Ubiquitin-Specific Protease 1 Promotes Bladder Cancer Progression by Stabilizing c-MYC
Abstract
1. Background
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Antibodies
2.3. Construction of USP1-Overexpressing and USP1-Knockout Cell Lines
2.4. Western Blot and Co-Immunoprecipitation
2.5. Immunohistochemistry
2.6. Cell Proliferation, Colony Formation, and Transwell Assays
2.7. Luciferase Assay
2.8. Immunofluorescence
2.9. Xenograft Animal Model
2.10. Statistical Analyses
3. Results
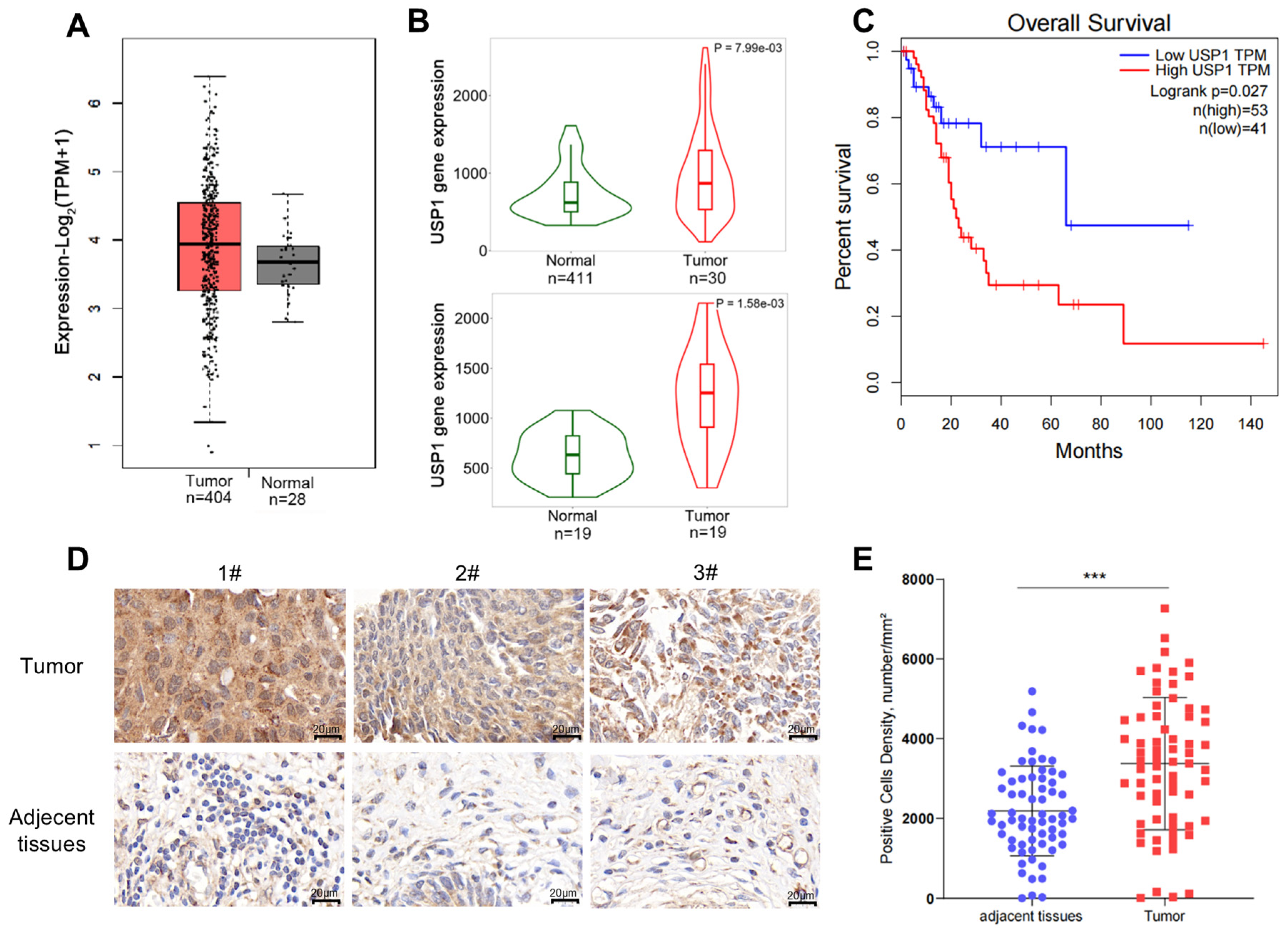
3.1. USP1 Expression Is Upregulated in Bladder Cancer
3.2. USP1 Overexpression Promotes Cell Proliferation, Migration, and Invasion
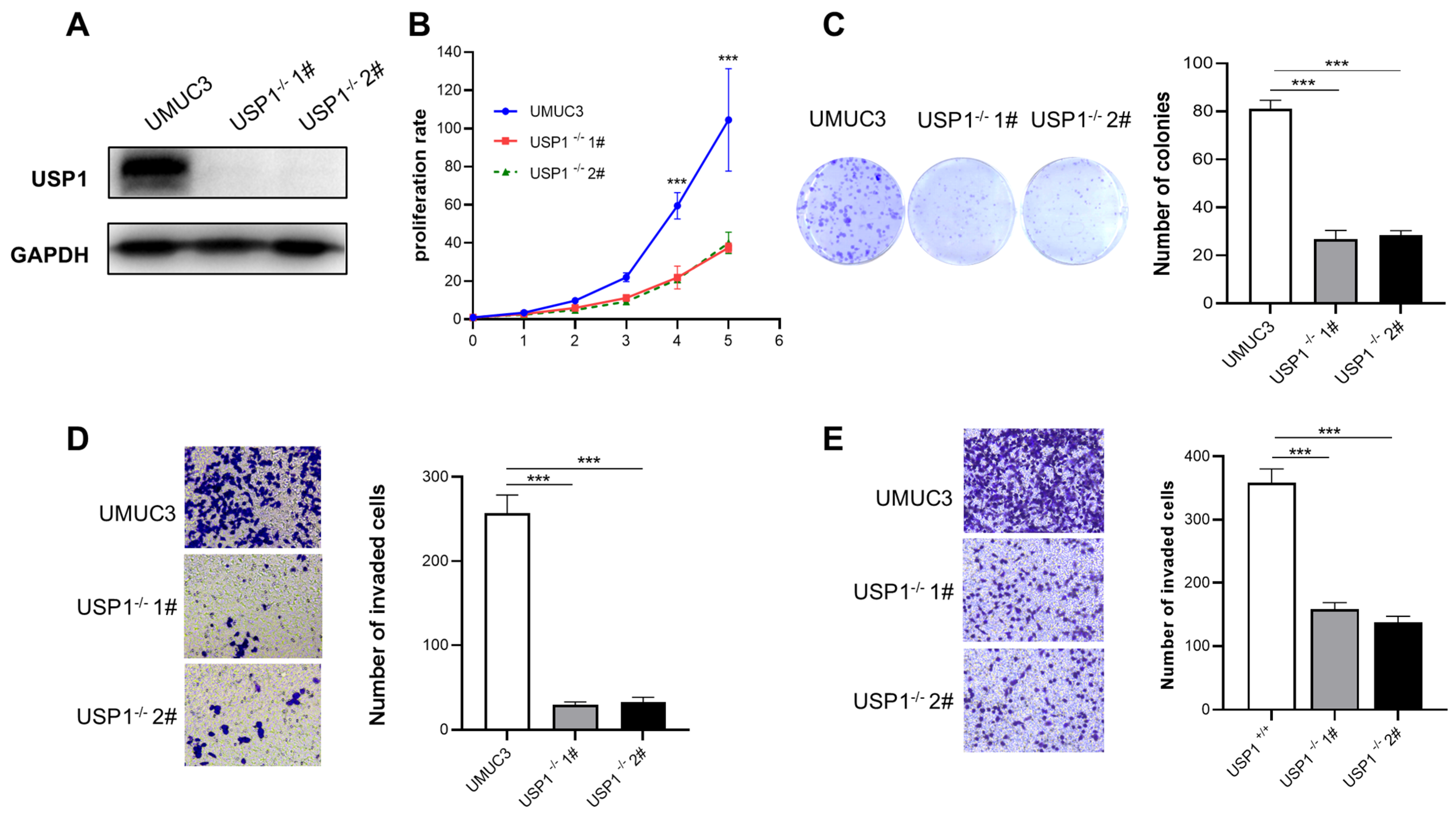
3.3. USP1-Knockout Represses Cell Proliferation, Migration, and Invasion
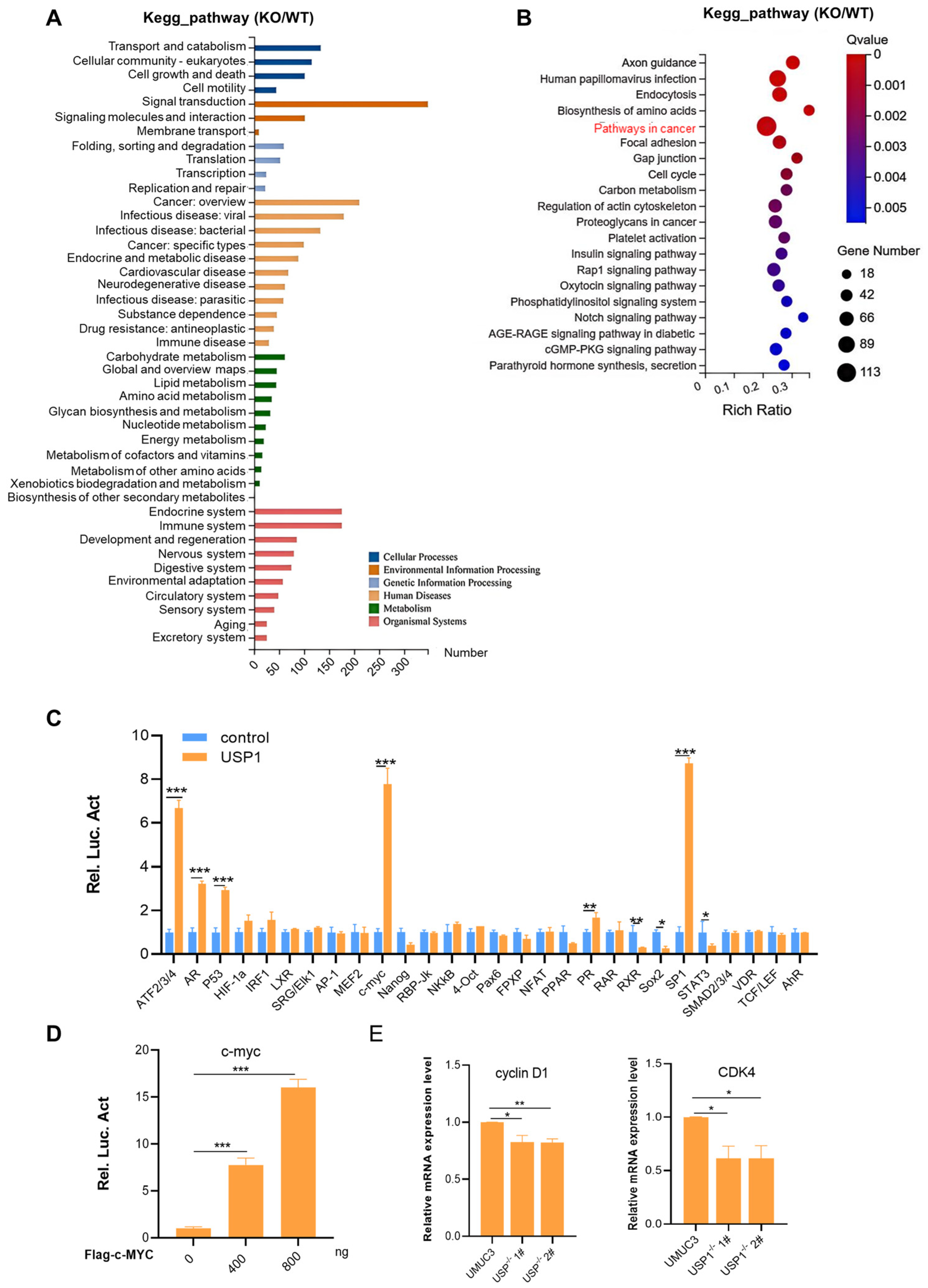
3.4. USP1 Upregulates the c-MYC Pathway
3.5. USP1 Deubiquitinates and Stabilizes c-MYC
3.6. USP1-Knockout Inhibits Tumor Formation In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Minoli, M.; Kiener, M.; Thalmann, G.N.; Kruithof-de Julio, M.; Seiler, R. Evolution of Urothelial Bladder Cancer in the Context of Molecular Classifications. Int. J. Mol. Sci. 2020, 21, 5670. [Google Scholar] [CrossRef]
- Al Hussein Al Awamlh, B.; Chang, S.S. Novel Therapies for High-Risk Non-Muscle Invasive Bladder Cancer. Curr. Oncol. Rep. 2023, 25, 83–91. [Google Scholar] [CrossRef]
- Mansour, M.A. Ubiquitination: Friend and foe in cancer. Int. J. Biochem. Cell Biol. 2018, 101, 80–93. [Google Scholar] [CrossRef]
- Darling, S.; Fielding, A.B.; Sabat-Pospiech, D.; Prior, I.A.; Coulson, J.M. Regulation of the cell cycle and centrosome biology by deubiquitylases. Biochem. Soc. Trans. 2017, 45, 1125–1136. [Google Scholar] [CrossRef]
- Ruan, J.; Schluter, D.; Wang, X. Deubiquitinating enzymes (DUBs): DoUBle-edged swords in CNS autoimmunity. J. Neuroinflamm. 2020, 17, 102. [Google Scholar] [CrossRef]
- Nanduri, B.; Suvarnapunya, A.E.; Venkatesan, M.; Edelmann, M.J. Deubiquitinating enzymes as promising drug targets for infectious diseases. Curr. Pharm. Des. 2013, 19, 3234–3247. [Google Scholar] [CrossRef]
- Zhang, H.H.; Li, C.; Ren, J.W.; Liu, L.; Du, X.H.; Gao, J.; Liu, T.; Li, S.Z. OTUB1 facilitates bladder cancer progression by stabilizing ATF6 in response to endoplasmic reticulum stress. Cancer Sci. 2021, 112, 2199–2209. [Google Scholar] [CrossRef]
- Nijman, S.M.; Huang, T.T.; Dirac, A.M.; Brummelkamp, T.R.; Kerkhoven, R.M.; D’Andrea, A.D.; Bernards, R. The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Mol. Cell 2005, 17, 331–339. [Google Scholar] [CrossRef]
- Huang, T.T.; Nijman, S.M.; Mirchandani, K.D.; Galardy, P.J.; Cohn, M.A.; Haas, W.; Gygi, S.P.; Ploegh, H.L.; Bernards, R.; D’Andrea, A.D. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat. Cell Biol. 2006, 8, 339–347. [Google Scholar] [CrossRef]
- Song, B.; Jiang, Y.; Jiang, Y.; Lin, Y.; Liu, J. ML323 suppresses the progression of ovarian cancer via regulating USP1-mediated cell cycle. Front. Genet. 2022, 13, 917481. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Li, D. Ubiquitin-specific protease 1 overexpression indicates poor prognosis and promotes proliferation, migration, and invasion of gastric cancer cells. Tissue Cell 2022, 74, 101723. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, T.; Shen, Z.; Zheng, Y.; Geng, Q.; Li, L.; Sha, B.; Li, M.; Sun, Y.; Guo, Y.; et al. Inhibition of USP1 activates ER stress through Ubi-protein aggregation to induce autophagy and apoptosis in HCC. Cell Death Dis. 2022, 13, 951. [Google Scholar] [CrossRef]
- Woo, S.M.; Kim, S.; Seo, S.U.; Kim, S.; Park, J.W.; Kim, G.; Choi, Y.R.; Hur, K.; Kwon, T.K. Inhibition of USP1 enhances anticancer drugs-induced cancer cell death through downregulation of survivin and miR-216a-5p-mediated upregulation of DR5. Cell Death Dis. 2022, 13, 821. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Li, X.; Feng, S.; Huang, Q.; Zhuang, T.; Yan, C.; Qian, H.; Ding, Y.; Zhu, J.; Xu, W. The deubiquitinating enzyme USP1 modulates ERalpha and modulates breast cancer progression. J. Cancer 2020, 11, 6992–7000. [Google Scholar] [CrossRef]
- Li, X.Y.; Wu, J.C.; Liu, P.; Li, Z.J.; Wang, Y.; Chen, B.Y.; Hu, C.L.; Fei, M.Y.; Yu, P.C.; Jiang, Y.L.; et al. Inhibition of USP1 reverses the chemotherapy resistance through destabilization of MAX in the relapsed/refractory B-cell lymphoma. Leukemia 2023, 37, 164–177. [Google Scholar] [CrossRef]
- Xu, X.; Li, S.; Cui, X.; Han, K.; Wang, J.; Hou, X.; Cui, L.; He, S.; Xiao, J.; Yang, Y. Inhibition of Ubiquitin Specific Protease 1 Sensitizes Colorectal Cancer Cells to DNA-Damaging Chemotherapeutics. Front. Oncol. 2019, 9, 1406. [Google Scholar] [CrossRef]
- Sonego, M.; Pellarin, I.; Costa, A.; Vinciguerra, G.L.R.; Coan, M.; Kraut, A.; D’Andrea, S.; Dall’Acqua, A.; Castillo-Tong, D.C.; Califano, D.; et al. USP1 links platinum resistance to cancer cell dissemination by regulating Snail stability. Sci. Adv. 2019, 5, eaav3235. [Google Scholar] [CrossRef]
- Shalem, O.; Sanjana, N.E.; Hartenian, E.; Shi, X.; Scott, D.A.; Mikkelson, T.; Heckl, D.; Ebert, B.L.; Root, D.E.; Doench, J.G.; et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 2014, 343, 84–87. [Google Scholar] [CrossRef]
- Sanjana, N.E.; Shalem, O.; Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 2014, 11, 783–784. [Google Scholar] [CrossRef]
- Zhang, H.H.; Zhang, A.Q.; Peng, P.; Huang, L.; Liu, C.Y.; Nie, X.R.; Hou, D.F.; Zhang, X.; Li, S.Z. USP5 facilitates bladder cancer progression by stabilizing the c-Jun protein. Cancer Cell Int. 2024, 24, 32. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, L.; Zuo, X.; Luo, H.; Sheng, Y.; Yan, J. USP1 Promotes GC Metastasis via Stabilizing ID2. Dis. Markers 2021, 2021, 3771990. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Y.; Gao, C.; Sun, Y.; Zhou, K.; Wang, P.; Cheng, J.; Guo, W.; Ya, C.; Fan, J.; et al. USP1 Maintains the Survival of Liver Circulating Tumor Cells by Deubiquitinating and Stabilizing TBLR1. Front. Oncol. 2020, 10, 554809. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Tang, M.; Zhang, L.; Wang, B.; Yang, Z.; Liu, Y.; Xu, G.; Wu, L.; Jing, T.; Xu, X.; et al. Correction to: USP1 inhibition destabilizes KPNA2 and suppresses breast cancer metastasis. Oncogene 2022, 41, 1673. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, H.; Zhong, N.; Jiang, Z.; Xu, L.; Deng, Y.; Jiang, Z.; Wang, H.; Wang, J. Gene silencing of USP1 by lentivirus effectively inhibits proliferation and invasion of human osteosarcoma cells. Int. J. Oncol. 2016, 49, 2549–2557. [Google Scholar] [CrossRef]
- Yuan, P.; Feng, Z.; Huang, H.; Wang, G.; Chen, Z.; Xu, G.; Xie, Z.; Jie, Z.; Zhao, X.; Ma, Q.; et al. USP1 inhibition suppresses the progression of osteosarcoma via destabilizing TAZ. Int. J. Biol. Sci. 2022, 18, 3122–3136. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Guo, Z.; Song, D.; Chen, Z.; Sun, M. Ubiquitin-specific protease 1 acts as an oncogene and promotes lenvatinib efficacy in hepatocellular carcinoma by stabilizing c-kit. Ann. Hepatol. 2022, 27, 100669. [Google Scholar] [CrossRef]
- Liu, D.; Li, Q.; Zang, Y.; Li, X.; Li, Z.; Zhang, P.; Feng, C.; Yang, P.; Cui, J.; Sun, Y.; et al. USP1 modulates hepatocellular carcinoma progression via the Hippo/TAZ axis. Cell Death Dis. 2023, 14, 264. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Zhu, Y.; Wu, Q.; Ma, S.; Ma, Y.; Shen, Z.C.; Wang, Z.; Sun, W.; Zhou, Y.C.; Wang, D.; et al. USP1 promotes cholangiocarcinoma progression by deubiquitinating PARP1 to prevent its proteasomal degradation. Cell Death Dis. 2023, 14, 669. [Google Scholar] [CrossRef]
- Macek, P.; Cliff, M.J.; Embrey, K.J.; Holdgate, G.A.; Nissink, J.W.M.; Panova, S.; Waltho, J.P.; Davies, R.A. Myc phosphorylation in its basic helix-loop-helix region destabilizes transient alpha-helical structures, disrupting Max and DNA binding. J. Biol. Chem. 2018, 293, 9301–9310. [Google Scholar] [CrossRef]
- Popov, N.; Wanzel, M.; Madiredjo, M.; Zhang, D.; Beijersbergen, R.; Bernards, R.; Moll, R.; Elledge, S.J.; Eilers, M. The ubiquitin-specific protease USP28 is required for MYC stability. Nat. Cell Biol. 2007, 9, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.X.; He, X.; Yin, L.; Komada, M.; Sears, R.C.; Dai, M.S. The nucleolar ubiquitin-specific protease USP36 deubiquitinates and stabilizes c-Myc. Proc. Natl. Acad. Sci. USA 2015, 112, 3734–3739. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Young, M.A.; Donato, N.J. Emerging Potential of Therapeutic Targeting of Ubiquitin-Specific Proteases in the Treatment of Cancer. Cancer Res. 2014, 74, 4955–4966. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Shao, Z.; Liu, Y.; Xia, X.; Deng, Y.; Yu, C.; Sun, W.; Kong, W.; He, X.; Liu, F.; et al. USP1-dependent RPS16 protein stability drives growth and metastasis of human hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. 2021, 40, 201. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sha, B.; Huang, W.; Li, M.; Zhao, S.; Zhang, Y.; Yan, J.; Li, Z.; Tang, J.; Duan, P.; et al. ML323, a USP1 inhibitor triggers cell cycle arrest, apoptosis and autophagy in esophageal squamous cell carcinoma cells. Apoptosis 2022, 27, 545–560. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Yin, J.; Gan, Y.; Xu, S.; Gu, Y.; Huang, W. Alternative approaches to target Myc for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 117. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Peng, P.; Bao, L.-W.; Zhang, A.-Q.; Yu, B.; Li, T.; Lei, J.; Zhang, H.-H.; Li, S.-Z. Ubiquitin-Specific Protease 1 Promotes Bladder Cancer Progression by Stabilizing c-MYC. Cells 2024, 13, 1798. https://doi.org/10.3390/cells13211798
Zhang X, Peng P, Bao L-W, Zhang A-Q, Yu B, Li T, Lei J, Zhang H-H, Li S-Z. Ubiquitin-Specific Protease 1 Promotes Bladder Cancer Progression by Stabilizing c-MYC. Cells. 2024; 13(21):1798. https://doi.org/10.3390/cells13211798
Chicago/Turabian StyleZhang, Xia, Peng Peng, Li-Wei Bao, An-Qi Zhang, Bo Yu, Tao Li, Jing Lei, Hui-Hui Zhang, and Shang-Ze Li. 2024. "Ubiquitin-Specific Protease 1 Promotes Bladder Cancer Progression by Stabilizing c-MYC" Cells 13, no. 21: 1798. https://doi.org/10.3390/cells13211798
APA StyleZhang, X., Peng, P., Bao, L.-W., Zhang, A.-Q., Yu, B., Li, T., Lei, J., Zhang, H.-H., & Li, S.-Z. (2024). Ubiquitin-Specific Protease 1 Promotes Bladder Cancer Progression by Stabilizing c-MYC. Cells, 13(21), 1798. https://doi.org/10.3390/cells13211798





