Chicken Embryo Fibroblast Viability and Trans-Differentiation Potential for Cultured Meat Production Across Passages
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Research Ethics Statements
2.2. Isolation of Chicken Embryo Fibroblasts (CEFs)
2.3. Purification of Chicken Embryo Fibroblasts (CEFs)
2.4. Culture Condition of Chicken Embryo Fibroblasts (CEFs)
2.5. Immunofluorescence Staining for Isolating and Identifying Chicken Embryo Fibroblasts (CEFs)
2.6. Proliferation Analysis of Chicken Embryo Fibroblasts (CEFs)
2.6.1. Cumulative Cell Numbers
2.6.2. Population Doubling Time
2.6.3. EdU Assay
2.6.4. Proliferation Capacity Assay Using Cell Counting Kit-8
2.7. Adipogenic Trans-Differentiation Analysis over Passages
2.7.1. Size Analysis of Transdifferentiated Adipocytes
2.7.2. Neutral Lipid Staining
2.7.3. Flow Cytometry for Adipogenesis Analysis
2.8. Statistical Analysis
3. Results
3.1. Isolation of Chicken Embryo Fibroblasts by Pre-Plating
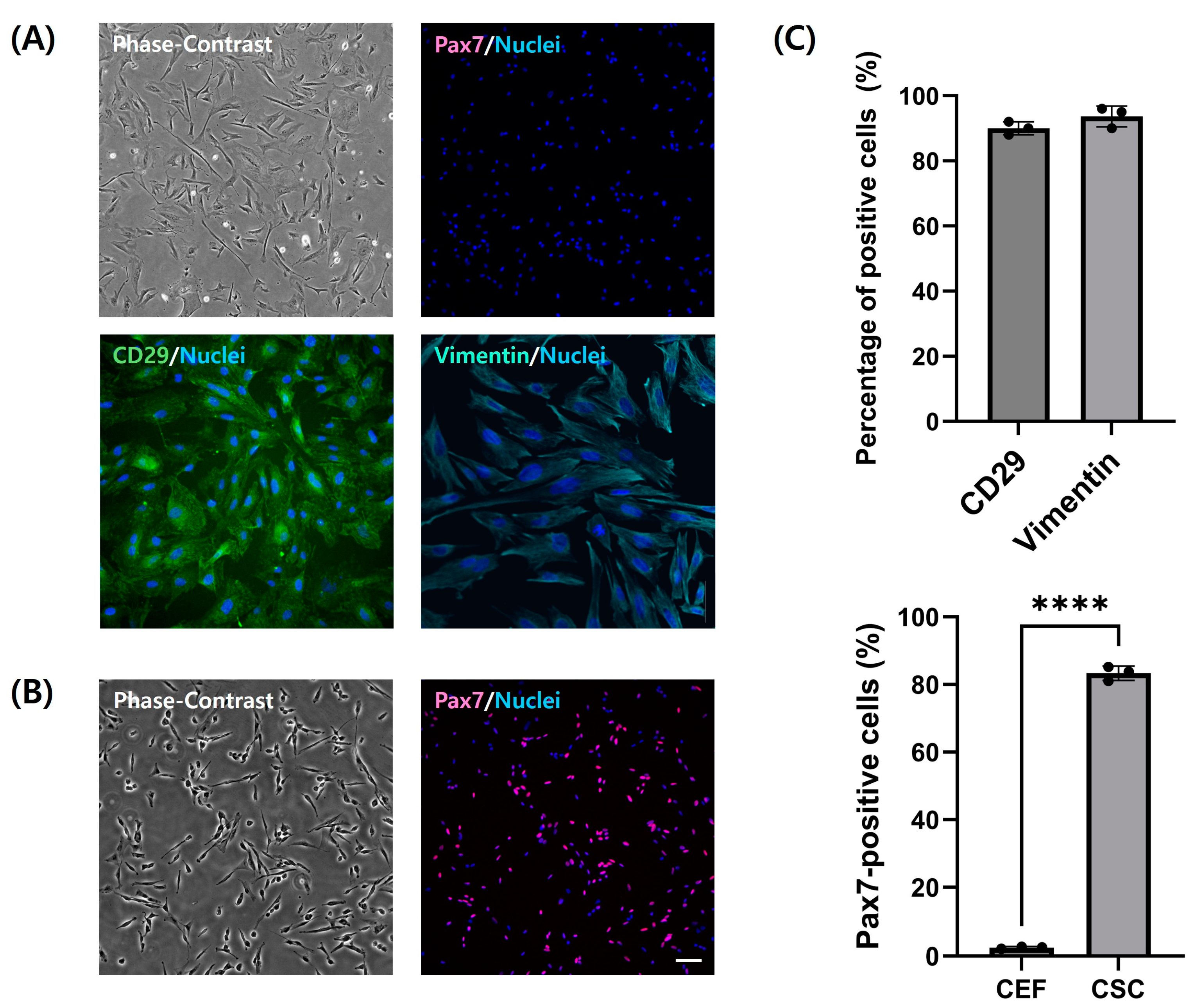
3.2. Identification of Chicken Embryo Fibroblasts
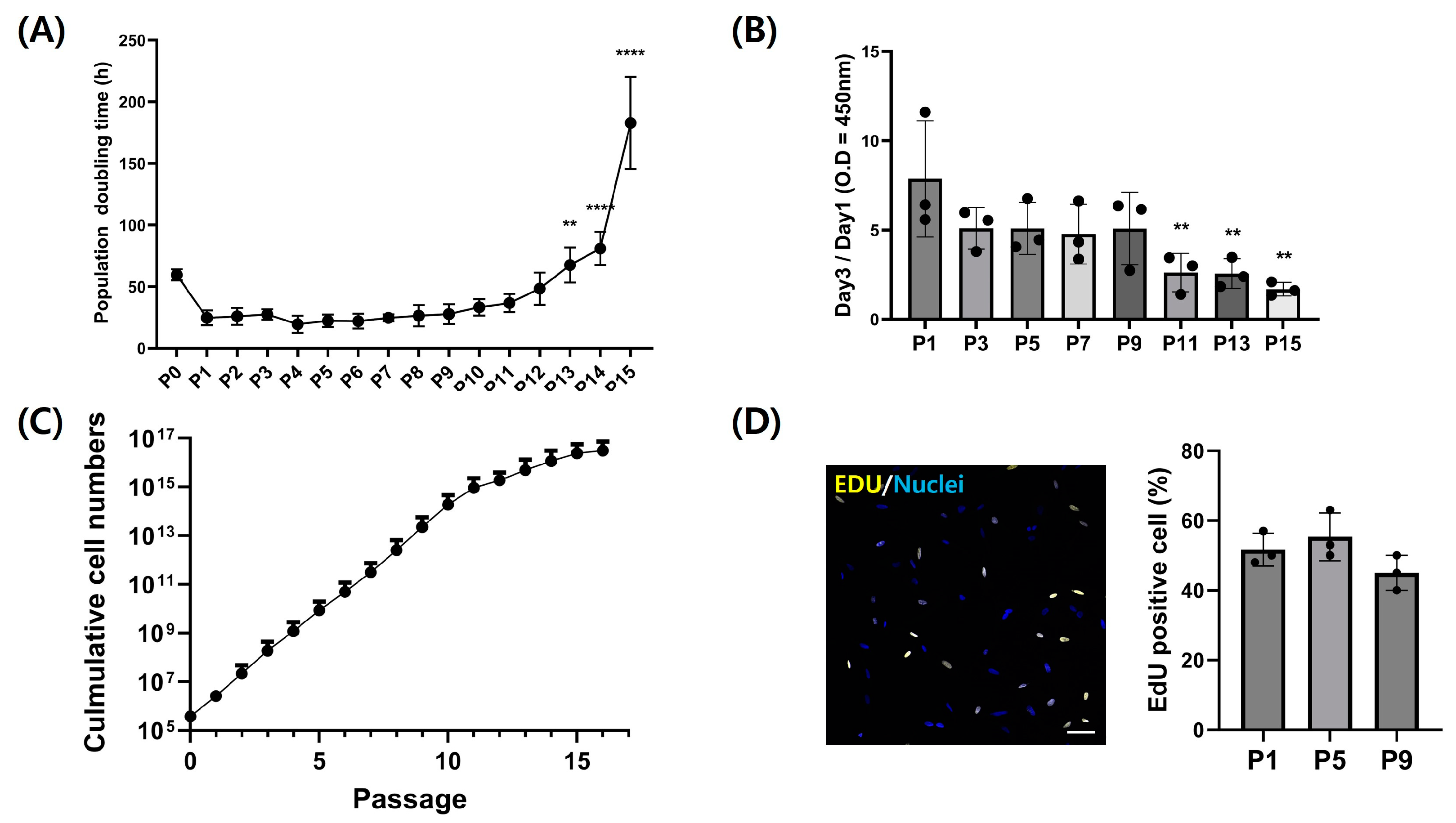
3.3. Analysis of Proliferation Capacity of Chicken Embryo Fibroblasts
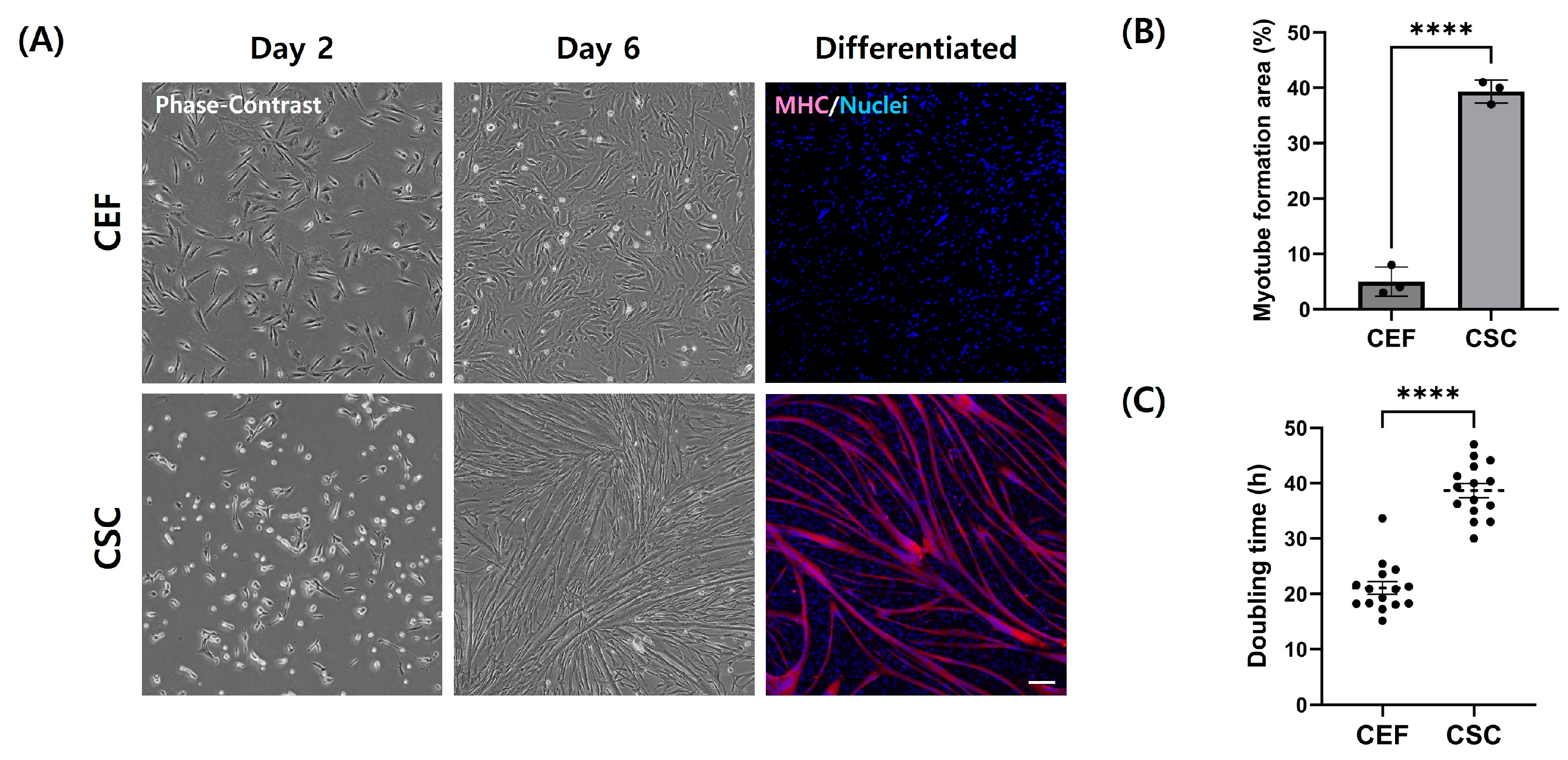
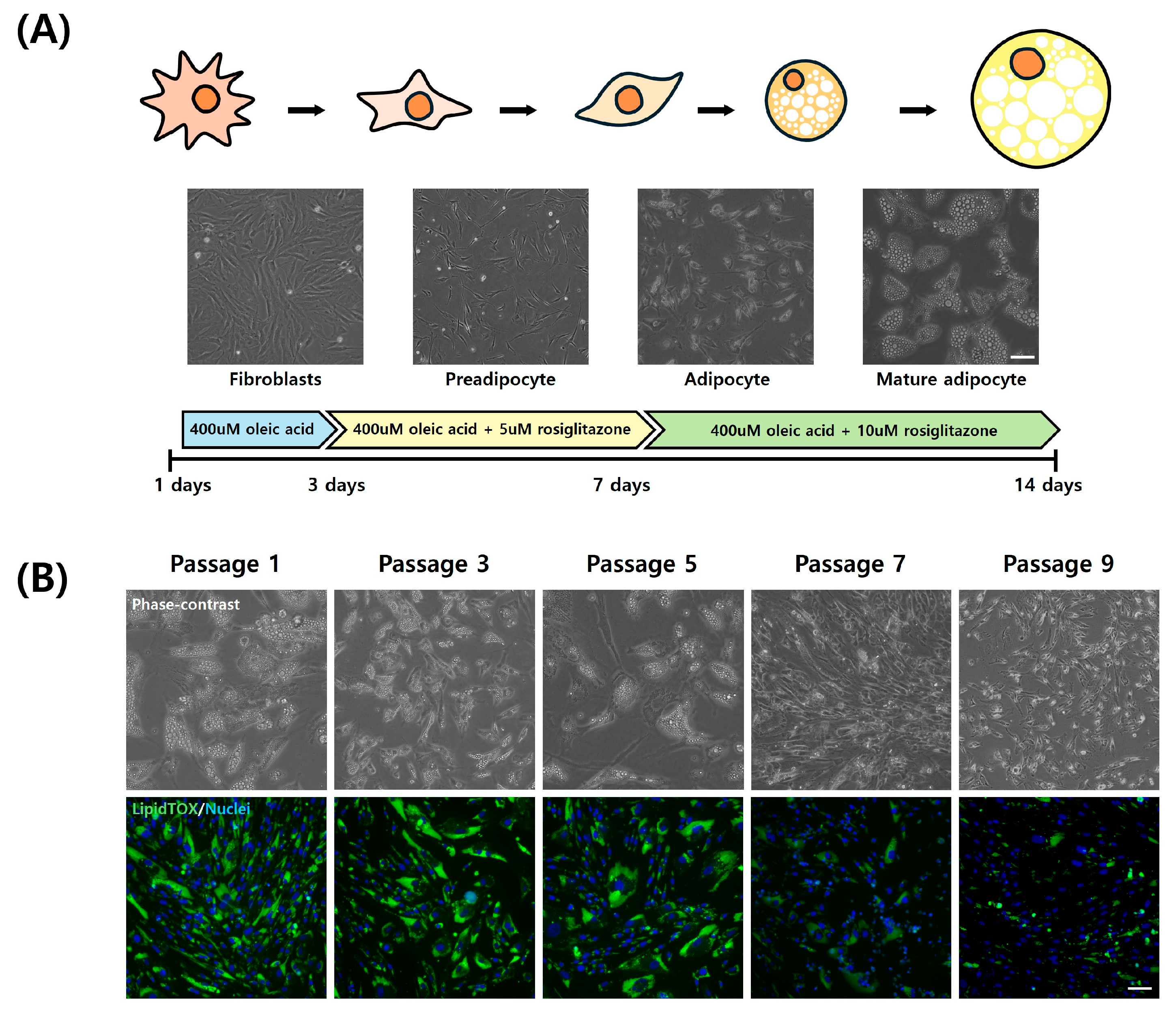
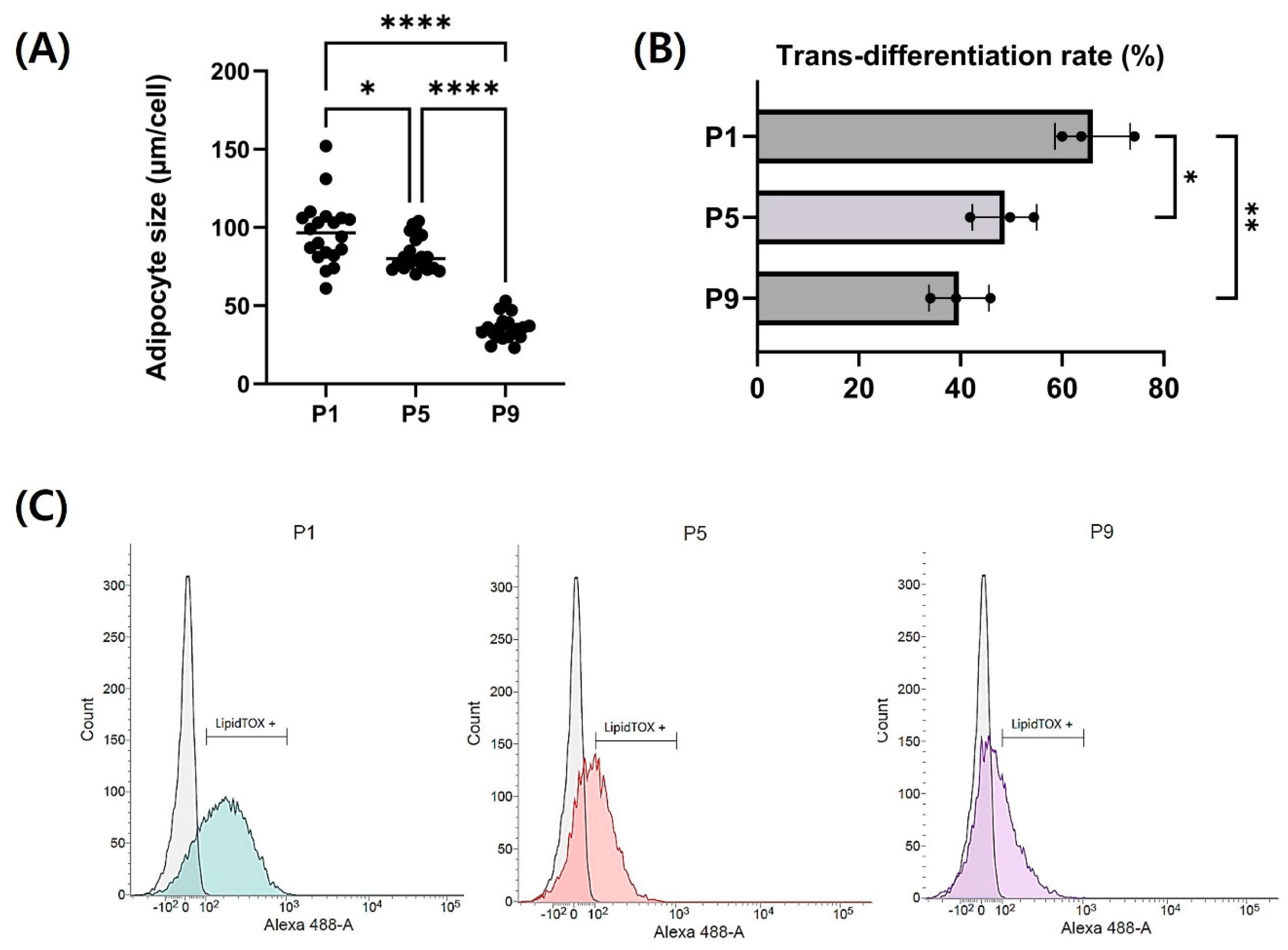
3.4. Adipogenic Trans-Differentiation of Chicken Embryo Fibroblasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Sugii, S.; Wong, C.Y.Q.; Lwin, A.K.O.; Chew, L.J.M. Alternative fat: Redefining adipocytes for biomanufacturing cultivated meat. Trends Biotechnol. 2023, 41, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.M.M.; Kim, C.J.; Kim, S.H.; Kumari, S.; Lee, E.Y.; Hwang, Y.H.; Joo, S.T. Scaffolding fundamentals and recent advances in sustainable scaffolding techniques for cultured meat development. Food Res. Int. 2024, 189, 114549. [Google Scholar] [CrossRef] [PubMed]
- Bomkamp, C.; Skaalure, S.C.; Fernando, G.F.; Ben-Arye, T.; Swartz, E.W.; Specht, E.A. Scaffolding Biomaterials for 3D Cultivated Meat: Prospects and Challenges. Adv. Sci. 2022, 9, 2102908. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Kim, S.H.; Lee, E.Y.; Hwang, Y.H.; Joo, S.T. Effect of Chicken Age on the Proliferation and Differentiation Abilities of Muscle Stem Cells and Nutritional Characteristics of Cultured Meat Tissue. Food Sci. Anim. Resour. 2024, 44, 1167. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Lee, D.Y.; Lee, J.; Mariano, E.; Choi, Y.; Park, J.; Han, D.; Kim, J.S.; Hur, S.J. Current Research, Industrialization Status, and Future Perspective of Cultured Meat. Food Sci. Anim. Resour. 2024, 44, 326–355. [Google Scholar] [CrossRef]
- Joo, S.T.; Choi, J.S.; Hur, S.J.; Kim, G.D.; Kim, C.J.; Lee, E.Y.; Bakhsh, A.; Hwang, Y.H. A Comparative Study on the Taste Characteristics of Satellite Cell Cultured Meat Derived from Chicken and Cattle Muscles. Food Sci. Anim. Resour. 2022, 42, 175–185. [Google Scholar] [CrossRef]
- Kang, K.M.; Lee, D.B.; Kim, H.Y. Industrial Research and Development on the Production Process and Quality of Cultured Meat Hold Significant Value: A Review. Food Sci. Anim. Resour. 2024, 44, 499–514. [Google Scholar] [CrossRef]
- Jr, J.S.K.Y.; Saad, M.K.; Xiang, N.; Barrick, B.M.; DiCindio, H.; Li, C.; Zhang, S.W.; Rittenberg, M.; Lew, E.T.; Zhang, K.L.; et al. Aggregating in vitro-grown adipocytes to produce macroscale cell-cultured fat tissue with tunable lipid compositions for food applications. eLife 2023, 12, e82120. [Google Scholar] [CrossRef]
- Jr, J.S.Y.; Stout, A.J.; Kawecki, N.S.; Letcher, S.M.; Theodossiou, S.K.; Cohen, J.M.; Barrick, B.M.; Saad, M.K.; Rubio, N.R.; Pietropinto, J.A.; et al. Perspectives on scaling production of adipose tissue for food applications. Biomaterials 2022, 280, 121273. [Google Scholar] [CrossRef]
- Pasitka, L.; Cohen, M.; Ehrlich, A.; Gildor, B.; Reuveni, E.; Ayyash, M.; Wissotsky, G.; Herscovici, A.; Kaminker, R.; Niv, A.; et al. Spontaneous immortalization of chicken fibroblasts generates stable, high-yield cell lines for serum-free production of cultured meat. Nat. Food 2022, 4, 35–50. [Google Scholar] [CrossRef]
- Zagury, Y.; Ianovici, I.; Landau, S.; Lavon, N.; Levenberg, S. Engineered marble-like bovine fat tissue for cultured meat. Commun. Biol. 2022, 5, 927. [Google Scholar] [CrossRef] [PubMed]
- Cieślar-Pobuda, A.; Knoflach, V.; Ringh, M.V.; Stark, J.; Likus, W.; Siemianowicz, K.; Ghavami, S.; Hudecki, A.; Green, J.L.; Łos, M.J. Transdifferentiation and reprogramming: Overview of the processes, their similarities and differences. Biochimica et Biophysica. Acta (BBA) Mol. Cell Res. 2017, 1864, 1359–1369. [Google Scholar] [CrossRef]
- Merrell, A.J.; Stanger, B.Z. Adult cell plasticity in vivo: De-differentiation and transdifferentiation are back in style. Nat. Rev. Mol. Cell Biol. 2016, 17, 413–425. [Google Scholar] [CrossRef]
- Clabaut, A.; Grare, C.; Rolland-Valognes, G.; Letarouilly, J.-G.; Bourrier, C.; Andersen, T.L.; Sikjær, T.; Rejnmark, L.; Ejersted, C.; Pastoureau, P.; et al. Adipocyte-induced transdifferentiation of osteoblasts and its potential role in age-related bone loss. PLoS ONE 2021, 16, e0245014. [Google Scholar] [CrossRef]
- Hu, E.; Tontonoz, P.; Spiegelman, B.M. Transdifferentiation of myoblasts by the adipogenic transcription factors PPARy and C/EBPa! Proc. Natl. Acad. Sci. USA 1995, 92, 9856–9860. [Google Scholar] [CrossRef]
- Ding, S.; Wang, F.; Liu, Y.; Li, S.; Zhou, G.; Hu, P. Characterization and isolation of highly purified porcine satellite cells. Cell Death Discov. 2017, 3, 17003. [Google Scholar] [CrossRef] [PubMed]
- Baquero-Perez, B.; Kuchipudi, S.V.; Nelli, R.K.; Chang, K.-C. A simplified but robust method for the isolation of avian and mammalijan muscle satellite cells. BMC Cell Biol. 2012, 13, 16. [Google Scholar] [CrossRef]
- Choi, K.H.; Kim, M.; Yoon, J.W.; Jeong, J.; Ryu, M.; Jo, C.; Lee, C.K. Purification of Pig Muscle Stem Cells Using Magnetic-Activated Cell Sorting (MACS) Based on the Expression of Cluster of Differentiation 29 (CD29). Food Sci. Anim. Resour. 2020, 40, 852–859. [Google Scholar] [CrossRef]
- Matsuyoshi, Y.; Akahoshi, M.; Nakamura, M.; Tatsumi, R.; Mizunoya, W. Isolation and Purification of Satellite Cells from Young Rats by Percoll Density Gradient Centrifugation. In Myogenesis; Rønning, S.B., Ed.; Springer: New York, NY, USA, 2019; Volume 1889, pp. 81–93. [Google Scholar] [CrossRef]
- Yoshioka, K.; Kitajima, Y.; Okazaki, N.; Chiba, K.; Yonekura, A.; Ono, Y. A Modified Pre-plating Method for High-Yield and High-Purity Muscle Stem Cell Isolation From Human/Mouse Skeletal Muscle Tissues. Front. Cell Dev. Biol. 2020, 8, 793. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, C.J.; Lee, E.Y.; Son, Y.M.; Hwang, Y.H.; Joo, S.T. Optimal Pre-Plating Method of Chicken Satellite Cells for Cultured Meat Production. Food Sci. Anim. Resour. 2022, 42, 942–952. [Google Scholar] [CrossRef]
- Benedetti, A.; Cera, G.; De Meo, D.; Villani, C.; Bouche, M.; Lozanoska-Ochser, B. A novel approach for the isolation and long-term expansion of pure satellite cells based on ice-cold treatment. Skelet. Muscle 2021, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Song, W.; Bassey, A.P.; Tang, C.; Li, H.; Ding, S.; Zhou, G. Preparation and Quality Evaluation of Cultured Fat. J. Agric. Food Chem. 2023, 71, 4113–4122. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Wang, L.; Wang, N.; Li, Y.; Li, H. Transdifferentiation of fibroblasts into adipocyte-like cells by chicken adipogenic transcription factors. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, L.; Wang, N.; Wang, Y.; Shi, H.; Li, H. Oleate induces transdifferentiation of chicken fibroblasts into adipocyte-like cells. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 154, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Sugathan, S.; Lee, S.-J.; Shiwani, S.; Singh, N.K. Transdifferentiation of bovine epithelial cells towards adipocytes in the presence of myoepithelium. Asian-Australas. J. Anim. Sci. 2020, 33, 349–359. [Google Scholar] [CrossRef]
- Fernandes, I.R.; Russo, F.B.; Pignatari, G.C.; Evangelinellis, M.M.; Tavolari, S.; Muotri, A.R.; Beltrão-Braga, P.C.B. Fibroblast sources: Where can we get them? Cytotechnology 2016, 68, 223–228. [Google Scholar] [CrossRef]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef]
- Saxena, S.; Hanwate, M.; Deb, K.; Sharma, V.; Totey, S. FGF2 secreting human fibroblast feeder cells: A novel culture system for human embryonic stem cells. Mol. Reprod. Dev. 2008, 75, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Kim, S.H.; Lee, E.Y.; Son, Y.M.; Bakhsh, A.; Hwang, Y.H.; Joo, S.T. Optimal temperature for culturing chicken satellite cells to enhance production yield and umami intensity of cultured meat. Food Chem. Adv. 2023, 2, 100307. [Google Scholar] [CrossRef]
- Allouh, M.Z.; Yablonka-Reuveni, Z.; Rosser, B.W.C. Pax7 Reveals a Greater Frequency and Concentration of Satellite Cells at the Ends of Growing Skeletal Muscle Fibers. J. Histochem. Cytochem. 2008, 56, 77–87. [Google Scholar] [CrossRef]
- Zammit, P.S.; Relaix, F.; Nagata, Y.; Ruiz, A.P.; Collins, C.A.; Partridge, T.A.; Beauchamp, J.R. Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Sci. 2006, 119, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Schüler, S.C.; Hüttner, S.S.; von Eyss, B.; von Maltzahn, J. Adult stem cells at work: Regenerating skeletal muscle. Cell. Mol. Life Sci. 2019, 76, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.Y.; Cao, J.; Ou-Yang, P.; Tsai, C.Y.; Lin, C.W.; Chen, C.C.; Tsai, M.K.; Lee, C.Y. Different methods of detaching adherent cells and their effects on the cell surface expression of Fas receptor and Fas ligand. Sci. Rep. 2022, 12, 5713. [Google Scholar] [CrossRef]
- Kim, D.K. Estimating doubling time of cells in vitro. Vitr. Cell. Dev. Biol. Anim. 1995, 31, 419–420. [Google Scholar] [CrossRef]
- Zhao, R.; Jin, J.; Sun, X.; Jin, K.; Wang, M.; Ahmed, M.F.; Zuo, Q.; Zhang, Y.; Zhao, Z.; Chen, G.; et al. The establishment of clonally derived chicken embryonic fibroblast cell line (CSC) with high transfection efficiency and ability as a feeder cell. J. Cell. Biochem. 2018, 119, 8841–8850. [Google Scholar] [CrossRef]
- Hubalek, S.; Post, M.J.; Moutsatsou, P. Towards resource-efficient and cost-efficient cultured meat. Curr. Opin. Food Sci. 2022, 47, 100885. [Google Scholar] [CrossRef]
- Matsubara, Y.; Sato, K.; Ishii, H.; Akiba, Y. Changes in mRNA expression of regulatory factors involved in adipocyte differentiation during fatty acid induced adipogenesis in chicken. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 141, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, Q.; Wang, Y.; Yang, P.; Wang, Q.; Li, H. Chicken adipocyte fatty acid-binding protein knockdown affects expression of peroxisome proliferator-activated receptor γ gene during oleate-induced adipocyte differentiation. Poult. Sci. 2011, 90, 1037–1044. [Google Scholar] [CrossRef]
- Rizzatti, V.; Boschi, F.; Pedrotti, M.; Zoico, E.; Sbarbati, A.; Zamboni, M. Lipid droplets characterization in adipocyte differentiated 3T3-L1 cells: Size and optical density distribution. Eur. J. Histochem. 2013, 57, 24. [Google Scholar] [CrossRef]
- Cheng, B.; Wu, M.; Xu, S.; Zhang, X.; Wang, Y.; Wang, N.; Leng, L.; Li, H. Cocktail supplement with rosiglitazone: A novel inducer for chicken preadipocyte differentiation in vitro. Biosci. Rep. 2016, 36, e00401. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, C.; Sheng, X.; Gong, Z.; Zang, Y.Q. Lecithin promotes adipocyte differentiation and hepatic lipid accumulation. Int. J. Mol. Med. 2009, 23, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Santibañez, G.; Cho, K.W.; Lumeng, C.N. Imaging White Adipose Tissue with Confocal Microscopy. Methods Enzymol. 2014, 537, 17–30. [Google Scholar] [CrossRef]
- Chen, J.H.; Ozanne, S.E.; Hales, C.N. Methods of Cellular Senescence Induction Using Oxidative Stress. In Biological Aging. Methods in Molecular Biology™; Tollefsbol, T.O., Ed.; Humana Press: Totowa, NJ, USA, 2007; Volume 371. [Google Scholar] [CrossRef]
- Xu, M.; Palmer, A.K.; Ding, H.; Weivoda, M.M.; Pirtskhalava, T.; White, T.A.; Sepe, A.; Johnson, K.O.; Stout, M.B.; Giorgadze, N.; et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife 2015, 4, e12997. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Kim, C.-J.; Lee, E.-Y.; Hwang, Y.-H.; Joo, S.-T. Chicken Embryo Fibroblast Viability and Trans-Differentiation Potential for Cultured Meat Production Across Passages. Cells 2024, 13, 1734. https://doi.org/10.3390/cells13201734
Kim S-H, Kim C-J, Lee E-Y, Hwang Y-H, Joo S-T. Chicken Embryo Fibroblast Viability and Trans-Differentiation Potential for Cultured Meat Production Across Passages. Cells. 2024; 13(20):1734. https://doi.org/10.3390/cells13201734
Chicago/Turabian StyleKim, So-Hee, Chan-Jin Kim, Eun-Yeong Lee, Young-Hwa Hwang, and Seon-Tea Joo. 2024. "Chicken Embryo Fibroblast Viability and Trans-Differentiation Potential for Cultured Meat Production Across Passages" Cells 13, no. 20: 1734. https://doi.org/10.3390/cells13201734
APA StyleKim, S.-H., Kim, C.-J., Lee, E.-Y., Hwang, Y.-H., & Joo, S.-T. (2024). Chicken Embryo Fibroblast Viability and Trans-Differentiation Potential for Cultured Meat Production Across Passages. Cells, 13(20), 1734. https://doi.org/10.3390/cells13201734







