The Role of Mesenchymal Stem Cells in Modulating Adaptive Immune Responses in Multiple Sclerosis
Abstract
1. Introduction
2. Pathophysiology of Multiple Sclerosis
3. Mesenchymal Stem Cells: Characteristics and Mechanisms of Action
4. Preclinical Studies Investigating MSC Therapy in MS
4.1. Overview of Preclinical Studies Using Animal Models of MS
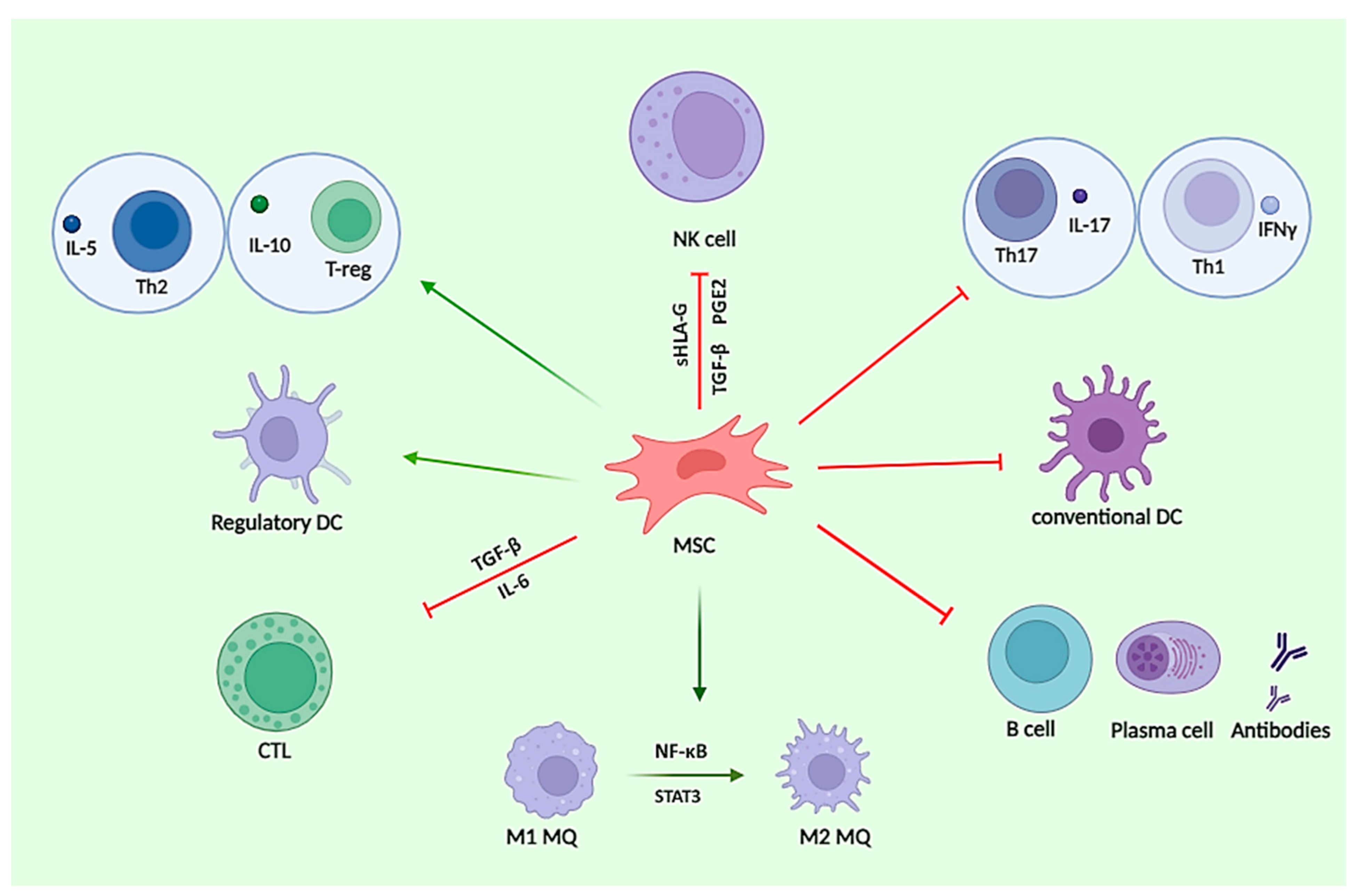
4.2. Findings on the Effects of MSCs on Adaptive Immune Responses in MS
5. Clinical Trials Evaluating MSC Therapy for MS
5.1. Overview of Clinical Trials Using MSCs in MS Patients
5.2. Safety and Efficacy of MSC Therapy in MS Patients
6. Challenges and Future Directions
6.1. The Effectiveness and Heterogeneity of Various MSC Populations
6.2. Migratory and Homing Capacity of MSCs
6.3. Route of Administration
6.4. Immunological Compatibility of Mesenchymal Stem Cells
6.5. Restricted Proliferation of MSCs
6.6. Long-Term Side Effects of MSCs Therapy
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qureshi, M.; Al-Suhaimi, E.A.; Wahid, F.; Shehzad, O.; Shehzad, A. Therapeutic potential of curcumin for multiple sclerosis. Neurol. Sci. 2018, 39, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; Brück, W.; Lucchinetti, C.F. The immunopathology of multiple sclerosis: An overview. Brain Pathol. 2007, 17, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; Van Der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.; Al-Suhaimi, E.; Shehzad, A. Chapter 20—Curcumin Impact on Multiple Sclerosis. In Curcumin for Neurological and Psychiatric Disorders; Farooqui, T., Farooqui, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 365–380. [Google Scholar]
- Genc, B.; Bozan, H.R.; Genc, S.; Genc, K. Stem Cell Therapy for Multiple Sclerosis. Adv. Exp. Med. Biol. 2019, 1084, 145–174. [Google Scholar] [PubMed]
- Rezaee, M.; Keshavarz, K.; Izadi, S.; Jafari, A.; Ravangard, R. Economic burden of multiple sclerosis: A cross-sectional study in Iran. Health Econ. Rev. 2022, 12, 2. [Google Scholar] [CrossRef]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2016, 13, 25–36. [Google Scholar] [CrossRef]
- Murúa, S.R.; Farez, M.F.; Quintana, F.J. The Immune Response in Multiple Sclerosis. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 121–139. [Google Scholar] [CrossRef]
- Lazibat, I.; Majdak, M.R.; Županić, S. Multiple Sclerosis: New Aspects of Immunopathogenesis. Acta Clin. Croat. 2018, 57, 352–360. [Google Scholar] [CrossRef]
- Schaeffer, J.; Cossetti, C.; Mallucci, G.; Pluchino, S. Chapter 30—Multiple Sclerosis. In Neurobiology of Brain Disorders; Zigmond, M.J., Rowland, L.P., Coyle, J.T., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 497–520. [Google Scholar]
- Al Jumah, M.A.; Abumaree, M.H. The Immunomodulatory and Neuroprotective Effects of Mesenchymal Stem Cells (MSCs) in Experimental Autoimmune Encephalomyelitis (EAE): A Model of Multiple Sclerosis (MS). Int. J. Mol. Sci. 2012, 13, 9298–9331. [Google Scholar] [CrossRef]
- Owens, T.; Sriram, S. The Immunology of Multiple Sclerosis and its Animal Model, Experimental Allergic Encephalomyelitis. Neurol. Clin. 1995, 13, 51–73. [Google Scholar] [CrossRef]
- Mosayebi, G.; Haghmorad, D.; Namaki, S.; Ghazavi, A.; Ekhtiari, P.; Mirshafiey, A. Therapeutic effect of EDTA in experimental model of multiple sclerosis. Immunopharmacol. Immunotoxicol. 2010, 32, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Mesenchymal Stem Cells in Multiple Sclerosis: Recent Evidence from Pre-Clinical to Clinical Studies. Int. J. Mol. Sci. 2020, 21, 8662. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Alam, S.S.; Kundu, S.; Ahmed, S.; Sultana, S.; Patar, A.; Hossan, T. Mesenchymal Stem Cell Therapy in Multiple Sclerosis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 6311. [Google Scholar] [CrossRef] [PubMed]
- la Rosa, R.S.-D.; Sabater, E.; Casado, M. PND30 cost analysis of glatiramer acetate versus fingolimod for the treatment of patients with relapsing-remitting multiple sclerosis in spain. Value Health 2013, 15, A551. [Google Scholar] [CrossRef][Green Version]
- Moura, R.P.; Sarmento, B. Therapeutic Approaches toward Multiple Sclerosis: Where Do We Stand and Where Are We Headed? Adv. Ther. 2019, 2, 1900070. [Google Scholar] [CrossRef]
- Ansboro, S.; Roelofs, A.J.; De Bari, C. Mesenchymal stem cells for the management of rheumatoid arthritis: Immune modulation, repair or both? Curr. Opin. Rheumatol. 2017, 29, 201–207. [Google Scholar] [CrossRef]
- Hazrati, A.; Malekpour, K.; Khorramdelazad, H.; Rajaei, S.; Hashemi, S.M. Therapeutic and immunomodulatory potentials of mesenchymal stromal/stem cells and immune checkpoints related molecules. Biomark. Res. 2024, 12, 35. [Google Scholar] [CrossRef]
- Zhao, Q.; Ren, H.; Han, Z. Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases. J. Cell. Immunother. 2016, 2, 3–20. [Google Scholar] [CrossRef]
- Klinker, M.W.; Wei, C.H. Mesenchymal stem cells in the treatment of inflammatory and autoimmune diseases in experimental animal models. World J. Stem Cells 2015, 7, 556–567. [Google Scholar] [CrossRef]
- Martin, R. Chapter 4 Immunology of Multiple Sclerosis. In Blue Books of Practical Neurology; McDonald, W.I., Noseworthy, J.H., Eds.; 27: Butterworth-Heinemann: Oxford, UK, 2003; pp. 33–58. [Google Scholar]
- Fletcher, J.M.; Lalor, S.J.; Sweeney, C.M.; Tubridy, N.; Mills, K.H.G. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2010, 162, 1–11. [Google Scholar] [CrossRef]
- Liu, R.; Du, S.; Zhao, L.; Jain, S.; Sahay, K.; Rizvanov, A.; Lezhnyova, V.; Khaibullin, T.; Martynova, E.; Khaiboullina, S.; et al. Autoreactive lymphocytes in multiple sclerosis: Pathogenesis and treatment target. Front. Immunol. 2022, 13, 996469. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, G.; Rossi, S.; Motta, C.; Macchiarulo, G.; Barbieri, F.; De Bardi, M.; Borsellino, G.; Finardi, A.; Grasso, M.G.; Ruggieri, S.; et al. T helper 9 cells induced by plasmacytoid dendritic cells regulate interleukin-17 in multiple sclerosis. Clin. Sci. 2015, 129, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerken, L. Role of Th1 and Th2 cells in autoimmune demyelinating disease. Braz. J. Med. Biol. Res. 1998, 31, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Prajeeth, C.K.; Kronisch, J.; Khorooshi, R.; Knier, B.; Toft-Hansen, H.; Gudi, V.; Floess, S.; Huehn, J.; Owens, T.; Korn, T.; et al. Effectors of Th1 and Th17 cells act on astrocytes and augment their neuroinflammatory properties. J. Neuroinflamm. 2017, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Domingues, H.S.; Mues, M.; Lassmann, H.; Wekerle, H.; Krishnamoorthy, G. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS ONE 2010, 5, e15531. [Google Scholar] [CrossRef]
- Lafaille, J.J.; Van de Keere, F.; Hsu, A.L.; Baron, J.L.; Haas, W.; Raine, C.S.; Tonegawa, S. Myelin basic protein–specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J. Exp. Med. 1997, 186, 307–312. [Google Scholar] [CrossRef]
- Panitch, H.S. Interferons in multiple sclerosis. A review of the evidence. Drugs 1992, 44, 946–962. [Google Scholar] [CrossRef]
- Panitch, H.S.; Hirsch, R.L.; Schindler, J.; Johnson, K.P. Treatment of multiple sclerosis with gamma interferon: Exacerbations associated with activation of the immune system. Neurology 1987, 37, 1097–1102. [Google Scholar] [CrossRef]
- Damsker, J.M.; Hansen, A.M.; Caspi, R.R. Th1 and Th17 cells: Adversaries and collaborators. Ann. N. Y. Acad. Sci. 2010, 1183, 211–221. [Google Scholar] [CrossRef]
- Manel, N.; Unutmaz, D.; Littman, D.R. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 2008, 9, 641–649. [Google Scholar] [CrossRef]
- Ma, J.; McCarl, C.; Khalil, S.; Lüthy, K.; Feske, S. T-cell-specific deletion of STIM1 and STIM2 protects mice from EAE by impairing the effector functions of Th1 and Th17 cells. Eur. J. Immunol. 2010, 40, 3028–3042. [Google Scholar] [CrossRef] [PubMed]
- Tzartos, J.S.; Friese, M.A.; Craner, M.J.; Palace, J.; Newcombe, J.; Esiri, M.M.; Fugger, L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008, 172, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, Y.; Nakae, S.; Matsuki, T.; Nambu, A.; Ishigame, H.; Kakuta, S.; Sudo, K.; Iwakura, Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006, 177, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Wang, C.; Zepp, J.; Wu, L.; Sun, K.; Zhao, J.; Chandrasekharan, U.; DiCorleto, P.E.; Trapp, B.D.; Ransohoff, R.M.; et al. Act1 mediates IL-17–induced EAE pathogenesis selectively in NG2+ glial cells. Nat. Neurosci. 2013, 16, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, P.; Yazdi, F.R.; Taghizadeh, F.; Khadivi, F.; Hamidabadi, H.G.; Kashani, I.R.; Zarini, D.; Mojaverrostami, S. Quercetin as a possible complementary therapy in multiple sclerosis: Anti-oxidative, anti-inflammatory and remyelination potential properties. Heliyon 2023, 9, e21741. [Google Scholar] [CrossRef]
- Kunkl, M.; Frascolla, S.; Amormino, C.; Volpe, E.; Tuosto, L. T Helper Cells: The Modulators of Inflammation in Multiple Sclerosis. Cells 2020, 9, 482. [Google Scholar] [CrossRef]
- Kroenke, M.A.; Carlson, T.J.; Andjelkovic, A.V.; Segal, B.M. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 2008, 205, 1535–1541. [Google Scholar] [CrossRef]
- Oreja-Guevara, C.; Ramos-Cejudo, J.; Aroeira, L.S.; Chamorro, B.; Diez-Tejedor, E. TH1/TH2 Cytokine profile in relapsing-remitting multiple sclerosis patients treated with Glatiramer acetate or Natalizumab. BMC Neurol. 2012, 12, 95. [Google Scholar] [CrossRef]
- Falcone, M.; Rajan, A.J.; Bloom, B.R.; Brosnan, C.F. A critical role for IL-4 in regulating disease severity in experimental allergic encephalomyelitis as demonstrated in IL-4-deficient C57BL/6 mice and BALB/c mice. J. Immunol. 1998, 160, 4822–4830. [Google Scholar] [CrossRef]
- Racke, M.K.; Bonomo, A.; Scott, D.E.; Cannella, B.; Levine, A.; Raine, C.S.; Shevach, E.M.; Röcken, M. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J. Exp. Med. 1994, 180, 1961–1966. [Google Scholar] [CrossRef]
- Kennedy, M.K.; Torrance, D.S.; Picha, K.S.; Mohler, K.M. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J. Immunol. 1992, 149, 2496–2505. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [PubMed]
- Dumont, F.J. Modulation of Th1 and Th2 responses for immunotherapy. Expert Opin. Ther. Pat. 2002, 12, 341–367. [Google Scholar] [CrossRef]
- Jäger, A.; Kuchroo, V.K. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand. J. Immunol. 2010, 72, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, O.; Bajénoff, M.; Guerder, S. IL-4-mediated inhibition of IFN-gamma production by CD4+ T cells proceeds by several developmentally regulated mechanisms. Int. Immunol. 2004, 16, 501–508. [Google Scholar] [CrossRef]
- Ghoryani, M.; Shariati-Sarabi, Z.; Tavakkol-Afshari, J.; Mohammadi, M. The Sufficient Immunoregulatory Effect of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation on Regulatory T Cells in Patients with Refractory Rheumatoid Arthritis. J. Immunol. Res. 2020, 2020, 3562753. [Google Scholar] [CrossRef]
- Koutrolos, M.; Berer, K.; Kawakami, N.; Wekerle, H.; Krishnamoorthy, G. Treg cells mediate recovery from EAE by controlling effector T cell proliferation and motility in the CNS. Acta Neuropathol. Commun. 2014, 2, 163. [Google Scholar] [CrossRef]
- Yang, T.-T.; Liu, P.-J.; Sun, Q.-Y.; Wang, Z.-Y.; Yuan, G.-B.; Fan, Z.-X.; Ma, L.; Lu, J.-F.; Yuan, B.-Y.; Zou, W.-L.; et al. CD4+CD25+ regulatory T cells ex vivo generated from autologous naïve CD4+ T cells suppress EAE progression. Sci. Rep. 2024, 14, 6262. [Google Scholar] [CrossRef]
- Denic, A.; Wootla, B.; Rodriguez, M. CD8(+) T cells in multiple sclerosis. Expert Opin. Ther. Targets 2013, 17, 1053–1066. [Google Scholar] [CrossRef]
- Wagner, C.A.; Roqué, P.J.; Mileur, T.R.; Liggitt, D.; Goverman, J.M. Myelin-specific CD8+ T cells exacerbate brain inflammation in CNS autoimmunity. J. Clin. Investig. 2020, 130, 203–213. [Google Scholar] [CrossRef]
- Deng, Q.; Luo, Y.; Chang, C.; Wu, H.; Ding, Y.; Xiao, R. The Emerging Epigenetic Role of CD8+ T Cells in Autoimmune Diseases: A Systematic Review. Front. Immunol. 2019, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.-H.; Lee, S.; Kwak, M.; Kim, B.-S.; Chung, Y. CD8 T-cell subsets: Heterogeneity, functions, and therapeutic potential. Exp. Mol. Med. 2023, 55, 2287–2299. [Google Scholar] [CrossRef] [PubMed]
- Dhaiban, S.; Al-Ani, M.; Elemam, N.M.; Al-Aawad, M.H.; Al-Rawi, Z.; Maghazachi, A.A. Role of Peripheral Immune Cells in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Sci 2021, 3, 12. [Google Scholar] [CrossRef]
- Saxena, A.; Martin-Blondel, G.; Mars, L.; Liblau, R. Role of CD8 T cell subsets in the pathogenesis of multiple sclerosis. FEBS Lett. 2011, 585, 3758–3763. [Google Scholar] [CrossRef]
- Huseby, E.S.; Huseby, P.G.; Shah, S.; Smith, R.; Stadinski, B.D. Pathogenic CD8 T cells in multiple sclerosis and its experimental models. Front. Immunol. 2012, 3, 22126. [Google Scholar] [CrossRef]
- Myhr, K.-M.; Torkildsen, Ø.; Lossius, A.; Bø, L.; Holmøy, T. B cell depletion in the treatment of multiple sclerosis. Expert Opin. Biol. Ther. 2019, 19, 261–271. [Google Scholar] [CrossRef]
- Chen, F.H.; Song, L.; Mauck, R.L.; Li, W.-J.; Tuan, R.S. Chapter Fifty-Five—Mesenchymal Stem Cells. In Principles of Tissue Engineering, 3rd ed.; Lanza, R., Langer, R., Vacanti, J., Eds.; Academic Press: Burlington, NJ, USA, 2007; pp. 823–843. [Google Scholar]
- Zhou, T.; Li, H.-Y.; Liao, C.; Lin, W.; Lin, S. Clinical Efficacy and Safety of Mesenchymal Stem Cells for Systemic Lupus Erythematosus. Stem Cells Int. 2020, 2020, 6518508. [Google Scholar] [CrossRef]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef]
- Shomali, N.; Gharibi, T.; Vahedi, G.; Mohammed, R.N.; Mohammadi, H.; Salimifard, S.; Marofi, F. Mesenchymal stem cells as carrier of the therapeutic agent in the gene therapy of blood disorders. J. Cell. Physiol. 2020, 235, 4120–4134. [Google Scholar] [CrossRef]
- Abbaszadeh, H.; Ghorbani, F.; Derakhshani, M.; Movassaghpour, A.A.; Yousefi, M.; Talebi, M.; Shamsasenjan, K. Regenerative potential of Wharton’s jelly-derived mesenchymal stem cells: A new horizon of stem cell therapy. J. Cell. Physiol. 2020, 235, 9230–9240. [Google Scholar] [CrossRef]
- Li, X.; Guan, Y.; Li, C.; Zhang, T.; Meng, F.; Zhang, J.; Li, J.; Chen, S.; Wang, Q.; Wang, Y.; et al. Immunomodulatory effects of mesenchymal stem cells in peripheral nerve injury. Stem Cell Res. Ther. 2022, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Gharibi, T.; Ahmadi, M.; Seyfizadeh, N.; Jadidi-Niaragh, F.; Yousefi, M. Immunomodulatory characteristics of mesenchymal stem cells and their role in the treatment of Multiple Sclerosis. Cell. Immunol. 2015, 293, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Refaie, A.F.; Elbassiouny, B.L.; Kloc, M.; Sabek, O.M.; Khater, S.M.; Ismail, A.M.; Mohamed, R.H.; Ghoneim, M.A. From Mesenchymal Stromal/Stem Cells to Insulin-Producing Cells: Immunological Considerations. Front. Immunol. 2021, 12, 690623. [Google Scholar] [CrossRef]
- Ke, F.; Zhang, L.; Liu, Z.; Yan, S.; Xu, Z.; Bai, J.; Zhu, H.; Lou, F.; Cai, W.; Sun, Y.; et al. Soluble Tumor Necrosis Factor Receptor 1 Released by Skin-Derived Mesenchymal Stem Cells Is Critical for Inhibiting Th17 Cell Differentiation. Stem Cells Transl. Med. 2016, 5, 301–313. [Google Scholar] [CrossRef]
- Barati, S.; Tahmasebi, F.; Faghihi, F. Effects of mesenchymal stem cells transplantation on multiple sclerosis patients. Neuropeptides 2020, 84, 102095. [Google Scholar] [CrossRef]
- Akhter, W.; Nakhle, J.; Vaillant, L.; Garcin, G.; Le Saout, C.; Simon, M.; Crozet, C.; Djouad, F.; Jorgensen, C.; Vignais, M.-L.; et al. Transfer of mesenchymal stem cell mitochondria to CD4+ T cells contributes to repress Th1 differentiation by downregulating T-bet expression. Stem Cell Res. Ther. 2023, 14, 12. [Google Scholar] [CrossRef]
- Zheng, G.; Ge, M.; Qiu, G.; Shu, Q.; Xu, J. Mesenchymal Stromal Cells Affect Disease Outcomes via Macrophage Polarization. Stem Cells Int. 2015, 2015, 989473. [Google Scholar] [CrossRef]
- Dabrowska, S.; Andrzejewska, A.; Janowski, M.; Lukomska, B. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases. Front. Immunol. 2020, 11, 591065. [Google Scholar] [CrossRef] [PubMed]
- Radmanesh, F.; Mahmoudi, M.; Yazdanpanah, E.; Keyvani, V.; Kia, N.; Nikpoor, A.R.; Zafari, P.; Esmaeili, S. The immunomodulatory effects of mesenchymal stromal cell-based therapy in human and animal models of systemic lupus erythematosus. IUBMB Life 2020, 72, 2366–2381. [Google Scholar] [CrossRef] [PubMed]
- Angoulvant, D.; Clerc, A.; Benchalal, S.; Galambrun, C.; Farre, A.; Bertrand, Y.; Eljaafari, A. Human mesenchymal stem cells suppress induction of cytotoxic response to alloantigens. Biorheology 2004, 41, 469–476. [Google Scholar]
- Rosado, M.M.; Bernardo, M.E.; Scarsella, M.; Conforti, A.; Giorda, E.; Biagini, S.; Cascioli, S.; Rossi, F.; Guzzo, I.; Vivarelli, M.; et al. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015, 24, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Sun, J.; Tian, Y.; Li, H.; Zhang, L.; Yang, J.; Wang, J.; Zhang, J.; Yan, S.; Xu, D. Immunomodulatory Effect of MSCs and MSCs-Derived Extracellular Vesicles in Systemic Lupus Erythematosus. Front. Immunol. 2021, 12, 714832. [Google Scholar] [CrossRef]
- Li, N.; Hua, J. Interactions between mesenchymal stem cells and the immune system. Cell. Mol. Life Sci. 2017, 74, 2345–2360. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Chen, X. The Immunomodulatory Effects of Mesenchymal Stem Cells on Regulatory B Cells. Front. Immunol. 2020, 11, 1843. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood 2009, 113, 6576–6583. [Google Scholar] [CrossRef]
- Zhang, W.; Ge, W.; Li, C.; You, S.; Liao, L.; Han, Q.; Deng, W.; Zhao, R.C. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004, 13, 263–271. [Google Scholar] [CrossRef]
- Lu, D.; Xu, Y.; Liu, Q.; Zhang, Q. Mesenchymal Stem Cell-Macrophage Crosstalk and Maintenance of Inflammatory Microenvironment Homeostasis. Front. Cell Dev. Biol. 2021, 9, 681171. [Google Scholar] [CrossRef]
- Gao, S.; Mao, F.; Zhang, B.; Zhang, L.; Zhang, X.; Wang, M.; Yan, Y.; Yang, T.; Zhang, J.; Zhu, W.; et al. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-κB and signal transducer and activator of transcription 3 pathways. Exp. Biol. Med. 2014, 239, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, P.A.; Perez, S.A.; Gritzapis, A.D.; Baxevanis, C.N.; Papamichail, M. Interactions Between Human Mesenchymal Stem Cells and Natural Killer Cells. Stem Cells 2005, 24, 74–85. [Google Scholar] [CrossRef] [PubMed]
- El-Akabawy, G.; Rashed, L.A. Beneficial effects of bone marrow-derived mesenchymal stem cell transplantation in a non-immune model of demyelination. Ann. Anat.-Anat. Anz. 2015, 198, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef] [PubMed]
- Weiner, H.L. Immunosuppressive treatment in multiple sclerosis. J. Neurol. Sci. 2004, 223, 1–11. [Google Scholar] [CrossRef]
- Xin, Y.; Gao, J.; Hu, R.; Li, H.; Li, Q.; Han, F.; He, Z.; Lai, L.; Su, M. Changes of immune parameters of T lymphocytes and macrophages in EAE mice after BM-MSCs transplantation. Immunol. Lett. 2020, 225, 66–73. [Google Scholar] [CrossRef]
- Sadeghnejad, A.; Pazoki, A.; Yazdanpanah, E.; Esmaeili, S.; Yousefi, B.; Sadighi-Moghaddam, B.; Baharlou, R.; Haghmorad, D. Exploring the role of mesenchymal stem cells in modulating immune responses via Treg and Th2 cell activation: Insights from mouse model of multiple sclerosis. APMIS 2024. [Google Scholar] [CrossRef]
- Liu, G.Y.; Wu, Y.; Kong, F.Y.; Ma, S.; Fu, L.Y.; Geng, J. BMSCs differentiated into neurons, astrocytes and oligodendrocytes alleviated the inflammation and demyelination of EAE mice models. PLoS ONE 2021, 16, e0243014. [Google Scholar] [CrossRef]
- Haghmorad, D.; Khaleghian, A.; Eslami, M.; Sadeghnejad, A.; Tarahomi, M.; Yousefi, B. Bone marrow mesenchymal stem cells to ameliorate experimental autoimmune encephalomyelitis via modifying expression patterns of miRNAs. Mol. Biol. Rep. 2023, 50, 9971–9984. [Google Scholar] [CrossRef]
- Zargarani, S.; Tavaf, M.J.; Soltanmohammadi, A.; Yazdanpanah, E.; Baharlou, R.; Yousefi, B.; Sadighimoghaddam, B.; Esmaeili, S.A.; Haghmorad, D. Adipose-derived mesenchymal stem cells ameliorates experimental autoimmune encephalomyelitis via modulation of Th1/Th17 and expansion of Th2/Treg responses. Cell Biol. Int. 2024, 48, 1124–1137. [Google Scholar] [CrossRef]
- Bai, L.; Lennon, D.P.; Caplan, A.I.; DeChant, A.; Hecker, J.; Kranso, J.; Zaremba, A.; Miller, R.H. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat. Neurosci. 2012, 15, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; Giacoppo, S.; Trubiani, O.; Diomede, F.; Piattelli, A.; Bramanti, P.; Mazzon, E. Conditioned medium of periodontal ligament mesenchymal stem cells exert anti-inflammatory effects in lipopolysaccharide-activated mouse motoneurons. Exp. Cell Res. 2016, 349, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Harris, V.K.; Zigelbaum, A.; Goossens, A.M.; Lu, A.; Rosenthal, H.; Sadiq, S.A. Mesenchymal stem cells enhance the engraftment and myelinating ability of allogeneic oligodendrocyte progenitors in dysmyelinated mice. Stem Cells Dev. 2011, 20, 2065–2076. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Wang, T.; Han, C.; Wang, P.; Liu, X.; Zheng, C.; Bi, J.; Zhou, X. IFN-γ-Primed hUCMSCs Significantly Reduced Inflammation via the Foxp3/ROR-γt/STAT3 Signaling Pathway in an Animal Model of Multiple Sclerosis. Front. Immunol. 2022, 13, 835345. [Google Scholar] [CrossRef] [PubMed]
- Singer, N.G.; Caplan, A.I. Mesenchymal stem cells: Mechanisms of inflammation. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 457–478. [Google Scholar] [CrossRef]
- Li, J.-F.; Zhang, D.-J.; Geng, T.; Chen, L.; Huang, H.; Yin, H.-L.; Zhang, Y.-Z.; Lou, J.-Y.; Cao, B.; Wang, Y.-L. The potential of human umbilical cord-derived mesenchymal stem cells as a novel cellular therapy for multiple sclerosis. Cell Transplant. 2014, 23, 113–122. [Google Scholar] [CrossRef]
- Meng, M.; Liu, Y.; Wang, W.; Wei, C.; Liu, F.; Du, Z.; Xie, Y.; Tang, W.; Hou, Z.; Li, Q. Umbilical cord mesenchymal stem cell transplantation in the treatment of multiple sclerosis. Am. J. Transl. Res. 2018, 10, 212–223. [Google Scholar]
- Petrou, P.; Kassis, I.; Ginzberg, A.; Halimi, M.; Yaghmour, N.; Abramsky, O.; Karussis, D. Long-term clinical and immunological effects of repeated mesenchymal stem cell injections in patients with progressive forms of multiple sclerosis. Front. Neurol. 2021, 12, 639315. [Google Scholar] [CrossRef]
- Harris, V.K.; Faroqui, R.; Vyshkina, T.; Sadiq, S.A. Characterization of autologous mesenchymal stem cell-derived neural progenitors as a feasible source of stem cells for central nervous system applications in multiple sclerosis. Stem Cells Transl. Med. 2012, 1, 536–547. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef]
- Alanazi, A.; Alassiri, M.; Jawdat, D.; Almalik, Y. Mesenchymal stem cell therapy: A review of clinical trials for multiple sclerosis. Regen. Ther. 2022, 21, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A.; Imrey, P.B.; Planchon, S.M.; Bermel, R.A.; Fisher, E.; Fox, R.J.; Bar-Or, A.; Sharp, S.L.; Skaramagas, T.T.; Jagodnik, P.; et al. Pilot trial of intravenous autologous culture-expanded mesenchymal stem cell transplantation in multiple sclerosis. Mult. Scler. J. 2018, 24, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhu, L.; Liu, Z.; Wu, J.; Xu, Y.; Zhang, C.J. IV/IT hUC-MSCs Infusion in RRMS and NMO: A 10-Year Follow-Up Study. Front. Neurol. 2020, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.K.; Stark, J.; Vyshkina, T.; Blackshear, L.; Joo, G.; Stefanova, V.; Sara, G.; Sadiq, S.A. Phase I Trial of Intrathecal Mesenchymal Stem Cell-derived Neural Progenitors in Progressive Multiple Sclerosis. EBioMedicine 2018, 29, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.K.; Vyshkina, T.; Sadiq, S.A. Clinical safety of intrathecal administration of mesenchymal stromal cell–derived neural progenitors in multiple sclerosis. Cytotherapy 2016, 18, 1476–1482. [Google Scholar] [CrossRef]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef]
- Le Blanc, K.; Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef]
- Wagner, W.; Bork, S.; Horn, P.; Krunic, D.; Walenda, T.; Diehlmann, A.; Benes, V.; Blake, J.; Huber, F.-X.; Eckstein, V.; et al. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS ONE 2009, 4, e5846. [Google Scholar] [CrossRef]
- Pachón-Peña, G.; Serena, C.; Ejarque, M.; Petriz, J.; Duran, X.; Oliva-Olivera, W.; Simó, R.; Tinahones, F.J.; Fernández-Veledo, S.; Vendrell, J. Obesity Determines the Immunophenotypic Profile and Functional Characteristics of Human Mesenchymal Stem Cells From Adipose Tissue. Stem Cells Transl. Med. 2016, 5, 464–475. [Google Scholar] [CrossRef]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, 9628536. [Google Scholar] [CrossRef] [PubMed]
- Eggenhofer, E.; Luk, F.; Dahlke, M.H.; Hoogduijn, M.J. The Life and Fate of Mesenchymal Stem Cells. Front. Immunol. 2014, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, A.; Dabrowska, S.; Nowak, B.; Walczak, P.; Lukomska, B.; Janowski, M. Mesenchymal stem cells injected into carotid artery to target focal brain injury home to perivascular space. Theranostics 2020, 10, 6615–6628. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Park, S.-N.; Suh, H. Adipose tissue engineering using mesenchymal stem cells attached to injectable PLGA spheres. Biomaterials 2005, 26, 5855–5863. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gao, L.-N.; An, Y.; Hu, C.-H.; Jin, F.; Zhou, J.; Jin, Y.; Chen, F.-M. Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials 2013, 34, 7033–7047. [Google Scholar] [CrossRef]
- Ducret, M.; Farges, J.C.; Pasdeloup, M.; Perrier-Groult, E.; Mueller, A.; Mallein-Gerin, F.; Fabre, H. Phenotypic Identification of Dental Pulp Mesenchymal Stem/Stromal Cells Subpopulations with Multiparametric Flow Cytometry. Odontogenesis Methods Protoc. 2019, 1922, 77–90. [Google Scholar]
- Pogozhykh, O.; Pogozhykh, D.; Neehus, A.-L.; Hoffmann, A.; Blasczyk, R.; Müller, T. Molecular and cellular characteristics of human and non-human primate multipotent stromal cells from the amnion and bone marrow during long term culture. Stem Cell Res. Ther. 2015, 6, 150. [Google Scholar] [CrossRef][Green Version]
- Roura, S.; Farré, J.; Soler-Botija, C.; Llach, A.; Hove-Madsen, L.; Cairó, J.J.; Gòdia, F.; Cinca, J.; Bayes-Genis, A. Effect of aging on the pluripotential capacity of human CD105+ mesenchymal stem cells. Eur. J. Heart Fail. 2006, 8, 555–563. [Google Scholar] [CrossRef]
- Holan, V.; Trosan, P.; Cejka, C.; Javorkova, E.; Zajicova, A.; Hermankova, B.; Chudickova, M.; Cejkova, J. A Comparative Study of the Therapeutic Potential of Mesenchymal Stem Cells and Limbal Epithelial Stem Cells for Ocular Surface Reconstruction. Stem Cells Transl. Med. 2015, 4, 1052–1063. [Google Scholar] [CrossRef]
- Sarkar, D.; Spencer, J.A.; Phillips, J.A.; Zhao, W.; Schäfer, S.; Spelke, D.S.; Mortensen, L.M.; Ruiz, J.P.; Vemula, P.K.; Sridharan, R.; et al. Engineered cell homing. Blood 2011, 118, 184–191. [Google Scholar] [CrossRef]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Ries, C.; Egea, V.; Karow, M.; Kolb, H.; Jochum, M.; Neth, P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: Differential regulation by inflammatory cytokines. Blood 2007, 109, 4055–4063. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Tammik, L.; Sundberg, B.; Haynesworth, S.E.; Ringdén, O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand. J. Immunol. 2003, 57, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, W.J.C.; Ploemacher, R.E. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 2003, 17, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Pallarès, V.; Unzueta, U.; Falgàs, A.; Sánchez-García, L.; Serna, N.; Gallardo, A.; Morris, G.A.; Alba-Castellón, L.; Álamo, P.; Sierra, J.; et al. An Auristatin nanoconjugate targeting CXCR4+ leukemic cells blocks acute myeloid leukemia dissemination. J. Hematol. Oncol. 2020, 13, 36. [Google Scholar] [CrossRef]
- Won, Y.-W.; Patel, A.N.; Bull, D.A. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials 2014, 35, 5627–5635. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Z.; Guo, J.; Ni, A.; Deb, A.; Zhang, L.; Mirotsou, M.; Pratt, R.E.; Dzau, V.J. Genetic modification of mesenchymal stem cells overexpressing ccr1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ. Res. 2010, 106, 1753–1762. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, W.; Chen, X.; Lian, Y.; Wang, J.; Cai, C.; Huang, L.; Wang, T.; Ren, J.; Xiang, A.P. CXCR5-Overexpressing Mesenchymal Stromal Cells Exhibit Enhanced Homing and Can Decrease Contact Hypersensitivity. Mol. Ther. 2017, 25, 1434–1447. [Google Scholar] [CrossRef][Green Version]
- Robles, J.D.; Liu, Y.P.; Cao, J.; Xiang, Z.; Cai, Y.; Manio, M.; Tang, E.H.; Chan, G.C.-F. Immunosuppressive mechanisms of human bone marrow derived mesenchymal stromal cells in BALB/c host graft versus host disease murine models. Exp. Hematol. Oncol. 2015, 4, 13. [Google Scholar] [CrossRef]
- Li, H.; Jiang, Y.; Jiang, X.; Guo, X.; Ning, H.; Li, Y.; Liao, L.; Yao, H.; Wang, X.; Liu, Y.; et al. CCR7 guides migration of mesenchymal stem cell to secondary lymphoid organs: A novel approach to separate GvHD from GvL effect. Stem Cells 2014, 32, 1890–1903. [Google Scholar] [CrossRef]
- Abramowski, P.; Krasemann, S.; Ernst, T.; Lange, C.; Ittrich, H.; Schweizer, M.; Zander, A.R.; Martin, R.; Fehse, B. Mesenchymal Stromal/Stem Cells Do Not Ameliorate Experimental Autoimmune Encephalomyelitis and Are Not Detectable in the Central Nervous System of Transplanted Mice. Stem Cells Dev. 2016, 25, 1134–1148. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.J.; Guttman, M.A.; Raman, V.K.; Peters, D.C.; Pessanha, B.S.; Hill, J.M.; Smith, S.; Scott, G.; McVeigh, E.R.; Lederman, R.J. Magnetic resonance fluoroscopy allows targeted delivery of mesenchymal stem cells to infarct borders in swine. Circulation 2003, 108, 2899–2904. [Google Scholar] [CrossRef] [PubMed]
- Augello, A.; Tasso, R.; Negrini, S.M.; Cancedda, R.; Pennesi, G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007, 56, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef]
- Chan, J.L.; Tang, K.C.; Patel, A.P.; Bonilla, L.M.; Pierobon, N.; Ponzio, N.M.; Rameshwar, P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood 2006, 107, 4817–4824. [Google Scholar] [CrossRef]
- Joswig, A.J.; Mitchell, A.; Cummings, K.J.; Levine, G.J.; Gregory, C.A.; Smith, R.; Watts, A.E. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res. Ther. 2017, 8, 42. [Google Scholar] [CrossRef]
- Skrahin, A.; Ahmed, R.K.; Ferrara, G.; Rane, L.; Poiret, T.; Isaikina, Y.; Skrahina, A.; Zumla, A.; Maeurer, M.J. Autologous mesenchymal stromal cell infusion as adjunct treatment in patients with multidrug and extensively drug-resistant tuberculosis: An open-label phase 1 safety trial. Lancet Respir. Med. 2014, 2, 108–122. [Google Scholar] [CrossRef]
- Giuliani, M.; Fleury, M.; Vernochet, A.; Ketroussi, F.; Clay, D.; Azzarone, B.; Lataillade, J.J.; Durrbach, A. Long-lasting inhibitory effects of fetal liver mesenchymal stem cells on T-lymphocyte proliferation. PLoS ONE 2011, 6, e19988. [Google Scholar] [CrossRef]
- Li, X.-Y.; Ding, J.; Zheng, Z.-H.; Li, X.-Y.; Wu, Z.-B.; Zhu, P. Long-term culture in vitro impairs the immunosuppressive activity of mesenchymal stem cells on T cells. Mol. Med. Rep. 2012, 6, 1183–1189. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, L.; Wei, Y.; Yu, H.; Zou, L.; Huo, J.; Yang, H.; Song, B.; Wei, T.; Wu, D.; et al. Systematic comparison of hUC-MSCs at various passages reveals the variations of signatures and therapeutic effect on acute graft-versus-host disease. Stem Cell Res. Ther. 2019, 10, 354. [Google Scholar] [CrossRef]
- Ryan, A.E.; Lohan, P.; O’Flynn, L.; Treacy, O.; Chen, X.; Coleman, C.; Shaw, G.; Murphy, M.; Barry, F.; Griffin, M.D.; et al. Chondrogenic differentiation increases antidonor immune response to allogeneic mesenchymal stem cell transplantation. Mol. Ther. 2014, 22, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Karussis, D.; Karageorgiou, C.; Vaknin-Dembinsky, A.; Gowda-Kurkalli, B.; Gomori, J.M.; Kassis, I.; Bulte, J.W.; Petrou, P.; Ben-Hur, T.; Abramsky, O.; et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 2010, 67, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J.; Canadian critical care trials group. safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yi, H.; Song, Y. The safety of MSC therapy over the past 15 years: A meta-analysis. Stem Cell Res. Ther. 2021, 12, 545. [Google Scholar] [CrossRef] [PubMed]


| NCT | MSCs | Phase | Enrollment | Summary |
|---|---|---|---|---|
| NCT02239393 | Autologous | 2 | 31 | Efficacy is assessed by counting the number of GELs on MRI scans. |
| NCT04823000 | Autologous | 1&2 | 24 | Evaluates the safety and tolerability of MSCs repeated treatment. |
| NCT01854957 | Autologous | 1&2 | 20 | Efficacy and safety are assessed by counting the number of GELs on MRI scans. |
| NCT04749667 | Autologous | 1&2 | 18 | Efficacy is assessed by measuring neurophysiological parameters. |
| NCT01228266 | Autologous | 2 | 9 | Efficacy is assessed by clinical variables, MRI, OCT, immunological analysis, and quality of life scales. |
| NCT03778333 | Autologous | 1 | 7 | Evaluates changes in EDSS. |
| NCT00813969 | Autologous | 1 | 24 | Evaluates safety and tolerability by measuring the number of Gd-enhancing brain MRI lesions. |
| NCT01377870 | Autologous | 1&2 | 22 | Efficacy is assessed by MRI, quality of life scales, and RAO test. |
| NCT03799718 | Autologous | 2 | 23 | Assesses safety and efficacy using the T25FW and 9-HPT tests. |
| NCT00395200 | Autologous-BM | 1&2 | 10 | Assessment of visual function following injection. |
| NCT00781872 | Autologous-BM | 1&2 | 24 | Evaluates changes in EDSS, the proportion of T-regs and activated cells, and the proliferation ability of lymphocytes. |
| NCT02403947 | Autologous-BM | 1&2 | 1 | Evaluates safety by monitoring for adverse effects and efficacy through MRI of GELs. |
| NCT01895439 | Autologous-BM | 1&2 | 13 | Evaluates safety by monitoring for adverse effects and efficacy through MRI and ophthalmological tests. |
| NCT01745783 | Autologous-BM | 1&2 | 26 | Evaluates safety by monitoring for adverse effects and efficacy through MRI tests. |
| NCT02495766 | Autologous-BM | 1&2 | 8 | Evaluates safety and efficacy by measuring the number of Gd-enhancing brain MRI lesions and EDSS changes. |
| NCT02035514 | Autologous-BM | 1&2 | 9 | Evaluates safety by monitoring for adverse effects and efficacy through MRI tests. |
| NCT02326935 | Autologous-AD | 1 | 2 | Evaluates safety by monitoring for adverse effects and efficacy through MSIS tests. |
| NCT01730547 | Autologous-AD | 1&2 | 2 | Evaluates safety by monitoring for adverse effects and efficacy through MRI and clinical tests. |
| NCT05116540 | Autologous-AD | 2 | 24 | Evaluates changes in EDSS and quality of life. |
| NCT01056471 | Autologous-AD | 1&2 | 30 | Efficacy is assessed by clinical variables, MRI, neurophysiological and immunological analysis, and quality of life scales. |
| NCT01933802 | Autologous-NP | 1 | 20 | Evaluates safety and efficiency by experimental tests like MRI. |
| NCT06360861 | Allogenic-UC | 1 | 5 | Evaluates changes in EDSS. |
| NCT03326505 | Allogenic-UC | 1&2 | 60 | Safety and efficacy assessment pre- and post-treatment. |
| NCT01364246 | Allogenic-UC | 1&2 | 20 | Evaluates safety and efficiency by experimental tests like MRI. |
| NCT05532943 | Allogenic-UC | 1&2 | 41 | Evaluates changes in EDSS and quality of life. |
| NCT05003388 | Allogenic-UC | 1 | 15 | Evaluates safety by monitoring for adverse effects and efficacy through EDSS tests. |
| NCT02587715 | Allogenic-UC | 1&2 | 69 | Evaluates efficacy through EDSS and MRI tests. |
| NCT02418325 | Allogenic-UC | 1&2 | 69 | Evaluates efficacy through EDSS and MRI tests. |
| NCT04956744 | hESC | 1 | 30 | Assesses the safety and tolerability by monitoring for any adverse effects. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadfar, S.; Yazdanpanah, E.; Pazoki, A.; Nemati, M.H.; Eslami, M.; Haghmorad, D.; Oksenych, V. The Role of Mesenchymal Stem Cells in Modulating Adaptive Immune Responses in Multiple Sclerosis. Cells 2024, 13, 1556. https://doi.org/10.3390/cells13181556
Dadfar S, Yazdanpanah E, Pazoki A, Nemati MH, Eslami M, Haghmorad D, Oksenych V. The Role of Mesenchymal Stem Cells in Modulating Adaptive Immune Responses in Multiple Sclerosis. Cells. 2024; 13(18):1556. https://doi.org/10.3390/cells13181556
Chicago/Turabian StyleDadfar, Sepehr, Esmaeil Yazdanpanah, Alireza Pazoki, Mohammad Hossein Nemati, Majid Eslami, Dariush Haghmorad, and Valentyn Oksenych. 2024. "The Role of Mesenchymal Stem Cells in Modulating Adaptive Immune Responses in Multiple Sclerosis" Cells 13, no. 18: 1556. https://doi.org/10.3390/cells13181556
APA StyleDadfar, S., Yazdanpanah, E., Pazoki, A., Nemati, M. H., Eslami, M., Haghmorad, D., & Oksenych, V. (2024). The Role of Mesenchymal Stem Cells in Modulating Adaptive Immune Responses in Multiple Sclerosis. Cells, 13(18), 1556. https://doi.org/10.3390/cells13181556








