The Formation of Human Arteriovenous Malformation Organoids and Their Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Cell Culture
2.1.1. Isolation and Culture of Human Fibroblasts
2.1.2. Isolation and Two-Dimensional (2D) Culture of Human Endothelial Cells (ECs)
2.1.3. Reprogramming of Human Fibroblasts and Culture
2.2. Generation of Blood Vessel Organoids (BVOs)
2.3. Immunofluorescence Staining
2.4. Whole-Mount Immunostaining of Organoids
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. TaqMan Assay (mir)
2.7. Statistical Analysis
3. Results
3.1. Identification of Two-Dimensional Cells
3.2. Whole-Mount Immunostaining of Organoids
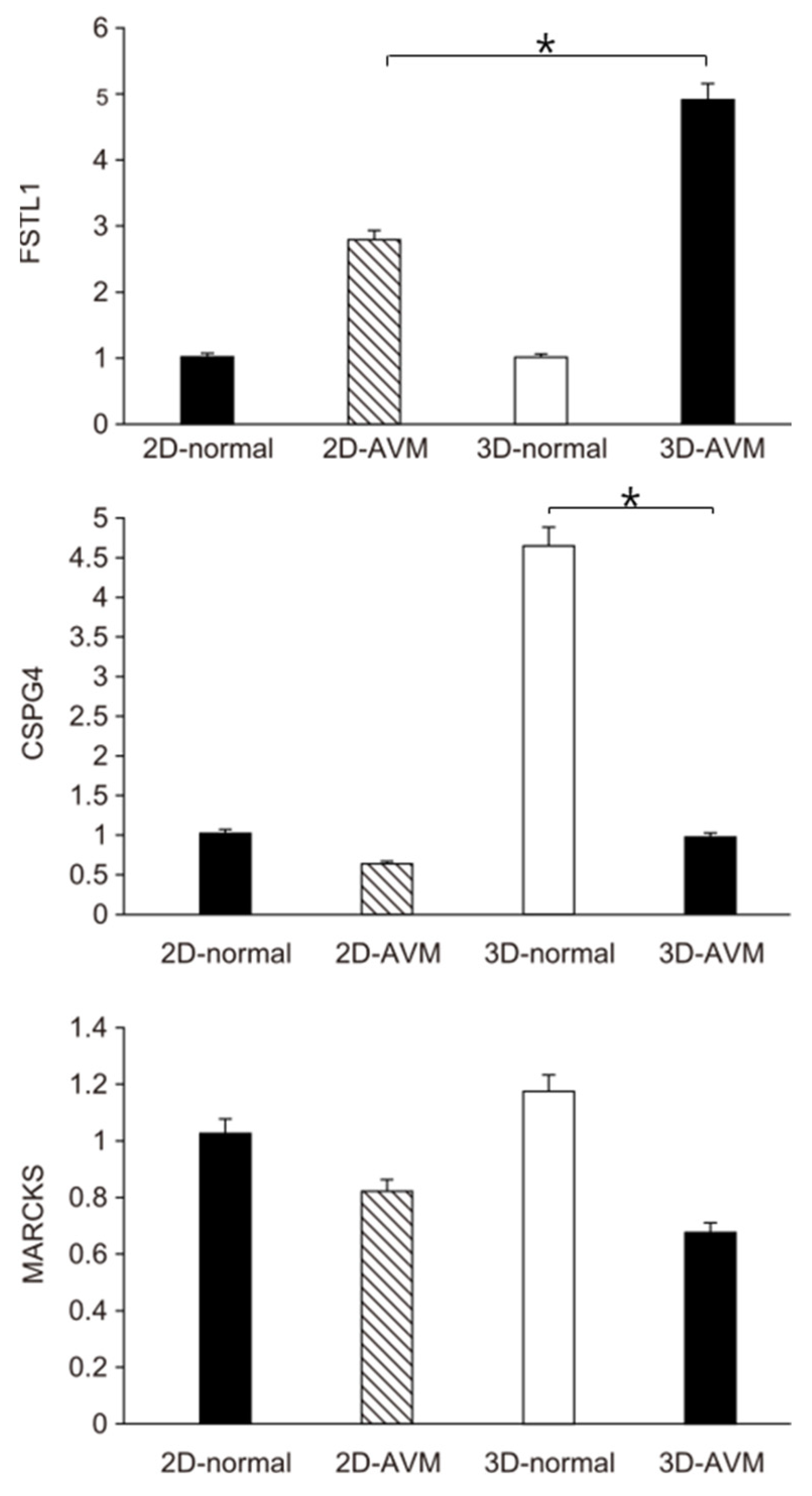
3.3. Real-Time PCR
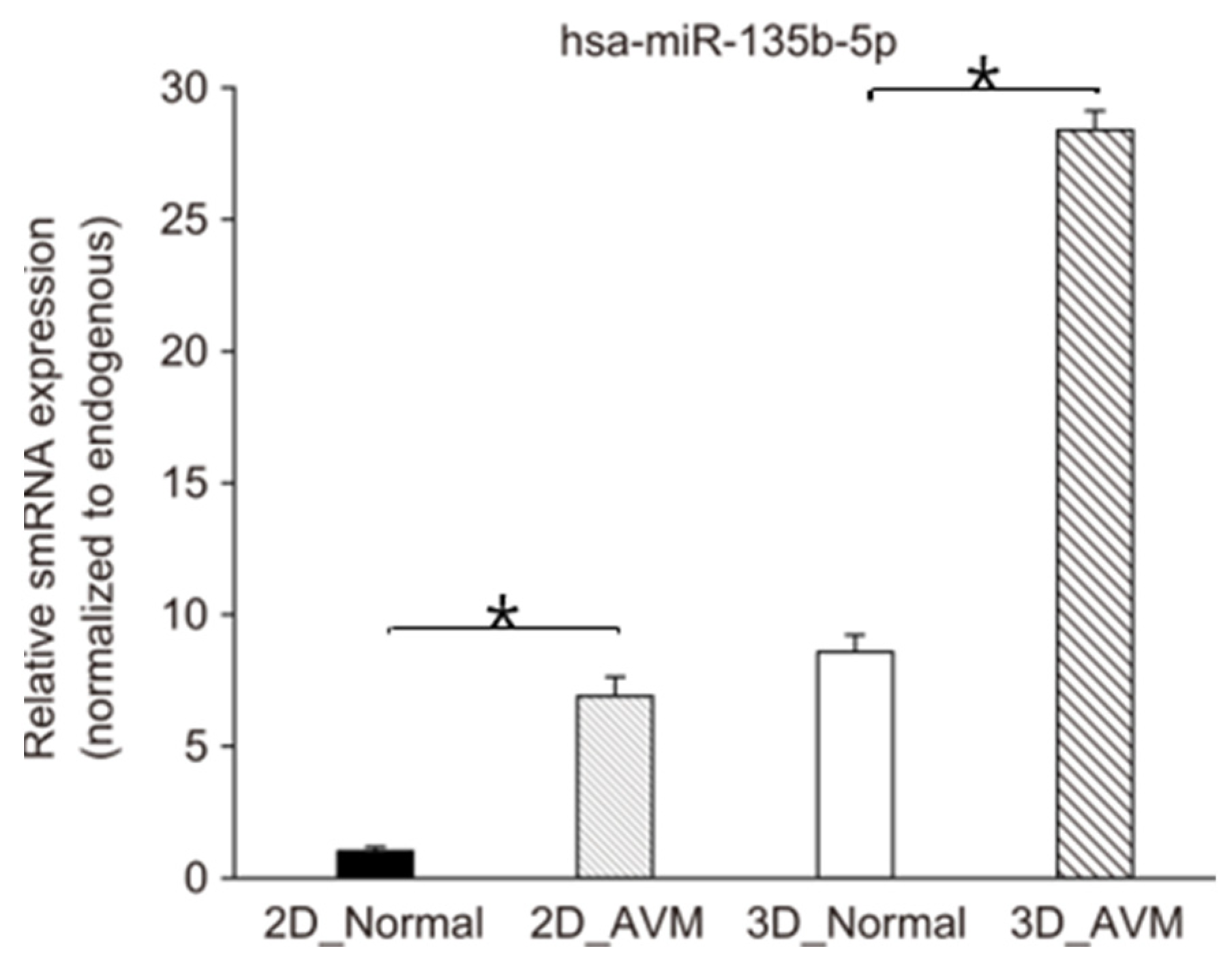
3.4. TaqMan Real-Time PCR Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, D.M. Practical genetic and biologic therapeutic considerations in vascular anomalies. Tech. Vasc. Interv. Radiol. 2019, 22, 100629. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, H.Y.; Kim, B.S.; Chung, H.Y.; Lee, J.M.; Huh, S.; Bae, H.I. Capillary malformation of port-wine stain: Differentiation from early arteriovenous malformation by histopathological clues. Am. J. Dermatopathol. 2012, 34, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Lee, J.W.; Choi, K.Y.; Yang, J.D.; Cho, B.C.; Lee, S.J.; Kim, Y.S.; Lee, J.M.; Huh, S.; Chung, H.Y. Clinical Characteristics of Arteriovenous Malformations of the Head and Neck. Dermatol. Surg. 2017, 43, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Chen, G.; Li, J.; Qin, K.; Ding, X.; Peng, C.; Zhou, D.; Lin, X. Three-dimensional brain arteriovenous malformation models for clinical use and resident training. Medicine 2018, 97, e9516. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Pontoriero, A.; Iatì, G.; Marino, D.; La Torre, D.; Vinci, S.; Germanò, A.; Pergolizzi, S.; Francesco, T. 3D-Printing of Arteriovenous Malformations for Radiosurgical Treatment: Pushing Anatomy Understanding to Real Boundaries. Cureus 2016, 8, e594. [Google Scholar] [CrossRef]
- Boon, L.M.; Dekeuleneer, V.; Coulie, J.; Marot, L.; Bataille, A.C.; Hammer, F.; Clapuyt, P.; Jeanjean, A.; Dompmartin, A.; Vikkula, M. Case report study of thalidomide therapy in 18 patients with severe arteriovenous malformation s. Nat. Cardiovasc. Res. 2022, 1, 562–567. [Google Scholar] [CrossRef]
- Liu, A.S.; Mulliken, J.B.; Zurakowski, D.; Fishman, S.J.; Greene, A.K. Extracranial Arteriovenous Malformations: Natural Progression and Recurrence after Treatment. Plast. Reconstr. Surg. 2010, 125, 1185–1194. [Google Scholar] [CrossRef]
- Alghuwainem, A.; Alshareeda, A.T.; Alsowayan, B. Scaffold-free 3-D cell sheet technique bridges the gap between 2-D cell culture and animal models. Int. J. Mol. Sci. 2019, 20, 4926. [Google Scholar] [CrossRef]
- Lee, J.S.; Cho, H.G.; Ryu, J.Y.; Oh, E.J.; Kim, H.M.; Kwak, S.I.; Lee, S.J.; Lee, J.M.; Lee, S.Y.; Huh, S.; et al. Hypoxia Promotes Angiogenic Effect in Extracranial Arteriovenous Malformation Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 9109. [Google Scholar] [CrossRef]
- Seebauer, C.T.; Wiens, B.; Hintschich, C.A.; Platz Batista da Silva, N.; Evert, K.; Haubner, F.; Kapp, F.G.; Wendl, C.; Renner, K.; Bohr, C.; et al. Targeting the microenvironment in the treatment of arteriovenous malformations. Angiogenesis 2024, 27, 91–103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, W.-H.; Melnychenko, I.; Eschenhagen, T. Engineered heart tissue for regeneration of diseased hearts. Biomaterials 2004, 25, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Dye, B.R.; Hill, D.R.; Ferguson, M.A.; Tsai, Y.H.; Nagy, M.S.; Dyal, R.; Wells, J.M.; Mayhew, C.N.; Nattiv, R.; Klein, O.D.; et al. In vitro generation of human pluripotent stem cell derived lung organoids. eLife 2015, 4, e05098. [Google Scholar] [CrossRef]
- Kauffman, A.L.; Ekert, J.E.; Gyurdieva, A.V.; Rycyzyn, M.A.; Hornby, P.J. Directed differentiation protocols for successful human intestinal organoids derived from multiple induced pluripotent stem cell lines. Stem Cell Biol. Res. 2015, 2, 1. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Kerjaschki, D.; Penninger, J.M. Generation of blood vessel organoids from human pluripotent stem cells. Nat. Protoc. 2019, 14, 3082–3100. [Google Scholar] [CrossRef] [PubMed]
- Shalek, A.K.; Satija, R.; Shuga, J.; Trombetta, J.J.; Gennert, D.; Lu, D.; Chen, P.; Gertner, R.S.; Gaublomme, J.T.; Yosef, N.; et al. Singlecell RNA-seq reveals dynamic paracrine control of cellular variation. Nature 2014, 510, 363–369. [Google Scholar] [CrossRef]
- Ricard, N.; Bailly, S.; Guignabert, C.; Simons, M. The quiescent endothelium: Signalling pathways regulating organ-specifc endothelial normalcy. Nat. Rev. Cardiol. 2021, 18, 565–580. [Google Scholar] [CrossRef]
- Kalucka, J.; de Rooij, L.P.; Goveia, J.; Rohlenova, K.; Dumas, S.J.; Meta, E.; Conchinha, N.V.; Taverna, F.; Teuwen, L.A.; Veys, K.; et al. Single-cell transcriptome atlas of murine endothelial cells. Cell 2020, 180, 764–779.e20. [Google Scholar] [CrossRef]
- Pasut, A.; Becker, L.M.; Cuypers, A.; Carmeliet, P. Endothelial cell plasticity at the single-cell level. Angiogenesis 2021, 24, 311–326. [Google Scholar] [CrossRef]
- Gurevich, D.B.; David, D.T.; Sundararaman, A.; Patel, J. Endothelial heterogeneity in development and wound healing. Cells 2021, 10, 2338. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, G.H.; Lee, J.H.; Ryu, J.Y.; Oh, E.J.; Kim, H.M.; Kwak, S.I.; Hur, K.; Chung, H.Y. MicroRNA-365a/b-3p as a Potential Biomarker for Hypertrophic Scars. Int. J. Mol. Sci. 2022, 23, 6117. [Google Scholar] [CrossRef] [PubMed]
- Mulliken, J.B.; Glowacki, J. Hemangiomas and vascular malformations in infants and children: A classification based on endothelial characteristics. Plast. Reconstr. Surg. 1982, 69, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Koo, B.-K. Modeling mouse and human development using organoid cultures. Development 2015, 142, 3113–3125. [Google Scholar] [CrossRef]
- Clevers, H. Modeling development and disease with organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- McCracken, K.W.; Catá, E.M.; Crawford, C.M.; Sinagoga, K.L.; Schumacher, M.; Rockich, B.E.; Tsai, Y.H.; Mayhew, C.N.; Spence, J.R.; Zavros, Y.; et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014, 516, 400–404. [Google Scholar] [CrossRef]
- Bigorgne, A.E.; Farin, H.F.; Lemoine, R.; Mahlaoui, N.; Lambert, N.; Gil, M.; Schulz, A.; Philippet, P.; Schlesser, P.; Abrahamsen, T.G.; et al. TTC7A mutations disrupt intestinal epithelial apicobasal polarity. J. Clin. Investig. 2014, 124, 328–337. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van Den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef]
- Dekkers, J.F.; Berkers, G.; Kruisselbrink, E.; Vonk, A.; De Jonge, H.R.; Janssens, H.M.; Bronsveld, I.; van de Graaf, E.A.; Nieuwenhuis, E.E.; Houwen, R.H.; et al. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci. Transl. Med. 2016, 8, 344ra84. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, G.; Lee, J.H.; Ryu, J.Y.; Oh, E.J.; Kim, H.M.; Kwak, S.; Hur, K.; Chung, H.Y. MicroRNA-135b-5p Is a Pathologic Biomarker in the Endothelial Cells of Arteriovenous Malformations. Int. J. Mol. Sci. 2024, 25, 4888. [Google Scholar] [CrossRef] [PubMed]



| No. | Age | Sex | AVM Status | Sample Type | Location | No. | Age | Sex | AVM Status | Sample Type | Location |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | M | None | Skin | Lt. Back | 13 | 6y4m | F | AVM | Skin | Lt. Cheek |
| 2 | 4y9m | M | None | Skin | Lt. Ear | 14 | 7y3m | F | AVM | Skin | Rt. Ear |
| 3 | 5y11m | M | None | Skin | Rt. Forearm | 15 | 44 | F | AVM | Skin | Rt. Ear |
| 4 | 59 | F | None | Skin | Rt. Inguinal | 16 | 18y4m | F | AVM | Skin | Lt. Chest |
| 5 | 65 | M | None | Skin | Lt. Forearm | 17 | 23 | F | AVM | Skin | Lt. Cheek |
| 6 | 11y6m | F | None | Skin | Lt. Cheek | 18 | 29 | M | AVM | Skin | Rt. Heel |
| 7 | 47 | F | None | Blood vessel | Lt. Axilla | 19 | 7y3m | F | AVM | Blood vessel | Rt. Ear |
| 8 | 75 | M | None | Blood vessel | Lt. Axilla | 20 | 27 | F | AVM | Blood vessel | Rt. Ear |
| 9 | 47 | M | None | Blood vessel | Lt. Axilla | 21 | 66 | M | AVM | Blood vessel | Rt. Trunk |
| 10 | 29 | M | None | Blood vessel | Lt. Thigh | 22 | 52 | M | AVM | Blood vessel | Rt. Glabella |
| 11 | 45 | M | None | Blood vessel | Rt. Inguinal | 23 | 29 | M | AVM | Blood vessel | Rt. Heel |
| 12 | 8y3m | F | None | Blood vessel | Rt. Inguinal | 24 | 23 | F | AVM | Blood vessel | Lt. Cheek |
| Primer Sequence | ||
|---|---|---|
| FSTL1 | Forward sequence | TCGCATCATCCAGTGGCTGGAA |
| Reverse sequence | TCACTGGAGTCCAGGCGAGAAT | |
| MARCKS | Forward sequence | CTCCTCGACTTCTTCGCCCAAG |
| Reverse sequence | TCTTGAAGGAGAAGCCGCTCAG | |
| CSPG4 | Forward sequence | GTCCTGCCTGTCAATGACCAAC |
| Reverse sequence | CGATGGTGTAGACCAGATCCTC | |
| Title | Mature miRNA Sequence (5′-3′) |
|---|---|
| Endogenous | UUAUCAGAAUCUCCAGGGGUAC |
| hsa-miR-135b-5p | UAUGGCUUUUCAUUCCUAUGUGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, E.J.; Kim, H.M.; Kwak, S.; Huh, C.; Chung, H.Y. The Formation of Human Arteriovenous Malformation Organoids and Their Characteristics. Cells 2024, 13, 1955. https://doi.org/10.3390/cells13231955
Oh EJ, Kim HM, Kwak S, Huh C, Chung HY. The Formation of Human Arteriovenous Malformation Organoids and Their Characteristics. Cells. 2024; 13(23):1955. https://doi.org/10.3390/cells13231955
Chicago/Turabian StyleOh, Eun Jung, Hyun Mi Kim, Suin Kwak, Chanhoe Huh, and Ho Yun Chung. 2024. "The Formation of Human Arteriovenous Malformation Organoids and Their Characteristics" Cells 13, no. 23: 1955. https://doi.org/10.3390/cells13231955
APA StyleOh, E. J., Kim, H. M., Kwak, S., Huh, C., & Chung, H. Y. (2024). The Formation of Human Arteriovenous Malformation Organoids and Their Characteristics. Cells, 13(23), 1955. https://doi.org/10.3390/cells13231955








