Cardiomyocyte Differentiation from Mouse Embryonic Stem Cells by WNT Switch Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
- CHIR99021 (APExBIO, Houston, TX, USA, cat.no. A3011);
- XAV939 (Stemcell Technologies, Vancouver, BC, Canada, cat.no. 72672);
- Trypsin-EDTA (0.25%, w/v; Life Technologies, Carlsbad, CA, USA, cat.no. 25200-056);
- FBS (Fisher Scientific, Waltham, MA, USA, cat.no. MT35015CV);
- E14 mouse embryonic stem cells (E14 Tg2A.4, cat.no. 015890-UCD-CELL);
- NEAA (Thermofisher Scientific, Waltham, MA, USA, cat.no. 11140050);
- Sodium pyruvate (Thermofisher Scientific, cat.no. 11360070);
- Penicillin-Streptomycin (Thermofisher Scientific, cat.no. 15140122);
- L-Glutamine (Thermofisher Scientific, cat.no. 25030081);
- Nonfat dry milk (can be purchased from a local grocery store);
- Trypan blue solution (Fisher Scientific, cat.no. 15250061);
- β-mercaptoethanol (Thermofisher Scientific, cat.no. 21985023);
- IMDM (Thermofisher Scientific, cat.no. 12440053);
- DMEM (Thermofisher Scientific, cat.no. 11995065);
- Evagreen master mix (Fisher Scientific, cat.no. NC1787870);
- cDNA synthesis kit (Meridian Bioscience, Cincinnati, OH, USA, cat.no. BIO-65043);
- TRIzol® (Thermofisher Scientific, cat.no. 15596026);
- Chloroform (Thermofisher Scientific, cat.no. 022920.K2);
- DNase I (Thermofisher Scientific, cat.no. 18068015);
- DPBS (Thermofisher Scientific, cat.no. 14200075);
- Triton X-100 (Millipore, Burlington, MA, USA, cat.no. T9284);
- Ethanol (Millipore, cat.no. 493511);
- HEPES (Millipore, cat.no. H4034-25G);
- Milli-Q water;
- Primers for quantitative RT-PCR (Table S1);
- DAPI solution (Thermofisher Scientific, cat.no. 62248);
- cTnT antibody (Abcam, Cambridge, UK, cat.no. ab8295);
- Mouse IgG Alexa Fluor® 488 (Santa Cruz, Dallas, TX, USA, cat.no. sc-3890);
- Gelatin (Sigma, St. Louis, MO, USA, cat.no. G1393-100 mL);
- RNase AWAY (Fisher Scientific, cat.no. 501978158);
- Formaldehyde (Millipore Sigma, Burlington, MA, USA, cat.no. 252549-100 mL);
- PureLink RNA Mini Kit (ThermoFisher Scientific, cat.no 12183018A).
2.2. Equipment
- Steriflip filtration system (50 mL; Fisher Scientific, cat.no. SCGP00525);
- Stericup filtration system (500 mL; Fisher Scientific, cat.no. S2GPT05RE);
- Zeiss phase-contrast microscope (Spectra Services, Ontario, NY, USA; cat.no. SKU Axiovert35);
- Fluorescence microscope (Biocompare, South San Francisco, CA, USA cat.no. EVOS®FL);
- Cell culture plates 100 mm (Corning, Glendale, AZ, USA, cat.no. 430167);
- Petri plates 60 mm (Sigma-Aldrich, St. Louis, MO, USA, cat.no. P5481-500EA);
- Six-well tissue culture plates (Corning, cat.no. 3516);
- Tissue culture flask-T25 (Thermofisher Scientific, cat.no. 130189);
- Hybex bottle flasks (1000 mL, 500 mL; Benchmark Scientific, Sayreville, NJ, USA, cat.no. B3000-100-B, B3000-500-B);
- Falcon conical tubes (50 mL, 15 mL; Fisher Scientific, cat.no. 14-432-22, 14-959-53A);
- Cell lifters (Fisher Scientific, cat.no. 08100240);
- Centrifuge (Eppendorf, Enfield, CT, USA cat.no. 022625080);
- Biosafety cabinet (Fisher Scientific, cat.no. 13-261-222);
- Vacuum liquid waste disposal system (Welch, Ilmenau, Germany, cat.no. 2511B-75);
- Glass Pasteur pipettes (Fisher Scientific, cat.no. 1-678-20D);
- Serological pipettes (25 mL, 10 mL, 5 mL; Fisher Scientific, cat.no. 13-676-10M, 13-678-11E, 13-676-10H);
- Humidified tissue culture incubators (37 °C, 5% CO2; Eppendorf, cat.no. c170i);
- Cell counter slides (BIO-RAD, Hercules, CA, USA, cat.no. 1450011);
- Block heater (Thermofisher Scientific, cat.no. 88870001);
- Automated cell counter (BIO-RAD, cat.no. 1450102);
- Water bath incubators (Fisher Scientific, cat.no. 15-462-20Q);
- Microcentrifuge tubes (1.5 mL; VWR, Batavia, IL, USA, cat.no. 87003-294);
- Water purification system (Millipore Sigma, cat.no. ZRQSVP3WW);
- Quantitative-PCR detection system (BIO-RAD, cat.no. 1855201);
- qPCR 96-well plates (USA Scientific, Ocala, FL, USA, cat.no. 1402-8590).
2.3. Reagent Setup
2.4. Procedure
2.5. Thawing Feeder-Free ESCs
- A total of 2 mL of 0.1% gelatin was added to coat the surface of T25 tissue culture flasks. The excess gelatin solution was aspirated off before seeding ESCs.
- We added 9.5 mL of 37 °C ES + LIF medium into a 15 mL sterile conical tube.
- A frozen vial of mESCs was removed from liquid nitrogen and the vial was thawed in a 37 °C water bath. The vial was swirled gently until only a small ice crystal remained in the core of the vial.
- The vial was swabbed with 70% ethanol and placed in the tissue culture hood. Using a sterile 1 mL pipette, we gently transferred the cells into the 15 mL conical tube containing ES + LIF medium.
- The cells were pelleted by centrifugation for 3 min at 1200 rpm in a benchtop centrifuge at room temperature. The supernatant was aspirated and discarded with a sterilized glass Pasteur pipette.
- The cell pellet was gently resuspended in 10 mL pre-warmed ES + LIF medium and we transferred the cell suspension into a 25 cm2 tissue culture flask. The cells were grown at 37 °C in a humidified 5% CO2 incubator.
- The cells were distributed evenly in the flask by rocking the flask in a gentle left, right, up, and down motions.
- The next day, the medium was changed with 10 mL ES + LIF medium to remove dead cells and residual DMSO (footnote: pre-warmed medium was added perpendicular to the plate to prevent dislodging cells from the tissue culture plate).
2.6. Passaging ESCs Using Trypsin
- When ESCs reached about 80–90% confluency (usually about 2–3 days after thawing), we aspirated the old ES + LIF medium and washed with 10 mL room temperature 1× PBS. The PBS was aspirated and the wash was repeated one more time (footnote: the aspirator pipette was positioned away from the cells at the corner of the flask to prevent dislodging the cells).
- The cells were covered with 1 mL of trypsin solution and incubated at 37 °C for 1–2 min for the cells to be uniformly dispersed into small clumps and detached from the plate surface.
- We added 9 mL of pre-warmed ES + LIF medium to inactivate the trypsin and gently mixed it about 10 times by pipetting up and down using a 10 mL sterile serological pipette.
- The cells were counted by mixing 10 μL cell suspension with 10 μL trypan blue solution at a 1:1 ratio. In total, 10 μL of the mixture was transferred into the cell counter slide and cells counted using the automated cell counter.
- In total, 3.0 × 106 ESCs were transferred into a gelatin-coated 10 cm tissue culture dish in 10 mL ES + LIF medium.
- After plating the cells, the plate was returned to the incubator. The plate was moved in quick, short, left, right, up, and down motions to evenly disperse the cells across the surface of the plate.
- The medium was changed the next day by aspirating it and replacing it with a pre-warmed ES + LIF medium.
- The cells were passaged again after the cells reached about 80–90% confluency by repeating steps 8–14 before proceeding with cardiomyocyte differentiation.
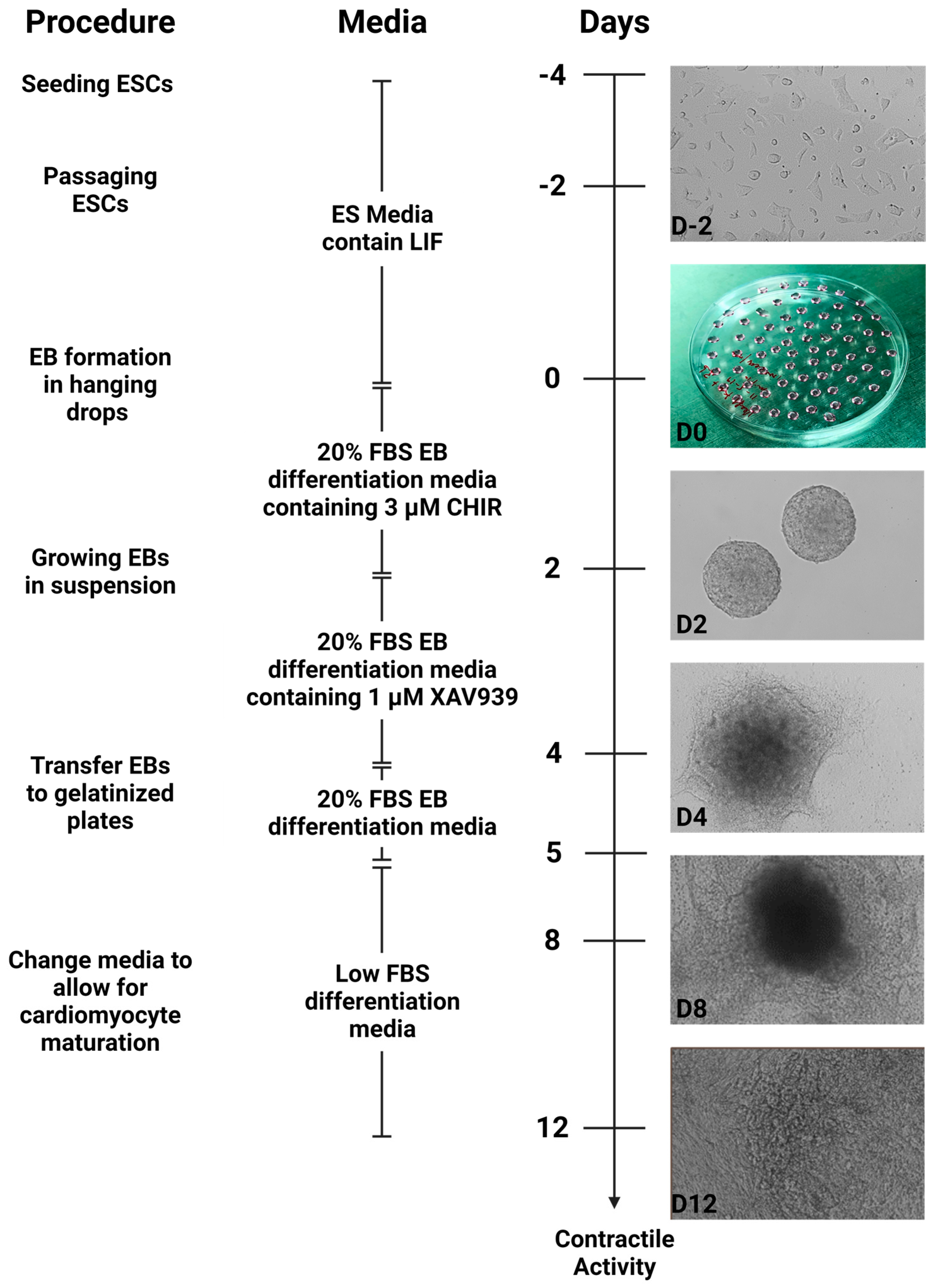
2.7. Differentiation of ESCs into Cardiomyocytes
- After the cells have reached 80–90% confluency, they can be differentiated into cardiomyocytes. Before performing the steps below, the 20% FBS differentiation medium was prewarmed at 37 °C and supplemented with CHIR990221. This medium was protected from light.
- Three sterile 10 cm petri plates were filled with about 15 mL of 1× PBS each to flood the base.
- Steps 8–9 were repeated to detach ESCs from the tissue culture plate. The detached cells were resuspended in 10 mL of 20% FBS differentiation medium supplemented with CHIR99021 by gently pipetting up and down 10 times.
- Cells were counted by repeating step 11 and diluted in the differentiation medium such that a 20 µL droplet contained 500 cells.
- Using a 100 µL multichannel pipette with 6 sterile pipette tips attached, we dispensed 20 µL droplets into the lid of the untreated petri plate. The droplets were evenly spaced so they did not touch each other. Each 10 cm petri plate lid contained approximately 60 droplets and were about 1 cm from the edge.
- The lid was carefully inverted over the base of the petri plate containing PBS. A quick flip-over was done to prevent the drops from running into each other.
- The plates were moved gently into the cell culture incubator for 48 h. While moving plates, we ensured the PBS solution at the base did not dislodge droplets from the lid.
- Exactly after 48 h, the plates were returned to the tissue culture hood. We pre-warmed the 20% FBS differentiation medium supplemented with XAV939.
- Using a 1000 µL pipette, the embryoid bodies were collected into a sterile 15 mL conical tube. These embryoid bodies are very delicate and were gently pipetted to maintain their structural integrity. The embryoid bodies were allowed to settle to the bottom of the conical tube by gravity.
- The supernatant was gently aspirated and discarded without disturbing the settled embryoid bodies.
- A total of 2 mL of pre-warmed 20% FBS differentiation medium supplemented with XAV939 was dispensed into 6 cm sterile petri plates and 1 mL of the medium was gently added to the embryoid bodies.
- Using a 1000 µL sterile pipette, embryoid bodies were gently resuspended and transferred into the 6 cm plate containing the pre-warmed medium.
- The plate was gently moved into the cell culture incubator for 48 h, and the plate moved in short left, right, up, and down motions to evenly disperse the embryoid bodies across the surface of the plate.
- After 48 h, the well of the 6-well tissue culture plate was flooded with 2 mL of 0.1% gelatin and placed in the cell culture incubator for about 30 min. Following incubation, the excess gelatin solution was aspirated and discarded.
- Using a 1000 µL pipette, embryoid bodies were transferred into a 15 mL conical tube and allowed to settle by gravity. After settling, the supernatant was aspirated and discarded without dislodging the embryoid bodies.
- The embryoid bodies were gently resuspended in 2 mL 20% FBS differentiation medium and transferred into the gelatin-coated well in the 6-well tissue culture plate.
- The plates were returned to the cell culture incubator at 37 °C for 24 h. *The embryoid bodies will attach and undergo morphological changes*.
- After 24 h, the 20% FBS differentiation medium was replaced with 3 mL low FBS differentiation medium. This will reduce the growth of other cell types.
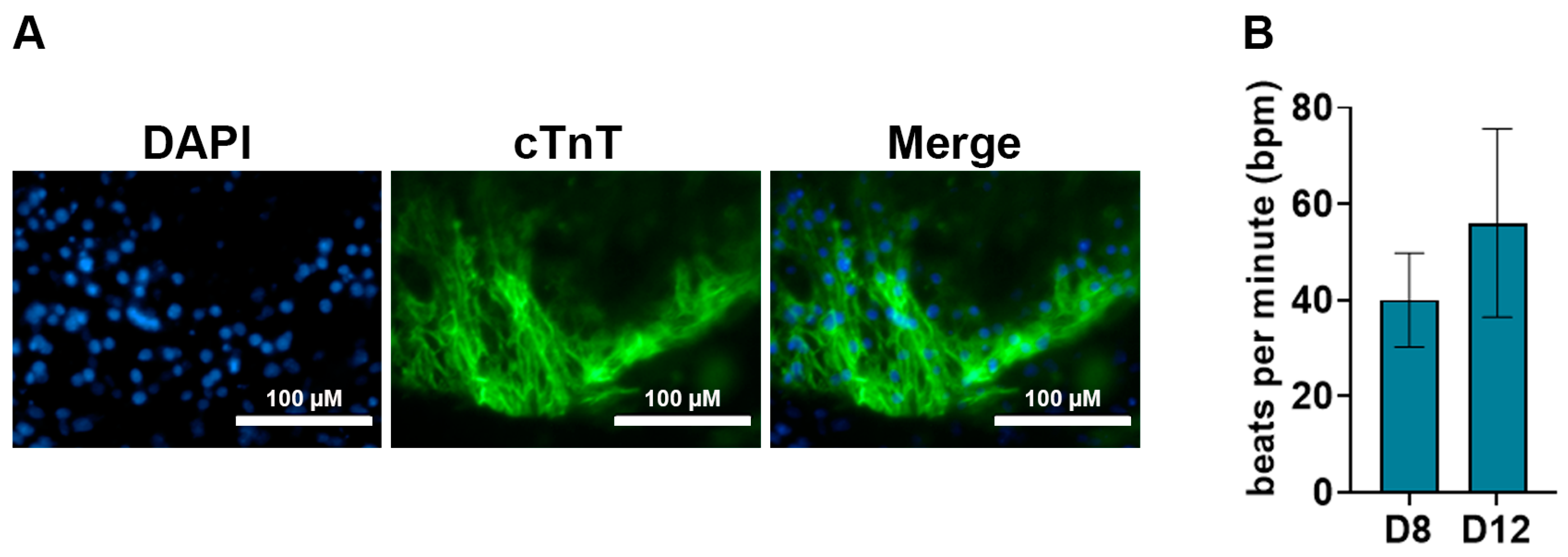
- The medium was changed with fresh pre-warmed low FBS differentiation medium every 24 h and cells were monitored for contractile activity.
- Typically, contractile activity was observed after 8 days post-differentiation.
2.8. Characterization of Cardiomyocytes and Differentiation
2.8.1. RT-QPCR for Stage-Specific Gene Markers during Cardiomyocyte Differentiation
- For undifferentiated cells, 5 × 106 cells were pelleted by centrifugation at 1200 rpm for 3 min, supernatant discarded, cells washed with 1× PBS, and 1 mL of TRIzol® reagent added. For embryoid bodies on day 2 and day 4, embryoid bodies were pelleted by gravity, washed with 1× PBS, and 1 mL of TRIzol® reagent added. For differentiation days 6, 8, and 12, cells were detached using a cell lifter and pelleted by centrifugation at 1200 rpm for 3 min or by washing attached cells gently with PBS and directly adding 1 mL of TRIzol® reagent.
- Using a 1 mL pipette, the TRIzol® reagent was mixed with the sample until there were no visible cells by pipetting up and down about 10 times. The samples were then incubated for 5 min at room temperature.
- A total of 200 µL of chloroform was added to the tubes and shaken vigorously by hand for 15 s and allowed to settle for 3 min at room temperature.
- After incubation, the phases began to separate. The tube was gently placed in a centrifuge and spun at 12,000× g for 15 min at 4 °C.
- At this point, the phases were well separated, and care was taken when removing tubes from the centrifuge to prevent mixing the two phases. The top aqueous phase (~500 µL) was collected into another clean 1.5 mL tube.
- A total of 500 µL of 100% isopropanol was added to the aqueous phase, mixed a few times by inverting the tubes up and down, and incubated at room temperature for 10 min. The tubes were centrifuged at 12,000× g for 10 min at 4 °C.
- The supernatant was discarded from the tube and the RNA pellet washed with 1 mL of 75% ethanol. The tube was centrifuged at 7500× g for 5 min at 4 °C, the wash discarded, and the RNA pellet air dried for 10 min.
- The RNA pellet was resuspended in 85 µL RNAse-free water, 4 µL DNAse, 1 µL RNase inhibitor, and 10 µL of 10× DNase Buffer was added. The resuspended RNA was mixed properly and incubated at 37 °C for 2 h.
- Following DNase treatment, the RNA was cleaned up by either (A) repeating steps (1–7) or following the instructor manual of the PureLink RNA Mini Kit.
- After final purification and resuspension of RNA in ~100 µL of RNase-free water, 0.5 µL of RNase inhibitor was added.
- The integrity of RNA was assessed by running 1 µg of RNA on an agarose gel. The RNA was quantified before proceeding to the next steps.
- cDNA was synthesized using 1 µg of RNA and random hexamers by following the instructor’s manual of the RT-cDNA synthesis kit.
- For quantitative PCR, 1:50 cDNA dilution was prepared. In total, 6 µL of the cDNA dilution, 1.5 µL of primer mix (forward and reverse), and 7.5 µL of Evagreen® mix was used per well. The qPCR reaction was performed in the quantitative-PCR detection system following the Evagreen® instructor’s manual.
2.8.2. cTnT Immunostaining Analysis for Cardiomyocytes
- Day 12 differentiated cardiomyocytes were washed with 1 mL PBS in the 6-well tissue culture plates.
- In the dark, 1 mL of freshly prepared 4% (v/v) formaldehyde was added and incubated for 20 min at room temperature to fix cells.
- Following incubation, we washed the cells 3 times with 1× PBS, aspirating PBS between each wash.
- We prepared the primary antibody solution by adding 1 µL of cTnT antibody to 1 mL of blocking solution, mixed it properly, and added it to the well containing fixed cardiomyocytes.
- The cells were incubated with cTnT antibody overnight at 4 °C or room temperature for 2 h.
- Following incubation, the cTnT antibody solution was discarded and the cells washed with 1× PBS three times, aspirating PBS between each wash.
- In the dark, the secondary antibody solution was prepared by diluting 1 µL of mouse IgG Alexa Fluor® 488 in 1 mL blocking solution. This solution was added to the well containing fixed cells and incubated for 30 min at room temperature in the dark.
- The secondary antibody solution was aspirated in the dark and step 6 repeated.
- Following washes, 1 mL of 0.5 µg/mL DAPI solution in PBS was added to the well in the dark.
- Immunofluorescence images were taken using the EVOS®FL fluorescence microscope.
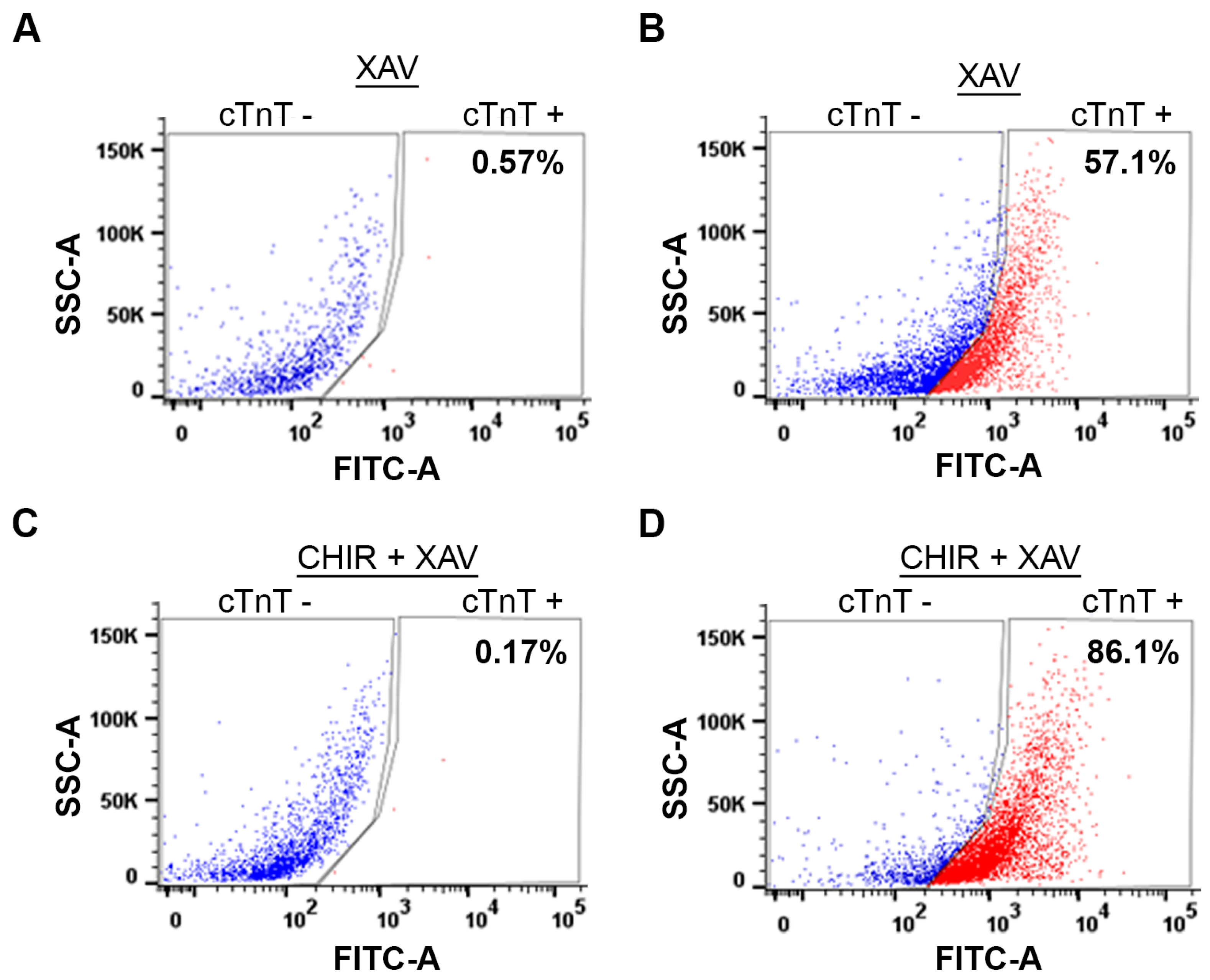
2.9. Fluorescence-Assisted Cell Sorting (FACS) Analysis of Cardiomyocytes
2.9.1. Preparation of Cells for FACS
2.9.2. FACS Analysis
2.10. Summary of Cardiomyocyte Differentiation Using WNT Switch Method
3. Results
3.1. Identification of Optimum CHIR99021 Concentration for WNT Switch Method
3.2. WNT Switch Method Causes Pluripotency Genes’ Repression during Cardiomyocyte Differentiation
3.3. WNT Signaling Gene Expression Responds to CHIR99021 and XAV939 Treatments
3.4. The WNT Switch Method Increases Cardiomyocyte Yields
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nichols, J.; Smith, A. Naive and primed pluripotent states. Cell Stem Cell 2009, 4, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Rossant, J. Stem Cells and Early Lineage Development. Cell 2008, 132, 527–531. [Google Scholar] [CrossRef]
- Parmacek, M.S.; Epstein, J.A. Cardiomyocyte renewal. N. Engl. J. Med. 2009, 361, 86–88. [Google Scholar] [CrossRef]
- Porrello, E.R.; Olson, E.N. A neonatal blueprint for cardiac regeneration. Stem Cell Res. 2014, 13, 556–570. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Castellanos, A.; Myerburg, R.J. Sudden death due to cardiac arrhythmias. N. Engl. J. Med. 2001, 345, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Kokkinopoulos, I.; Ishida, H.; Saba, R.; Coppen, S.; Suzuki, K.; Yashiro, K. Cardiomyocyte differentiation from mouse embryonic stem cells using a simple and defined protocol. Dev. Dyn. 2016, 245, 157–165. [Google Scholar] [CrossRef]
- Mazzotta, S.; Neves, C.; Bonner, R.J.; Bernardo, A.S.; Docherty, K.; Hoppler, S. Distinctive Roles of Canonical and Noncanonical Wnt Signaling in Human Embryonic Cardiomyocyte Development. Stem Cell Rep. 2016, 7, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Pahnke, A.; Conant, G.; Huyer, L.D.; Zhao, Y.; Feric, N.; Radisic, M. The role of Wnt regulation in heart development, cardiac repair and disease: A tissue engineering perspective. Biochem. Biophys. Res. Commun. 2016, 473, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Cohen, E.D.; Morrisey, E.E. The importance of Wnt signaling in cardiovascular development. Pediatr. Cardiol. 2010, 31, 342–348. [Google Scholar] [CrossRef]
- Buikema, J.W.; Zwetsloot, P.-P.M.; Doevendans, P.A.; Domian, I.J.; Sluijter, J.P.G. Wnt/β-Catenin Signaling during Cardiac Development and Repair. J. Cardiovasc. Dev. Dis. 2014, 1, 98–110. [Google Scholar] [CrossRef]
- Fu, W.-B.; Wang, W.E.; Zeng, C.-Y. Wnt signaling pathways in myocardial infarction and the therapeutic effects of Wnt pathway inhibitors. Acta Pharmacol. Sin. 2019, 40, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Brade, T.; Männer, J.; Kühl, M. The role of Wnt signalling in cardiac development and tissue remodelling in the mature heart. Cardiovasc. Res. 2006, 72, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Merrill, B.J. Wnt pathway regulation of embryonic stem cell self-renewal. Cold Spring Harb. Perspect. Biol. 2012, 4, a007971. [Google Scholar] [CrossRef] [PubMed]
- Kreuser, U.; Buchert, J.; Haase, A.; Richter, W.; Diederichs, S. Initial WNT/β-Catenin Activation Enhanced Mesoderm Commitment, Extracellular Matrix Expression, Cell Aggregation and Cartilage Tissue Yield From Induced Pluripotent Stem Cells. Front. Cell Dev. Biol. 2020, 8, 581331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Tang, Y.; Zhou, Y.; Zhang, J. Deciphering Role of Wnt Signalling in Cardiac Mesoderm and Cardiomyocyte Differentiation from Human iPSCs: Four-dimensional control of Wnt pathway for hiPSC-CMs differentiation. Sci. Rep. 2019, 9, 19389. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Weidinger, G.; Osugi, T.; Kohn, A.D.; Golob, J.L.; Pabon, L.; Reinecke, H.; Moon, R.T.; Murry, C.E. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 9685–9690. [Google Scholar] [CrossRef] [PubMed]
- Naito, A.T.; Shiojima, I.; Akazawa, H.; Hidaka, K.; Morisaki, T.; Kikuchi, A.; Komuro, I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc. Natl. Acad. Sci. USA 2006, 103, 19812–19817. [Google Scholar] [CrossRef]
- Kwon, C.; Cordes, K.R.; Srivastava, D. Wnt/beta-catenin signaling acts at multiple developmental stages to promote mammalian cardiogenesis. Cell Cycle 2008, 7, 3815–3818. [Google Scholar] [CrossRef]
- Gessert, S.; Kühl, M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ. Res. 2010, 107, 186–199. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, J.; Wu, Y.; Huang, T.L.; Wang, G. Small-molecule inhibitors of Wnt signaling pathway: Towards novel anticancer therapeutics. Future Med. Chem. 2015, 7, 2485–2505. [Google Scholar] [CrossRef]
- Tran, F.H.; Zheng, J.J. Modulating the wnt signaling pathway with small molecules. Protein Sci. 2017, 26, 650–661. [Google Scholar] [CrossRef]
- Wang, B.; Khan, S.; Wang, P.; Wang, X.; Liu, Y.; Chen, J.; Tu, X. A Highly Selective GSK-3β Inhibitor CHIR99021 Promotes Osteogenesis by Activating Canonical and Autophagy-Mediated Wnt Signaling. Front. Endocrinol. 2022, 13, 926622. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; DeRan, M.T.; Ignatius, M.S.; Grandinetti, K.B.; Clagg, R.; McCarthy, K.M.; Lobbardi, R.M.; Brockmann, J.; Keller, C.; Wu, X.; et al. Glycogen synthase kinase 3 inhibitors induce the canonical WNT/β-catenin pathway to suppress growth and self-renewal in embryonal rhabdomyosarcoma. Proc. Natl. Acad. Sci. USA 2014, 111, 5349–5354. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zheng, X.; Han, Y.; Lv, Y.; Lan, F.; Zhao, J. XAV939 inhibits the proliferation and migration of lung adenocarcinoma A549 cells through the WNT pathway. Oncol. Lett. 2018, 15, 8973–8982. [Google Scholar] [CrossRef]
- Halevy, T.; Urbach, A. Comparing ESC and iPSC-Based Models for Human Genetic Disorders. J. Clin. Med. 2014, 3, 1146–1162. [Google Scholar] [CrossRef]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef]
- Lian, X.; Bao, X.; Zilberter, M.; Westman, M.; Fisahn, A.; Hsiao, C.; Hazeltine, L.B.; Dunn, K.K.; Kamp, T.J.; Palecek, S.P. Chemically defined, albumin-free human cardiomyocyte generation. Nat. Methods 2015, 12, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M.; et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef]
- Wang, H.; Hao, J.; Hong, C.C. Cardiac induction of embryonic stem cells by a small molecule inhibitor of Wnt/β-catenin signaling. ACS Chem. Biol. 2011, 6, 192–197. [Google Scholar] [CrossRef]
- Heo, H.J.; Kim, H.K.; Youm, J.B.; Cho, S.W.; Song, I.S.; Lee, S.Y.; Ko, T.H.; Kim, N.; Ko, K.S.; Rhee, B.D.; et al. Mitochondrial pyruvate dehydrogenase phosphatase 1 regulates the early differentiation of cardiomyocytes from mouse embryonic stem cells. Exp. Mol. Med. 2016, 48, e254. [Google Scholar] [CrossRef]
- Van Vliet, P.; Wu, S.M.; Zaffran, S.; Pucéat, M. Early cardiac development: A view from stem cells to embryos. Cardiovasc. Res. 2012, 96, 352–362. [Google Scholar] [CrossRef]
- Tran, T.H.; Wang, X.; Browne, C.; Zhang, Y.; Schinke, M.; Izumo, S.; Burcin, M. Wnt3a-induced mesoderm formation and cardiomyogenesis in human embryonic stem cells. Stem Cells 2009, 27, 1869–1878. [Google Scholar] [CrossRef]
- Kemp, C.R.; Willems, E.; Wawrzak, D.; Hendrickx, M.; Agbor Agbor, T.; Leyns, L. Expression of Frizzled5, Frizzled7, and Frizzled10 during early mouse development and interactions with canonical Wnt signaling. Dev. Dyn. 2007, 236, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Schohl, A.; Fagotto, F. A role for maternal beta-catenin in early mesoderm induction in Xenopus. Embo J. 2003, 22, 3303–3313. [Google Scholar] [CrossRef] [PubMed]
- Engels, M.C.; Rajarajan, K.; Feistritzer, R.; Sharma, A.; Nielsen, U.B.; Schalij, M.J.; de Vries, A.A.; Pijnappels, D.A.; Wu, S.M. Insulin-like growth factor promotes cardiac lineage induction in vitro by selective expansion of early mesoderm. Stem Cells 2014, 32, 1493–1502. [Google Scholar] [CrossRef]
- Kattman, S.J.; Witty, A.D.; Gagliardi, M.; Dubois, N.C.; Niapour, M.; Hotta, A.; Ellis, J.; Keller, G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 2011, 8, 228–240. [Google Scholar] [CrossRef]
- Ferretti, E.; Hadjantonakis, A.K. Mesoderm specification and diversification: From single cells to emergent tissues. Curr. Opin. Cell Biol. 2019, 61, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Evseenko, D.; Zhu, Y.; Schenke-Layland, K.; Kuo, J.; Latour, B.; Ge, S.; Scholes, J.; Dravid, G.; Li, X.; MacLellan, W.R.; et al. Mapping the first stages of mesoderm commitment during differentiation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 13742–13747. [Google Scholar] [CrossRef]
- Davis, L.A.; Zur Nieden, N.I. Mesodermal fate decisions of a stem cell: The Wnt switch. Cell Mol. Life Sci. 2008, 65, 2658–2674. [Google Scholar] [CrossRef]
- Woll, P.S.; Morris, J.K.; Painschab, M.S.; Marcus, R.K.; Kohn, A.D.; Biechele, T.L.; Moon, R.T.; Kaufman, D.S. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood 2008, 111, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.J.; Pohl, S.; Deshmukh, A.; Visweswaran, M.; Ward, N.C.; Arfuso, F.; Agostino, M.; Dharmarajan, A. The Role of Wnt Signalling in Angiogenesis. Clin. Biochem. Rev. 2017, 38, 131–142. [Google Scholar] [PubMed]
- Manukjan, N.; Ahmed, Z.; Fulton, D.; Blankesteijn, W.M.; Foulquier, S. A Systematic Review of WNT Signaling in Endothelial Cell Oligodendrocyte Interactions: Potential Relevance to Cerebral Small Vessel Disease. Cells 2020, 9, 1545. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Lederer, W.J.; Cannell, M.B. Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science 1993, 262, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Guatimosim, S.; Guatimosim, C.; Song, L.S. Imaging calcium sparks in cardiac myocytes. Methods Mol. Biol. 2011, 689, 205–214. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Zhao, S.R.; Tu, C.; Pang, P.; Zhang, M.; Wu, J.C. Protocol to measure contraction, calcium, and action potential in human-induced pluripotent stem cell-derived cardiomyocytes. STAR Protoc. 2021, 2, 100859. [Google Scholar] [CrossRef] [PubMed]
- Laurila, E.; Ahola, A.; Hyttinen, J.; Aalto-Setälä, K. Methods for in vitro functional analysis of iPSC derived cardiomyocytes—Special focus on analyzing the mechanical beating behavior. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 1864–1872. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensah, I.K.; Emerson, M.L.; Tan, H.J.; Gowher, H. Cardiomyocyte Differentiation from Mouse Embryonic Stem Cells by WNT Switch Method. Cells 2024, 13, 132. https://doi.org/10.3390/cells13020132
Mensah IK, Emerson ML, Tan HJ, Gowher H. Cardiomyocyte Differentiation from Mouse Embryonic Stem Cells by WNT Switch Method. Cells. 2024; 13(2):132. https://doi.org/10.3390/cells13020132
Chicago/Turabian StyleMensah, Isaiah K., Martin L. Emerson, Hern J. Tan, and Humaira Gowher. 2024. "Cardiomyocyte Differentiation from Mouse Embryonic Stem Cells by WNT Switch Method" Cells 13, no. 2: 132. https://doi.org/10.3390/cells13020132
APA StyleMensah, I. K., Emerson, M. L., Tan, H. J., & Gowher, H. (2024). Cardiomyocyte Differentiation from Mouse Embryonic Stem Cells by WNT Switch Method. Cells, 13(2), 132. https://doi.org/10.3390/cells13020132






