The Role of MicroRNA in the Pathogenesis of Acute Kidney Injury
Abstract
1. Introduction
2. The Role of MicroRNA in the Pathogenesis of Acute Kidney Injury—In Vitro and Animal Models
2.1. Brief Overview of miRNA Biogenesis and Functionality
2.2. miRNA in Sepsis-Induced AKI
2.3. miRNAs in AKI Induced by Nephrotoxic Agents
2.4. Ischemia-Reperfusion AKI
3. microRNA in Clinical Studies and Their Role as Diagnostic Biomarkers
4. miRNA-Based Therapeutics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levey, A.S.; James, M.T. Acute Kidney Injury. Ann. Intern. Med. 2017, 167, ITC66–ITC80. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Ounci, E.; Boukabous, S.; Bkiyar, H.; Abda, N.; Bentata, Y.; Housni, B. Acute kidney injury in critically ill patients with COVID-19: Prevalence, risk factors and mortality in eastern Morocco. J. Nephrol. 2022, 35, 2383–2386. [Google Scholar] [CrossRef]
- Gaudry, S.; Palevsky, P.M.; Dreyfuss, D. Extracorporeal Kidney-Replacement Therapy for Acute Kidney Injury. N. Engl. J. Med. 2022, 386, 964–975. [Google Scholar] [CrossRef]
- Kiełbowski, K.; Ptaszyński, K.; Wójcik, J.; Wojtyś, M.E. The role of selected non-coding RNAs in the biology of non-small cell lung cancer. Adv. Med. Sci. 2023, 68, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xu, L.; Geng, Z.; Liu, J.; Zhang, L.; Wu, Y.; He, D.; Qu, P. The role of non-coding RNA network in atherosclerosis. Life Sci. 2021, 265, 118756. [Google Scholar] [CrossRef]
- Baker, M.A.; Davis, S.J.; Liu, P.; Pan, X.; Williams, A.M.; Iczkowski, K.A.; Gallagher, S.T.; Bishop, K.; Regner, K.R.; Liu, Y.; et al. Tissue-Specific MicroRNA Expression Patterns in Four Types of Kidney Disease. J. Am. Soc. Nephrol. 2017, 28, 2985–2992. [Google Scholar] [CrossRef]
- Farrell, C.E.; Liu, X.; Yagan, N.O.; Suda, A.C.; Cerqueira, D.M.; Bodnar, A.J.; Kashlan, O.B.; Subramanya, A.R.; Ho, J.; Butterworth, M.B. MicroRNA-19 is regulated by aldosterone in a sex-specific manner to alter kidney sodium transport. Am. J. Physiol. Cell Physiol. 2024, 326, C282–C293. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Rådmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003, 425, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Qin, Y.; Macara, I.G.; Cullen, B.R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes. Dev. 2003, 17, 3011–3016. [Google Scholar] [CrossRef]
- Bohnsack, M.T.; Czaplinski, K.; Gorlich, D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 2004, 10, 185–191. [Google Scholar] [CrossRef]
- Zhang, H.; Kolb, F.A.; Brondani, V.; Billy, E.; Filipowicz, W. Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J. 2002, 21, 5875–5885. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- Reichholf, B.; Herzog, V.A.; Fasching, N.; Manzenreither, R.A.; Sowemimo, I.; Ameres, S.L. Time-Resolved Small RNA Sequencing Unravels the Molecular Principles of MicroRNA Homeostasis. Mol. Cell 2019, 75, 756–768.e757. [Google Scholar] [CrossRef]
- Ender, C.; Meister, G. Argonaute proteins at a glance. J. Cell Sci. 2010, 123, 1819–1823. [Google Scholar] [CrossRef]
- Swolin-Eide, D.; Forsander, G.; Pundziute Lyckå, A.; Novak, D.; Grillari, J.; Diendorfer, A.B.; Hackl, M.; Magnusson, P. Circulating microRNAs in young individuals with long-duration type 1 diabetes in comparison with healthy controls. Sci. Rep. 2023, 13, 11634. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.M.; Bhakta, N.R.; Barczak, A.J.; Cakmak, H.; Fisher, S.; MacKenzie, T.C.; Patel, T.; Price, R.W.; Smith, J.F.; Woodruff, P.G.; et al. Large Differences in Small RNA Composition Between Human Biofluids. Cell Rep. 2018, 25, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Place, R.F.; Li, L.C.; Pookot, D.; Noonan, E.J.; Dahiya, R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc. Natl. Acad. Sci. USA 2008, 105, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, C.; Liu, W.; Liu, Y.; Li, L.; Chen, Q. Comprehensive analysis of differentially expressed miRNAs in mice with kidney injury induced by chronic intermittent hypoxia. Front. Genet. 2022, 13, 918728. [Google Scholar] [CrossRef]
- Shihana, F.; Wong, W.K.M.; Joglekar, M.V.; Mohamed, F.; Gawarammana, I.B.; Isbister, G.K.; Hardikar, A.A.; Seth, D.; Buckley, N.A. Urinary microRNAs as non-invasive biomarkers for toxic acute kidney injury in humans. Sci. Rep. 2021, 11, 9165. [Google Scholar] [CrossRef]
- Akcay, A.; Nguyen, Q.; Edelstein, C.L. Mediators of inflammation in acute kidney injury. Mediat. Inflamm. 2009, 2009, 137072. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, R.S.; Shi, Z.; Li, J.Q. Nuclear factor-κB in rheumatoid arthritis. Int. J. Rheum. Dis. 2020, 23, 1627–1635. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, Y.; Liang, J.; Ying, P.; Situ, Q.; Tan, X.; Zhu, J. Upregulation of NF-κB by USP24 aggravates ferroptosis in diabetic cardiomyopathy. Free. Radic. Biol. Med. 2024, 210, 352–366. [Google Scholar] [CrossRef]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 2020, 122, 109772. [Google Scholar] [CrossRef]
- Ge, Q.M.; Huang, C.M.; Zhu, X.Y.; Bian, F.; Pan, S.M. Differentially expressed miRNAs in sepsis-induced acute kidney injury target oxidative stress and mitochondrial dysfunction pathways. PLoS ONE 2017, 12, e0173292. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Z. MicroRNA-23a-3p ameliorates acute kidney injury by targeting FKBP5 and NF-κB signaling in sepsis. Cytokine 2022, 155, 155898. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Ji, J.L.; Wen, Y.; Cao, J.Y.; Kharbuja, N.; Ni, W.J.; Yin, D.; Feng, S.T.; Liu, H.; Lv, L.L.; et al. HIF-1α is transcriptionally regulated by NF-κB in acute kidney injury. Am. J. Physiol. Renal Physiol. 2021, 321, F225–F235. [Google Scholar] [CrossRef]
- Ye, J.; Feng, H.; Peng, Z. miR-23a-3p inhibits sepsis-induced kidney epithelial cell injury by suppressing Wnt/β-catenin signaling by targeting wnt5a. Braz. J. Med. Biol. Res. 2022, 55, e11571. [Google Scholar] [CrossRef] [PubMed]
- Huffstater, T.; Merryman, W.D.; Gewin, L.S. Wnt/β-Catenin in Acute Kidney Injury and Progression to Chronic Kidney Disease. Semin. Nephrol. 2020, 40, 126–137. [Google Scholar] [CrossRef]
- Sheng, S.; Zou, M.; Yang, Y.; Guan, M.; Ren, S.; Wang, X.; Wang, L.; Xue, Y. miR-23a-3p regulates the inflammatory response and fibrosis in diabetic kidney disease by targeting early growth response 1. Vitr. Cell. Dev. Biol. Anim. 2021, 57, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Huang, D. lncRNA GAS5-mediated miR-23a-3p promotes inflammation and cell apoptosis by targeting TLR4 in a cell model of sepsis. Mol. Med. Rep. 2021, 24, 510. [Google Scholar] [CrossRef]
- Yang, X.; Lu, L.; Wu, C.; Zhang, F. ATP2B1-AS1 exacerbates sepsis-induced cell apoptosis and inflammation by regulating miR-23a-3p/TLR4 axis. Allergol. Immunopathol. (Madr) 2023, 51, 17–26. [Google Scholar] [CrossRef]
- Kuzmich, N.N.; Sivak, K.V.; Chubarev, V.N.; Porozov, Y.B.; Savateeva-Lyubimova, T.N.; Peri, F. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines 2017, 5, 34. [Google Scholar] [CrossRef]
- Zhong, Y.; Wu, S.; Yang, Y.; Li, G.Q.; Meng, L.; Zheng, Q.Y.; Li, Y.; Xu, G.L.; Zhang, K.Q.; Peng, K.F. LIGHT aggravates sepsis-associated acute kidney injury via TLR4-MyD88-NF-κB pathway. J. Cell. Mol. Med. 2020, 24, 11936–11948. [Google Scholar] [CrossRef]
- Vázquez-Carballo, C.; Guerrero-Hue, M.; García-Caballero, C.; Rayego-Mateos, S.; Opazo-Ríos, L.; Morgado-Pascual, J.L.; Herencia-Bellido, C.; Vallejo-Mudarra, M.; Cortegano, I.; Gaspar, M.L.; et al. Toll-Like Receptors in Acute Kidney Injury. Int. J. Mol. Sci. 2021, 22, 816. [Google Scholar] [CrossRef]
- Wang, L.; Lin, J.; Yu, T.; Zuo, Q.; Shen, B.; Zhang, H.; Liu, B.; Cai, D.; Mao, H.; Zhao, H.; et al. Identification of plasma miR-106a-5p and miR-30a-5p as potential biomarkers for mesangial proliferative glomerulonephritis. Clin. Biochem. 2020, 84, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yu, J.; Jing, Y.; Zhang, J. MiR-106a aggravates sepsis-induced acute kidney injury by targeting THBS2 in mice model. Acta Cir. Bras. 2019, 34, e201900602. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.P.; Ma, X.Y.; Yang, C. Circular RNA TLK1 Promotes Sepsis-Associated Acute Kidney Injury by Regulating Inflammation and Oxidative Stress Through miR-106a-5p/HMGB1 Axis. Front. Mol. Biosci. 2021, 8, 660269. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Lu, L.; Chen, X. Release of HMGB1 in Podocytes Exacerbates Lipopolysaccharide-Induced Acute Kidney Injury. Mediat. Inflamm. 2021, 2021, 5220226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Q.; Dai, Z.; Pan, H. miR-22 alleviates sepsis-induced acute kidney injury via targeting the HMGB1/TLR4/NF-κB signaling pathway. Int. Urol. Nephrol. 2023, 55, 409–421. [Google Scholar] [CrossRef]
- Nechemia-Arbely, Y.; Barkan, D.; Pizov, G.; Shriki, A.; Rose-John, S.; Galun, E.; Axelrod, J.H. IL-6/IL-6R axis plays a critical role in acute kidney injury. J. Am. Soc. Nephrol. 2008, 19, 1106–1115. [Google Scholar] [CrossRef]
- Liu, K.D.; Altmann, C.; Smits, G.; Krawczeski, C.D.; Edelstein, C.L.; Devarajan, P.; Faubel, S. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: A case-control study. Crit. Care 2009, 13, R104. [Google Scholar] [CrossRef]
- Shimazui, T.; Nakada, T.A.; Tateishi, Y.; Oshima, T.; Aizimu, T.; Oda, S. Association between serum levels of interleukin-6 on ICU admission and subsequent outcomes in critically ill patients with acute kidney injury. BMC Nephrol. 2019, 20, 74. [Google Scholar] [CrossRef]
- Mitazaki, S.; Kato, N.; Suto, M.; Hiraiwa, K.; Abe, S. Interleukin-6 deficiency accelerates cisplatin-induced acute renal failure but not systemic injury. Toxicology 2009, 265, 115–121. [Google Scholar] [CrossRef]
- Su, H.; Lei, C.T.; Zhang, C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front. Immunol. 2017, 8, 405. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X.; Wu, Y. The miR-26a-5p/IL-6 axis alleviates sepsis-induced acute kidney injury by inhibiting renal inflammation. Ren. Fail. 2022, 44, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jia, Y.; Xue, M.; Hu, F.; Zheng, Z.; Zhang, S.; Ren, S.; Yang, Y.; Si, Z.; Wang, L.; et al. Inhibiting Rab27a in renal tubular epithelial cells attenuates the inflammation of diabetic kidney disease through the miR-26a-5p/CHAC1/NF-kB pathway. Life Sci. 2020, 261, 118347. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Huang, G.K.; Kang, C.H.; Cheng, Y.T.; Kao, Y.H.; Chien, Y.S. MicroRNA-26a-5p Restoration Ameliorates Unilateral Ureteral Obstruction-Induced Renal Fibrosis in Mice Through Modulating TGF-β Signaling. Lab. Investig. 2023, 103, 100131. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, M.; Guo, X.; Wang, P.; Zeng, F.; Wang, H.; Tang, J.; Qin, Z.; Tao, T. miR-26a-5p alleviates CFA-induced chronic inflammatory hyperalgesia through Wnt5a/CaMKII/NFAT signaling in mice. CNS Neurosci. Ther. 2023, 29, 1254–1271. [Google Scholar] [CrossRef]
- Bian, J.; Ge, W.; Jiang, Z. miR-26a-5p Attenuates Oxidative Stress and Inflammation in Diabetic Retinopathy through the USP14/NF-. J. Ophthalmol. 2024, 2024, 1470898. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, T.; Fei, Z. miR-26a-5p alleviates lipopolysaccharide-induced acute lung injury by targeting the connective tissue growth factor. Mol. Med. Rep. 2021, 23, 5. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Kong, M.; Yang, J. MiR-22-3p suppresses sepsis-induced acute kidney injury by targeting PTEN. Biosci. Rep. 2020, 40, BSR20200527. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhu, M.; Liu, S.; Lu, J.; Ni, Z.; Cai, H.; Zhang, W. MicroRNA-93 inhibits the apoptosis and inflammatory response of tubular epithelial cells via the PTEN/AKT/mTOR pathway in acute kidney injury. Mol. Med. Rep. 2021, 24, 666. [Google Scholar] [CrossRef]
- Sang, Z.; Dong, S.; Zhang, P.; Wei, Y. miR-214 ameliorates sepsis-induced acute kidney injury via PTEN/AKT/mTOR-regulated autophagy. Mol. Med. Rep. 2021, 24, 683. [Google Scholar] [CrossRef]
- Xie, X.; Qu, P.; Wu, H.; Liu, P.; Luo, J.; Chi, J.; Liu, X.; Chen, X.; Xu, C. Circulating exosomal miR-21 mediates HUVEC proliferation and migration through PTEN/PI3K/AKT in Crohn’s disease. Ann. Transl. Med. 2022, 10, 258. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhang, Y.Y.; Zhu, B.L.; Feng, F.Z.; Yan, H.; Zhang, H.Y.; Zhou, B. miR-21 regulates the proliferation and apoptosis of ovarian cancer cells through PTEN/PI3K/AKT. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4149–4155. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, B.; Wang, Z.; Wang, D.; Ni, H.; Zhang, L.; Wang, Y. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics 2019, 9, 6901–6919. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Dong, J.; Li, P.; Tang, C.; Cheng, W.; Xu, Z.; Zhou, W.; Ge, J.; Xia, C.; Zhang, Z. MiRNA-21 has effects to protect kidney injury induced by sepsis. Biomed. Pharmacother. 2017, 94, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Jia, P.; Chen, N.; Fang, Y.; Liang, Y.; Guo, M.; Ding, X. Delayed Remote Ischemic Preconditioning ConfersRenoprotection against Septic Acute Kidney Injury via Exosomal miR-21. Theranostics 2019, 9, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yao, Y.Y.; Bi, H.Y.; Zhai, Z.; Gao, Y. miR-21 protects against lipopolysaccharide-stimulated acute kidney injury and apoptosis by targeting CDK6. Ann. Transl. Med. 2020, 8, 303. [Google Scholar] [CrossRef]
- Zhao, S.; Li, W.; Yu, W.; Rao, T.; Li, H.; Ruan, Y.; Yuan, R.; Li, C.; Ning, J.; Li, S.; et al. Exosomal miR-21 from tubular cells contributes to renal fibrosis by activating fibroblasts via targeting PTEN in obstructed kidneys. Theranostics 2021, 11, 8660–8673. [Google Scholar] [CrossRef]
- Wang, L.; Wang, K.; Tian, Z. miR-128-3p Inhibits NRP1 Expression and Promotes Inflammatory Response to Acute Kidney Injury in Sepsis. Inflammation 2020, 43, 1772–1779. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, G.; Guo, C.; Zhao, X.; Shen, D.; Yang, N. MiR-128-3p mediates TNF-α-induced inflammatory responses by regulating Sirt1 expression in bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2020, 521, 98–105. [Google Scholar] [CrossRef]
- Pang, Y.; Luo, D.; Wang, S. miR-128-3p inhibits the inflammation by targeting MAPK6 in penicillin-induced astrocytes. Neuroreport 2022, 33, 742–749. [Google Scholar] [CrossRef]
- Liu, X.; Cui, H.; Bai, Q.; Piao, H.; Song, Y.; Yan, G. miR-128-3p alleviates airway inflammation in asthma by targeting SIX1 to regulate mitochondrial fission and fusion. Int. Immunopharmacol. 2024, 130, 111703. [Google Scholar] [CrossRef]
- Yang, P.; Han, J.; Li, S.; Luo, S.; Tu, X.; Ye, Z. miR-128-3p inhibits apoptosis and inflammation in LPS-induced sepsis by targeting TGFBR2. Open Med. 2021, 16, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Borkan, S.C. The Role of BCL-2 Family Members in Acute Kidney Injury. Semin. Nephrol. 2016, 36, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Duan, J.; Zhang, T.; Fu, Y.; Xu, Y.; Miao, H.; Ge, X. miR-16-5p aggravates sepsis-associated acute kidney injury by inducing apoptosis. Ren. Fail. 2024, 46, 2322688. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Wang, H.J.; Li, Y.Z.; Du, X.F.; Hao, X.L.; Jiang, H.M.; Yang, L.H. Inhibition of microRNA-543 alleviates sepsis-induced acute kidney injury via targeting Bcl-2. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Wu, D.; Shi, S.; Wang, L. miR-34b-5p promotes renal cell inflammation and apoptosis by inhibiting aquaporin-2 in sepsis-induced acute kidney injury. Ren. Fail. 2021, 43, 291–301. [Google Scholar] [CrossRef]
- Yi, H.X.; Jiang, S.Y.; Yu, L.H.; Chen, K.; Yang, Z.X.; Wu, Q. MicroRNA 181a-2-3p Alleviates the Apoptosis of Renal Tubular Epithelial Cells via Targeting GJB2 in Sepsis-Induced Acute Kidney Injury. Mol. Cell. Biol. 2021, 41, e0001621. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, Y.; Xia, Y.; Li, C.; Song, Z.; Zhu, J. MiR-150-5p protects against septic acute kidney injury via repressing the MEKK3/JNK pathway. Cell. Signal. 2021, 86, 110101. [Google Scholar] [CrossRef]
- Mao, W.; Wang, X.; Zhang, Y.; Zhu, H.; Dai, L.; Chen, J. Nuclear factor-kappa B p50-induced microRNA-20a-3p plays a detrimental role in sepsis-induced acute kidney injury. Cell. Mol. Biol. 2023, 69, 198–202. [Google Scholar] [CrossRef]
- Wu, J.; Li, D.D.; Li, J.Y.; Yin, Y.C.; Li, P.C.; Qiu, L.; Chen, L.M. Identification of microRNA-mRNA networks involved in cisplatin-induced renal tubular epithelial cells injury. Eur. J. Pharmacol. 2019, 851, 1–12. [Google Scholar] [CrossRef]
- Xia, Y.; Pan, W.; Xiao, X.; Zhou, X.; Gu, W.; Liu, Y.; Zhao, Y.; Li, L.; Zheng, C.; Liu, J.; et al. MicroRNA-483-5p accentuates cisplatin-induced acute kidney injury by targeting GPX3. Lab. Investig. 2022, 102, 589–601. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, H.; Xu, Y.; Shu, G.; Xiao, Y.; Hong, G.; Xu, S. Targeted Restoration of GPX3 Attenuates Renal Ischemia/Reperfusion Injury by Balancing Selenoprotein Expression and Inhibiting ROS-mediated Mitochondrial Apoptosis. Transplantation 2024. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xiao, C.; Zheng, S.; Wang, Q.; Zhu, H.; Zhang, Y.; Wang, R. MicroRNA-214-3p aggravates ferroptosis by targeting GPX4 in cisplatin-induced acute kidney injury. Cell Stress. Chaperones 2022, 27, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021, 28, 231–243. [Google Scholar] [CrossRef]
- Zhu, B.; He, J.; Ye, X.; Pei, X.; Bai, Y.; Gao, F.; Guo, L.; Yong, H.; Zhao, W. Role of Cisplatin in Inducing Acute Kidney Injury and Pyroptosis in Mice via the Exosome miR-122/ELAVL1 Regulatory Axis. Physiol. Res. 2023, 72, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Tang, C.; Ma, Z.; Huang, S.; Dong, Z. DNA damage response in nephrotoxic and ischemic kidney injury. Toxicol. Appl. Pharmacol. 2016, 313, 104–108. [Google Scholar] [CrossRef]
- Taguchi, K.; Sugahara, S.; Elias, B.C.; Pabla, N.S.; Canaud, G.; Brooks, C.R. IL-22 is secreted by proximal tubule cells and regulates DNA damage response and cell death in acute kidney injury. Kidney Int. 2024, 105, 99–114. [Google Scholar] [CrossRef]
- Yamashita, N.; Nakai, K.; Nakata, T.; Nakamura, I.; Kirita, Y.; Matoba, S.; Humphreys, B.D.; Tamagaki, K.; Kusaba, T. Cumulative DNA damage by repeated low-dose cisplatin injection promotes the transition of acute to chronic kidney injury in mice. Sci. Rep. 2021, 11, 20920. [Google Scholar] [CrossRef]
- Yin, Q.; Zhao, Y.J.; Ni, W.J.; Tang, T.T.; Wang, Y.; Cao, J.Y.; Yin, D.; Wen, Y.; Li, Z.L.; Zhang, Y.L.; et al. MiR-155 deficiency protects renal tubular epithelial cells from telomeric and genomic DNA damage in cisplatin-induced acute kidney injury. Theranostics 2022, 12, 4753–4766. [Google Scholar] [CrossRef]
- Fu, S.; Hu, X.; Ma, Z.; Wei, Q.; Xiang, X.; Li, S.; Wen, L.; Liang, Y.; Dong, Z. p53 in Proximal Tubules Mediates Chronic Kidney Problems after Cisplatin Treatment. Cells 2022, 11, 712. [Google Scholar] [CrossRef]
- Jiang, M.; Wei, Q.; Wang, J.; Du, Q.; Yu, J.; Zhang, L.; Dong, Z. Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene 2006, 25, 4056–4066. [Google Scholar] [CrossRef]
- Chen, G.; Xue, H.; Zhang, X.; Ding, D.; Zhang, S. p53 inhibition attenuates cisplatin-induced acute kidney injury through microRNA-142-5p regulating SIRT7/NF-κB. Ren. Fail. 2022, 44, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Miyasato, Y.; Yoshizawa, T.; Sato, Y.; Nakagawa, T.; Kakizoe, Y.; Kuwabara, T.; Adachi, M.; Ianni, A.; Braun, T.; Komohara, Y.; et al. Sirtuin 7 Deficiency Ameliorates Cisplatin-induced Acute Kidney Injury through Regulation of the Inflammatory Response. Sci. Rep. 2018, 8, 5927. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Liu, F.; Guan, B.; Luo, Z.; Lin, J.; Fang, W.; Liu, L.; Zuo, W. p53 induces miR-199a-3p to suppress mechanistic target of rapamycin activation in cisplatin-induced acute kidney injury. J. Cell. Biochem. 2019, 120, 17625–17634. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Shu, S.; Cai, J.; Tang, C.; Dong, Z. AMPK/mTOR Signaling in Autophagy Regulation During Cisplatin-Induced Acute Kidney Injury. Front. Physiol. 2020, 11, 619730. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, K.; Zhou, L.; Mi, Q.S.; Huang, S.; She, J.X.; Dong, Z. MicroRNA-34a is induced via p53 during cisplatin nephrotoxicity and contributes to cell survival. Mol. Med. 2010, 16, 409–416. [Google Scholar] [CrossRef]
- Qin, W.; Xie, W.; Yang, X.; Xia, N.; Yang, K. Inhibiting microRNA-449 Attenuates Cisplatin-Induced Injury in NRK-52E Cells Possibly via Regulating the SIRT1/P53/BAX Pathway. Med. Sci. Monit. 2016, 22, 818–823. [Google Scholar] [CrossRef]
- Nagasaka, M.; Miyajima, C.; Aoki, H.; Aoyama, M.; Morishita, D.; Inoue, Y.; Hayashi, H. Insights into Regulators of p53 Acetylation. Cells 2022, 11, 3825. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Zhang, L.; Wang, Y.; Sun, S.; Wu, J.; Sun, J.; Zou, X.; Chen, M.; Zhang, G. Cisplatin induces acute kidney injury by downregulating miR-30e-5p that targets Galnt3 to activate the AMPK signaling pathway. Environ. Toxicol. 2024, 39, 1567–1580. [Google Scholar] [CrossRef]
- de Almeida, D.C.; Bassi, E.J.; Azevedo, H.; Anderson, L.; Origassa, C.S.; Cenedeze, M.A.; de Andrade-Oliveira, V.; Felizardo, R.J.; da Silva, R.C.; Hiyane, M.I.; et al. A Regulatory miRNA-mRNA Network Is Associated with Tissue Repair Induced by Mesenchymal Stromal Cells in Acute Kidney Injury. Front. Immunol. 2016, 7, 645. [Google Scholar] [CrossRef]
- Nørgård, M.; Svenningsen, P. Acute Kidney Injury by Ischemia/Reperfusion and Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 15312. [Google Scholar] [CrossRef]
- Du, Y.; Ning, J.Z. MiR-182 Promotes Ischemia/Reperfusion-Induced Acute Kidney Injury in Rat by Targeting FoxO3. Urol. Int. 2021, 105, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kang, H.; Zhang, Q.; D’Agati, V.D.; Al-Awqati, Q.; Lin, F. FoxO3 activation in hypoxic tubules prevents chronic kidney disease. J. Clin. Investig. 2019, 129, 2374–2389. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, Z.; Chen, C.; Liu, Y.; Yuan, D.; Hei, Z.; Luo, G. PI3K/AKT activation attenuates acute kidney injury following liver transplantation by inducing FoxO3a nuclear export and deacetylation. Life Sci. 2021, 272, 119119. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Han, S.; Zhu, J.; Cheng, F. MiR-132-3p activation aggravates renal ischemia-reperfusion injury by targeting Sirt1/PGC1alpha axis. Cell. Signal. 2023, 110, 110801. [Google Scholar] [CrossRef]
- Song, N.; Zhang, T.; Xu, X.; Lu, Z.; Yu, X.; Fang, Y.; Hu, J.; Jia, P.; Teng, J.; Ding, X. miR-21 Protects against Ischemia/Reperfusion-Induced Acute Kidney Injury by Preventing Epithelial Cell Apoptosis and Inhibiting Dendritic Cell Maturation. Front. Physiol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Jia, P.; Pan, T.; Xu, S.; Fang, Y.; Song, N.; Guo, M.; Liang, Y.; Xu, X.; Ding, X. Depletion of miR-21 in dendritic cells aggravates renal ischemia-reperfusion injury. FASEB J. 2020, 34, 11729–11740. [Google Scholar] [CrossRef]
- Zhang, W.; Shu, L. Upregulation of miR-21 by Ghrelin Ameliorates Ischemia/Reperfusion-Induced Acute Kidney Injury by Inhibiting Inflammation and Cell Apoptosis. DNA Cell Biol. 2016, 35, 417–425. [Google Scholar] [CrossRef]
- Hoppe, B.; Pietsch, S.; Franke, M.; Engel, S.; Groth, M.; Platzer, M.; Englert, C. MiR-21 is required for efficient kidney regeneration in fish. BMC Dev. Biol. 2015, 15, 43. [Google Scholar] [CrossRef]
- Shang, J.; Sun, S.; Zhang, L.; Hao, F.; Zhang, D. miR-211 alleviates ischaemia/reperfusion-induced kidney injury by targeting TGFβR2/TGF-β/SMAD3 pathway. Bioengineered 2020, 11, 547–557. [Google Scholar] [CrossRef]
- Zhang, C.; Guan, G.; Wang, J.; Wei, H.; Cai, J. MicroRNA-192-5p downregulates Fat Mass and Obesity-associated Protein to aggravate renal ischemia/reperfusion injury. Ren. Fail. 2023, 45, 2285869. [Google Scholar] [CrossRef]
- Yue, J.; Si, Y.; Zhu, T.; Yang, J.; Xu, X.; Fang, Y.; Fu, W. MicroRNA-187 Reduces Acute Ischemic Renal Podocyte Injury via Targeting Acetylcholinesterase. J. Surg. Res. 2019, 244, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhao, Y.; Wang, L.; Zhang, W.; Liu, C.; Yin, A. MicroRNA-194 overexpression protects against hypoxia/reperfusion-induced HK-2 cell injury through direct targeting Rheb. J. Cell. Biochem. 2019, 120, 8311–8318. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fang, H.; Li, X.; Yu, P.; Guan, Y.; Xiao, C.; Deng, Z.; Hei, Z.; Chen, C.; Luo, C. Connexin32 gap junction channels deliver miR155-3p to mediate pyroptosis in renal ischemia-reperfusion injury. Cell Commun. Signal. 2024, 22, 121. [Google Scholar] [CrossRef]
- Vinas, J.L.; Burger, D.; Zimpelmann, J.; Haneef, R.; Knoll, W.; Campbell, P.; Gutsol, A.; Carter, A.; Allan, D.S.; Burns, K.D. Transfer of microRNA-486-5p from human endothelial colony forming cell-derived exosomes reduces ischemic kidney injury. Kidney Int. 2016, 90, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Vinas, J.L.; Spence, M.; Porter, C.J.; Douvris, A.; Gutsol, A.; Zimpelmann, J.A.; Campbell, P.A.; Burns, K.D. micro-RNA-486-5p protects against kidney ischemic injury and modifies the apoptotic transcriptome in proximal tubules. Kidney Int. 2021, 100, 597–612. [Google Scholar] [CrossRef]
- Ma, M.; Fu, L.; Jia, Z.; Zhong, Q.; Huang, Z.; Wang, X.; Fan, Y.; Lin, T.; Song, T. miR-17-5p attenuates kidney ischemia-reperfusion injury by inhibiting the PTEN and BIM pathways. Ann. Transl. Med. 2021, 9, 1545. [Google Scholar] [CrossRef]
- Zhou, J.; Jia, L.; Hu, Z.; Wang, Y. Pharmacological Inhibition of PTEN Aggravates Acute Kidney Injury. Sci. Rep. 2017, 7, 9503. [Google Scholar] [CrossRef]
- Mogilyansky, E.; Rigoutsos, I. The miR-17/92 cluster: A comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013, 20, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Cerqueira, D.M.; Li, Y.; Bodnar, A.J.; Mukherjee, E.; Pfister, K.; Phua, Y.L.; Shaikh, K.; Sanders, B.T.; Hemker, S.L.; et al. Endothelial-Derived miR-17 approximately 92 Promotes Angiogenesis to Protect against Renal Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2021, 32, 553–562. [Google Scholar] [CrossRef]
- Sethi, K.; Rao, K.; Bolton, D.; Patel, O.; Ischia, J. Targeting HIF-1alpha to Prevent Renal Ischemia-Reperfusion Injury: Does It Work? Int. J. Cell Biol. 2018, 2018, 9852791. [Google Scholar] [CrossRef]
- Wei, Q.; Sun, H.; Song, S.; Liu, Y.; Liu, P.; Livingston, M.J.; Wang, J.; Liang, M.; Mi, Q.S.; Huo, Y.; et al. MicroRNA-668 represses MTP18 to preserve mitochondrial dynamics in ischemic acute kidney injury. J. Clin. Investig. 2018, 128, 5448–5464. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Liu, Y.; Liu, P.; Hao, J.; Liang, M.; Mi, Q.S.; Chen, J.K.; Dong, Z. MicroRNA-489 Induction by Hypoxia-Inducible Factor-1 Protects against Ischemic Kidney Injury. J. Am. Soc. Nephrol. 2016, 27, 2784–2796. [Google Scholar] [CrossRef] [PubMed]
- Aomatsu, A.; Kaneko, S.; Yanai, K.; Ishii, H.; Ito, K.; Hirai, K.; Ookawara, S.; Kobayashi, Y.; Sanui, M.; Morishita, Y. MicroRNA expression profiling in acute kidney injury. Transl. Res. 2022, 244, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Cao, Y.; Huang, T.; Sang, Y.; Dai, Y.; Tao, Z. Comprehensive analysis of fifteen hub genes to identify a promising diagnostic model, regulated networks, and immune cell infiltration in acute kidney injury. J. Clin. Lab. Anal. 2022, 36, e24709. [Google Scholar] [CrossRef]
- Zhang, H.; Che, L.; Wang, Y.; Zhou, H.; Gong, H.; Man, X.; Zhao, Q. Deregulated microRNA-22-3p in patients with sepsis-induced acute kidney injury serves as a new biomarker to predict disease occurrence and 28-day survival outcomes. Int. Urol. Nephrol. 2021, 53, 2107–2116. [Google Scholar] [CrossRef]
- Yu, R.; Wu, C.; Xiao, Y.; Li, Q.; Chen, J.; Song, J.; Chen, H.; Wang, Z.; Wang, W. The clinical predictive value and regulation mechanism of microRNA-188-5p in contrast-induced acute kidney injury. Biochem. Biophys. Res. Commun. 2023, 679, 215–223. [Google Scholar] [CrossRef]
- Schulze, P.C.; Bogoviku, J.; Westphal, J.; Aftanski, P.; Haertel, F.; Grund, S.; von Haehling, S.; Schumacher, U.; Mobius-Winkler, S.; Busch, M. Effects of Early Empagliflozin Initiation on Diuresis and Kidney Function in Patients With Acute Decompensated Heart Failure (EMPAG-HF). Circulation 2022, 146, 289–298. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Li, X.; Wang, L.; Yu, H.; Huang, J.; Liu, Q.; Wang, C.; Jiang, A. Diagnostic and prognostic significance of aberrant miR-652-3p levels in patients with acute decompensated heart failure and acute kidney injury. J. Int. Med. Res. 2020, 48, 300060520967829. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Li, X.; Wang, L.; Yu, H.; Huang, J.; Liu, Q.; Wang, C.; Jiang, A. MicroRNA-423-5p as a biomarker for early diagnosis and outcome prediction of acute kidney injury in patients with acute decompensated heart failure. Int. J. Urol. 2021, 28, 25–32. [Google Scholar] [CrossRef]
- Pei, J.; Zhang, J.; Yu, C.; Luo, J.; Wen, S.; Hua, Y.; Wei, G. Transcriptomics-based exploration of shared M1-type macrophage-related biomarker in acute kidney injury after kidney transplantation and acute rejection after kidney transplantation. Transpl. Immunol. 2024, 85, 102066. [Google Scholar] [CrossRef]
- Gaede, L.; Liebetrau, C.; Blumenstein, J.; Troidl, C.; Dörr, O.; Kim, W.K.; Gottfried, K.; Voss, S.; Berkowitsch, A.; Walther, T.; et al. Plasma microRNA-21 for the early prediction of acute kidney injury in patients undergoing major cardiac surgery. Nephrol. Dial. Transplant. 2016, 31, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Chen, L.; Wen, B.; Wang, X.; Wang, L.; Yang, F.; Chen, Y.; Bao, J.; Zhang, G.; Ji, K.; et al. MicroRNA-206 functions as a potential oligonucleotide therapeutics in preterm birth. Chin. Med. J. 2024, 137, 1000–1002. [Google Scholar] [CrossRef]
- van der Ree, M.H.; van der Meer, A.J.; van Nuenen, A.C.; de Bruijne, J.; Ottosen, S.; Janssen, H.L.; Kootstra, N.A.; Reesink, H.W. Miravirsen dosing in chronic hepatitis C patients results in decreased microRNA-122 levels without affecting other microRNAs in plasma. Aliment. Pharmacol. Ther. 2016, 43, 102–113. [Google Scholar] [CrossRef] [PubMed]
- De Santi, C.; Greene, C.M. Challenges facing microRNA therapeutics for cystic fibrosis lung disease. Epigenomics 2020, 12, 179–181. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Alswailem, R.; Alqahtani, F.Y.; Aleanizy, F.S.; Alrfaei, B.M.; Badran, M.; Alqahtani, Q.H.; Abdelhady, H.G.; Alsarra, I. MicroRNA-219 loaded chitosan nanoparticles for treatment of glioblastoma. Artif. Cells Nanomed. Biotechnol. 2022, 50, 198–207. [Google Scholar] [CrossRef]
- Thibonnier, M.; Ghosh, S. Strategy for Pre-Clinical Development of Active Targeting MicroRNA Oligonucleotide Therapeutics for Unmet Medical Needs. Int. J. Mol. Sci. 2023, 24, 7126. [Google Scholar] [CrossRef]
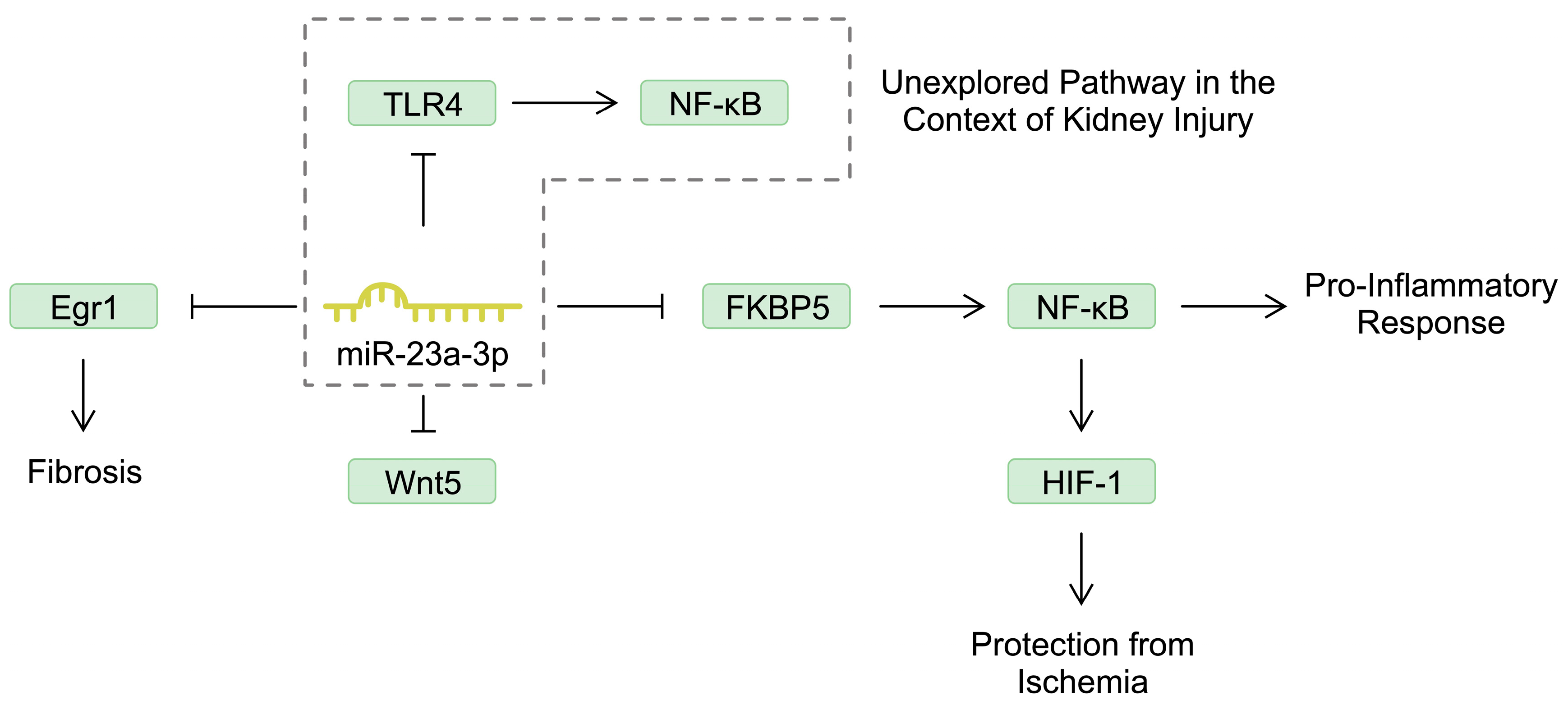
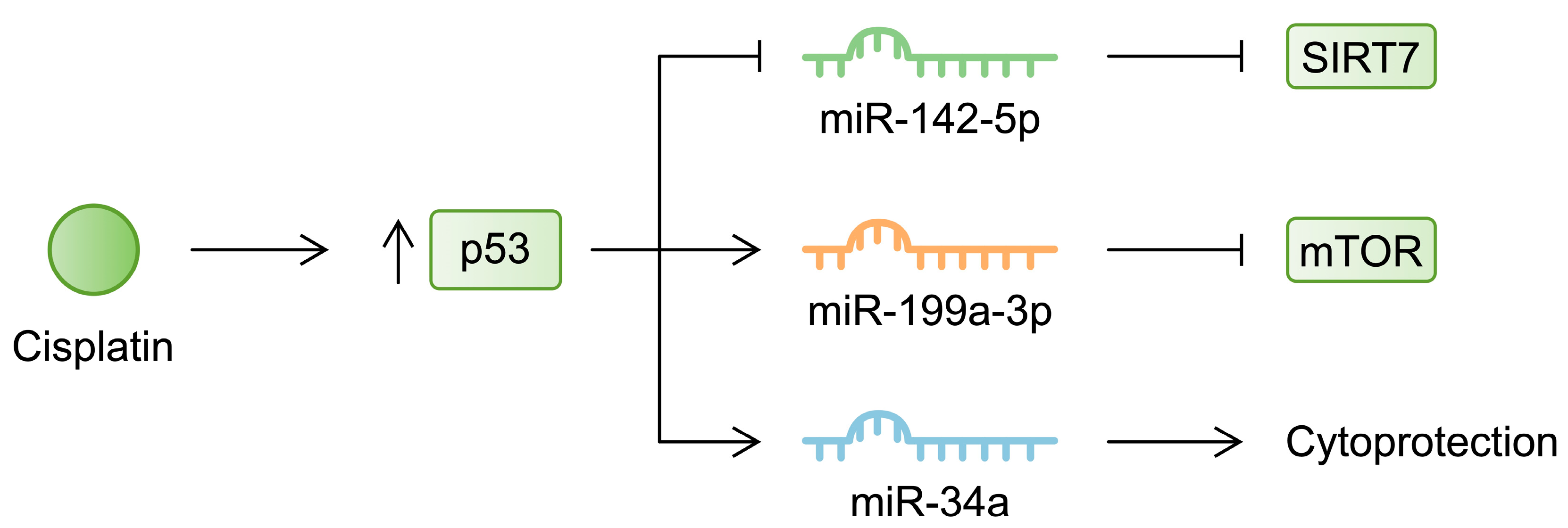
| MicroRNA | Mechanism Associated with Acute Kidney Injury | References |
|---|---|---|
| miR-23a-3p | miR-23a-3p negatively regulates FKBP5, thus suppressing the activity of the NF-κB and reducing pro-inflammatory response. | [31] |
| miR-23a-3p | miR-23a-3p suppresses kidney injury by targeting Wnt5, a member of the Wnt/β-catenin signaling pathway. | [33] |
| miR-106a | miR-106a further stimulates kidney cell injury by targeting the anti-angiogenic thrombospondin 2. | [42] |
| miR-106a-5p | miR-106a-5p targets HMGB1, which is associated with podocyte injury and the expression of which is elevated in mouse models of AKI. | [43,44] |
| miR-22 | miR-22 suppresses septic AKI due to inhibiting the HMGB1/TLR4/NF-κB signaling pathway. | [45] |
| miR-26a-5p | miR-26a-5p induced protective mechanisms in septic models of AKI by targeting IL-6. | [51] |
| miR-22-3p | miR-22-3p suppresses inflammatory responses in animal sepsis AKI models and LPS-stimulated HK-2 cells by targeting PTEN. | [57] |
| miR-93 | miR-93 targets PTEN and activates the signaling of the AKT/mTOR pathway, which is associated with improved viability in LPS-pretreated renal cells. | [58] |
| miR-214 | By targeting PTEN, miR-214 improved renal parameters, suppressed inflammation, and improves renal histopathology in septic mice. | [59] |
| miR-21 | miR-21 can suppress renal injury induced by sepsis by targeting PTEN. | [63] |
| miR-21 | miR-21 contributes to the kidney damage induced by LPS by targeting CDK6. | [65] |
| miR-128-3p | miR-128-3p contributes to sepsis-associated kidney damage by targeting NRP1 and enhancing inflammatory response. | [67] |
| miR-16-5p | By targeting BCL-2, miR-16-5p regulates apoptosis and further stimulates LPS-induced inflammation. | [73] |
| miR-543 | Downregulating miR-543, which targets BCL-2, reduces inflammatory response and apoptosis. | [74] |
| MicroRNA | Expression in Cisplatin-Induced AKI (In Vitro or In Vivo) | Mechanism | References |
|---|---|---|---|
| miR-483-5p | Increased | miR-483-5p disrupted apoptosis and autophagy in renal cells through targeting GPX3, a member of the glutathione peroxidase family. | [80] |
| miR-214-3p | Increased | miR-214-3p regulates ferroptosis by targeting GPX4. | [82] |
| miR-155 | Increased | Targeting miR-155 suppresses cisplatin-induced DNA damage. | [88] |
| miR-142-5p | Decreased | Suppression of p53 enhances the expression of miR-142-5p, which inhibits apoptosis induced by cisplatin. | [91] |
| miR-199a-3p | Increased | Cisplatin-induced expression of miR-199a-3p was p53-dependent, and the molecule regulated the mTOR gene. | [93] |
| miR-34a | Increased | miR-34a has cytoprotective features as its inhibition further enhanced cisplatin-induced cell damage. | [95] |
| miR-449 | Increased | Suppression of miR-449 enhances renal cell viability. | [96] |
| miR-30e-5p | Decreased | Overexpression of miR-30e-5p improves cell viability in cisplatin-treated renal cells. The molecule regulates the AMPK pathway. | [98] |
| MicroRNA | Impact of Ischemia-Reperfusion Injury on Expression in Renal Tissue/Cells | Mechanism Linking to Acute Kidney Injury | References |
|---|---|---|---|
| miR-182 | Increased | miR-182 enhances apoptosis of tubular epithelial cells through targeting FoxO3. | [101] |
| miR-132-3p | Increased | miR-132-3p targets SIRT1 and further deteriorates oxidative balance in ischemia. | [104] |
| miR-21 | Increased | miR-21 plays a protective role in IRI-associated AKI by suppressing dendritic cell maturation, thus limiting the inflammatory responses. | [105] |
| miR-211 | Decreased | Through inhibiting TGF-β/SMAD signaling, miR-211 improves cell viability. | [109] |
| miR-192-5p | Increased | Suppression of miR-192-5p improved viability of renal cells under hypoxia/reoxygenation conditions. The molecule targets FTO. | [110] |
| miR-187 | Decreased | Increasing the expression of miR-187 reduces podocyte damage. | [111] |
| miR-194 | Decreased | By targeting Rheb, miR-194 reduces inflammation and oxidative stress in renal cells stimulated by hypoxia and reoxygenation. | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakinowska, E.; Kiełbowski, K.; Pawlik, A. The Role of MicroRNA in the Pathogenesis of Acute Kidney Injury. Cells 2024, 13, 1559. https://doi.org/10.3390/cells13181559
Bakinowska E, Kiełbowski K, Pawlik A. The Role of MicroRNA in the Pathogenesis of Acute Kidney Injury. Cells. 2024; 13(18):1559. https://doi.org/10.3390/cells13181559
Chicago/Turabian StyleBakinowska, Estera, Kajetan Kiełbowski, and Andrzej Pawlik. 2024. "The Role of MicroRNA in the Pathogenesis of Acute Kidney Injury" Cells 13, no. 18: 1559. https://doi.org/10.3390/cells13181559
APA StyleBakinowska, E., Kiełbowski, K., & Pawlik, A. (2024). The Role of MicroRNA in the Pathogenesis of Acute Kidney Injury. Cells, 13(18), 1559. https://doi.org/10.3390/cells13181559





