17β-Estradiol Stimulates Oxidative Stress Components and Thyroid Specific Genes in Porcine Thyroid Follicular Cells: Potential Differences Between Sexes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Cell Culture
2.4. Cell Treatment
2.5. Cell Viability Assay
2.6. Evaluation of ROS Level
2.7. mRNA Analysis by qRT-PCR
2.8. Statistical Analysis
3. Results
3.1. Stimulation of Thyroid NADPH Oxidases Expression in Male/Female Thyroid Cells
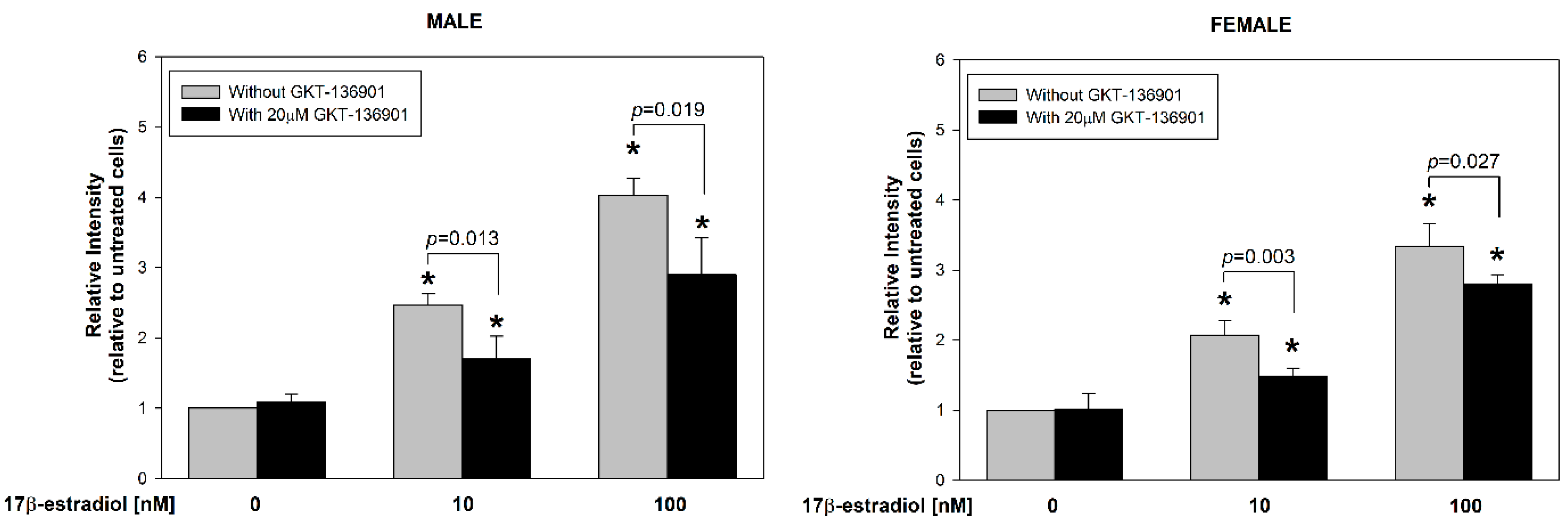
3.2. Stimulation of ROS Formation by 17β-Estradiol in Male/Female Thyroid Cells
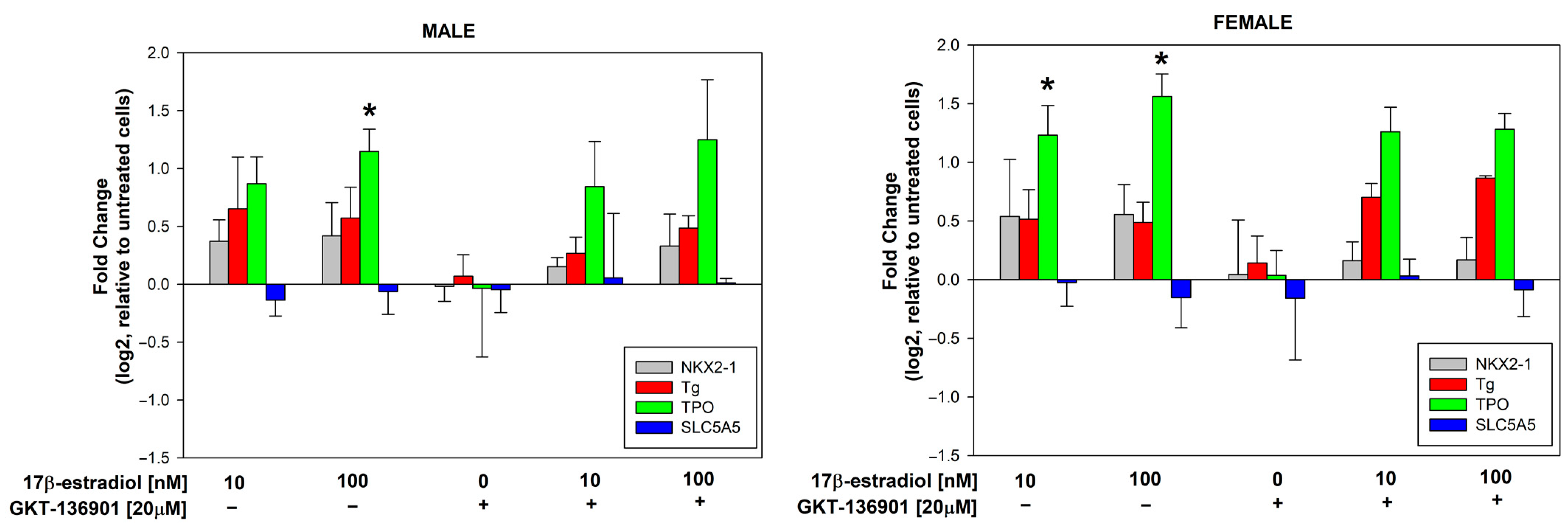
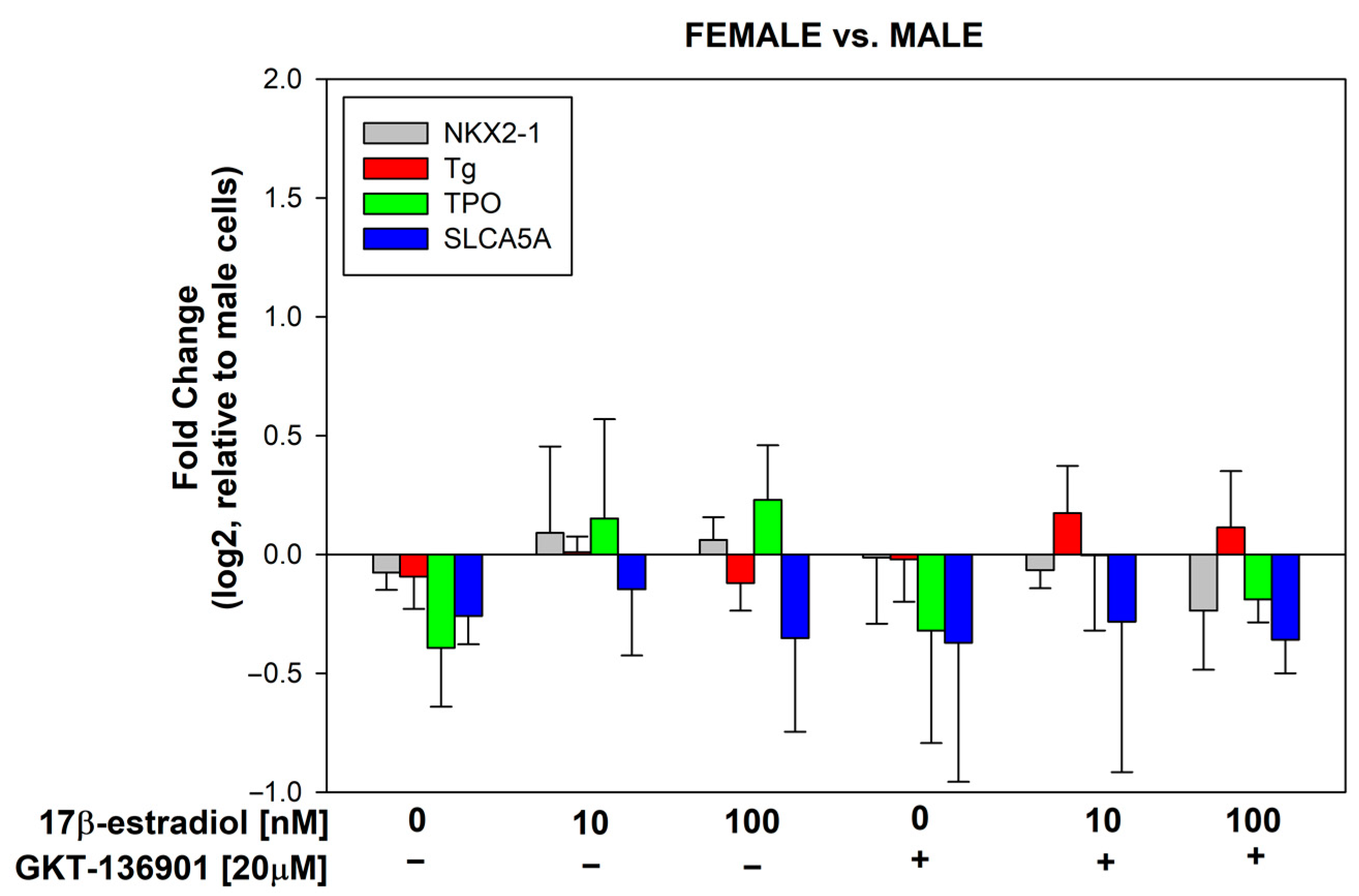
3.3. Stimulation of Thyroid Specific Genes Expression by 17β-Estradiol in Male/Female Thyroid Cells
3.4. Stimulation of ER Stress/UPR Initiation Markers Expression by 17β-Estradiol in Male/Female Thyroid Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barakat, R.; Oakley, O.; Kim, H.; Jin, J.; Ko, C.J. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2016, 49, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Banerjee, A.; Singh, D.; Thakur, G.; Kasarpalkar, N.; Gavali, S.; Gadkar, S.; Madan, T.; Mahale, S.D.; Balasinor, N.H.; et al. Estradiol: A Steroid with Multiple Facets. Horm. Metab. Res. 2018, 50, 359–374. [Google Scholar] [CrossRef]
- Angeloni, C.; Teti, G.; Barbalace, M.C.; Malaguti, M.; Falconi, M.; Hrelia, S. 17β-Estradiol enhances sulforaphane cardioprotection against oxidative stress. J. Nutr. Biochem. 2017, 42, 26–36. [Google Scholar] [CrossRef]
- Nilsen, J. Estradiol and neurodegenerative oxidative stress. Front. Neuroendocrinol. 2008, 29, 463–475. [Google Scholar] [CrossRef]
- Rynkowska, A.; Stępniak, J.; Karbownik-Lewińska, M. Fenton Reaction-Induced Oxidative Damage to Membrane Lipids and Protective Effects of 17β-Estradiol in Porcine Ovary and Thyroid Homogenates. Int. J. Environ. Res. Public Health 2020, 17, 6841. [Google Scholar] [CrossRef] [PubMed]
- Stepniak, J.; Karbownik-Lewinska, M. 17β-estradiol prevents experimentally-induced oxidative damage to membrane lipids and nuclear DNA in porcine ovary. Syst. Biol. Reprod. Med. 2016, 62, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Stępniak, J.; Koziróg, E.; Karbownik-Lewińska, M. The Protective Effect of Exogenous 17β-Estradiol against Experimentally Induced Oxidative Damage to Membrane Lipids Is Stronger in Male vs. Female Porcine Thyroids: Preliminary Results. Toxics 2023, 11, 746. [Google Scholar] [CrossRef]
- Ramesh, S.S.; Christopher, R.; Indira Devi, B.; Bhat, D.I. The vascular protective role of oestradiol: A focus on postmenopausal oestradiol deficiency and aneurysmal subarachnoid haemorrhage. Biol. Rev. Camb. Philos. Soc. 2019, 94, 1897–1917. [Google Scholar] [CrossRef]
- Bukato, K.; Kostrzewa, T.; Gammazza, A.M.; Gorska-Ponikowska, M.; Sawicki, S. Endogenous estrogen metabolites as oxidative stress mediators and endometrial cancer biomarkers. Cell Commun. Signal. 2024, 22, 205. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, G.; Vlantis, A.; Tse, G.; van Hasselt, C. The contributions of oestrogen receptor isoforms to the development of papillary and anaplastic thyroid carcinomas. J. Pathol. 2008, 214, 425–433. [Google Scholar] [CrossRef]
- Kumar, A.; Klinge, C.M.; Goldstein, R.E. Estradiol-induced proliferation of papillary and follicular thyroid cancer cells is mediated by estrogen receptors alpha and beta. Int. J. Oncol. 2010, 36, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Derwahl, M.; Nicula, D. Estrogen and its role in thyroid cancer. Endocr. Relat. Cancer 2014, 21, T273–T283. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.Z.; Zheng, L.L.; Chen, K.H.; Wang, R.; Yi, D.D.; Jiang, C.Y.; Liu, Z.J.; Shi, X.B.; Sang, J.F. Serum sex hormones correlate with pathological features of papillary thyroid cancer. Endocrine 2024, 84, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Garmendia Madariaga, A.; Santos Palacios, S.; Guillén-Grima, F.; Galofré, J.C. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef]
- Fortunato, R.S.; Braga, W.M.; Ortenzi, V.H.; Rodrigues, D.C.; Andrade, B.M.; Miranda-Alves, L.; Rondinelli, E.; Dupuy, C.; Ferreira, A.C.; Carvalho, D.P. Sexual dimorphism of thyroid reactive oxygen species production due to higher NADPH oxidase 4 expression in female thyroid glands. Thyroid 2013, 23, 111–119. [Google Scholar] [CrossRef]
- Stepniak, J.; Lewinski, A.; Karbownik-Lewinska, M. Sexual Dimorphism of NADPH Oxidase/H2O2 System in Rat Thyroid Cells; Effect of Exogenous 17β-Estradiol. Int. J. Mol. Sci. 2018, 19, 4063. [Google Scholar] [CrossRef] [PubMed]
- Burek, C.L.; Rose, N.R. Autoimmune thyroiditis and ROS. Autoimmun. Rev. 2008, 7, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Ameziane-El-Hassani, R.; Schlumberger, M.; Dupuy, C. NADPH oxidases: New actors in thyroid cancer? Nat. Rev. Endocrinol. 2016, 12, 485–494. [Google Scholar] [CrossRef]
- Song, Y.; Driessens, N.; Costa, M.; De Deken, X.; Detours, V.; Corvilain, B.; Maenhaut, C.; Miot, F.; Van Sande, J.; Many, M.C.; et al. Roles of hydrogen peroxide in thyroid physiology and disease. J. Clin. Endocrinol. Metab. 2007, 92, 3764–3773. [Google Scholar] [CrossRef]
- Oglio, R.; Salvarredi, L.; Rossich, L.; Copelli, S.; Pisarev, M.; Juvenal, G.; Thomasz, L. Participation of NADPH 4 oxidase in thyroid regulation. Mol. Cell. Endocrinol. 2019, 480, 65–73. [Google Scholar] [CrossRef]
- Wu, R.F.; Ma, Z.; Liu, Z.; Terada, L.S. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol. Cell. Biol. 2010, 30, 3553–3568. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Körner, H.; Wu, H.; Wei, W. Endoplasmic reticulum stress in autoimmune diseases. Immunobiology 2020, 225, 151881. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Sheng, J.; Tang, P.; Peng, X.; Zhang, R.; Zhao, X.; Hu, J.; Xu, T. The role and mechanism of NADPH oxidase in the development and progression of thyroid carcinoma. Am. J. Cancer Res. 2023, 13, 4366–4375. [Google Scholar]
- Cammarota, F.; Fiscardi, F.; Esposito, T.; de Vita, G.; Salvatore, M.; Laukkanen, M.O. Clinical relevance of thyroid cell models in redox research. Cancer Cell Int. 2015, 15, 113. [Google Scholar] [CrossRef]
- Walters, E.M.; Prather, R.S. Advancing swine models for human health and diseases. Mo. Med. 2013, 110, 212–215. [Google Scholar]
- James, B.C.; Mitchell, J.M.; Jeon, H.D.; Vasilottos, N.; Grogan, R.H.; Aschebrook-Kilfoy, B. An update in international trends in incidence rates of thyroid cancer, 1973–2007. Cancer Causes Control 2018, 29, 465–473. [Google Scholar] [CrossRef]
- Hollowell, J.G.; Staehling, N.W.; Flanders, W.D.; Hannon, W.H.; Gunter, E.W.; Spencer, C.A.; Braverman, L.E. Serum TSH, T4, and thyroid antibodies in the United States population (1988–1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. [Google Scholar] [CrossRef]
- Santin, A.P.; Furlanetto, T.W. Role of estrogen in thyroid function and growth regulation. J. Thyroid Res. 2011, 2011, 875125. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ye, Q.; Lu, H.; Wu, Z.; Chen, S.; Zheng, R. Estrogen-mediated DNMT1 and DNMT3A recruitment by EZH2 silences miR-570-3p that contributes to papillary thyroid malignancy through DPP4. Clin. Epigenet. 2024, 16, 81. [Google Scholar] [CrossRef]
- Mo, X.M.; Li, L.; Zhu, P.; Dai, Y.J.; Zhao, T.T.; Liao, L.Y.; Chen, G.G.; Liu, Z.M. Up-regulation of Hsp27 by ERα/Sp1 facilitates proliferation and confers resistance to apoptosis in human papillary thyroid cancer cells. Mol. Cell. Endocrinol. 2016, 431, 71–87. [Google Scholar] [CrossRef]
- Lv, P.P.; Tian, S.; Feng, C.; Li, J.Y.; Yu, D.Q.; Jin, L.; Shen, Y.; Yu, T.T.; Meng, Y.; Ding, G.L.; et al. Maternal High Estradiol Exposure is Associated with Elevated Thyroxine and Pax8 in Mouse Offspring. Sci. Rep. 2016, 6, 36805. [Google Scholar] [CrossRef] [PubMed]
- Kordi, F.; Khazali, H. The effect of ghrelin and estradiol on mean concentration of thyroid hormones. Int. J. Endocrinol. Metab. 2015, 13, e17988. [Google Scholar] [CrossRef] [PubMed]
- Hima, S.; Sreeja, S. Modulatory role of 17β-estradiol in the tumor microenvironment of thyroid cancer. IUBMB Life 2016, 68, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Arduc, A.; Aycicek Dogan, B.; Bilmez, S.; Imga Nasiroglu, N.; Tuna, M.M.; Isik, S.; Berker, D.; Guler, S. High prevalence of Hashimoto’s thyroiditis in patients with polycystic ovary syndrome: Does the imbalance between estradiol and progesterone play a role? Endocr. Res. 2015, 40, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Fenniche, S.; Oukabli, M.; Oubaddou, Y.; Chahdi, H.; Damiri, A.; Alghuzlan, A.; Laraqui, A.; Dakka, N.; Bakri, Y.; Dupuy, C.; et al. A Comparative Analysis of NOX4 Protein Expression in Malignant and Non-Malignant Thyroid Tumors. Curr. Issues Mol. Biol. 2023, 45, 5811–5823. [Google Scholar] [CrossRef]
- He, L.; Li, X.; Chen, Z.; Wang, W.; Wang, K.; Huang, X.; Yang, Q.; Ke, W.; Liu, J.; Zha, B. High concentration of estradiol has a negative correlation with free thyroxine during the second trimester of pregnancy. Endocr. Connect. 2022, 11, e220236. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Zhou, L.; Lei, Y.; Zhang, Y.; Huang, C. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biol. 2019, 25, 101047. [Google Scholar] [CrossRef]
- Dicks, N.; Gutierrez, K.; Michalak, M.; Bordignon, V.; Agellon, L.B. Endoplasmic reticulum stress, genome damage, and cancer. Front. Oncol. 2015, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2019, 9, 3083. [Google Scholar] [CrossRef]
- Ma, D.J.; Hwang, J.S.; Noh, K.B.; Oh, S.H.; Kim, K.W.; Shin, Y.J. Role of NADPH Oxidase 4 in Corneal Endothelial Cells Is Mediated by Endoplasmic Reticulum Stress and Autophagy. Antioxidants 2023, 12, 1228. [Google Scholar] [CrossRef]
- Pedruzzi, E.; Guichard, C.; Ollivier, V.; Driss, F.; Fay, M.; Prunet, C.; Marie, J.C.; Pouzet, C.; Samadi, M.; Elbim, C.; et al. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell. Biol. 2004, 24, 10703–10717. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Q.; Wang, H.; Xia, S. Endoplasmic reticulum stress in T cell-mediated diseases. Scand. J. Immunol. 2023, 98, e13307. [Google Scholar] [CrossRef] [PubMed]
- Barrera, M.J.; Aguilera, S.; Castro, I.; González, S.; Carvajal, P.; Molina, C.; Hermoso, M.A.; González, M.J. Endoplasmic reticulum stress in autoimmune diseases: Can altered protein quality control and/or unfolded protein response contribute to autoimmunity? A critical review on Sjögren’s syndrome. Autoimmun. Rev. 2018, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Kuzmuk, K.N.; Schook, L.B. Pigs as a Model for Biomedical Sciences. In The Genetics of the Pig, 2nd ed.; Rothschild, M.F., Ruvinsky, A., Eds.; CAB International: Wallingford, UK, 2011; pp. 426–444. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępniak, J.; Karbownik-Lewińska, M. 17β-Estradiol Stimulates Oxidative Stress Components and Thyroid Specific Genes in Porcine Thyroid Follicular Cells: Potential Differences Between Sexes. Cells 2024, 13, 1769. https://doi.org/10.3390/cells13211769
Stępniak J, Karbownik-Lewińska M. 17β-Estradiol Stimulates Oxidative Stress Components and Thyroid Specific Genes in Porcine Thyroid Follicular Cells: Potential Differences Between Sexes. Cells. 2024; 13(21):1769. https://doi.org/10.3390/cells13211769
Chicago/Turabian StyleStępniak, Jan, and Małgorzata Karbownik-Lewińska. 2024. "17β-Estradiol Stimulates Oxidative Stress Components and Thyroid Specific Genes in Porcine Thyroid Follicular Cells: Potential Differences Between Sexes" Cells 13, no. 21: 1769. https://doi.org/10.3390/cells13211769
APA StyleStępniak, J., & Karbownik-Lewińska, M. (2024). 17β-Estradiol Stimulates Oxidative Stress Components and Thyroid Specific Genes in Porcine Thyroid Follicular Cells: Potential Differences Between Sexes. Cells, 13(21), 1769. https://doi.org/10.3390/cells13211769






