Biophys. Res. Commun. 2016, 470, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Schewe, M.; Franken, P.F.; Sacchetti, A.; Schmitt, M.; Joosten, R.; Böttcher, R.; van Royen, M.E.; Jeammet, L.; Payré, C.; Scott, P.M.; et al. Secreted Phospholipases A2 Are Intestinal Stem Cell Niche Factors with Distinct Roles in Homeostasis, Inflammation, and Cancer. Cell Stem Cell 2016, 19, 38–51. [Google Scholar] [CrossRef]
- Lu, S.; Dong, Z. Overexpression of secretory phospholipase A2-IIa supports cancer stem cell phenotype via HER/ERBB-elicited signaling in lung and prostate cancer cells. Int. J. Oncol. 2017, 50, 2113–2122. [Google Scholar] [CrossRef]
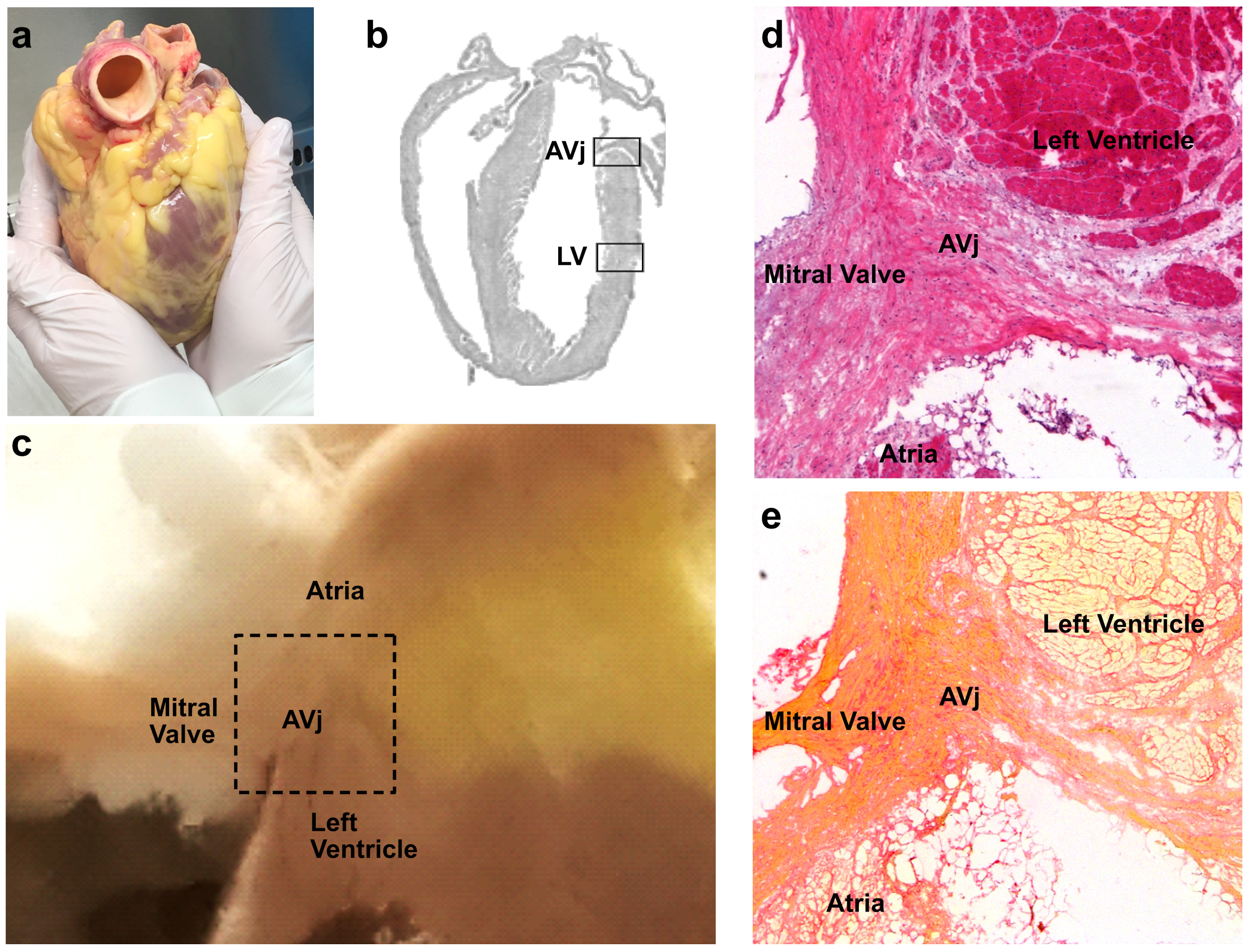

| Donor | Sex | Age | Cause of Death | Other Diseases | Tissue Used for |
|---|---|---|---|---|---|
| 1 | F | 63 | Ischemic cerebral oedema, due to cardiac arrest | Ischemic heart disease, hypertension, obesity, hypothyroidism, diabetes type 2, renal insufficiency, emphysema | Proteomics, RNAseq |
| 2 | F | 19 | Ischemic cerebral oedema, due to cardiac arrest | Anorexia | Proteomics, RNAseq; IHC |
| 3 | F | 43 | Ischemic cerebral oedema, due to cardiac arrest | None | Proteomics, RNAseq |
| 4 | M | 19 | Ischemic cerebral oedema, due to cardiac arrest | HF in the acute setting | Proteomics |
| 5 | M | 21 | Ischemic cerebral oedema, due to cardiac arrest | HF in the acute setting | Proteomics |
| 6 | M | 46 | Ischemic cerebral oedema, due to cardiac arrest | None | Proteomics |
| 7 | F | 42 | Intracerebral haemorrhage | Takotsubo cardiomyopathy in the acute setting | RNAseq |
| 8 | M | 52 | Cardiac arrest | HF in the acute setting | RNAseq |
| 9 | F | 75 | Intracerebral haemorrhage | Atrial fibrillation, ischemic heart disease, previous AMI | RNAseq |
| 10 | M | 74 | Intracerebral haemorrhage | Previous stroke | RNAseq |
| 11 | M | 63 | Traumatic brain injury | Hypertension | IHC |
| 12 | F | 75 | Cerebral haemorrhage | Atrial fibrillation, previous AMI | IHC |
| 13 | M | 54 | Cardiac arrest | Suspected LAD dissection | IHC |
| 14 | M | 69 | Traffic accident | None | IHC |
| 15 | F | 53 | Cardiac arrest | None | IHC |
| Gene ID | Accession | Protein Description | Log2 FC | FDR | Biological Function or Pathways * |
|---|---|---|---|---|---|
| NPPA | P01160 | Natriuretic peptide A | 6.86 | 3.22 × 10−3 | Cardiac system development, stem cell marker, Wnt signalling |
| CILP2 | Q8IUL8 | Cartilage intermediate layer protein 2 | 6.63 | 3.30 × 10−3 | ECM organization |
| COMP | P49747 | Cartilage oligomeric matrix protein | 6.61 | 2.61 × 10−3 | ECM component, developmental processes |
| FMOD | Q06828 | Fibromodulin | 6.08 | 4.56 × 10−3 | TGF-β signalling |
| PLIN1 | O60240 | Perilipin-1 | 5.69 | 1.13 × 10−3 | Lipid metabolism |
| POSTN | Q15063 | Periostin | 5.16 | 2.38 × 10−3 | ECM organization, developmental processes, stem cell marker |
| BGN | P21810 | Biglycan | 4.91 | 6.23 × 10−3 | Cartilage development, developmental processes |
| COL1A2 | P08123 | Collagen alpha-2(I) chain | 4.89 | 1.77 × 10−3 | TGF-β signalling, developmental processes |
| HAPLN1 | P10915 | Hyaluronan and proteoglycan link protein 1 | 4.81 | 3.84 × 10−3 | ECM component, developmental processes |
| PRELP | P51888 | Prolargin | 4.60 | 3.11 × 10−3 | ECM component, developmental processes |
| ASPN | Q9BXN1 | Asporin | 4.45 | 3.48 × 10−3 | ECM component, TGF-β signalling |
| ABI3BP | Q7Z7G0 | Target of Nesh-SH3 | 4.39 | 2.02 × 10−3 | ECM organization |
| MAMDC2 | Q7Z304 | MAM domain-containing protein 2 | 4.38 | 4.70 × 10−3 | ECM component, developmental processes |
| OGN | P20774 | Mimecan | 4.33 | 2.38 × 10−3 | Cartilage development, ECM component |
| PLA2G2A | P14555 | Phospholipase A2 | 4.33 | 1.13 × 10−3 | Inflammatory response, stem cell homeostasis |
| FRZB | Q92765 | Secreted frizzled-related protein 3 | 4.28 | 3.39 × 10−3 | Developmental processes, Wnt signalling |
| MYL7 | Q01449 | Myosin regulatory light chain 2 | 4.18 | 2.74 × 10−3 | Cardiac muscle tissue development |
| COL1A1 | P02452 | Collagen alpha-1(I) chain | 4.15 | 3.15 × 10−3 | Cartilage development, ECM organization, Wnt signalling |
| MYOZ1 | Q9NP98 | Myozenin-1 | 4.14 | 1.13 × 10−3 | Muscle tissue development |
| PCOLCE2 | Q9UKZ9 | Procollagen C-endopeptidase enhancer 2 | 4.06 | 4.21 × 10−3 | Response to leukaemia inhibitory factor |
| COL12A1 | Q99715 | Collagen alpha-1(XII) chain | 3.98 | 2.21 × 10−3 | Cell adhesion, ECM component |
| COL5A2 | P05997 | Collagen alpha-2(V) chain | 3.97 | 2.38 × 10−3 | Developmental processes, ECM organization |
| CCDC80 | Q76M96 | Coiled-coil domain- containing protein 80 | 3.94 | 7.96 × 10−3 | ECM organization |
| VCAN | P13611 | Versican core protein | 3.93 | 3.48 × 10−3 | Developmental processes, ECM component |
| CILP | O75339 | Cartilage intermediate layer protein 1 | 3.86 | 1.13 × 10−3 | TGF-β signalling |
| Gene ID | Accession | Protein Description | Log2 FC | FDR | Biological Function or Pathways * |
|---|---|---|---|---|---|
| MT-ND2 | P03891 | NADH-ubiquinone oxidoreductase chain 2 | −2.99 | 2.19 × 10−3 | Mitochondrial respiratory chain |
| CD300LG | Q6UXG3 | CMRF35-like molecule 9 | −2.76 | 4.04 × 10−3 | Immune system |
| SLC2A4 | P14672 | Solute carrier family 2, facilitated glucose transporter member 4 | −2.24 | 7.20 × 10−3 | Glucose homeostasis, response to hypoxia |
| SDHD | O14521 | Succinate dehydrogenase [ubiquinone] cytochrome b small subunit, mitochondrial | −2.12 | 1.40 × 10−3 | Mitochondrial electron transport, TCA cycle, response to hypoxia |
| FHL2 | Q14192 | Four and a half LIM domains protein 2 | −2.09 | 1.70 × 10−3 | Ventricular cardiac muscle, cell development |
| PDE1C | Q14123 | Dual specificity calcium/calmodulin-dependent 3′,5′-cyclic nucleotide phosphodiesterase 1C | −2.05 | 3.76 × 10−3 | Signal transduction |
| SDHC | Q99643 | Succinate dehydrogenase cytochrome b560 subunit, mitochondrial | −2.01 | 1.48 × 10−3 | Mitochondrial electron transport, TCA cycle |
| ANKRD2 | Q9GZV1 | Ankyrin repeat domain-containing protein 2 | −1.94 | 1.36 × 10−2 | Muscle contraction, muscle development |
| MYL1 | P05976 | Myosin light chain 1/3, skeletal muscle isoform | −1.92 | 1.61 × 10−2 | Cardiac muscle contraction |
| MYL3 | P08590 | Myosin light chain 3 | −1.90 | 4.78 × 10−3 | Cardiac muscle contraction |
| ATP5MJ | P56378 | ATP synthase subunit ATP5MJ, mitochondrial | −1.90 | 1.65 × 10−3 | ATP synthesis |
| CA4 | P22748 | Carbonic anhydrase 4 | −1.73 | 1.77 × 10−3 | Metabolic process |
| ACSS1 | Q9NUB1 | Acetyl-coenzyme A synthetase 2-like, mitochondrial | −1.70 | 1.83 × 10−3 | Acetyl-CoA synthesis |
| IDH2 | P48735 | Isocitrate dehydrogenase [NADP], mitochondrial | −1.69 | 1.41 × 10−3 | Metabolic process, TCA cycle |
| MT-ATP6 | P00846 | ATP synthase subunit a | −1.69 | 1.13 × 10−3 | ATP synthesis, response to hyperoxia |
| FAM210A | Q96ND0 | Protein FAM210A | −1.68 | 1.37 × 10−3 | Mitochondrial homeostasis, cardiac muscle contraction |
| TUBA8 | Q9NY65 | Tubulin alpha-8 chain | −1.68 | 2.88 × 10−3 | Microtubule organization |
| C4orf54 | D6RIA3 | Uncharacterized protein C4orf54 | −1.64 | 2.36 × 10−2 | |
| MICOS10 | Q5TGZ0 | MICOS complex subunit MIC10 | −1.62 | 2.68 × 10−3 | Mitochondrial membrane organization |
| BNIP3 | Q12983 | BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 | −1.62 | 3.95 × 10−3 | Mitochondrial membrane potential, apoptosis |
| MLYCD | O95822 | Malonyl-CoA decarboxylase, mitochondrial | −1.60 | 1.54 × 10−3 | Acetyl-CoA synthesis, fatty acid oxidation |
| PROB1 | E7EW31 | Proline-rich basic protein 1 | −1.59 | 3.55 × 10−3 | Cardiac muscle contraction |
| MT-CO1 | P00395 | Cytochrome c oxidase subunit 1 | −1.59 | 1.25 × 10−3 | Mitochondrial electron transport |
| MT-ND5 | P03915 | NADH-ubiquinone oxidoreductase chain 5 | −1.58 | 1.63 × 10−3 | Mitochondrial electron transport, response to hypoxia |
| SLC25A4 | P12235 | ADP/ATP translocase 1 | −1.57 | 1.59 × 10−3 | Mitochondrial transport |
| Gene ID | Accession | Protein Description | FC | Log2 FC | FDR |
|---|---|---|---|---|---|
| COMP | P49747 | Cartilage oligomeric matrix protein | 115 | 4.2 | 0.001 |
| PLA2G2A | P14555 | Phospholipase A2, membrane-associated | 12 | 3.6 | 0.04 |
| FMOD | Q06828 | Fibromodulin | 11 | 3.5 | 0.02 |
| PRELP | P51888 | Prolargin | 8 | 3.0 | 0.02 |
| CFH | P08603 | Complement factor H | 6 | 2.6 | 0.04 |
| AEBP1 | Q8IUX7 | Adipocyte enhancer-binding protein 1 | 5 | 2.3 | 0.04 |
| COL14A1 | Q05707 | Collagen alpha-1(XIV) chain | 5 | 2.3 | 0.04 |
| FBLN1 | P23142 | Fibulin-1 | 5 | 2.3 | 0.03 |
| LTBP2 | Q14767 | Latent-transforming growth factor beta-binding protein 2 | 2 | 1.0 | 0.03 |
| SERPINF1 | P36955 | Pigment epithelium-derived factor | 2 | 1.0 | 0.04 |
| S100A6 | P06703 | Protein S100-A6 | 2 | 1.0 | 0.03 |
| MMP2 | P08253 | 72 kDa type IV collagenase | 2 | 1.0 | 0.04 |
| GSN | P06396 | Gelsolin | 2 | 1.0 | 0.04 |
| DCTN4 | Q9UJW0 | Dynactin subunit 4 | 0.8 | −0.3 | 0.04 |
| PPP6C | O00743 | Serine/threonine-protein phosphatase 6 catalytic subunit | 0.7 | −0.5 | 0.02 |
| ABRAXAS2 | Q15018 | BRISC complex subunit Abraxas 2 | 0.7 | −0.5 | 0.03 |
| PRKAR2A | P13861 | cAMP-dependent protein kinase type II-alpha regulatory subunit | 0.7 | −0.5 | 0.04 |
| USP10 | Q14694 | Ubiquitin carboxyl-terminal hydrolase 10 | 0.7 | −0.5 | 0.03 |
| SCN5A | Q14524 | Sodium channel protein type 5 subunit alpha | 0.6 | −0.7 | 0.04 |
| NCEH1 | Q6PIU2 | Neutral cholesterol ester hydrolase 1 | 0.5 | −1.0 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorsell, A.; Sjölin, L.; Berger, E.; Jeppsson, A.; Oldfors, A.; Rotter Sopasakis, V.; Vukusic, K. Stem Cell-Associated Proteins and Extracellular Matrix Composition of the Human Atrioventricular Junction. Cells 2024, 13, 2048. https://doi.org/10.3390/cells13242048
Thorsell A, Sjölin L, Berger E, Jeppsson A, Oldfors A, Rotter Sopasakis V, Vukusic K. Stem Cell-Associated Proteins and Extracellular Matrix Composition of the Human Atrioventricular Junction. Cells. 2024; 13(24):2048. https://doi.org/10.3390/cells13242048
Chicago/Turabian StyleThorsell, Annika, Linnéa Sjölin, Evelin Berger, Anders Jeppsson, Anders Oldfors, Victoria Rotter Sopasakis, and Kristina Vukusic. 2024. "Stem Cell-Associated Proteins and Extracellular Matrix Composition of the Human Atrioventricular Junction" Cells 13, no. 24: 2048. https://doi.org/10.3390/cells13242048
APA StyleThorsell, A., Sjölin, L., Berger, E., Jeppsson, A., Oldfors, A., Rotter Sopasakis, V., & Vukusic, K. (2024). Stem Cell-Associated Proteins and Extracellular Matrix Composition of the Human Atrioventricular Junction. Cells, 13(24), 2048. https://doi.org/10.3390/cells13242048






