Serotonin Inhibition of Claustrum Projection Neurons: Ionic Mechanism, Receptor Subtypes and Consequences for Claustrum Computation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Brain Slice Preparation
2.3. Electrophysiological Recordings
2.4. Classification of Claustral Neurons
2.5. Responses to 5-HT Application
2.6. 5-HT Uncaging
2.7. Immunohistochemistry
2.8. Image Acquisition
2.9. Calculations and Analyses
3. Results
3.1. Identification of Claustral PNs

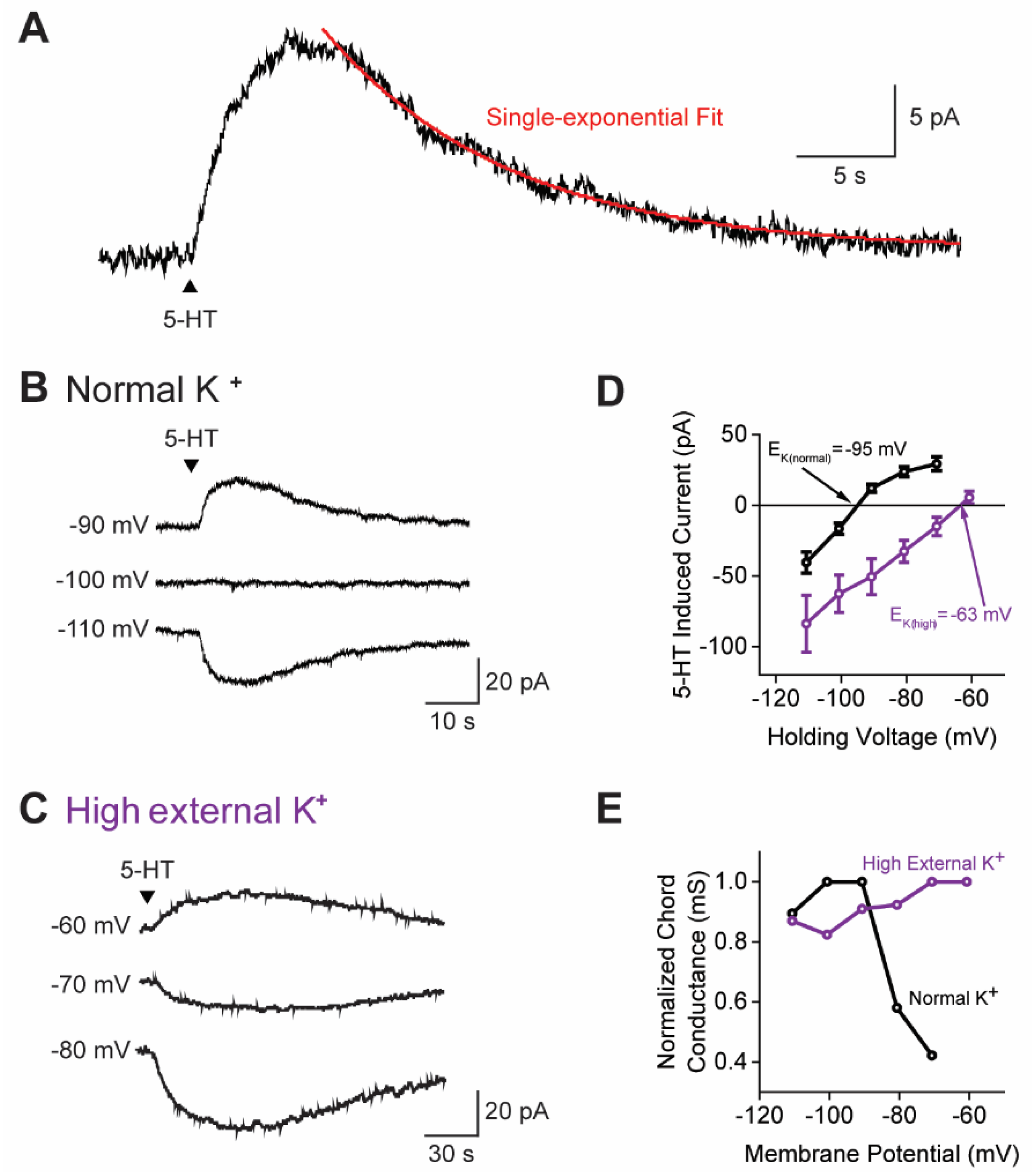
3.2. Claustral PNs Are Inhibited by a K+ Conductance Increase
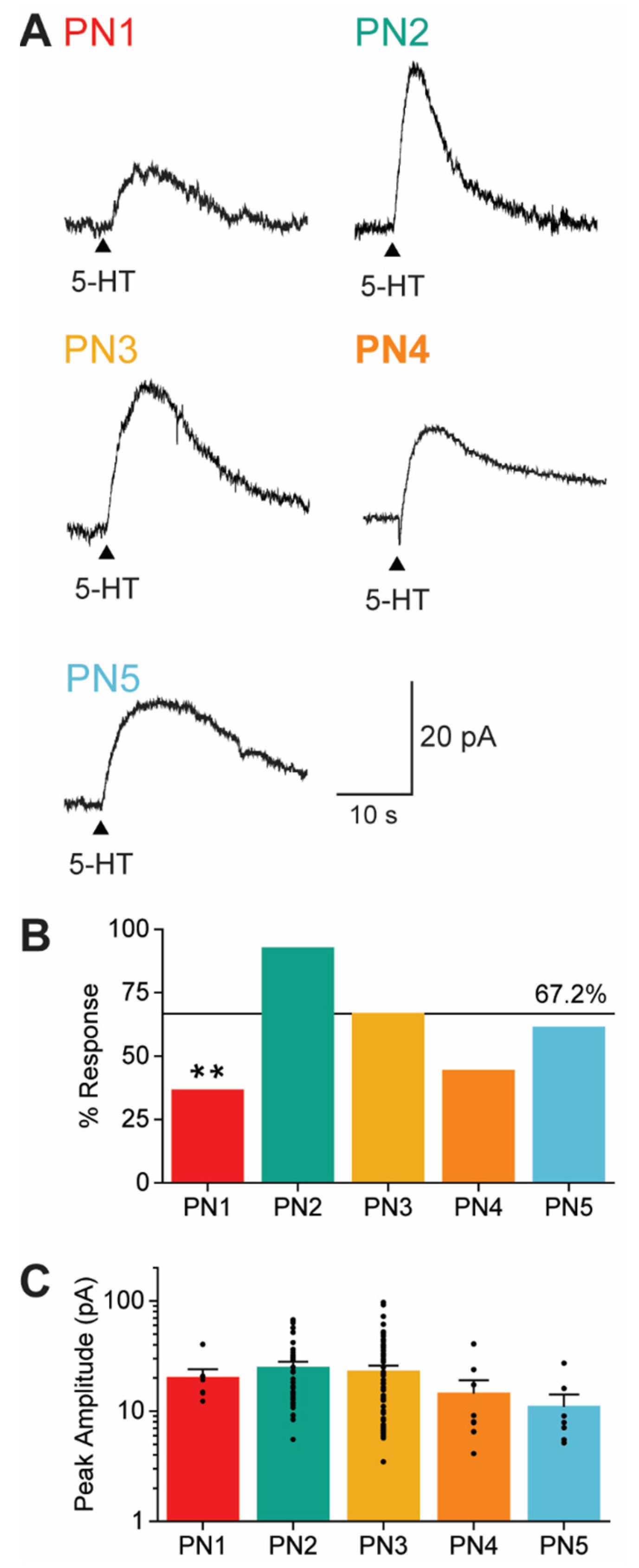
3.3. Claustral PN Subtypes Differ in Probability of 5-HT Responses
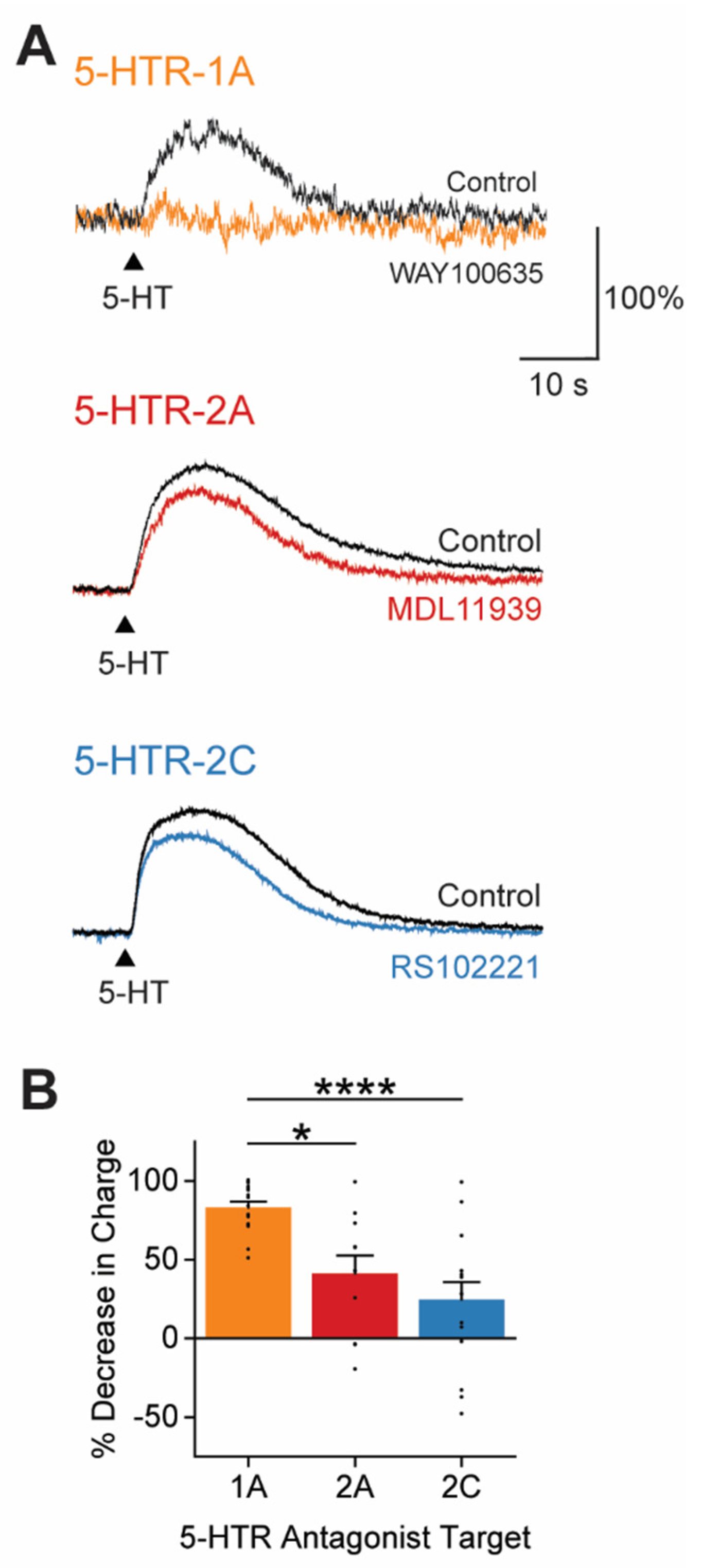
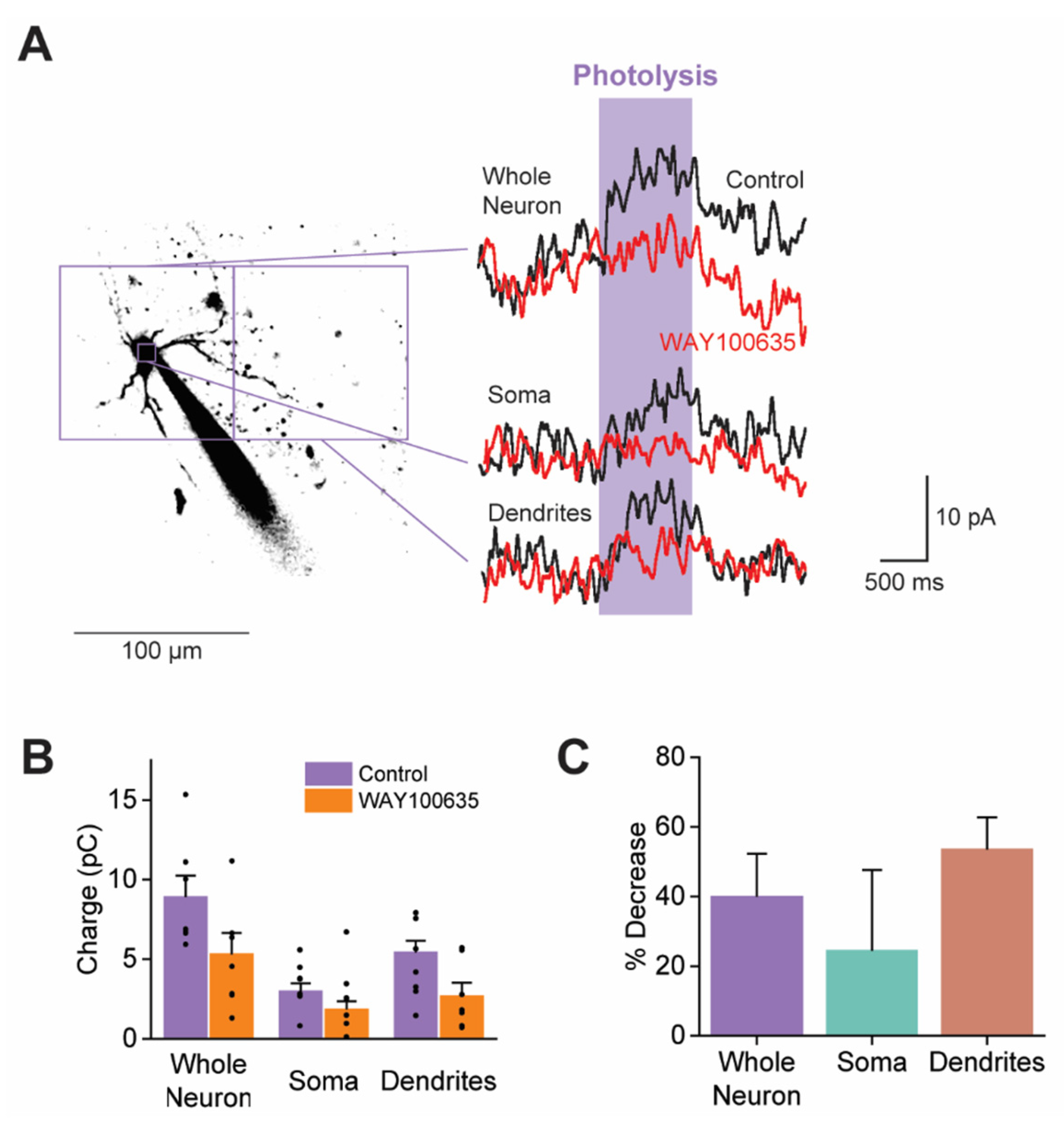
3.4. 5-HT Responses Are Generated by Multiple Types of 5-HTRs
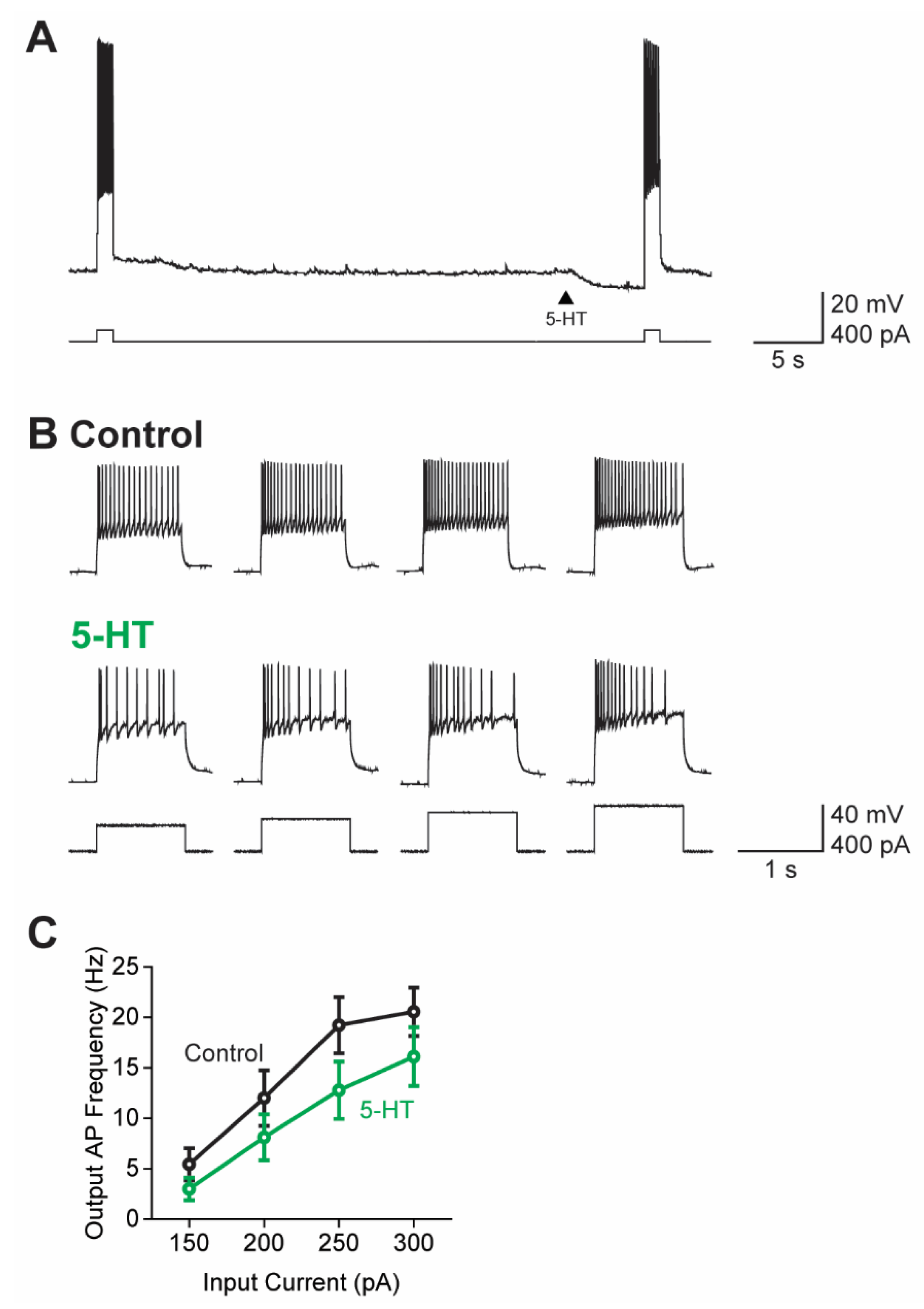
3.5. Actions of 5-HT on Action Potential Firing
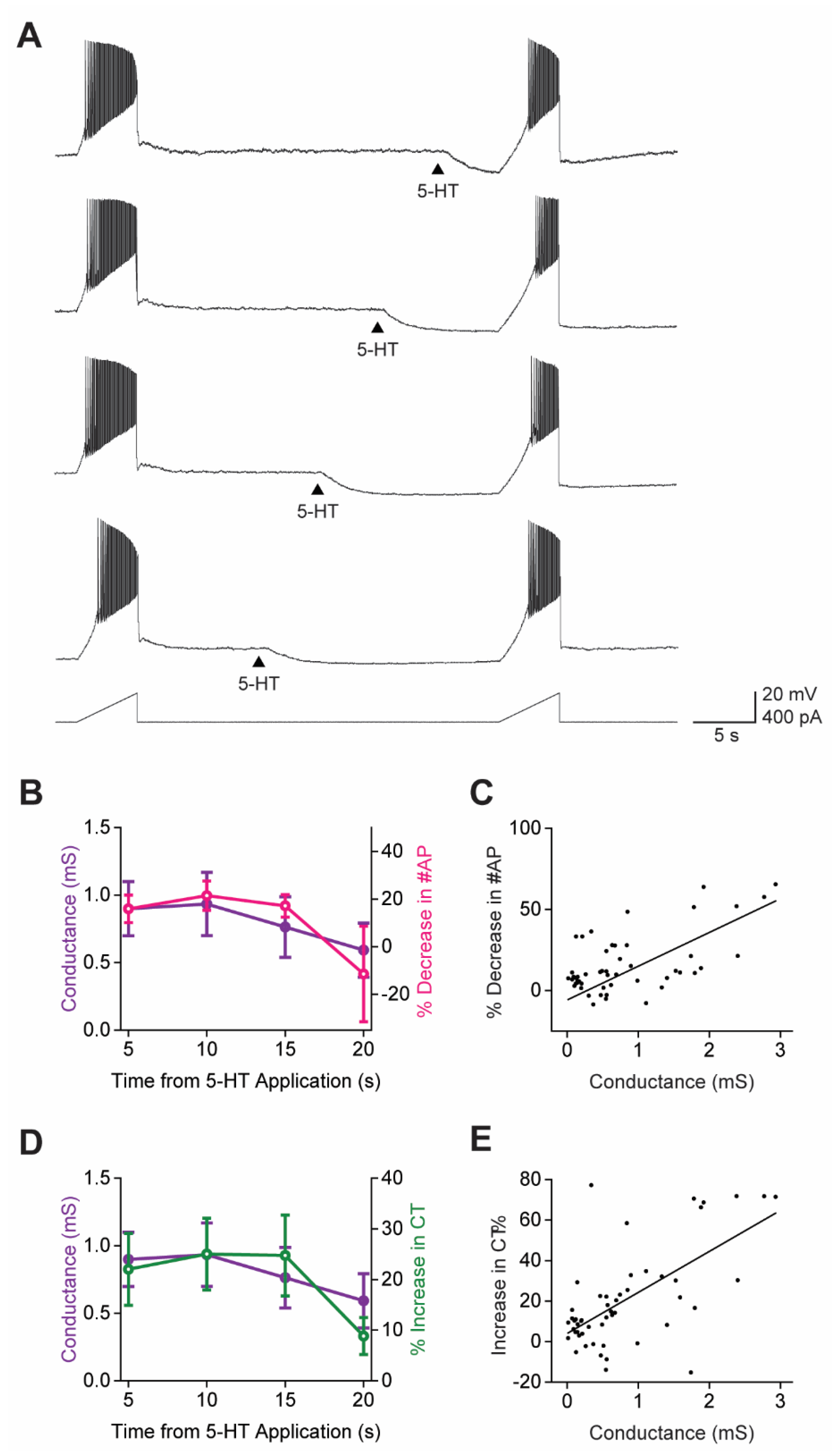
3.6. 5-HT Causes a Subtractive Reduction in PN Output
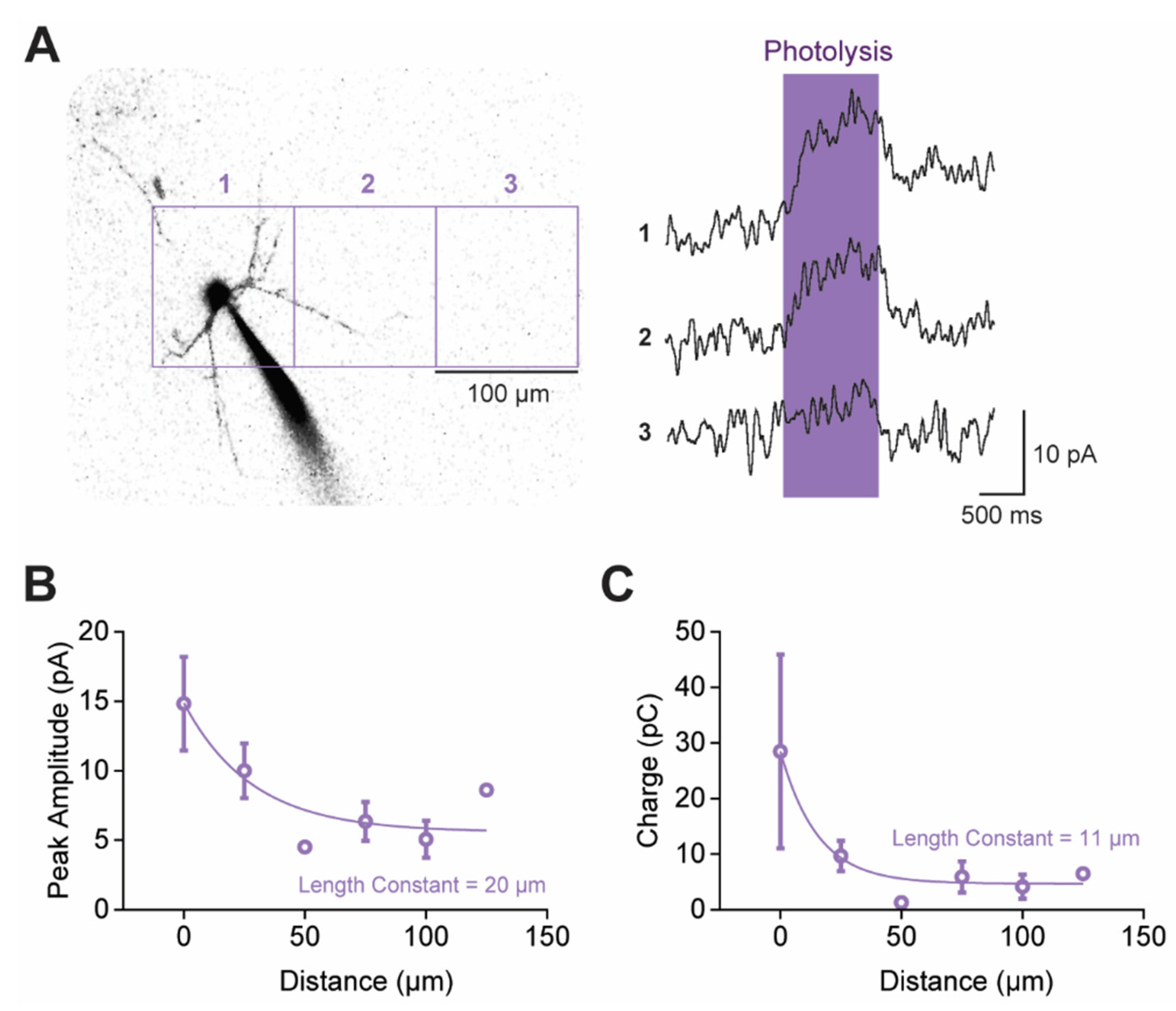
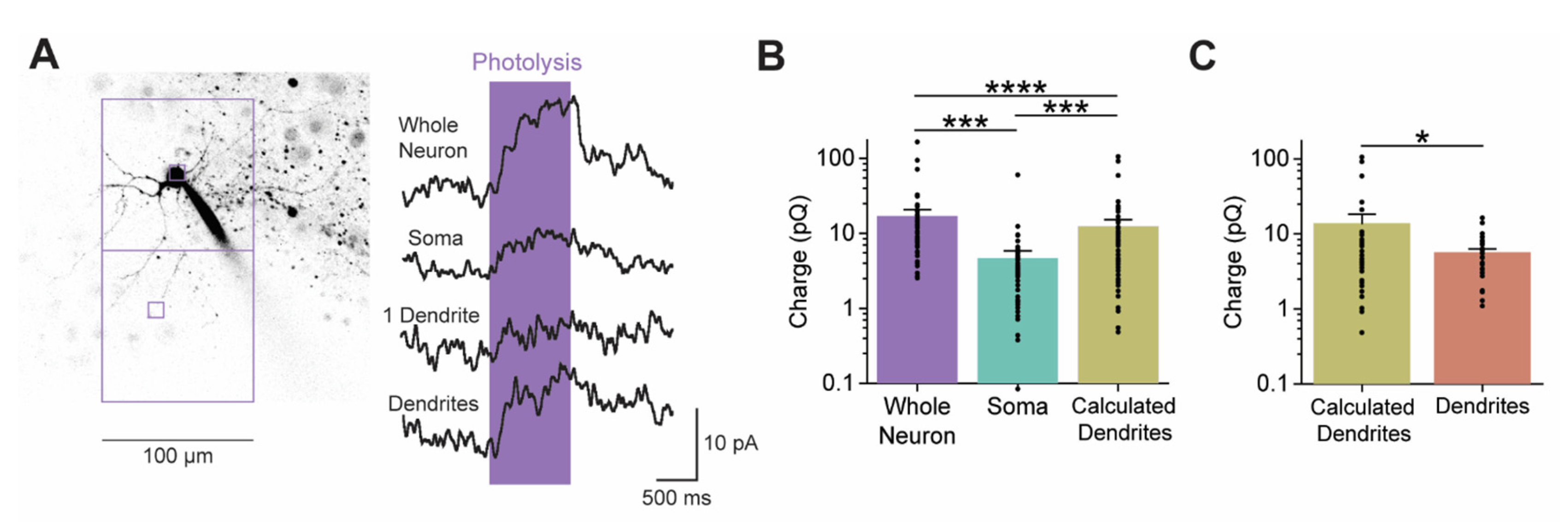
3.7. 5-HTRs Are Distributed Throughout Claustral PN Compartments
4. Discussion
4.1. Ionic Mechanism of 5-HT Inhibition of Claustrum PNs
4.2. Receptors Mediating 5-HT Inhibition
4.3. Location of 5-HT Receptors on Claustrum PNs
4.4. Implications for Higher Brain Function
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torgerson, C.M.; Irimia, A.; Goh, S.Y.M.; Van Horn, J.D. The DTI connectivity of the human claustrum. Hum. Brain Mapp. 2015, 36, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ng, L.; Harris, J.A.; Feng, D.; Li, Y.; Royall, J.J.; Oh, S.W.; Bernard, A.; Sunkin, S.M.; Koch, C.; et al. Organization of the connections between claustrum and cortex in the mouse. J. Comp. Neurol. 2017, 525, 1317–1346. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Xie, P.; Liu, L.; Kuang, X.; Wang, Y.; Qu, L.; Gong, H.; Jiang, S.; Li, A.; Ruan, Z.; et al. Morphological diversity of single neurons in molecularly defined cell types. Nature 2021, 598, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Zingg, B.; Dong, H.-W.; Tao, H.W.; Zhang, L.I. Input–output organization of the mouse claustrum. J. Comp. Neurol. 2018, 526, 2428–2443. [Google Scholar] [CrossRef]
- Sherk, H. The claustrum and the cerebral cortex. In Sensory-Motor Areas and Aspects of Cortical Connectivity; Jones, E.G., Peters, A., Eds.; Springer: Boston, MA, USA, 1986; pp. 467–499. [Google Scholar]
- Atlan, G.; Terem, A.; Peretz-Rivlin, N.; Groysman, M.; Citri, A. Mapping synaptic cortico-claustral connectivity in the mouse. J. Comp. Neurol. 2017, 525, 1381–1402. [Google Scholar] [CrossRef]
- Crick, F.C.; Koch, C. What is the function of the claustrum? Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1271–1279. [Google Scholar] [CrossRef]
- Atilgan, H.; Doody, M.; Oliver, D.K.; McGrath, T.M.; Shelton, A.M.; Echeverria-Altuna, I.; Tracey, I.; Vyazovskiy, V.V.; Manohar, S.G.; Packer, A.M. Human lesions and animal studies link the claustrum to perception, salience, sleep and pain. Brain 2022, 145, 1610–1623. [Google Scholar] [CrossRef]
- Jackson, J.; Smith, J.B.; Lee, A.K. The Anatomy and Physiology of Claustrum-Cortex Interactions. Annu. Rev. Neurosci. 2020, 43, 231–247. [Google Scholar] [CrossRef]
- Madden, M.B.; Stewart, B.W.; White, M.G.; Krimmel, S.R.; Qadir, H.; Barrett, F.S.; Seminowicz, D.A.; Mathur, B.N. A role for the claustrum in cognitive control. Trends Cogn. Sci. 2022, 26, 1133–1152. [Google Scholar] [CrossRef]
- Liaw, Y.S.; Augustine, G.J. The claustrum and consciousness: An update. Int. J. Clin. Health. Psychol. 2023, 23, 100405. [Google Scholar] [CrossRef]
- Wong, K.L.L.; Nair, A.; Augustine, G.J. Changing the cortical conductor’s tempo: Neuromodulation of the claustrum. Front. Neural Circuits 2021, 15, 658228. [Google Scholar] [CrossRef] [PubMed]
- Jouvet, M. Sleep and serotonin: An unfinished story. Neuropsychopharmacology 1999, 21, 24–27. [Google Scholar] [CrossRef]
- Monti, J.M. The role of dorsal raphe nucleus serotonergic and non-serotonergic neurons, and of their receptors, in regulating waking and rapid eye movement (REM) sleep. Sleep Med. Rev. 2010, 14, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Portas, C.M.; Bjorvatn, B.; Ursin, R. Serotonin and the sleep/wake cycle: Special emphasis on microdialysis studies. Prog. Neurobiol. 2000, 60, 13–35. [Google Scholar] [CrossRef]
- Atlan, G.; Matosevich, N.; Peretz-Rivlin, N.; Marsh-Yvgi, I.; Zelinger, N.; Chen, E.; Kleinman, T.; Bleistein, N.; Sheinbach, E.; Groysman, M.; et al. Claustrum neurons projecting to the anterior cingulate restrict engagement during sleep and behavior. Nat Commun. 2024, 15, 5415. [Google Scholar] [CrossRef]
- Narikiyo, K.; Mizuguchi, R.; Ajima, A.; Shiozaki, M.; Hamanaka, H.; Johansen, J.P.; Mori, K.; Yoshihara, Y. The claustrum coordinates cortical slow-wave activity. Nat. Neurosci. 2020, 23, 741–753. [Google Scholar] [CrossRef]
- Norimoto, H.; Fenk, L.A.; Li, H.H.; Tosches, M.A.; Gallego-Flores, T.; Hain, D.; Reiter, S.; Kobayashi, R.; Macias, A.; Arends, A.; et al. A claustrum in reptiles and its role in slow-wave sleep. Nature 2020, 578, 413–418. [Google Scholar] [CrossRef]
- Olaghere Da Silva, U.; Morabito, M.; Canal, C.; Airey, D.; Emeson, R.; Sanders-Bush, E. Impact of RNA editing on functions of the serotonin 2C receptor in vivo. Front. Neurosci. 2010, 4, 26. [Google Scholar] [CrossRef]
- Kinsey, A.M.; Wainwright, A.; Heavens, R.; Sirinathsinghji, D.J.; Oliver, K.R. Distribution of 5-ht(5A), 5-ht(5B), 5-ht(6) and 5-HT(7) receptor mRNAs in the rat brain. Brain Research. Mol. Brain Res. 2001, 88, 194–198. [Google Scholar] [CrossRef]
- Mengod, G.; Nguyen, H.; Le, H.; Waeber, C.; Lubbert, H.; Palacios, J.M. The distribution and cellular localization of the serotonin 1C receptor mRNA in the rodent brain examined by in situ hybridization histochemistry. Comparison with receptor binding distribution. Neuroscience 1990, 35, 577–591. [Google Scholar] [CrossRef]
- Pompeiano, M.; Palacios, J.M.; Mengod, G. Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Brain Res. Mol. Brain Res. 1994, 23, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Rioux, A.; Fabre, V.; Lesch, K.P.; Moessner, R.; Murphy, D.L.; Lanfumey, L.; Hamon, M.; Martres, M.P. Adaptive changes of serotonin 5-HT2A receptors in mice lacking the serotonin transporter. Neurosci. Lett. 1999, 262, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.P.; Dorsa, D.M. Colocalization of serotonin receptor subtypes 5-HT2A, 5-HT2C, and 5-HT6 with neuropeptides in rat striatum. J. Comp. Neurol. 1996, 370, 405–414. [Google Scholar] [CrossRef]
- Wright, D.E.; Seroogy, K.B.; Lundgren, K.H.; Davis, B.M.; Jennes, L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J. Comp. Neurol. 1995, 351, 357–373. [Google Scholar] [CrossRef]
- Hamada, S.; Senzaki, K.; Hamaguchi-Hamada, K.; Tabuchi, K.; Yamamoto, H.; Yamamoto, T.; Yoshikawa, S.; Okano, H.; Okado, N. Localization of 5-HT2A receptor in rat cerebral cortex and olfactory system revealed by immunohistochemistry using two antibodies raised in rabbit and chicken. Mol. Brain Res. 1998, 54, 199–211. [Google Scholar] [CrossRef]
- Dawson, T.; Gehlert, D.; McCabe, R.; Barnett, A.; Wamsley, J. D-1 dopamine receptors in the rat brain: A quantitative autoradiographic analysis. J. Neurosci. 1986, 6, 2352–2365. [Google Scholar] [CrossRef]
- Gawliński, D.; Smaga, I.; Zaniewska, M.; Gawlińska, K.; Faron-Górecka, A.; Filip, M. Adaptive mechanisms following antidepressant drugs: Focus on serotonin 5-HT2A receptors. Pharmacol. Rep. 2019, 71, 994–1000. [Google Scholar] [CrossRef]
- Vertes, R.P. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J. Comp. Neurol. 1991, 313, 643–668. [Google Scholar] [CrossRef]
- Zhang, X.; Hannesson, D.K.; Saucier, D.M.; Wallace, A.E.; Howland, J.; Corcoran, M.E. Susceptibility to kindling and neuronal connections of the anterior claustrum. J. Neurosci. 2001, 21, 3674–3687. [Google Scholar] [CrossRef]
- Peyron, C.; Petit, J.M.; Rampon, C.; Jouvet, M.; Luppi, P.H. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience 1998, 82, 443–468. [Google Scholar] [CrossRef]
- Muzerelle, A.; Scotto-Lomassese, S.; Bernard, J.F.; Soiza-Reilly, M.; Gaspar, P. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5–B9) to the forebrain and brainstem. Brain Struct. Funct. 2016, 221, 535–561. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.E.; Baizer, J.S. Neurochemically defined cell types in the claustrum of the cat. Brain Res. 2007, 1159, 94–111. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.A.; Nichols, C.D. Psychedelics recruit multiple cellular types and produce complex transcriptional responses within the brain. EBioMedicine 2016, 11, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef]
- Barrett, F.S.; Krimmel, S.R.; Griffiths, R.; Seminowicz, D.A.; Mathur, B.N. Psilocybin acutely alters the functional connectivity of the claustrum with brain networks that support perception, memory, and attention. NeuroImage 2020, 218, 116980. [Google Scholar] [CrossRef]
- Doss, M.K.; Madden, M.B.; Gaddis, A.; Nebel, M.B.; Griffiths, R.R.; Mathur, B.N.; Barrett, F.S. Models of psychedelic drug action: Modulation of cortical-subcortical circuits. Brain 2022, 145, 441–456. [Google Scholar] [CrossRef]
- Snider, S.B.; Hsu, J.; Darby, R.R.; Cooke, D.; Fischer, D.; Cohen, A.L.; Grafman, J.H.; Fox, M.D. Cortical lesions causing loss of consciousness are anticorrelated with the dorsal brainstem. Hum. Brain Mapp. 2020, 41, 1520–1531. [Google Scholar] [CrossRef]
- Chia, Z.; Silberberg, G.; Augustine, G.J. Functional properties, topological organization and sexual dimorphism of claustrum neurons projecting to anterior cingulate cortex. Claustrum 2017, 2, 1357412. [Google Scholar] [CrossRef]
- Graf, M.; Nair, A.; Wong, K.L.L.; Tang, Y.; Augustine, G.J. Identification of mouse claustral neuron types based on their intrinsic electrical properties. eNeuro 2020, 7, ENEURO.0216-20.2020. [Google Scholar] [CrossRef]
- Cabrera, R.; Filevich, O.; García-Acosta, B.; Athilingam, J.; Bender, K.J.; Poskanzer, K.E.; Etchenique, R. A visible-light-sensitive caged serotonin. ACS Chem. Neurosci. 2017, 8, 1036–1042. [Google Scholar] [CrossRef]
- Bellot-Saez, A.; Stevenson, R.; Kékesi, O.; Samokhina, E.; Ben-Abu, Y.; Morley, J.W.; Buskila, Y. Neuromodulation of astrocytic K+ clearance. Int. J. Mol. Sci. 2021, 22, 2520. [Google Scholar] [CrossRef] [PubMed]
- Gantz, S.C.; Levitt, E.S.; Llamosas, N.; Neve, K.A.; Williams, J.T. Depression of serotonin synaptic transmission by the dopamine precursor L-DOPA. Cell Rep. 2015, 12, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Rea, A.C.; Vandenberg, L.N.; Ball, R.E.; Snouffer, A.A.; Hudson, A.G.; Zhu, Y.; McLain, D.E.; Johnston, L.L.; Lauderdale, J.D.; Levin, M.; et al. Light-activated serotonin for exploring its action in biological systems. Chem. Biol. 2013, 20, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Blackiston, D.J.; Rea, A.C.; Dore, T.M.; Levin, M. Left-right patterning in Xenopus conjoined twin embryos requires serotonin signaling and gap junctions. Int. J. Dev. Biol. 2014, 58, 799–809. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 30 October 2024).
- Augustine, G.J.; Huettel, S.; LaMantia, A.-S.; White, L.L. Neuroscience, 7th ed.; Oxford University Press: Sunderland, MA, USA, 2023. [Google Scholar]
- Arshadi, C.; Günther, U.; Eddison, M.; Harrington, K.I.S.; Ferreira, T.A. SNT: A unifying toolbox for quantification of neuronal anatomy. Nat. Methods 2021, 18, 374–377. [Google Scholar] [CrossRef]
- Backstrom, J.R.; Chang, M.S.; Chu, H.; Niswender, C.M.; Sanders-Bush, E. Agonist-directed signaling of serotonin 5-HT2C receptors: Differences between serotonin and lysergic acid diethylamide (LSD). Neuropsychopharmacology 1999, 21, 77–81. [Google Scholar] [CrossRef][Green Version]
- Maeda, K.; Sugino, H.; Akazawa, H.; Amada, N.; Shimada, J.; Futamura, T.; Yamashita, H.; Ito, N.; McQuade, R.D.; Mørk, A.; et al. Brexpiprazole I: In vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J. Pharmacol. Exp. Ther. 2014, 350, 589–604. [Google Scholar] [CrossRef]
- Sodickson, D.L.; Bean, B.P. Neurotransmitter activation of inwardly rectifying potassium current in dissociated hippocampal CA3 neurons: Interactions among multiple receptors. J. Neurosci. 1998, 18, 8153–8162. [Google Scholar] [CrossRef]
- del Burgo, L.S.; Cortes, R.; Mengod, G.; Zarate, J.; Echevarria, E.; Salles, J. Distribution and neurochemical characterization of neurons expressing GIRK channels in the rat brain. J. Comp. Neurol. 2008, 510, 581–606. [Google Scholar] [CrossRef]
- Jaén, C.; Doupnik, C.A. Neuronal Kir3.1/Kir3.2a channels coupled to serotonin 1A and muscarinic m2 receptors are differentially modulated by the ‘short’ RGS3 isoform. Neuropharmacology 2005, 49, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, A.; Corradetti, R.; Mlinar, B. Pharmacological characterization of 5-HT1A autoreceptor-coupled GIRK channels in rat dorsal raphe 5-HT neurons. PLoS ONE 2015, 10, e0140369. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Camardo, J.; Kandel, E.R. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc. Natl. Acad. Sci. USA 1982, 79, 5713–5717. [Google Scholar] [CrossRef] [PubMed]
- Ciranna, L. Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: Implications in physiological functions and in pathology. Curr. Neuropharmacol. 2006, 4, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef]
- Palchaudhuri, M.; Flügge, G. 5-HT1A receptor expression in pyramidal neurons of cortical and limbic brain regions. Cell Tissue Res. 2005, 321, 159–172. [Google Scholar] [CrossRef]
- Pazos, A.; Probst, A.; Palacios, J.M. Serotonin receptors in the human brain—III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience 1987, 21, 97–122. [Google Scholar] [CrossRef]
- el Mansari, M.; Blier, P. In vivo electrophysiological characterization of 5-HT receptors in the guinea pig head of caudate nucleus and orbitofrontal cortex. Neuropharmacology 1997, 36, 577–588. [Google Scholar] [CrossRef]
- Johansson, L.; Sohn, D.; Thorberg, S.-O.; Jackson, D.M.; Kelder, D.; Larsson, L.-G.; Rényi, L.; Ross, S.B.; Wallsten, C.; Eriksson, H.; et al. The pharmacological characterization of a novel selective 5-hydroxytryptamine 1A receptor antagonist, NAD-299. J. Pharmacol. Exp. Ther. 1997, 283, 216–225. [Google Scholar]
- Tang, Z.-Q.; Trussell, L.O. Serotonergic regulation of excitability of principal cells of the dorsal cochlear nucleus. J. Neurosci. 2015, 35, 4540–4551. [Google Scholar] [CrossRef]
- Zhang, Z. Serotonin induces tonic firing in layer V pyramidal neurons of rat prefrontal cortex during postnatal development. J. Neurosci. 2003, 23, 3373–3384. [Google Scholar] [CrossRef] [PubMed]
- Austgen, J.R.; Dantzler, H.A.; Barger, B.K.; Kline, D.D. 5-Hydroxytryptamine 2C receptors tonically augment synaptic currents in the nucleus tractus solitarii. J. Neurophysiol. 2012, 108, 2292–2305. [Google Scholar] [CrossRef] [PubMed]
- Bocchio, M.; Fucsina, G.; Oikonomidis, L.; McHugh, S.B.; Bannerman, D.M.; Sharp, T.; Capogna, M. Increased serotonin transporter expression reduces fear and recruitment of parvalbumin interneurons of the amygdala. Neuropsychopharmacology 2015, 40, 3015–3026. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Liang, Y.-C.; Lee, C.-C.; Wu, M.-Y.; Hsu, K.-S. Repeated cocaine administration decreases 5-HT2A receptor-mediated serotonergic enhancement of synaptic activity in rat medial prefrontal cortex. Neuropsychopharmacology 2009, 34, 1979–1992. [Google Scholar] [CrossRef]
- Aloyo, V.J.; Harvey, J.A. Antagonist binding at 5-HT2A and 5-HT2C receptors in the rabbit: High correlation with the profile for the human receptors. Eur. J. Pharmacol. 2000, 406, 163–169. [Google Scholar] [CrossRef]
- Knight, A.R.; Misra, A.; Quirk, K.; Benwell, K.; Revell, D.; Kennett, G.; Bickerdike, M. Pharmacological characterisation of the agonist radioligand binding site of 5-HT2A, 5-HT2B and 5-HT2C receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 370, 114–123. [Google Scholar] [CrossRef]
- Pehek, E.A.; Nocjar, C.; Roth, B.L.; Byrd, T.A.; Mabrouk, O.S. Evidence for the Preferential Involvement of 5-HT2A Serotonin Receptors in Stress- and Drug-Induced Dopamine Release in the Rat Medial Prefrontal Cortex. Neuropsychopharmacology 2006, 31, 265–277. [Google Scholar] [CrossRef]
- Nair, A.; Teo, Y.Y.; Augustine, G.J.; Graf, M. A functional logic for neurotransmitter corelease in the cholinergic forebrain pathway. Proc. Natl. Acad. Sci USA 2023, 120, e2218830120. [Google Scholar] [CrossRef]
- White, M.G.; Mathur, B.N. Claustrum circuit components for top-down input processing and cortical broadcast. Brain Struct. Funct. 2018, 223, 3945–3958. [Google Scholar] [CrossRef]
- Ferguson, K.A.; Cardin, J.A. Mechanisms underlying gain modulation in the cortex. Nat. Rev. Neurosci. 2020, 21, 80–92. [Google Scholar] [CrossRef]
- Silver, R.A. Neuronal arithmetic. Nat. Rev. Neurosci. 2010, 11, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Celada, P.; Puig, M.V.; Artigas, F. Serotonin modulation of cortical neurons and networks. Front. Integr. Neurosc. 2013, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Savalia, N.K.; Shao, L.-X.; Kwan, A.C. A dendrite-focused framework for understanding the actions of ketamine and psychedelics. Trends Neurosci. 2021, 44, 260–275. [Google Scholar] [CrossRef]
- Ellis-Davies, G.C.R. Two-photon uncaging of glutamate. Front. Synaptic Neurosci. 2019, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Pettit, D.L.; Wang, S.S.; Gee, K.R.; Augustine, G.J. Chemical two-photon uncaging: A novel approach to mapping glutamate receptors. Neuron 1997, 19, 465–471. [Google Scholar] [CrossRef]
- Wang, S.S.; Augustine, G.J. Confocal imaging and local photolysis of caged compounds: Dual probes of synaptic function. Neuron 1995, 15, 755–760. [Google Scholar] [CrossRef]
- Kasai, H.; Ucar, H.; Morimoto, Y.; Eto, F.; Okazaki, H. Mechanical transmission at spine synapses: Short-term potentiation and working memory. Curr. Opin. Neurobiol. 2023, 80, 102706. [Google Scholar] [CrossRef]
- Escobar, C.; Salas, M. Dendritic branching of claustral neurons in neonatally undernourished rats. Biol. Neonate 1995, 68, 47–54. [Google Scholar] [CrossRef]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef]
- Jeremic, D.; Sanchez-Rodriguez, I.; Jimenez-Diaz, L.; Navarro-Lopez, J.D. Therapeutic potential of targeting G protein-gated inwardly rectifying potassium (GIRK) channels in the central nervous system. Pharmacol. Ther. 2021, 223, 107808. [Google Scholar] [CrossRef]
- Kubo, Y.; Adelman, J.P.; Clapham, D.E.; Jan, L.Y.; Karschin, A.; Kurachi, Y.; Lazdunski, M.; Nichols, C.G.; Seino, S.; Vandenberg, C.A. International Union of Pharmacology. LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels. Pharmacol. Rev. 2005, 57, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Erwin, S.R.; Bristow, B.N.; Sullivan, K.E.; Kendrick, R.M.; Marriott, B.; Wang, L.; Clements, J.; Lemire, A.L.; Jackson, J.; Cembrowski, M.S. Spatially patterned excitatory neuron subtypes and projections of the claustrum. eLife 2021, 10, e68967. [Google Scholar] [CrossRef] [PubMed]
- Luscher, C.; Jan, L.Y.; Stoffel, M.; Malenka, R.C.; Nicoll, R.A. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 1997, 19, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Llamosas, N.; Ugedo, L.; Torrecilla, M. Inactivation of GIRK channels weakens the pre- and postsynaptic inhibitory activity in dorsal raphe neurons. Physiol. Rep. 2017, 5, e13141. [Google Scholar] [CrossRef] [PubMed]
- Spauschus, A.; Lentes, K.; Wischmeyer, E.; Dissmann, E.; Karschin, C.; Karschin, A. A G-protein-activated inwardly rectifying K+ channel (GIRK4) from human hippocampus associates with other GIRK channels. J. Neurosci. 1996, 16, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Wickman, K.; Karschin, C.; Karschin, A.; Picciotto, M.R.; Clapham, D.E. Brain localization and behavioral impact of the G-protein-gated K+ channel subunit GIRK4. J. Neurosci. 2000, 20, 5608–5615. [Google Scholar] [CrossRef]
- Bijak, M.; Misgeld, U. Effects of serotonin through serotonin1A and serotonin4 receptors on inhibition in the guinea-pig dentate gyrus in vitro. Neuroscience 1997, 78, 1017–1026. [Google Scholar] [CrossRef]
- Davies, M.F.; Deisz, R.A.; Prince, D.A.; Peroutka, S.J. Two distinct effects of 5-hydroxytryptamine on single cortical neurons. Brain Res. 1987, 423, 347–352. [Google Scholar] [CrossRef]
- Perrier, J.-F.; Alaburda, A.; Hounsgaard, J. 5-HT1A receptors increase excitability of spinal motoneurons by inhibiting a TASK-1-like K+ current in the adult turtle. J. Physiol. 2003, 548, 485–492. [Google Scholar] [CrossRef]
- Raymond, J.R.; Mukhin, Y.V.; Gelasco, A.; Turner, J.; Collinsworth, G.; Gettys, T.W.; Grewal, J.S.; Garnovskaya, M.N. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol. Ther. 2001, 92, 179–212. [Google Scholar] [CrossRef]
- Peroutka, S.J. 5-HT receptors: Past, present and future. Trends Neurosci. 1995, 18, 68–69. [Google Scholar] [CrossRef] [PubMed]
- Azimi, Z.; Barzan, R.; Spoida, K.; Surdin, T.; Wollenweber, P.; Mark, M.D.; Herlitze, S.; Jancke, D. Separable gain control of ongoing and evoked activity in the visual cortex by serotonergic input. eLife 2020, 9, e53552. [Google Scholar] [CrossRef] [PubMed]
- Araneda, R.; Andrade, R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 1991, 40, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Puig, M.V.; Gulledge, A.T. Serotonin and prefrontal cortex function: Neurons, networks, and circuits. Mol. Neurobiol. 2011, 44, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, X.; Liu, P.; Jing, S.; Du, H.; Zhang, L.; Jia, F.; Li, A. Serotonergic afferents from the dorsal raphe decrease the excitability of pyramidal neurons in the anterior piriform cortex. Proc. Natl. Acad. Sci. USA 2020, 117, 3239–3247. [Google Scholar] [CrossRef]
- Tian, M.K.; Schmidt, E.F.; Lambe, E.K. Serotonergic suppression of mouse prefrontal circuits implicated in task attention. eNeuro 2016, 3, ENEURO.0269-0216.2016. [Google Scholar] [CrossRef]
- Vargas, M.V.; Dunlap, L.E.; Dong, C.; Carter, S.J.; Tombari, R.J.; Jami, S.A.; Cameron, L.P.; Patel, S.D.; Hennessey, J.J.; Saeger, H.N.; et al. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science 2023, 379, 700–706. [Google Scholar] [CrossRef]
- Burns, C.M.; Chu, H.; Rueter, S.M.; Hutchinson, L.K.; Canton, H.; Sanders-Bush, E.; Emeson, R.B. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature 1997, 387, 303–308. [Google Scholar] [CrossRef]
- Fitzgerald, L.W.; Iyer, G.; Conklin, D.S.; Krause, C.M.; Marshall, A.; Patterson, J.P.; Tran, D.P.; Jonak, G.J.; Hartig, P.R. Messenger RNA Editing of the Human Serotonin 5-HT2C Receptor. Neuropsychopharmacology 1999, 21, 82–90. [Google Scholar] [CrossRef]
- Okada, M.; Goldman, D.; Linnoila, M.; Iwata, N.; Ozaki, N.; Northup, J.K. Comparison of G-Protein selectivity of human 5-HT2C and 5-HT1A receptors. Ann. N. Y. Acad. Sci. 2004, 1025, 570–577. [Google Scholar] [CrossRef]
- Maroteaux, L.; Béchade, C.; Roumier, A. Dimers of serotonin receptors: Impact on ligand affinity and signaling. Biochimie 2019, 161, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Dahlström, A.B.; Jonsson, G.; Marcellino, D.; Guescini, M.; Dam, M.; Manger, P.; Agnati, L. The discovery of central monoamine neurons gave volume transmission to the wired brain. Prog. Neurobiol. 2010, 90, 82–100. [Google Scholar] [CrossRef] [PubMed]
- Jadi, M.; Polsky, A.; Schiller, J.; Mel, B.W. Location-dependent effects of inhibition on local spiking in pyramidal neuron dendrites. PLoS Comput. Biol. 2012, 8, e1002550. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, R.; Lee, S.; Rudy, B. Gabaergic interneurons in the neocortex: From cellular properties to circuits. Neuron 2016, 91, 260–292. [Google Scholar] [CrossRef]
- Wilson, N.R.; Runyan, C.A.; Wang, F.L.; Sur, M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 2012, 488, 343–348. [Google Scholar] [CrossRef]
- Mantas, I.; Flais, I.; Masarapu, Y.; Ionescu, T.; Frapard, S.; Jung, F.; Le Merre, P.; Saarinen, M.; Tiklova, K.; Salmani, B.Y.; et al. Claustrum and dorsal endopiriform cortex complex cell-identity is determined by Nurr1 and regulates hallucinogenic-like states in mice. Nat. Commun. 2024, 15, 8176. [Google Scholar] [CrossRef]
- Madden, M.; Mathur, B.N. Transclaustral circuit strength is attenuated by serotonin. Soc. Neurosci. Abstr. 2023, 53, PSTR456.20. [Google Scholar]
- Anderson, T.L.; Keady, J.V.; Songrady, J.; Tavakoli, N.S.; Asadipooya, A.; Neeley, R.E.; Turner, J.R.; Ortinski, P.I. Distinct 5-HT receptor subtypes regulate claustrum excitability by serotonin and the psychedelic, DOI. Prog. Neurobiol. 2024, 240, 102660. [Google Scholar] [CrossRef]
- Wong, K.L.L. Serotonergic Modulation of the Claustrum. Doctoral Thesis, Nanyang Technological University, Singapore, 2021. Available online: https://hdl.handle.net/10356/155926 (accessed on 30 October 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, K.L.L.; Graf, M.; Augustine, G.J. Serotonin Inhibition of Claustrum Projection Neurons: Ionic Mechanism, Receptor Subtypes and Consequences for Claustrum Computation. Cells 2024, 13, 1980. https://doi.org/10.3390/cells13231980
Wong KLL, Graf M, Augustine GJ. Serotonin Inhibition of Claustrum Projection Neurons: Ionic Mechanism, Receptor Subtypes and Consequences for Claustrum Computation. Cells. 2024; 13(23):1980. https://doi.org/10.3390/cells13231980
Chicago/Turabian StyleWong, Kelly Li Lin, Martin Graf, and George J. Augustine. 2024. "Serotonin Inhibition of Claustrum Projection Neurons: Ionic Mechanism, Receptor Subtypes and Consequences for Claustrum Computation" Cells 13, no. 23: 1980. https://doi.org/10.3390/cells13231980
APA StyleWong, K. L. L., Graf, M., & Augustine, G. J. (2024). Serotonin Inhibition of Claustrum Projection Neurons: Ionic Mechanism, Receptor Subtypes and Consequences for Claustrum Computation. Cells, 13(23), 1980. https://doi.org/10.3390/cells13231980






