Prognostic Significance of Tumor–Stroma Ratio (TSR) in Head and Neck Squamous Cell Carcinoma: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Article Screening
2.3. Data Extraction
2.4. Quality Assessment
2.5. Quantitative Synthesis and Statistical Analysis
3. Results
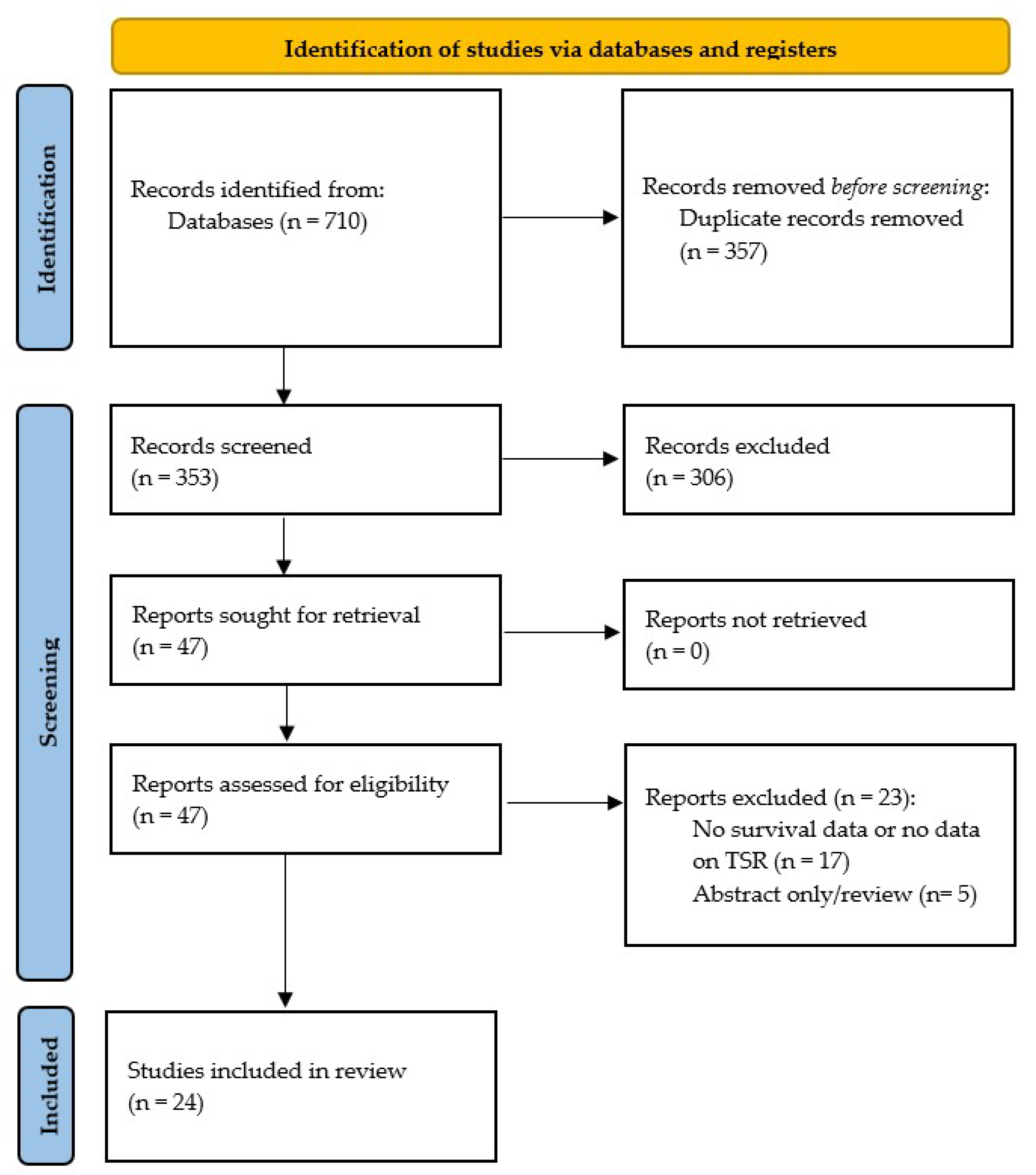
3.1. Literature Search
3.2. Characteristics of Included Studies and Patients
3.3. Quality Assessment
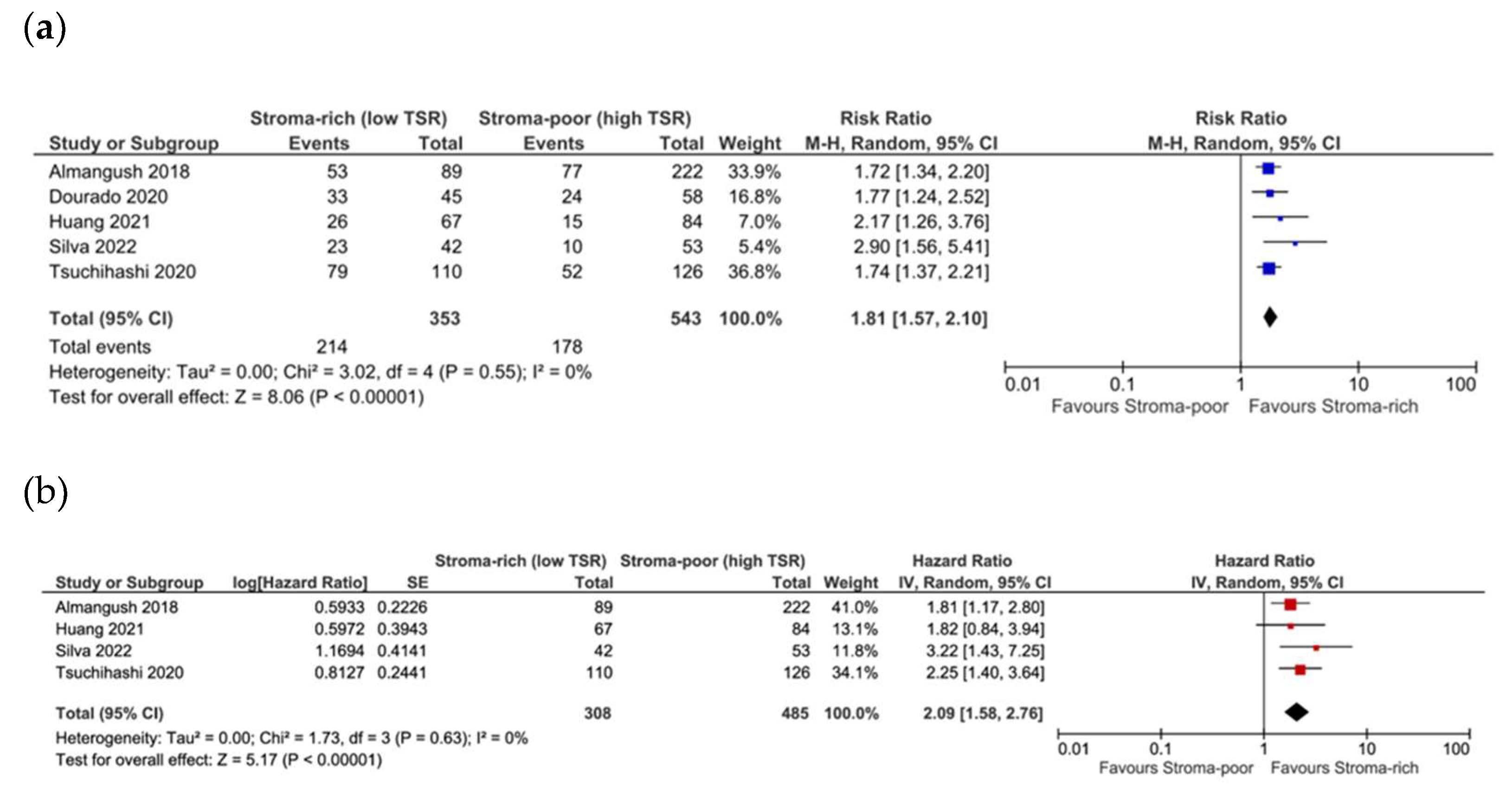
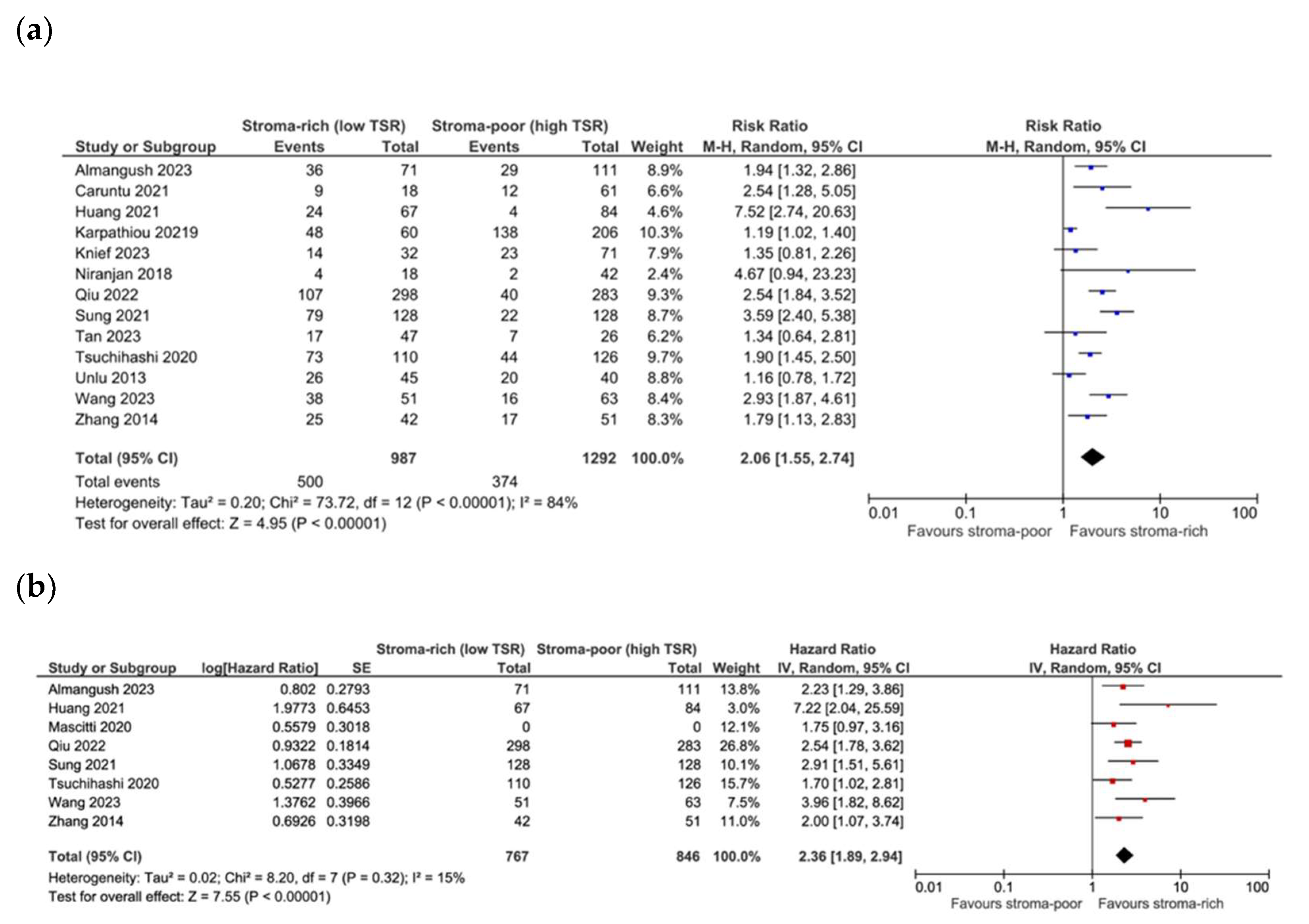
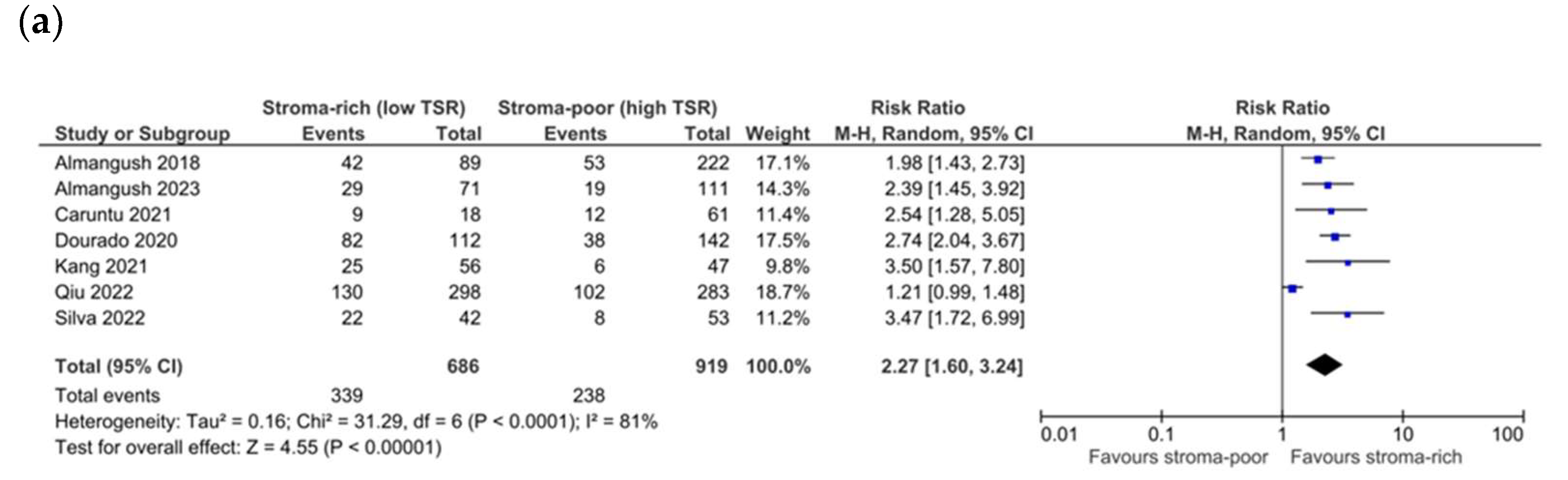
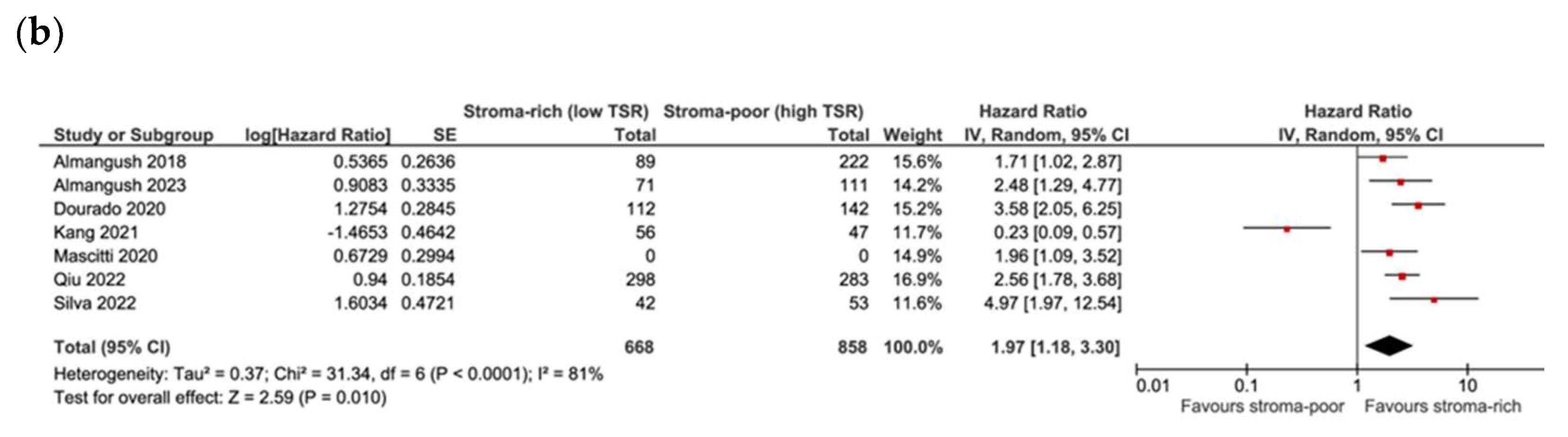
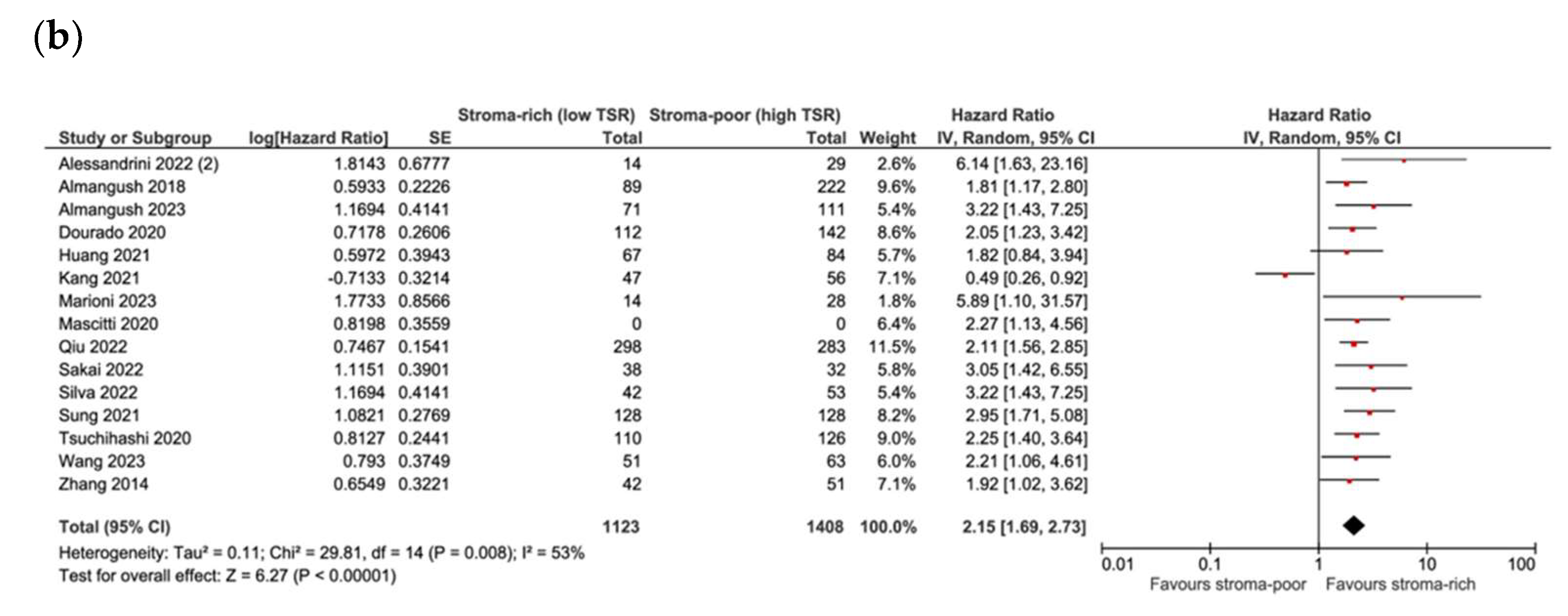
3.4. Association between TSR and Survival
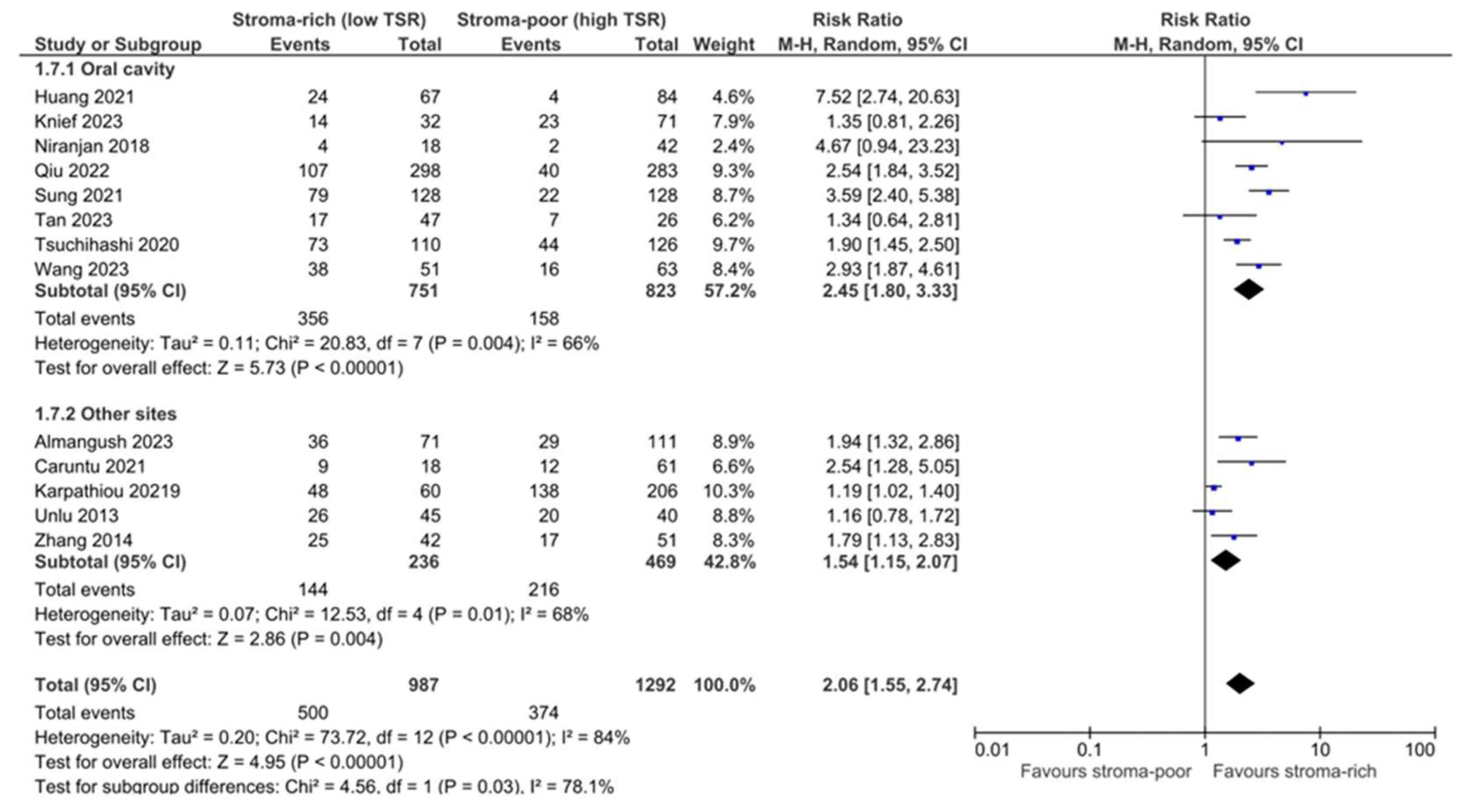
3.5. Association between TSR and Recurrence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Almangush, A.; Alabi, R.O.; Troiano, G.; Coletta, R.D.; Salo, T.; Pirinen, M.; Mäkitie, A.A.; Leivo, I. Clinical significance of tumor-stroma ratio in head and neck cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 480. [Google Scholar] [CrossRef] [PubMed]
- Dourado, M.R.; Guerra, E.N.S.; Salo, T.; Lambert, D.W.; Coletta, R.D. Prognostic value of the immunohistochemical detection of cancer-associated fibroblasts in oral cancer: A systematic review and meta-analysis. J. Oral Pathol. Med. 2018, 47, 443–453. [Google Scholar] [CrossRef]
- van Pelt, G.W.; Kjær-Frifeldt, S.; van Krieken, J.H.J.M.; Al Dieri, R.; Morreau, H.; Tollenaar, R.A.E.M.; Sørensen, F.B.; Mesker, W.E. Scoring the tumor-stroma ratio in colon cancer: Procedure and recommendations. Virchows Arch. 2018, 473, 405–412. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, T.; Xia, R.; Wei, Y.; Wei, X. Targeting the tumor stroma for cancer therapy. Mol. Cancer 2022, 21, 208. [Google Scholar] [CrossRef]
- Lou, E.; Clemente, V.; Grube, M.; Svedbom, A.; Nelson, A.C.; Blome, F.; Staebler, A.; Kommoss, S.; Bazzaro, M. Tumor-Stroma Proportion to Predict Chemoresistance in Patients With Ovarian Cancer. JAMA Netw. Open 2024, 7, e240407. [Google Scholar] [CrossRef]
- Jiang, P.; Chen, Y.; Liu, B. Prognostic Efficacy of Tumor-Stroma Ratio in Women With Breast Cancer: A Meta-Analysis of Cohort Studies. Front. Oncol. 2021, 11, 731409. [Google Scholar] [CrossRef]
- Sullivan, L.; Pacheco, R.R.; Kmeid, M.; Chen, A.; Lee, H. Tumor Stroma Ratio and Its Significance in Locally Advanced Colorectal Cancer. Curr. Oncol. 2022, 29, 3232–3241. [Google Scholar] [CrossRef]
- Kemi, N.; Eskuri, M.; Kauppila, J.H. Tumour-stroma ratio and 5-year mortality in gastric adenocarcinoma: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 16018. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonradomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 24 October 2024).
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Huang, S.; Cai, H.; Song, F.; Zhu, Y.; Hou, C.; Hou, J. Tumor–stroma ratio is a crucial histological predictor of occult cervical lymph node metastasis and survival in early-stage (cT1/2N0) oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2022, 51, 450–458. [Google Scholar] [CrossRef]
- Kang, J.; Su, M.; Xu, Q.; Wang, C.; Yuan, X.; Han, Z. Tumour-stroma ratio is a valuable prognostic factor for oral tongue squamous cell carcinoma. Oral Dis. 2023, 29, 628–638. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Jiang, C.; Zhang, Z.-X.; Liu, F.; Zhang, F.; Cheng, Y.-F. The Tumor-Stroma Ratio Is an Independent Predictor for Survival in Nasopharyngeal Cancer. Oncol. Res. Treat. 2014, 37, 480–484. [Google Scholar] [CrossRef]
- Niranjan, K.C.; Sarathy, N.A. Prognostic impact of tumor-stroma ratio in oral squamous cell carcinoma—A pilot study. Ann. Diagn. Pathol. 2018, 35, 56–61. [Google Scholar] [CrossRef]
- Qiu, J.; Jiang, E.; Shang, Z. Prognostic value of tumor–stroma ratio in oral carcinoma: Role of cancer-associated fibroblasts. Oral Dis. 2023, 29, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Saito, Y.; Tateishi, Y.; Yamazawa, S.; Fukuoka, O.; Kobayashi, K.; Omura, G.; Akashi, K.; Yoshida, M.; Ando, M.; et al. Tumor–stroma ratio can predict lymph-node metastasis in cT1/2N0 oral tongue squamous cell carcinoma independent of tumor budding grade. Int. J. Clin. Oncol. 2022, 27, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.E.; Kim, M.; Lee, Y.S. Proposal of a scoring system for predicting pathological risk based on a semiautomated analysis of whole slide images in oral squamous cell carcinoma. Head Neck 2021, 43, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Taskin, T. Tumor Budding Should Be in Oral Cavity Cancer Reporting: A Retrospective Cohort Study Based on Tumor Microenvironment. Cancers 2023, 15, 3905. [Google Scholar] [CrossRef]
- Tsuchihashi, K.; Nakatsugawa, M.; Kobayashi, J.-I.; Sasaya, T.; Morita, R.; Kubo, T.; Kanaseki, T.; Tsukahara, T.; Asanuma, H.; Hasegawa, T.; et al. Borderline Microenvironment Fibrosis Is a Novel Poor Prognostic Marker of Oral Squamous Cell Carcinoma. Anticancer Res. 2020, 40, 4319–4326. [Google Scholar] [CrossRef]
- Unlu, M.; Cetinayak, H.O.; Onder, D.; Ecevit, C.; Akman, F.; Ikiz, A.O.; Ada, E.; Karacali, B.; Sarioglu, S. The prognostic value of tumor-stroma proportion in laryngeal squamous cell carcinoma. Turkish J. Pathol. 2013, 29, 27. [Google Scholar] [CrossRef]
- Wang, S.; Si, Q.; Wu, Y.; Sun, Y.; Zhang, W.; Huang, X.; Zeng, T.; Chen, S.; Yang, X.; Ni, Y.; et al. Multiperspective quantitative tumor–stroma ratio reveals histological areas associated with poor outcomes in oral squamous cell carcinoma. Cancer Med. 2023, 12, 12161–12172. [Google Scholar] [CrossRef]
- Alessandrini, L.; Franz, L.; Sbaraglia, M.; Saccardo, T.; Cappello, F.; Drigo, A.; Frigo, A.C.; Marioni, G. Tumor-Stroma Ratio and Programmed Cell Death Ligand 1 Expression in Preoperative Biopsy and Matched Laryngeal Carcinoma Surgical Specimen. Int. J. Mol. Sci. 2022, 23, 8053. [Google Scholar] [CrossRef]
- Alessandrini, L.; Ferrari, M.; Taboni, S.; Sbaraglia, M.; Franz, L.; Saccardo, T.; Del Forno, B.M.; Agugiaro, F.; Frigo, A.C.; Dei Tos, A.P.; et al. Tumor-stroma ratio, neoangiogenesis and prognosis in laryngeal carcinoma. A pilot study on preoperative biopsies and matched surgical specimens. Oral Oncol. 2022, 132, 105982. [Google Scholar] [CrossRef]
- Almangush, A.; Heikkinen, I.; Bakhti, N.; Mäkinen, L.K.; Kauppila, J.H.; Pukkila, M.; Hagström, J.; Laranne, J.; Soini, Y.; Kowalski, L.P.; et al. Prognostic impact of tumour–stroma ratio in early-stage oral tongue cancers. Histopathology 2018, 72, 1128–1135. [Google Scholar] [CrossRef]
- Almangush, A.; Jouhi, L.; Haglund, C.; Hagström, J.; Mäkitie, A.A.; Leivo, I. Tumor-stroma ratio is a promising prognostic classifier in oropharyngeal cancer. Hum. Pathol. 2023, 136, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Caruntu, A.; Moraru, L.; Lupu, M.; Ciubotaru, D.A.; Dumitrescu, M.; Eftimie, L.; Hertzog, R.; Zurac, S.; Caruntu, C.; Voinea, O.C. Assessment of Histological Features in Squamous Cell Carcinoma Involving Head and Neck Skin and Mucosa. J. Clin. Med. 2021, 10, 2343. [Google Scholar] [CrossRef] [PubMed]
- Hyytiäinen, A.; Mroueh, R.; Peltonen, J.; Wennerstrand, P.; Mäkitie, A.; Al-Samadi, A.; Ventelä, S.; Salo, T. Prognostic histological markers in oral tongue squamous cell carcinoma patients treated with (chemo)radiotherapy. APMIS 2023, 131, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Karpathiou, G.; Vieville, M.; Gavid, M.; Camy, F.; Dumollard, J.M.; Magné, N.; Froudarakis, M.; Prades, J.M.; Peoc’h, M. Prognostic significance of tumor budding, tumor-stroma ratio, cell nests size, and stroma type in laryngeal and pharyngeal squamous cell carcinomas. Head Neck 2019, 41, 1918–1927. [Google Scholar] [CrossRef]
- Knief, J.; Herber, K.; Muenscher, A.; Thorns, C.; Moeckelmann, N. Tumor-stroma ratio in preoperative biopsies and matched surgical specimens in oral squamous cell carcinoma: Concordance and impact on recurrence-free and overall survival. Pathol. Res. Pract. 2024, 255, 155211. [Google Scholar] [CrossRef]
- Marioni, G.; Taboni, S.; Sbaraglia, M.; Franz, L.; Saccardo, T.; Colombo, A.; Zimello, C.; Frigo, A.C.; Ferrari, M.; Alessandrini, L. Tumor-Stroma Ratio in Basaloid and Conventional Laryngeal Squamous Cell Carcinoma: Prognostic Significance and Concordance in Paired Biopsies and Surgical Samples. Cancers 2023, 15, 1645. [Google Scholar] [CrossRef]
- Mascitti, M.; Zhurakivska, K.; Togni, L.; Caponio, V.C.A.; Almangush, A.; Balercia, P.; Balercia, A.; Rubini, C.; Lo Muzio, L.; Santarelli, A.; et al. Addition of the tumour–stroma ratio to the 8th edition American Joint Committee on Cancer staging system improves survival prediction for patients with oral tongue squamous cell carcinoma. Histopathology 2020, 77, 810–822. [Google Scholar] [CrossRef]
- Dourado, M.R.; Miwa, K.Y.M.; Hamada, G.B.; Paranaíba, L.M.R.; Sawazaki-Calone, Í.; Domingueti, C.B.; Ervolino de Oliveira, C.; Furlan, E.C.B.; Longo, B.C.; Almangush, A.; et al. Prognostication for oral squamous cell carcinoma patients based on the tumour–stroma ratio and tumour budding. Histopathology 2020, 76, 906–918. [Google Scholar] [CrossRef]
- Silva, G.V.D.; da Silva Dolens, E.; Paranaíba, L.M.R.; Ayroza, A.L.C.; Gurgel Rocha, C.A.; Almangush, A.; Salo, T.; Brennan, P.A.; Coletta, R.D. Exploring the combination of tumor-stroma ratio, tumor-infiltrating lymphocytes, and tumor budding with WHO histopathological grading on early-stage oral squamous cell carcinoma prognosis. J. Oral Pathol. Med. 2023, 52, 402–409. [Google Scholar] [CrossRef]
- Grawish, M.E.; Denewar, M.; Ahmed, R.A.; Abouzid, A.; Esmaeil, D.A.; Mourad, M.I. Tumor stroma ratio as a parameter for prognosis and clinicopathological behavior of oral squamous cell carcinoma: A retrospective cohort study. J. Solid Tumors 2021, 10, 36. [Google Scholar] [CrossRef]
- Dolens, E.d.S.; Dourado, M.R.; Almangush, A.; Salo, T.A.; Gurgel Rocha, C.A.; da Silva, S.D.; Brennan, P.A.; Coletta, R.D. The Impact of Histopathological Features on the Prognosis of Oral Squamous Cell Carcinoma: A Comprehensive Review and Meta-Analysis. Front. Oncol. 2021, 11, 784924. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, J.; Plesca, I.; Rothe, R.; Resag, A.; Löck, S.; Benešová, I.; Rupp, L.; Linge, A.; Wehner, R.; Krause, M.; et al. Type I conventional dendritic cells and CD8+ T cells predict favorable clinical outcome of head and neck squamous cell carcinoma patients. Front. Immunol. 2024, 15, 1414298. [Google Scholar] [CrossRef] [PubMed]
- Heng, Y.; Zhu, X.; Lin, H.; Jingyu, M.; Ding, X.; Tao, L.; Lu, L. CD206+ tumor-associated macrophages interact with CD4+ tumor-infiltrating lymphocytes and predict adverse patient outcome in human laryngeal squamous cell carcinoma. J. Transl. Med. 2023, 21, 167. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.; Alnemri, A.; Philips, R.; Fiorella, M.; Sussman, S.; Stapp, R.; Solomides, C.; Harshyne, L.; South, A.; Luginbuhl, A.; et al. CD8+ and FoxP3+ T-Cell Cellular Density and Spatial Distribution After Programmed Death-Ligand 1 Check Point Inhibition. Laryngoscope 2023, 133, 1875–1884. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinom. Adv. Anat. Pathol. 2017, 24, 311–335. [Google Scholar] [CrossRef]
- Secrier, M.; McGrath, L.; Ng, F.; Gulati, S.; Raymond, A.; Nuttall, B.R.B.; Berthe, J.; Jones, E.V.; Sidders, B.S.; Galon, J.; et al. Immune Cell Abundance and T-cell Receptor Landscapes Suggest New Patient Stratification Strategies in Head and Neck Squamous Cell Carcinoma. Cancer Res. Commun. 2023, 3, 2133–2145. [Google Scholar] [CrossRef]
- Galeano Niño, J.L.; Wu, H.; LaCourse, K.D.; Kempchinsky, A.G.; Baryiames, A.; Barber, B.; Futran, N.; Houlton, J.; Sather, C.; Sicinska, E.; et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 2022, 611, 810–817. [Google Scholar] [CrossRef]
- Zhao, H.; Shi, C.; Han, W.; Luo, G.; Huang, Y.; Fu, Y.; Lu, W.; Hu, Q.; Shang, Z.; Yang, X. Advanced progress of spatial metabolomics in head and neck cancer research. Neoplasia 2024, 47, 100958. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girolami, I.; Damiani, D.; Negro, R.; Abousiam, M.; Gazzini, L.; Calabrese, L.; Hanspeter, E. Prognostic Significance of Tumor–Stroma Ratio (TSR) in Head and Neck Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Cells 2024, 13, 1772. https://doi.org/10.3390/cells13211772
Girolami I, Damiani D, Negro R, Abousiam M, Gazzini L, Calabrese L, Hanspeter E. Prognostic Significance of Tumor–Stroma Ratio (TSR) in Head and Neck Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Cells. 2024; 13(21):1772. https://doi.org/10.3390/cells13211772
Chicago/Turabian StyleGirolami, Ilaria, Domenico Damiani, Rosa Negro, Monir Abousiam, Luca Gazzini, Luca Calabrese, and Esther Hanspeter. 2024. "Prognostic Significance of Tumor–Stroma Ratio (TSR) in Head and Neck Squamous Cell Carcinoma: Systematic Review and Meta-Analysis" Cells 13, no. 21: 1772. https://doi.org/10.3390/cells13211772
APA StyleGirolami, I., Damiani, D., Negro, R., Abousiam, M., Gazzini, L., Calabrese, L., & Hanspeter, E. (2024). Prognostic Significance of Tumor–Stroma Ratio (TSR) in Head and Neck Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Cells, 13(21), 1772. https://doi.org/10.3390/cells13211772