Inhibition of Chk1 with Prexasertib Enhances the Anticancer Activity of Ciclopirox in Non-Small Cell Lung Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines and Cultures
2.3. Cell Viability and Proliferation Assays
2.4. Drug Combination Analysis
2.5. Cell Cycle Analysis
2.6. Apoptosis Assay
2.7. Western Blot Analysis
2.8. BrdU Labeling and Staining
2.9. Immunofluorescence Staining for γH2AX
2.10. Statistical Analysis
3. Results
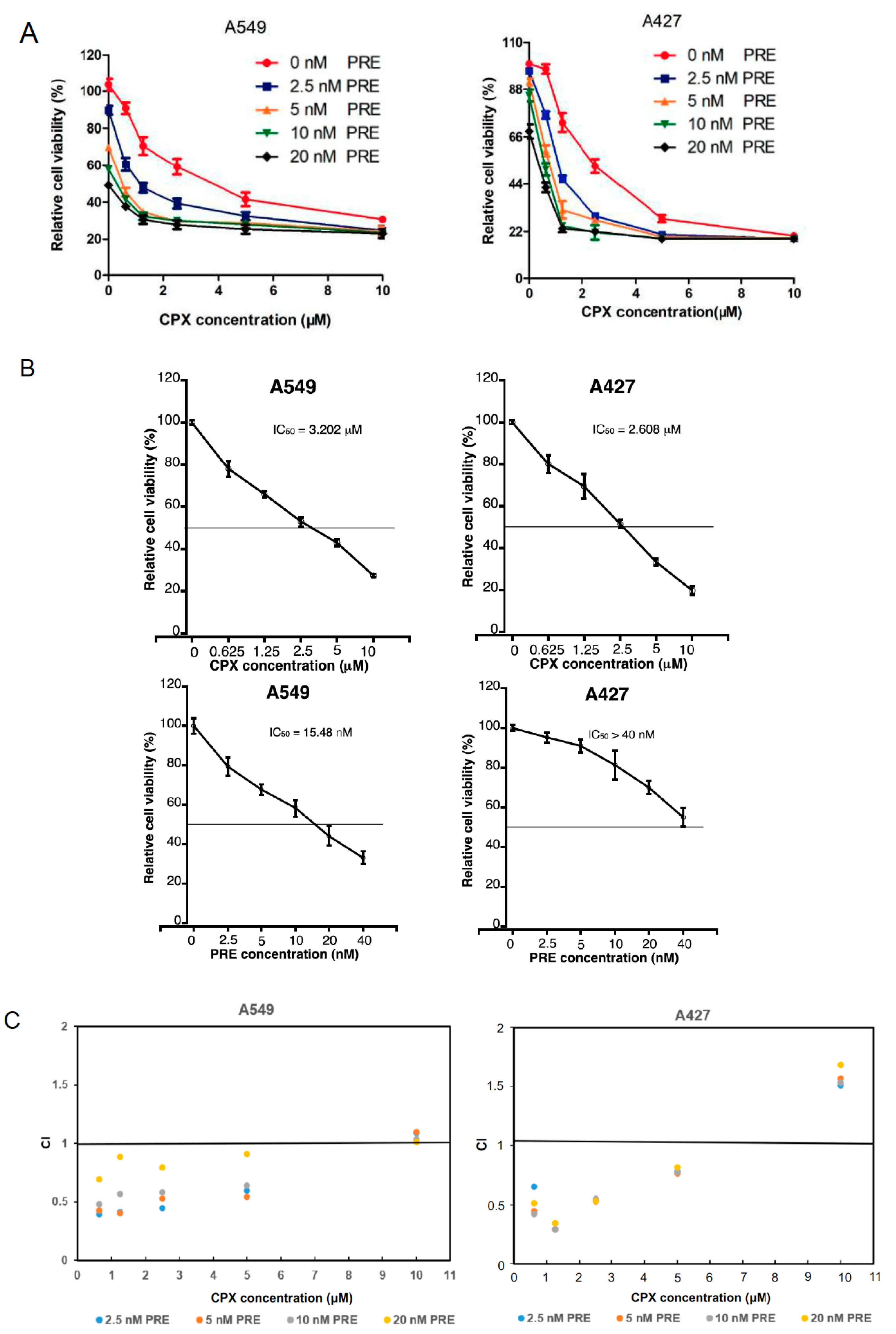
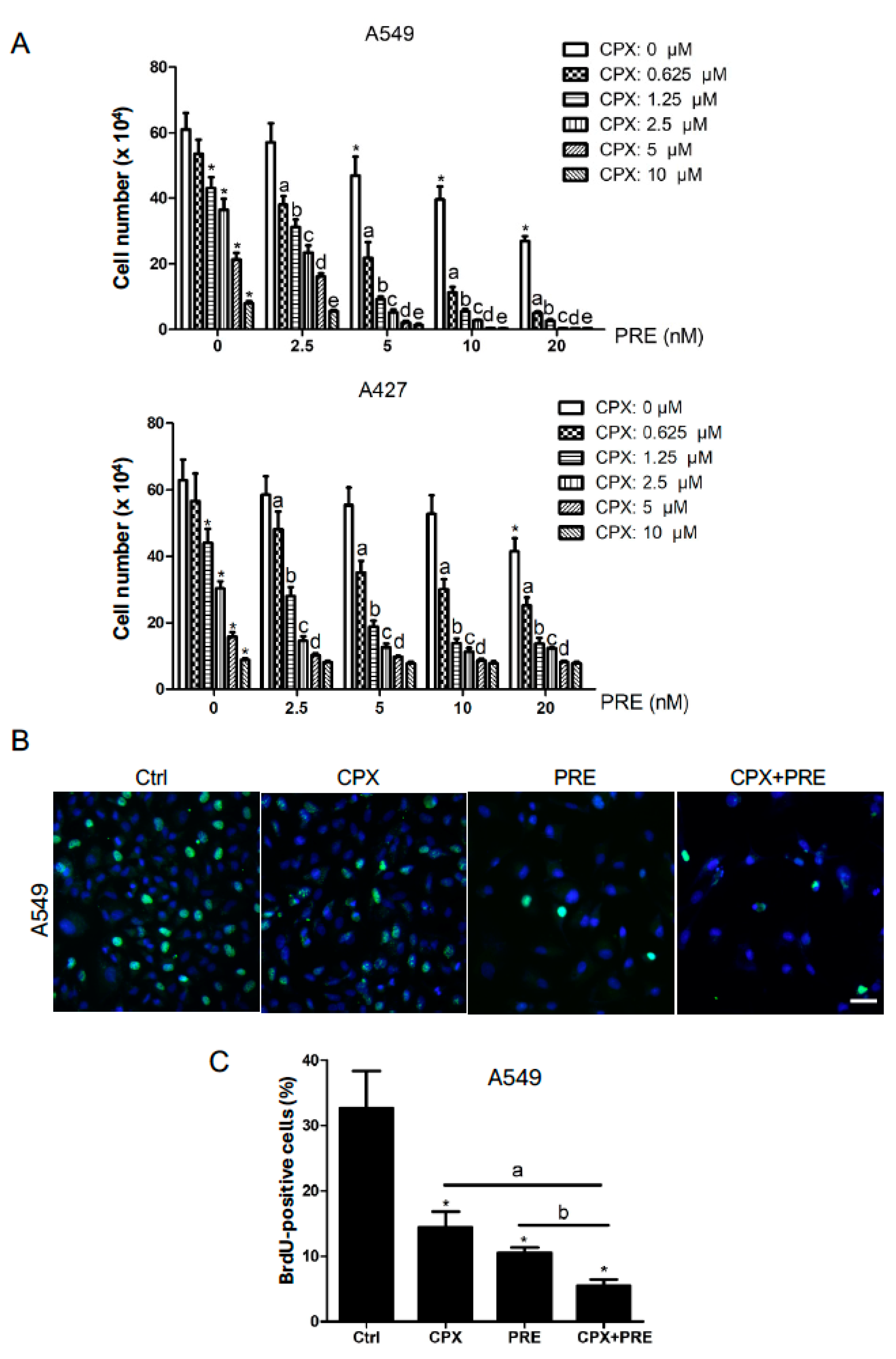
3.1. CPX Is Synergistic with PRE in Reducing Cell Viability and Inhibiting Cell Proliferation of NSCLC Cells
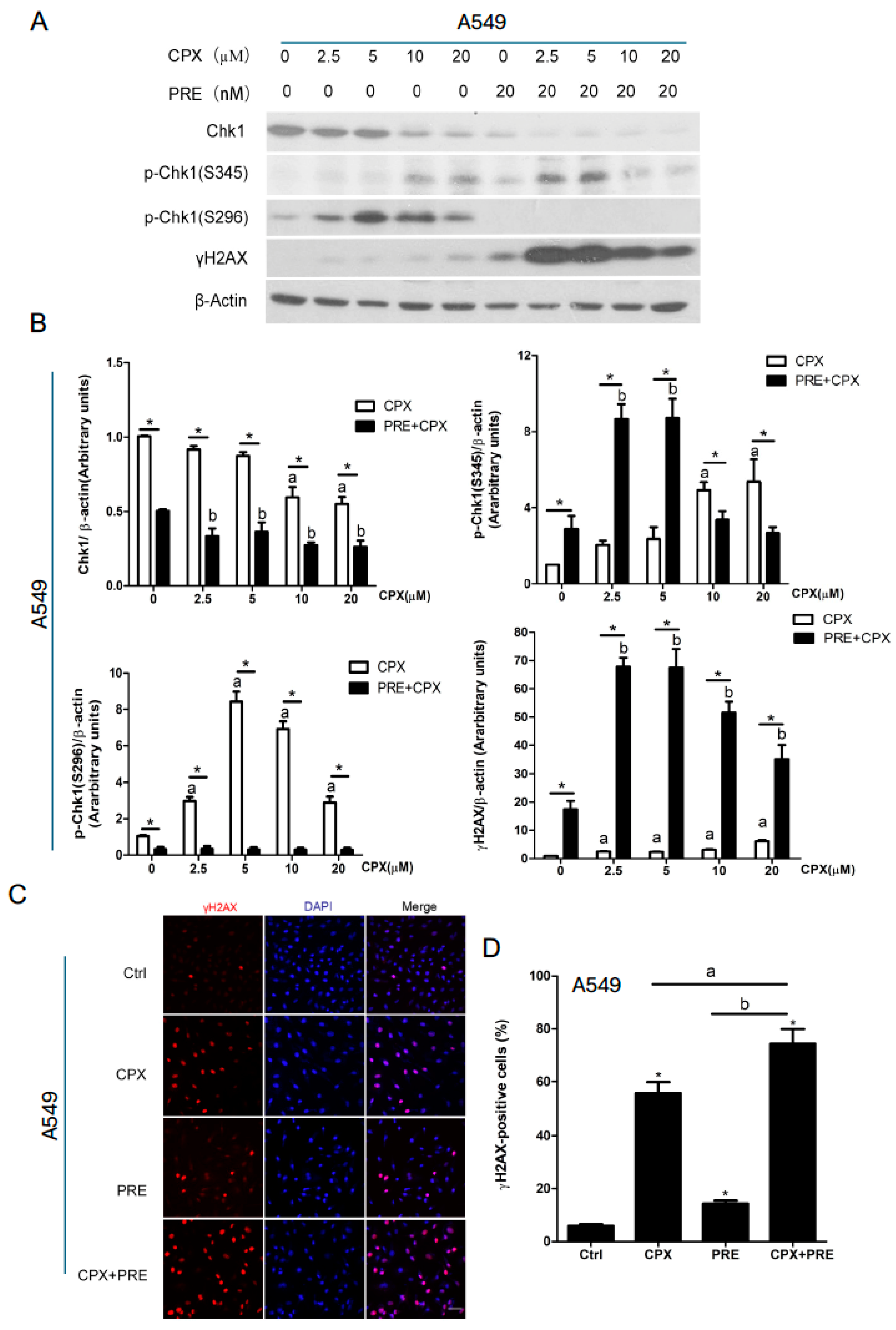
3.2. Combined Treatment with CPX and PRE Induces Excessive DNA Damage in NSCLC Cells
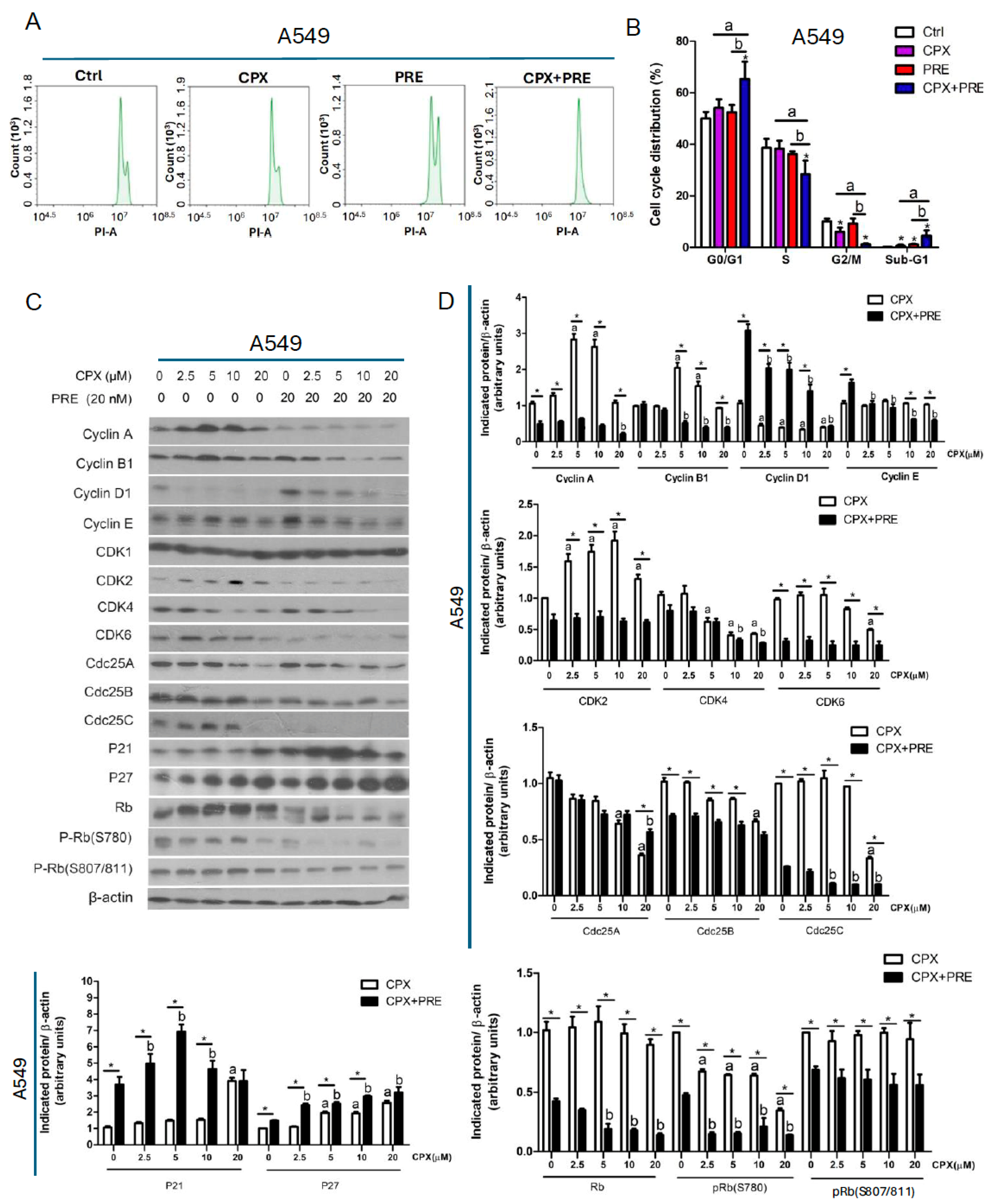
3.3. Combined Treatment of CPX and PRE Induces Cell Cycle Arrest in the G1/G0 Phase in NSCLC Cells
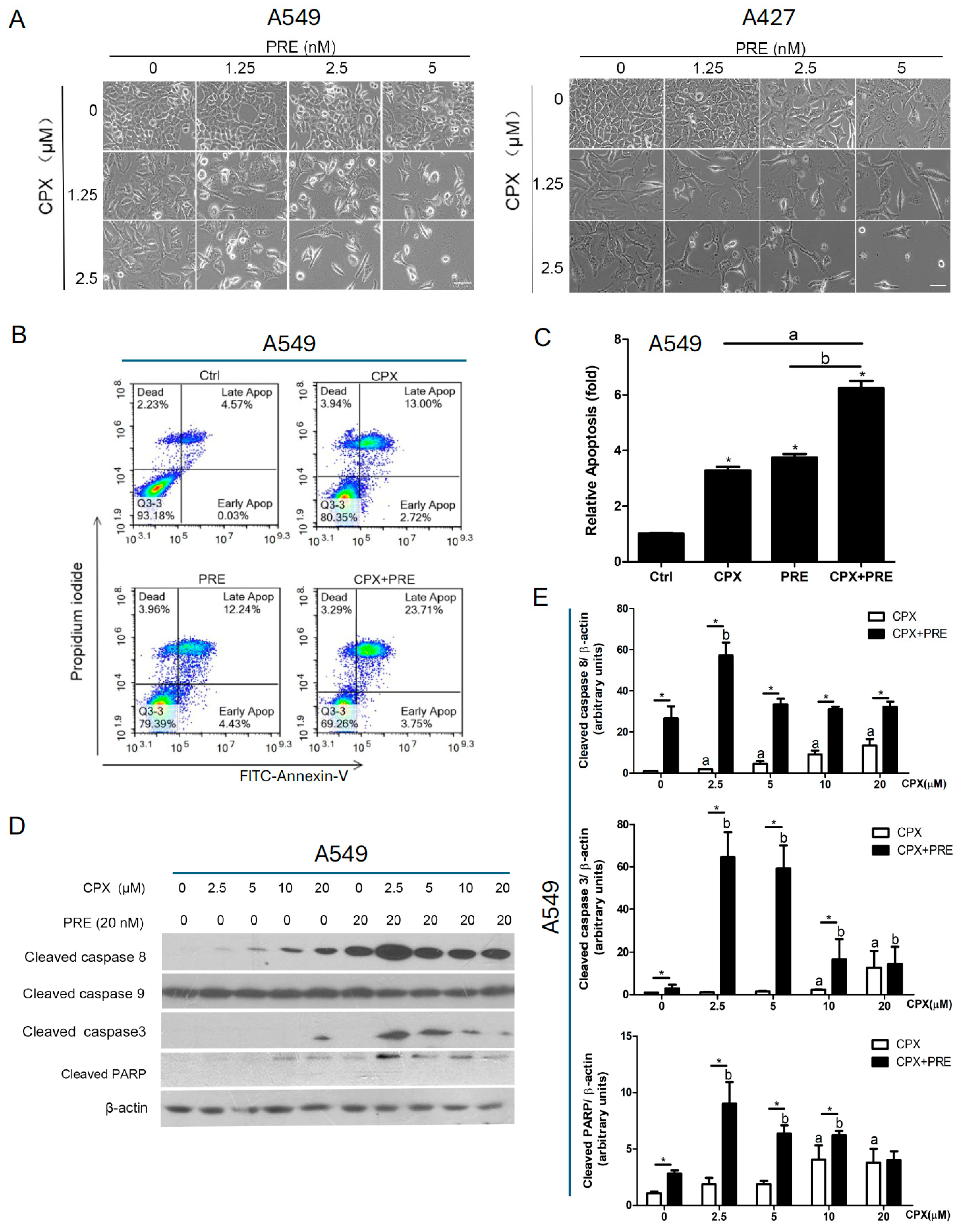
3.4. Combined Treatment with CPX and PRE Induces Apoptosis of NSCLC Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rotow, J.; Bivona, T.G. Understanding and targeting resistance mechanisms in NSCLC. Nat. Rev. Cancer 2017, 17, 637–658. [Google Scholar] [CrossRef]
- Cooper, A.J.; Sequist, L.V.; Lin, J.J. Third-generation EGFR and ALK inhibitors: Mechanisms of resistance and management. Nat. Rev. Clin. Oncol. 2022, 19, 499–514. [Google Scholar] [CrossRef]
- Otano, I.; Ucero, A.C.; Zugazagoitia, J.; Paz-Ares, L. At the crossroads of immunotherapy for oncogene-addicted subsets of NSCLC. Nat. Rev. Clin. Oncol. 2023, 20, 143–159. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Li, B.T.; Chaft, J.E.; Kris, M.G. Chemotherapy remains an essential element of personalized care for persons with lung cancers. Ann. Oncol. 2016, 27, 1829–1835. [Google Scholar] [CrossRef]
- Gupta, A.K. Ciclopirox: An overview. Int. J. Dermatol. 2001, 40, 305–310. [Google Scholar] [CrossRef]
- Subissi, A.; Monti, D.; Togni, G.; Mailland, F. Ciclopirox. Drugs 2010, 70, 2133–2152. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, S. Reposition of the Fungicide Ciclopirox for Cancer Treatment. Recent Pat. Anticancer Drug Discov. 2021, 16, 122–135. [Google Scholar] [CrossRef]
- Eberhard, Y.; McDermott, S.P.; Wang, X.; Gronda, M.; Venugopal, A.; Wood, T.E.; Hurren, R.; Datti, A.; Batey, R.A.; Wrana, J.; et al. Chelation of intracellular iron with the antifungal agent ciclopirox olamine induces cell death in leukemia and myeloma cells. Blood 2009, 114, 3064–3073. [Google Scholar] [CrossRef]
- Goss, K.L.; Gordon, D.J. Gene expression signature based screening identifies ribonucleotide reductase as a candidate therapeutic target in Ewing sarcoma. Oncotarget 2016, 7, 63003–63019. [Google Scholar] [CrossRef]
- Zhou, H.; Shen, T.; Luo, Y.; Liu, L.; Chen, W.; Xu, B.; Han, X.; Pang, J.; Rivera, C.A.; Huang, S. The antitumor activity of the fungicide ciclopirox. Int. J. Cancer 2010, 127, 2467–2477. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Zhang, G.; Gu, L.; Zhang, Y.; Gao, J.; Wei, Y.; Ma, Z. Antileukemia Effect of Ciclopirox Olamine Is Mediated by Downregulation of Intracellular Ferritin and Inhibition β-Catenin-c-Myc Signaling Pathway in Glucocorticoid Resistant T-ALL Cell Lines. PLoS ONE 2016, 11, e0161509. [Google Scholar] [CrossRef]
- Yang, J.; Milasta, S.; Hu, D.; AlTahan, A.M.; Interiano, R.B.; Zhou, J.; Davidson, J.; Low, J.; Lin, W.; Bao, J.; et al. Targeting Histone Demethylases in MYC-Driven Neuroblastomas with Ciclopirox. Cancer Res. 2017, 77, 4626–4638. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Wang, M.; Zhou, L.; Feng, X.; Yu, L.; Lan, J.; Gao, W.; Zhang, C.; Bu, Y.; et al. CPX Targeting DJ-1 Triggers ROS-induced Cell Death and Protective Autophagy in Colorectal Cancer. Theranostics 2019, 9, 5577–5594. [Google Scholar] [CrossRef]
- Kim, Y.; Schmidt, M.; Endo, T.; Lu, D.; Carson, D.; Schmidt-Wolf, I.G. Targeting the Wnt/beta-catenin pathway with the antifungal agent ciclopirox olamine in a murine myeloma model. In Vivo 2011, 25, 887–893. [Google Scholar]
- Schmeel, L.C.; Schmeel, F.C.; Kim, Y.; Endo, T.; Lu, D.; Schmidt-Wolf, I.G. Targeting the Wnt/beta-catenin pathway in multiple myeloma. Anticancer Res. 2013, 33, 4719–4726. [Google Scholar]
- Clement, P.M.; Hanauske-Abel, H.M.; Wolff, E.C.; Kleinman, H.K.; Park, M.H. The antifungal drug ciclopirox inhibits deoxyhypusine and proline hydroxylation, endothelial cell growth and angiogenesis in vitro. Int. J. Cancer 2002, 100, 491–498. [Google Scholar] [CrossRef]
- Mémin, E.; Hoque, M.; Jain, M.R.; Heller, D.S.; Li, H.; Cracchiolo, B.; Hanauske-Abel, H.M.; Pe’ery, T.; Mathews, M.B. Blocking eIF5A modification in cervical cancer cells alters the expression of cancer-related genes and suppresses cell proliferation. Cancer Res. 2014, 74, 552–562. [Google Scholar] [CrossRef]
- Fujimura, K.; Wright, T.; Strnadel, J.; Kaushal, S.; Metildi, C.; Lowy, A.M.; Bouvet, M.; Kelber, J.A.; Klemke, R.L. A hypusine–eIF5A–PEAK1 switch regulates the pathogenesis of pancreatic cancer. Cancer Res. 2014, 74, 6671–6681. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, H.; Liu, L.; Shen, T.; Chen, W.; Xu, B.; Han, X.; Zhang, F.; Scott, R.S.; Alexander, J.S.; et al. The fungicide ciclopirox inhibits lymphatic endothelial cell tube formation by suppressing VEGFR-3-mediated ERK signaling pathway. Oncogene 2011, 30, 2098–2107. [Google Scholar] [CrossRef]
- Shen, T.; Shang, C.; Zhou, H.; Luo, Y.; Barzegar, M.; Odaka, Y.; Wu, Y.; Huang, S. Ciclopirox inhibits cancer cell proliferation by suppression of Cdc25A. Genes Cancer 2017, 8, 505–516. [Google Scholar] [CrossRef]
- Shen, T.; Zhou, H.; Shang, C.; Luo, Y.; Wu, Y.; Huang, S. Ciclopirox activates ATR-Chk1 signaling pathway leading to Cdc25A protein degradation. Genes Cancer 2018, 9, 39–52. [Google Scholar] [CrossRef][Green Version]
- Zhou, H.; Shang, C.; Wang, M.; Shen, T.; Kong, L.; Yu, C.; Ye, Z.; Luo, Y.; Liu, L.; Li, Y.; et al. Ciclopirox olamine inhibits mTORC1 signaling by activation of AMPK. Biochem. Pharmacol. 2016, 116, 39–50. [Google Scholar] [CrossRef]
- Sen, S.; Hassane, D.C.; Corbett, C.; Becker, M.W.; Jordan, C.T.; Guzman, M.L. Novel mTOR inhibitory activity of ciclopirox enhances parthenolide antileukemia activity. Exp. Hematol. 2013, 41, 799–807.e4. [Google Scholar] [CrossRef]
- Shang, C.; Zhou, H.; Liu, W.; Shen, T.; Luo, Y.; Huang, S. Iron chelation inhibits mTORC1 signaling involving activation of AMPK and REDD1/Bnip3 pathways. Oncogene 2020, 39, 5201–5213. [Google Scholar] [CrossRef]
- Zhou, B.B.; Elledge, S.J. The DNA damage response: Putting checkpoints in perspective. Nature 2000, 408, 433–439. [Google Scholar] [CrossRef]
- Abraham, R.T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001, 15, 2177–2196. [Google Scholar] [CrossRef]
- Bartek, J.; Lukas, J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 2003, 3, 421–429. [Google Scholar] [CrossRef]
- Zhang, Y.; Hunter, T. Roles of Chk1 in cell biology and cancer therapy. Int. J. Cancer 2014, 134, 1013–1023. [Google Scholar] [CrossRef]
- Angius, G.; Tomao, S.; Stati, V.; Vici, P.; Bianco, V.; Tomao, F. Prexasertib, a checkpoint kinase inhibitor: From preclinical data to clinical development. Cancer Chemother. Pharmacol. 2020, 85, 9–20. [Google Scholar] [CrossRef]
- Lowery, C.D.; VanWye, A.B.; Dowless, M.; Blosser, W.; Falcon, B.L.; Stewart, J.; Stephens, J.; Beckmann, R.P.; Lin, A.B.; Stancato, L.F. The Checkpoint Kinase 1 Inhibitor Prexasertib Induces Regression of Preclinical Models of Human Neuroblastoma. Clin. Cancer Res. 2017, 23, 4354–4363. [Google Scholar] [CrossRef]
- Mani, C.; Jonnalagadda, S.; Lingareddy, J.; Awasthi, S.; Gmeiner, W.H.; Palle, K. Prexasertib treatment induces homologous recombination deficiency and synergizes with olaparib in triple-negative breast cancer cells. Breast Cancer Res. 2019, 21, 104. [Google Scholar] [CrossRef]
- Heidler, C.L.; Roth, E.K.; Thiemann, M.; Blattmann, C.; Perez, R.L.; Huber, P.E.; Kovac, M.; Amthor, B.; Neu-Yilik, G.; Kulozik, A.E. Prexasertib (LY2606368) reduces clonogenic survival by inducing apoptosis in primary patient-derived osteosarcoma cells and synergizes with cisplatin and talazoparib. Int. J. Cancer 2020, 147, 1059–1070. [Google Scholar] [CrossRef]
- Do, K.T.; Kochupurakkal, B.; Kelland, S.; de Jonge, A.; Hedglin, J.; Powers, A.; Quinn, N.; Gannon, C.; Vuong, L.; Parmar, K.; et al. Phase 1 Combination Study of the CHK1 Inhibitor Prexasertib and the PARP Inhibitor Olaparib in High-grade Serous Ovarian Cancer and Other Solid Tumors. Clin. Cancer Res. 2021, 27, 4710–4716. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Boutros, R.; Lobjois, V.; Ducommun, B. CDC25 phosphatases in cancer cells: Key players? Good targets? Nat. Rev. Cancer 2007, 7, 495–507. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Gong, S.; Wang, J.; Lu, X.; Jin, Q.; Lu, B.; Chen, Q. Ciclopirox targets cellular bioenergetics and activates ER stress to induce apoptosis in non-small cell lung cancer cells. Cell Commun. Signal. 2022, 20, 37. [Google Scholar] [CrossRef]
- Yin, J.; Che, G.; Jiang, K.; Zhou, Z.; Wu, L.; Xu, M.; Liu, J.; Yan, S. Ciclopirox Olamine Exerts Tumor-Suppressor Effects via Topoisomerase II Alpha in Lung Adenocarcinoma. Front. Oncol. 2022, 12, 791916. [Google Scholar] [CrossRef]
- Yuan, J.; Ofengeim, D. A guide to cell death pathways. Nat. Rev. Mol. Cell Biol. 2024, 25, 379–395. [Google Scholar] [CrossRef]
- Sen, T.; Tong, P.; Stewart, C.A.; Cristea, S.; Valliani, A.; Shames, D.S.; Redwood, A.B.; Fan, Y.H.; Li, L.; Glisson, B.S.; et al. CHK1 Inhibition in Small-Cell Lung Cancer Produces Single-Agent Activity in Biomarker-Defined Disease Subsets and Combination Activity with Cisplatin or Olaparib. Cancer Res. 2017, 77, 3870–3884. [Google Scholar] [CrossRef]
- Hsu, W.H.; Zhao, X.; Zhu, J.; Kim, I.K.; Rao, G.; McCutcheon, J.; Hsu, S.T.; Teicher, B.; Kallakury, B.; Dowlati, A.; et al. Checkpoint Kinase 1 Inhibition Enhances Cisplatin Cytotoxicity and Overcomes Cisplatin Resistance in SCLC by Promoting Mitotic Cell Death. J. Thorac. Oncol. 2019, 14, 1032–1045. [Google Scholar] [CrossRef]
- Byers, L.A.; Navarro, A.; Schaefer, E.; Johnson, M.; Özgüroğlu, M.; Han, J.Y.; Bondarenko, I.; Cicin, I.; Dragnev, K.H.; Abel, A.; et al. A Phase II Trial of Prexasertib (LY2606368) in Patients With Extensive-Stage Small-Cell Lung Cancer. Clin. Lung Cancer 2021, 22, 531–540. [Google Scholar] [CrossRef]
- Hoffman, B.D.; Hanauske-Abel, H.M.; Flint, A.; Lalande, M. A new class of reversible cell cycle inhibitors. Cytometry 1991, 12, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Levenson, V.; Hamlin, J.L. A general protocol for evaluating the specific effects of DNA replication inhibitors. Nucleic Acids Res. 1993, 21, 3997–4004. [Google Scholar] [CrossRef][Green Version]
- Szüts, D.; Krude, T. Cell cycle arrest at the initiation step of human chromosomal DNA replication causes DNA damage. J. Cell Sci. 2004, 117, 4897–4908. [Google Scholar] [CrossRef] [PubMed]
- Sidarovich, V.; Adami, V.; Gatto, P.; Greco, V.; Tebaldi, T.; Tonini, G.P.; Quattrone, A. Translational downregulation of HSP90 expression by iron chelators in neuroblastoma cells. Mol. Pharmacol. 2015, 87, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Xiang, J.; Fan, H.; Jiang, Y.; Lu, Y.; Zhang, C.; Zhang, Y.; Chen, Q.; Lei, Y. Ciclopirox Olamine Induces Proliferation Inhibition and Protective Autophagy in Hepatocellular Carcinoma. Pharmaceuticals 2023, 16, 113. [Google Scholar] [CrossRef]
- Sun, Q.; Guo, Y.; Liu, X.; Czauderna, F.; Carr, M.I.; Zenke, F.T.; Blaukat, A.; Vassilev, L.T. Therapeutic Implications of p53 Status on Cancer Cell Fate Following Exposure to Ionizing Radiation and the DNA-PK Inhibitor M3814. Mol. Cancer Res. 2019, 17, 2457–2468. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Li, W.; Wu, Y.; Cheng, B.; Huang, S. Inhibition of Chk1 with Prexasertib Enhances the Anticancer Activity of Ciclopirox in Non-Small Cell Lung Cancer Cells. Cells 2024, 13, 1752. https://doi.org/10.3390/cells13211752
Huang Z, Li W, Wu Y, Cheng B, Huang S. Inhibition of Chk1 with Prexasertib Enhances the Anticancer Activity of Ciclopirox in Non-Small Cell Lung Cancer Cells. Cells. 2024; 13(21):1752. https://doi.org/10.3390/cells13211752
Chicago/Turabian StyleHuang, Zhu, Wenjing Li, Yan Wu, Bing Cheng, and Shile Huang. 2024. "Inhibition of Chk1 with Prexasertib Enhances the Anticancer Activity of Ciclopirox in Non-Small Cell Lung Cancer Cells" Cells 13, no. 21: 1752. https://doi.org/10.3390/cells13211752
APA StyleHuang, Z., Li, W., Wu, Y., Cheng, B., & Huang, S. (2024). Inhibition of Chk1 with Prexasertib Enhances the Anticancer Activity of Ciclopirox in Non-Small Cell Lung Cancer Cells. Cells, 13(21), 1752. https://doi.org/10.3390/cells13211752





