DNA-Binding Protein A Is Actively Secreted in a Calcium-and Inflammasome-Dependent Manner and Negatively Influences Tubular Cell Survival
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Material
2.2. Animals
2.3. Cell Culture
2.4. DbpA Secretion Assays
2.5. Protein Precipitation and Cell Lysis
2.6. Immunoblotting
2.7. Cell Death Assays
2.8. Statistical Analysis
3. Results
3.1. Despite the Absence of a Bona Fide Secretory Motif, Databases on Body Fluid Composition Indicate Extracellular DbpA Occurrence

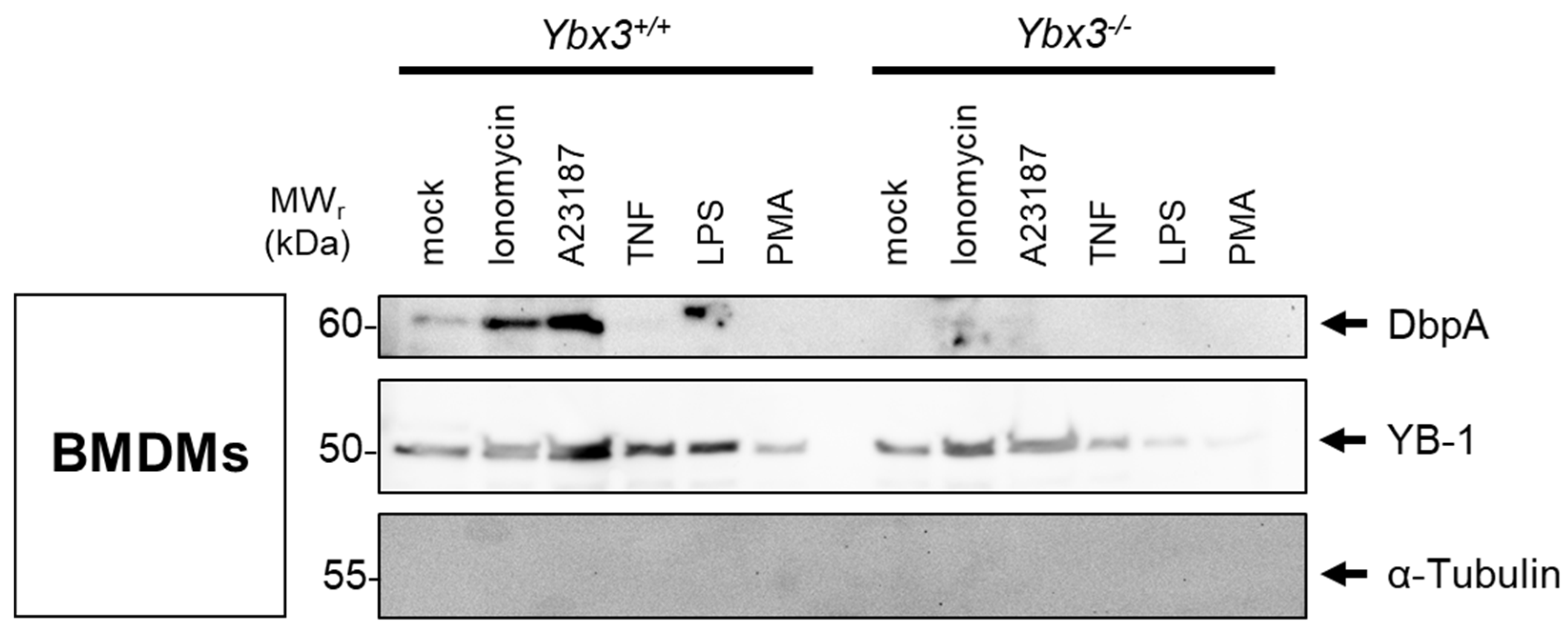
3.2. DbpA Expression in Kidney Disease and Secretion by Cells
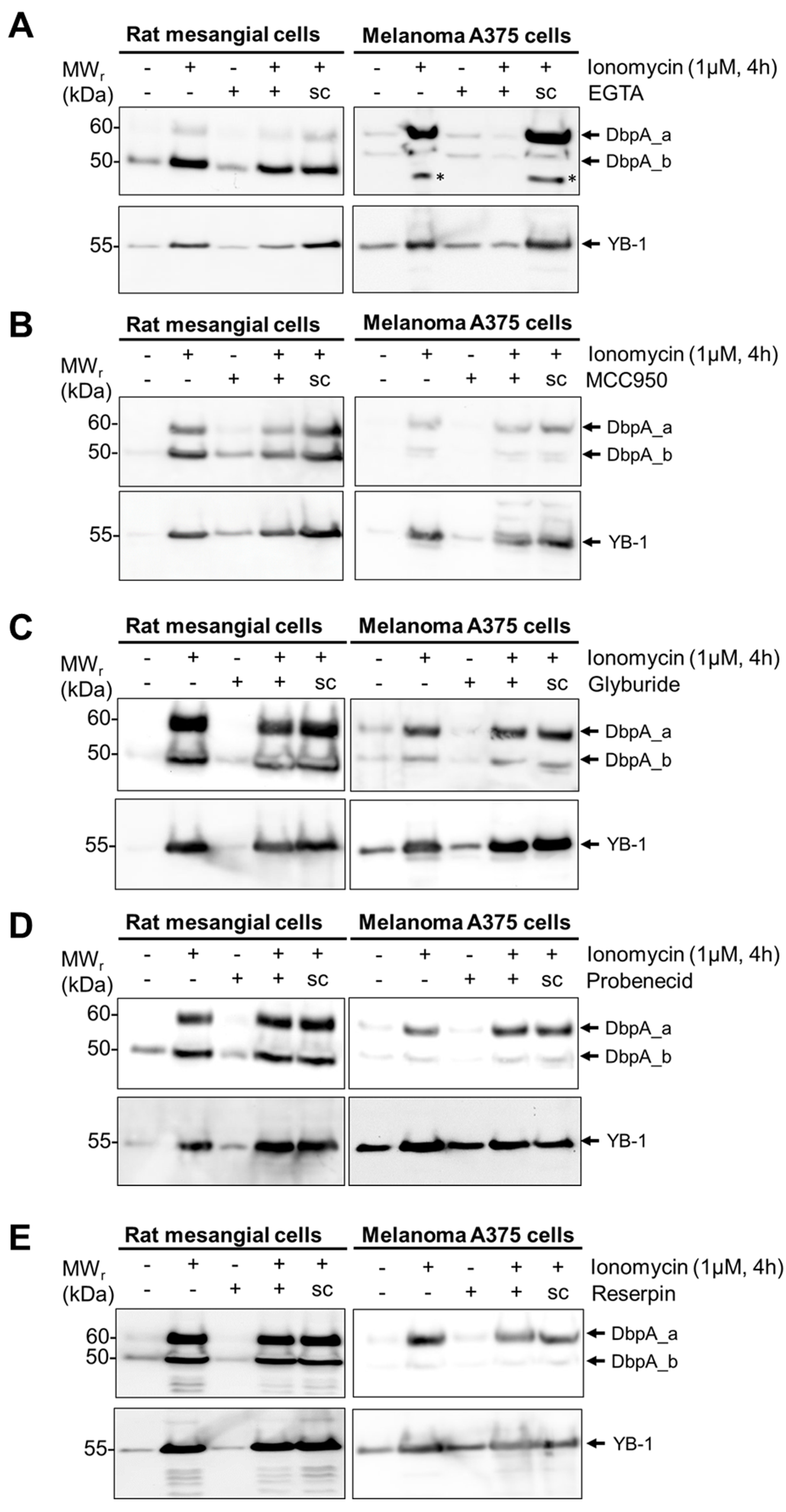
3.3. DbpA Is Secreted by a Non-Classical Secretion Pathway
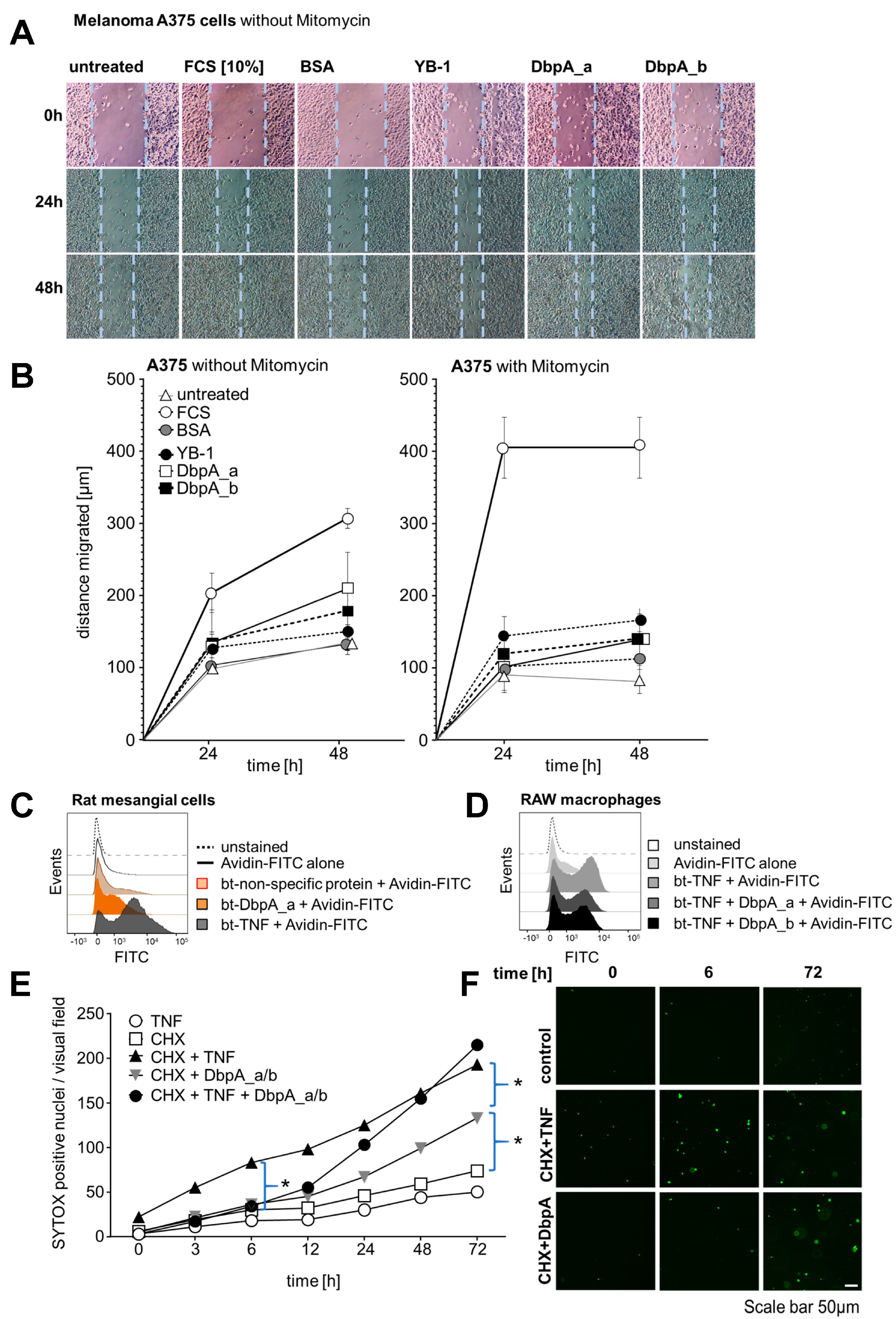
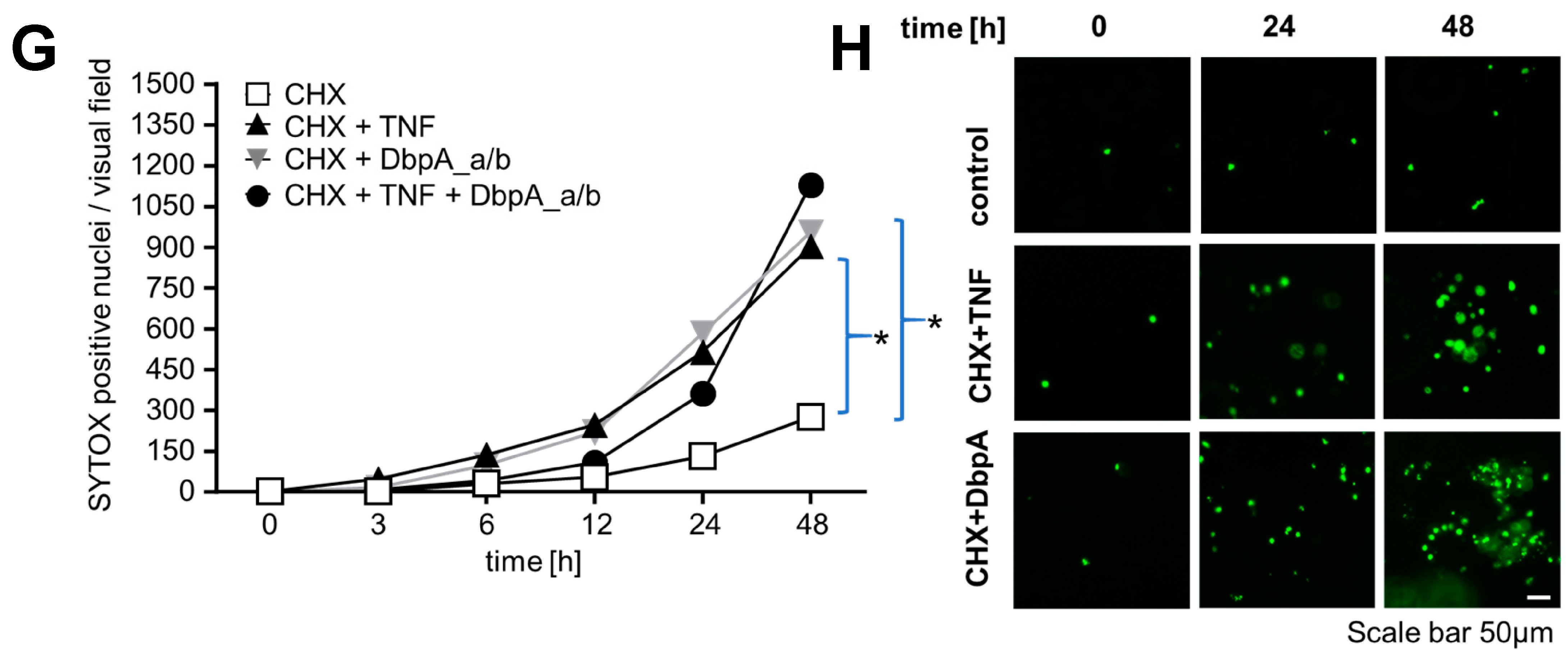
3.4. Extracellular DbpA Stimulates Migration and Proliferation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Espinosa-Cantu, A.; Cruz-Bonilla, E.; Noda-Garcia, L.; DeLuna, A. Multiple forms of multifunctional proteins in health and disease. Front. Cell Dev. Biol. 2020, 8, 451. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Niu, L.; Wu, L.; He, Y.; Liu, G.; Hong, K. Identification of an endoplasmic reticulum stress-associated gene signature to predict the immune status and prognosis of cutaneous melanoma. Medicine 2022, 101, e30280. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, J.A.; Mertens, P.R. Cold shock proteins: From cellular mechanisms to pathophysiology and disease. Cell Commun. Signal. 2018, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Izumi, H.; Uchiumi, T.; Ashizuka, M.; Kuwano, M. The pleiotropic functions of the y-box-binding protein, yb-1. Bioessays 2003, 25, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Wolffe, A.P. Structural and functional properties of the evolutionarily ancient y-box family of nucleic acid binding proteins. Bioessays 1994, 16, 245–251. [Google Scholar] [CrossRef]
- Graumann, P.L.; Marahiel, M.A. A superfamily of proteins that contain the cold-shock domain. Trends Biochem. Sci. 1998, 23, 286–290. [Google Scholar] [CrossRef]
- Sommerville, J. Activities of cold-shock domain proteins in translation control. Bioessays 1999, 21, 319–325. [Google Scholar] [CrossRef]
- Kudo, S.; Mattei, M.G.; Fukuda, M. Characterization of the gene for dbpa, a family member of the nucleic-acid-binding proteins containing a cold-shock domain. Eur. J. Biochem. 1995, 231, 72–82. [Google Scholar] [CrossRef]
- Sakura, H.; Maekawa, T.; Imamoto, F.; Yasuda, K.; Ishii, S. Two human genes isolated by a novel method encode DNA-binding proteins containing a common region of homology. Gene 1988, 73, 499–507. [Google Scholar]
- Balda, M.S.; Matter, K. The tight junction protein zo-1 and an interacting transcription factor regulate erbb-2 expression. EMBO J. 2000, 19, 2024–2033. [Google Scholar] [CrossRef]
- Balda, M.S.; Garrett, M.D.; Matter, K. The zo-1-associated y-box factor zonab regulates epithelial cell proliferation and cell density. J. Cell Biol. 2003, 160, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, J.A.; Bernhardt, A.; Reichardt, C.; Sauter, E.; Brandt, S.; Rana, R.; Lindenmeyer, M.T.; Philipsen, L.; Isermann, B.; Zhu, C.; et al. Cold shock domain protein dbpa orchestrates tubular cell damage and interstitial fibrosis in inflammatory kidney disease. Cells 2023, 12, 1426. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C.; Brandt, S.; Bernhardt, A.; Krause, A.; Lindquist, J.A.; Weinert, S.; Geffers, R.; Franz, T.; Kahlfuss, S.; Dudeck, A.; et al. DNA-binding protein-a promotes kidney ischemia/reperfusion injury and participates in mitochondrial function. Kidney Int. 2024, 106, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Frye, B.C.; Halfter, S.; Djudjaj, S.; Muehlenberg, P.; Weber, S.; Raffetseder, U.; En-Nia, A.; Knott, H.; Baron, J.M.; Dooley, S.; et al. Y-box protein-1 is actively secreted through a non-classical pathway and acts as an extracellular mitogen. EMBO Rep. 2009, 10, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Kosnopfel, C.; Sinnberg, T.; Sauer, B.; Niessner, H.; Muenchow, A.; Fehrenbacher, B.; Schaller, M.; Mertens, P.R.; Garbe, C.; Thakur, B.K.; et al. Tumour progression stage-dependent secretion of yb-1 stimulates melanoma cell migration and invasion. Cancers 2020, 12, 2328. [Google Scholar] [CrossRef]
- Guarino, A.M.; Troiano, A.; Pizzo, E.; Bosso, A.; Vivo, M.; Pinto, G.; Amoresano, A.; Pollice, A.; La Mantia, G.; Calabro, V. Oxidative stress causes enhanced secretion of yb-1 protein that restrains proliferation of receiving cells. Genes 2018, 9, 513. [Google Scholar] [CrossRef]
- Palicharla, V.R.; Maddika, S. Hace1 mediated k27 ubiquitin linkage leads to yb-1 protein secretion. Cell Signal 2015, 27, 2355–2362. [Google Scholar] [CrossRef]
- Tacke, F.; Galm, O.; Kanig, N.; Yagmur, E.; Brandt, S.; Lindquist, J.A.; Eberhardt, C.S.; Raffetseder, U.; Mertens, P.R. High prevalence of y-box protein-1/p18 fragment in plasma of patients with malignancies of different origin. BMC Cancer 2014, 14, 33. [Google Scholar] [CrossRef]
- Willis, W.L.; Hariharan, S.; David, J.J.; Strauch, A.R. Transglutaminase-2 mediates calcium-regulated crosslinking of the y-box 1 (yb-1) translation-regulatory protein in tgfbeta1-activated myofibroblasts. J. Cell. Biochem. 2013, 114, 2753–2769. [Google Scholar] [CrossRef]
- Rauen, T.; Raffetseder, U.; Frye, B.C.; Djudjaj, S.; Muhlenberg, P.J.; Eitner, F.; Lendahl, U.; Bernhagen, J.; Dooley, S.; Mertens, P.R. Yb-1 acts as a ligand for notch-3 receptors and modulates receptor activation. J. Biol. Chem. 2009, 284, 26928–26940. [Google Scholar] [CrossRef]
- Remijsen, Q.; Kuijpers, T.W.; Wirawan, E.; Lippens, S.; Vandenabeele, P.; Vanden Berghe, T. Dying for a cause: Netosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011, 18, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Gu, Y.; Gao, Y.; Jankowski, V.; Was, N.; Leitz, A.; Reiss, L.K.; Shi, Y.; Cai, J.; et al. DNA binding protein yb-1 is a part of the neutrophil extracellular trap mediation of kidney damage and cross-organ effects. Kidney Int. 2023, 104, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Sauter, E.; Schreiter, A.; van Roeyen, C.R.; Ostendorf, T.; Floege, J.; Gembardt, F.; Hugo, C.P.; Isermann, B.; Lindquist, J.A.; et al. Cold shock proteins mediate gn with mesangioproliferation. J. Am. Soc. Nephrol. 2016, 27, 3678–3689. [Google Scholar] [CrossRef] [PubMed]
- DeCaprio, J.; Kohl, T.O. Detergent lysis of tissue culture cells for immunoprecipitation. Cold Spring Harb. Protoc. 2017, 2017, pdb.err107714. [Google Scholar] [CrossRef]
- Li, C.; Wu, J.; Li, Y.; Xing, G. Cytoprotective effect of heat shock protein 27 against lipopolysaccharide-induced apoptosis of renal epithelial hk-2 cells. Cell Physiol. Biochem. 2017, 41, 2211–2220. [Google Scholar] [CrossRef]
- Hessman, C.L.; Hildebrandt, J.; Shah, A.; Brandt, S.; Bock, A.; Frye, B.C.; Raffetseder, U.; Geffers, R.; Brunner-Weinzierl, M.C.; Isermann, B.; et al. Yb-1 interferes with tnfalpha-tnfr binding and modulates progranulin-mediated inhibition of tnfalpha signaling. Int. J. Mol. Sci. 2020, 21, 7076. [Google Scholar] [CrossRef]
- Briesemeister, S.; Blum, T.; Brady, S.; Lam, Y.; Kohlbacher, O.; Shatkay, H. Sherloc2: A high-accuracy hybrid method for predicting subcellular localization of proteins. J. Proteome Res. 2009, 8, 5363–5366. [Google Scholar] [CrossRef]
- Imai, K.; Nakai, K. Tools for the recognition of sorting signals and the prediction of subcellular localization of proteins from their amino acid sequences. Front. Genet. 2020, 11, 607812. [Google Scholar] [CrossRef]
- Maricchiolo, E.; Panfili, E.; Pompa, A.; De Marchis, F.; Bellucci, M.; Pallotta, M.T. Unconventional pathways of protein secretion: Mammals vs. Plants. Front. Cell Dev. Biol. 2022, 10, 895853. [Google Scholar] [CrossRef]
- Leis, H.J.; Windischhofer, W. Ionomycin induces prostaglandin e2 formation in murine osteoblastic mc3t3-e1 cells via mechanisms independent of its ionophoric nature. Biochem. Cell Biol. 2016, 94, 236–240. [Google Scholar] [CrossRef]
- Artlett, C.M. The mechanism and regulation of the nlrp3 inflammasome during fibrosis. Biomolecules 2022, 12, 634. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Pang, I.K.; Iwasaki, A. Influenza virus activates inflammasomes via its intracellular m2 ion channel. Nat. Immunol. 2010, 11, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.V.; Miller, E.A.; Bhardwaj, N. Activation and measurement of nlrp3 inflammasome activity using il-1beta in human monocyte-derived dendritic cells. J. Vis. Exp. 2014, 87, e51284. [Google Scholar] [CrossRef]
- Wright, P.E.; Dyson, H.J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 2015, 16, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Holehouse, A.S.; Kragelund, B.B. The molecular basis for cellular function of intrinsically disordered protein regions. Nat. Rev. Mol. Cell Biol. 2023, 25, 187–211. [Google Scholar] [CrossRef] [PubMed]
- Fefilova, A.S.; Antifeeva, I.A.; Gavrilova, A.A.; Turoverov, K.K.; Kuznetsova, I.M.; Fonin, A.V. Reorganization of cell compartmentalization induced by stress. Biomolecules 2022, 12, 1441. [Google Scholar] [CrossRef]
- Lima, W.R.; Parreira, K.S.; Devuyst, O.; Caplanusi, A.; N’Kuli, F.; Marien, B.; Van Der Smissen, P.; Alves, P.M.; Verroust, P.; Christensen, E.I.; et al. Zonab promotes proliferation and represses differentiation of proximal tubule epithelial cells. J. Am. Soc. Nephrol. 2010, 21, 478–488. [Google Scholar] [CrossRef]
- Sourisseau, T.; Georgiadis, A.; Tsapara, A.; Ali, R.R.; Pestell, R.; Matter, K.; Balda, M.S. Regulation of pcna and cyclin d1 expression and epithelial morphogenesis by the zo-1-regulated transcription factor zonab/dbpa. Mol. Cell. Biol. 2006, 26, 2387–2398. [Google Scholar] [CrossRef]
- Ma, M.; Jiang, W.; Zhou, R. Damps and damp-sensing receptors in inflammation and diseases. Immunity 2024, 57, 752–771. [Google Scholar] [CrossRef]
- Mariani, L.H.; Eddy, S.; AlAkwaa, F.M.; McCown, P.J.; Harder, J.L.; Nair, V.; Eichinger, F.; Martini, S.; Ademola, A.D.; Boima, V.; et al. Precision nephrology identified tumor necrosis factor activation variability in minimal change disease and focal segmental glomerulosclerosis. Kidney Int. 2023, 103, 565–579. [Google Scholar] [CrossRef]
- Ladik, M.; Valenta, H.; Erard, M.; Vandenabeele, P.; Riquet, F.B. From tnf-induced signaling to nadph oxidase enzyme activity: Methods to investigate protein complexes involved in regulated cell death modalities. Front. Cell Death 2023, 2, 1127330. [Google Scholar] [CrossRef]
- Karathanasis, C.; Medler, J.; Fricke, F.; Smith, S.; Malkusch, S.; Widera, D.; Fulda, S.; Wajant, H.; van Wijk, S.J.L.; Dikic, I.; et al. Single-molecule imaging reveals the oligomeric state of functional tnfalpha-induced plasma membrane tnfr1 clusters in cells. Sci. Signal 2020, 13, eaax5647. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, U.; Roske, Y. Cold-shock domains-abundance, structure, properties, and nucleic-acid binding. Cancers 2021, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E. Damps, pamps and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef]




| Inhibitors | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cell Line | Stimulus | Protein Secretion | BFA | Monensin | EGTA | MCC950 | Glyburide | Probenecid | Reserpin | |
| rMC | LPS | DbpA_a | Yes | ⬆ | ⬆ | Not tested | ||||
| DbpA_b | Yes | ⬆ | ⬆ | |||||||
| YB-1 | No | ⚊ | ⚊ | |||||||
| Ionomycin | DbpA_a | Yes | ⚊ | Not tested | ⬇ | ⬇ | ⚊ | ⚊ | ⚊ | |
| DbpA_b | Yes | ⚊ | ⚊ | ⬇ | ⚊ | ⚊ | ⚊ | |||
| YB-1 | Yes | ⚊ | ⬇ | ⬇ | ⚊ | ⚊ | ⚊ | |||
| A375 | LPS | DbpA_a | No | No secretion, therefore not tested | ||||||
| DbpA_b | No | |||||||||
| YB-1 | No | |||||||||
| Ionomycin | DbpA_a | Yes | ⚊ | ⚊ | ⬇ | ⬇ | ⚊ | ⚊ | ⚊ | |
| DbpA_b | Yes | ⚊ | ⚊ | ⬇ | ⬇ | ⚊ | ⚊ | ⚊ | ||
| YB-1 | Yes | ⚊ | ⬆ | ⬇ | ⬇ | ⚊ | ⚊ | ⚊ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoppstock, G.; Lindquist, J.A.; Willems, A.; Becker, A.; Reichardt, C.; Morgenroth, R.; Stolze, S.; Zhu, C.; Brandt, S.; Mertens, P.R. DNA-Binding Protein A Is Actively Secreted in a Calcium-and Inflammasome-Dependent Manner and Negatively Influences Tubular Cell Survival. Cells 2024, 13, 1742. https://doi.org/10.3390/cells13201742
Hoppstock G, Lindquist JA, Willems A, Becker A, Reichardt C, Morgenroth R, Stolze S, Zhu C, Brandt S, Mertens PR. DNA-Binding Protein A Is Actively Secreted in a Calcium-and Inflammasome-Dependent Manner and Negatively Influences Tubular Cell Survival. Cells. 2024; 13(20):1742. https://doi.org/10.3390/cells13201742
Chicago/Turabian StyleHoppstock, Gregor, Jonathan A. Lindquist, Antonia Willems, Annika Becker, Charlotte Reichardt, Ronnie Morgenroth, Saskia Stolze, Cheng Zhu, Sabine Brandt, and Peter R. Mertens. 2024. "DNA-Binding Protein A Is Actively Secreted in a Calcium-and Inflammasome-Dependent Manner and Negatively Influences Tubular Cell Survival" Cells 13, no. 20: 1742. https://doi.org/10.3390/cells13201742
APA StyleHoppstock, G., Lindquist, J. A., Willems, A., Becker, A., Reichardt, C., Morgenroth, R., Stolze, S., Zhu, C., Brandt, S., & Mertens, P. R. (2024). DNA-Binding Protein A Is Actively Secreted in a Calcium-and Inflammasome-Dependent Manner and Negatively Influences Tubular Cell Survival. Cells, 13(20), 1742. https://doi.org/10.3390/cells13201742






