Potential Therapeutic Interventions Targeting NAD+ Metabolism for ALS
Abstract
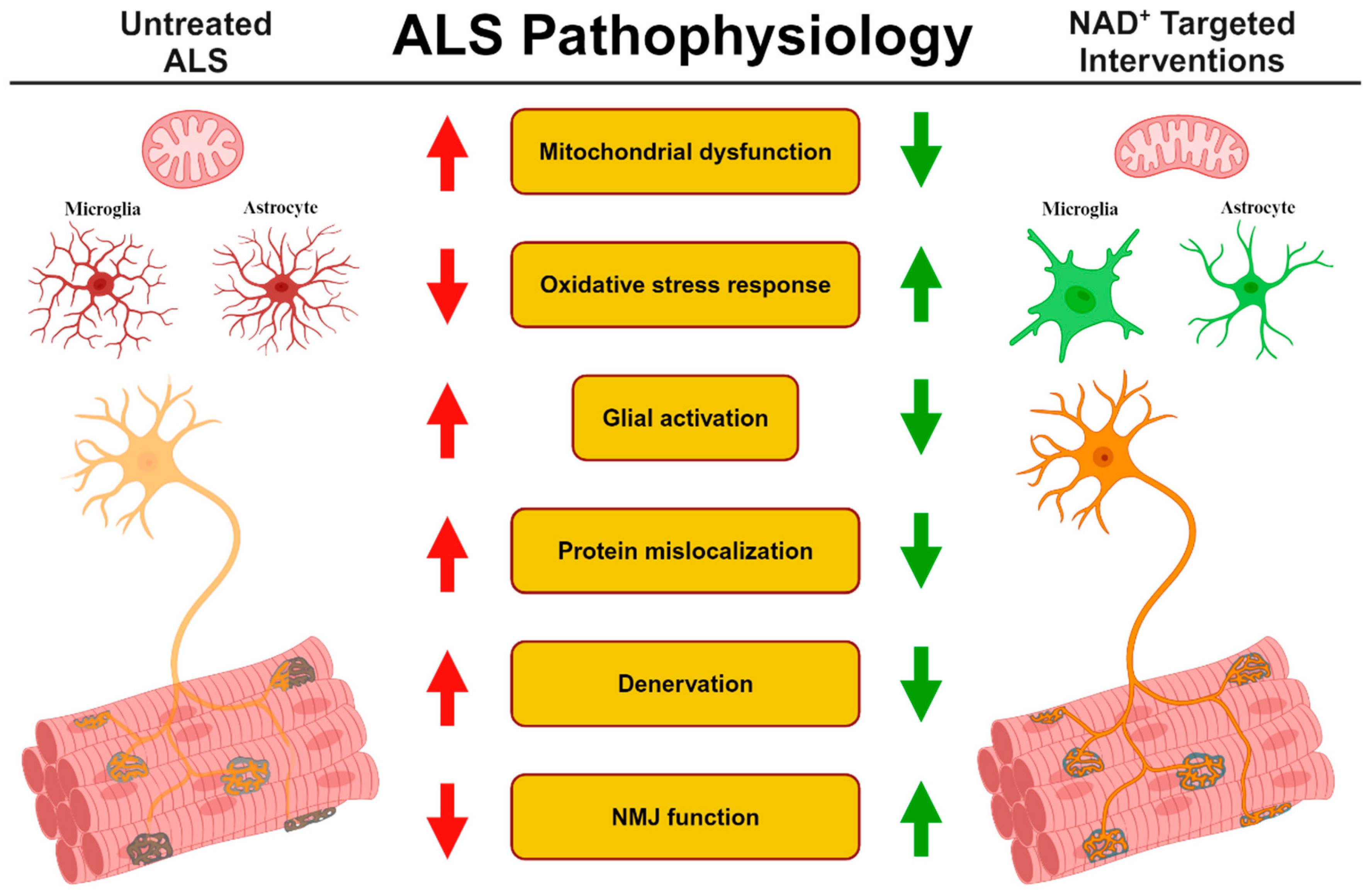
1. Introduction
2. NADase Activity and Cellular Function
3. ALS and NAD+ Metabolism
4. Therapeutic Interventions of ALS by NAD+ Precursors
| Model | Cell Type | Treatment | Dose | Duration | Benefits | Ref. |
|---|---|---|---|---|---|---|
| Sporadic | SC-MN | NAD+ | 10 mM | 14 days | Increased neurite length | [95] |
| TDP-43 KD | SC-MN | NAM | 0.1 mM | 6 days | Axon outgrowth and increased protein synthesis | [96] |
| SOD1G93A | VSC 4.1 cells | Resveratrol | 10 µM | 2 days | Improved survival; increased ATP, MFN2 and PGC-1α | [97] |
| SOD1G93A | Cortical neurons | Resveratrol | 250 nM | 2 days | Reduced SOD1G93A toxicity | [98] |
| Sporadic | hiPSC MN | NAM | 0.5 mM | 7 days | Improved survival; increased mitochondrial NAD+ and respiration; higher CHOP and sXBP1 expression | [99] |
| C12 | 5 µM | |||||
| SOD1G93A | SC-astrocytes | NR | 5 mM | 1 day | Increased NAD+; decreased astrocyte toxicity; reduced mitochondrial ROS production; higher HMOX1 and SRXN1 expression | [90,91] |
| NMN | 5 mM | |||||
| TDP-43A315T | CS-MN | NMN | 1 µM | 3 days | Increased axon length and neurite intersections and improved mitochondrial ultrastructure | [93] |
| SOD1G93A | SC-MN | NMN | 2 mM | 2 days | Elevated GSH and NAD+, increased neurite length/complexity; improved survival;higher nuclear/cytoplasmic TDP-43 ratio | [94] |
| SOD1D90A | hiPSC MN | |||||
| wtTDP-43 OE | SC-MN |
| Model | Route | Treatment | Dose | Duration | Benefits | Ref. |
|---|---|---|---|---|---|---|
| SOD1G93A | Sub-cutaneouspump | NAM | 7.4 mg/kg BW/day | 12 weeks | Longer rotarod retention; lower neurological score; increased NAM in CSF | [82] |
| SOD1G93A | i.p | P7C3 | 20 mg/kg BW/day | 7 weeks | Improved rotarod retention and stride length and reduced MN loss | [92] |
| SOD1G93A | Diet | Resveratrol | 160 mg/kg BW/day | 8 weeks | Faster treadmill walking speed; increased lifespan and MN survival; higher CMAP and MEP amplitude; decreased p53 acetylation | [103] |
| SOD1G93A | Diet | NR | 400 mg/kg BW/day | 9 weeks | Improved body weight and grip strength; decreased Cxcl10/Ccl5/Ptgs2/Tnf; reduced Chrna1 and Uchl1 | [85] |
| SOD1G93A | Drinking water | NR | 400 mg/kg BW/day | 10 weeks | Increased Vimentin+ and DCX+ neurons | [104] |
| SOD1G93A | Oral gavage and diet | NR (w/ and w/o PT/NAC) | 185 mg/kg BW/day | 14 weeks | Prolonged survival; higher nerve conduction amplitude and velocity; increased NAD+; decreased TNF-α/IL2/IL6 and mitochondrial Ca2+ | [77] |
| FUSS57Δ | Growth media | 3-AB | 20 µM | 9 days | Decreased axonal breaks, slower paralysis onset | [105] |
| SOD1G93A | Olaparib | 500 nM | ||||
| TDP-43A315T | Veliparib | 1 µM | ||||
| SOD1G93A | Oral gavage and diet | NR (w/ PT+Ibu) | 185 mg/kg BW/day | 14 weeks | Longer rotarod retention and lifespan; improved MN survival; reduced TNF-α/IFNγ/IL1-β in CSF | [89] |
| FUSR521C | ||||||
| SOD1G93A | Diet | NMN | 400 mg/kg BW/day | 10 weeks | Improved evoked and spontaneous EPP amplitude and increased NMJ plasticity and morphology | [76] |
5. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Taylor, J.P.; Brown, R.H., Jr.; Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Zufiria, M.; Gil-Bea, F.J.; Fernandez-Torron, R.; Poza, J.J.; Munoz-Blanco, J.L.; Rojas-Garcia, R.; Riancho, J.; Lopez de Munain, A. ALS: A bucket of genes, environment, metabolism and unknown ingredients. Prog. Neurobiol. 2016, 142, 104–129. [Google Scholar] [CrossRef]
- Mejzini, R.; Flynn, L.L.; Pitout, I.L.; Fletcher, S.; Wilton, S.D.; Akkari, P.A. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front. Neurosci. 2019, 13, 1310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, X.; Huo, Z.; Chen, Y.; Liu, J.; Zhao, Z.; Meng, F.; Su, Q.; Bao, W.; Zhang, L.; et al. The Impact of Mitochondrial Dysfunction in Amyotrophic Lateral Sclerosis. Cells 2022, 11, 2049. [Google Scholar] [CrossRef] [PubMed]
- Petri, S.; Korner, S.; Kiaei, M. Nrf2/ARE Signaling Pathway: Key Mediator in Oxidative Stress and Potential Therapeutic Target in ALS. Neurol. Res. Int. 2012, 2012, 878030. [Google Scholar] [CrossRef]
- Evans, C.S.; Holzbaur, E.L.F. Autophagy and mitophagy in ALS. Neurobiol. Dis. 2019, 122, 35–40. [Google Scholar] [CrossRef]
- Amin, A.; Perera, N.D.; Beart, P.M.; Turner, B.J.; Shabanpoor, F. Amyotrophic Lateral Sclerosis and Autophagy: Dysfunction and Therapeutic Targeting. Cells 2020, 9, 2413. [Google Scholar] [CrossRef]
- Nguyen, D.K.H.; Thombre, R.; Wang, J. Autophagy as a common pathway in amyotrophic lateral sclerosis. Neurosci. Lett. 2019, 697, 34–48. [Google Scholar] [CrossRef]
- Williamson, T.L.; Cleveland, D.W. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat. Neurosci. 1999, 2, 50–56. [Google Scholar] [CrossRef]
- Bilsland, L.G.; Sahai, E.; Kelly, G.; Golding, M.; Greensmith, L.; Schiavo, G. Deficits in axonal transport precede ALS symptoms in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 20523–20528. [Google Scholar] [CrossRef]
- Berth, S.H.; Lloyd, T.E. Disruption of axonal transport in neurodegeneration. J. Clin. Investig. 2023, 133, e168554. [Google Scholar] [CrossRef]
- Katsyuba, E.; Romani, M.; Hofer, D.; Auwerx, J. NAD(+) homeostasis in health and disease. Nat. Metab. 2020, 2, 9–31. [Google Scholar] [CrossRef]
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 2009, 324, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Lundt, S.; Zhang, N.; Li, J.L.; Zhang, Z.; Zhang, L.; Wang, X.; Bao, R.; Cai, F.; Sun, W.; Ge, W.P.; et al. Metabolomic and transcriptional profiling reveals bioenergetic stress and activation of cell death and inflammatory pathways in vivo after neuronal deletion of NAMPT. J. Cereb. Blood Flow Metab. 2021, 41, 2116–2131. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E. NAD(+) in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Katsyuba, E.; Auwerx, J. Modulating NAD(+) metabolism, from bench to bedside. EMBO J. 2017, 36, 2670–2683. [Google Scholar] [CrossRef]
- Mori, V.; Amici, A.; Mazzola, F.; Di Stefano, M.; Conforti, L.; Magni, G.; Ruggieri, S.; Raffaelli, N.; Orsomando, G. Metabolic profiling of alternative NAD biosynthetic routes in mouse tissues. PLoS ONE 2014, 9, e113939. [Google Scholar] [CrossRef]
- Fujigaki, H.; Yamamoto, Y.; Saito, K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Revollo, J.R.; Korner, A.; Mills, K.F.; Satoh, A.; Wang, T.; Garten, A.; Dasgupta, B.; Sasaki, Y.; Wolberger, C.; Townsend, R.R.; et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007, 6, 363–375. [Google Scholar] [CrossRef]
- Luk, T.; Malam, Z.; Marshall, J.C. Pre-B cell colony-enhancing factor (PBEF)/visfatin: A novel mediator of innate immunity. J. Leukoc. Biol. 2008, 83, 804–816. [Google Scholar] [CrossRef]
- Revollo, J.R.; Grimm, A.A.; Imai, S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 2004, 279, 50754–50763. [Google Scholar] [CrossRef]
- Lautrup, S.; Sinclair, D.A.; Mattson, M.P.; Fang, E.F. NAD(+) in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019, 30, 630–655. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, Y.; Wang, T.; Bi, J.; Li, H.; Zhang, L.Q.; Ye, S.Q.; Ding, S. Neuronal protective role of PBEF in a mouse model of cerebral ischemia. J. Cereb. Blood Flow Metab. 2010, 30, 1962–1971. [Google Scholar] [CrossRef]
- Kitani, T.; Okuno, S.; Fujisawa, H. Growth phase-dependent changes in the subcellular localization of pre-B-cell colony-enhancing factor. FEBS Lett. 2003, 544, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.; Zhang, N.; Li, H.; Zhang, L.; Baines, C.P.; Ding, S. Subcellular NAMPT-mediated NAD(+) salvage pathways and their roles in bioenergetics and neuronal protection after ischemic injury. J. Neurochem. 2019, 151, 732–748. [Google Scholar] [CrossRef] [PubMed]
- Berger, F.; Lau, C.; Dahlmann, M.; Ziegler, M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J. Biol. Chem. 2005, 280, 36334–36341. [Google Scholar] [CrossRef]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018, 27, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Baur, J.A.; Imai, S.I. NAD(+) Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.; Kataura, T.; Korsgen, M.E.; Sun, C.; Sarkar, S.; Korolchuk, V.I. The autophagy-NAD axis in longevity and disease. Trends Cell Biol. 2023, 33, 788–802. [Google Scholar] [CrossRef]
- Massudi, H.; Grant, R.; Guillemin, G.J.; Braidy, N. NAD+ metabolism and oxidative stress: The golden nucleotide on a crown of thorns. Redox Rep. 2012, 17, 28–46. [Google Scholar] [CrossRef]
- Waddell, J.; Khatoon, R.; Kristian, T. Cellular and Mitochondrial NAD Homeostasis in Health and Disease. Cells 2023, 12, 1329. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Park, J.H.; Lu, H.C. Axonal energy metabolism, and the effects in aging and neurodegenerative diseases. Mol. Neurodegener. 2023, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Hou, Y.; Lautrup, S.; Jensen, M.B.; Yang, B.; SenGupta, T.; Caponio, D.; Khezri, R.; Demarest, T.G.; Aman, Y.; et al. NAD(+) augmentation restores mitophagy and limits accelerated aging in Werner syndrome. Nat. Commun. 2019, 10, 5284. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Zhou, R.; Xia, B.; Dang, W.; Yang, Z.; Chen, X. NAMPT maintains mitochondria content via NRF2-PPARalpha/AMPKalpha pathway to promote cell survival under oxidative stress. Cell. Signal. 2020, 66, 109496. [Google Scholar] [CrossRef]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; D’Amico, D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R.; et al. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 2016, 352, 1436–1443. [Google Scholar] [CrossRef]
- Yang, S.; Niou, Z.X.; Enriquez, A.; LaMar, J.; Huang, J.Y.; Ling, K.; Jafar-Nejad, P.; Gilley, J.; Coleman, M.P.; Tennessen, J.M.; et al. NMNAT2 supports vesicular glycolysis via NAD homeostasis to fuel fast axonal transport. Mol. Neurodegener. 2024, 19, 13. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD(+) metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef]
- Garten, A.; Schuster, S.; Penke, M.; Gorski, T.; de Giorgis, T.; Kiess, W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat. Rev. Endocrinol. 2015, 11, 535–546. [Google Scholar] [CrossRef]
- McReynolds, M.R.; Chellappa, K.; Chiles, E.; Jankowski, C.; Shen, Y.; Chen, L.; Descamps, H.C.; Mukherjee, S.; Bhat, Y.R.; Lingala, S.R.; et al. NAD(+) flux is maintained in aged mice despite lower tissue concentrations. Cell Syst. 2021, 12, 1160–1172.e1164. [Google Scholar] [CrossRef]
- Camacho-Pereira, J.; Tarrago, M.G.; Chini, C.C.S.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A.; et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef]
- Zhu, X.H.; Lu, M.; Lee, B.Y.; Ugurbil, K.; Chen, W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc. Natl. Acad. Sci. USA 2015, 112, 2876–2881. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.; Wong, M.; Poljak, A.; Sachdev, P.; Braidy, N. The Plasma NAD(+) Metabolome Is Dysregulated in “Normal” Aging. Rejuvenation Res. 2019, 22, 121–130. [Google Scholar] [CrossRef]
- Corpas, R.; Grinan-Ferre, C.; Rodriguez-Farre, E.; Pallas, M.; Sanfeliu, C. Resveratrol Induces Brain Resilience Against Alzheimer Neurodegeneration Through Proteostasis Enhancement. Mol. Neurobiol. 2019, 56, 1502–1516. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Qian, L.; Zhang, J.; Zhang, W.; Morrison, A.; Hayes, P.; Wilson, S.; Chen, T.; Zhao, J. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J. Neurosci. Res. 2011, 89, 1723–1736. [Google Scholar] [CrossRef]
- Zou, X.D.; Guo, S.Q.; Hu, Z.W.; Li, W.L. NAMPT protects against 6-hydroxydopamine-induced neurotoxicity in PC12 cells through modulating SIRT1 activity. Mol. Med. Rep. 2016, 13, 4058–4064. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Ageta-Ishihara, N.; Nagatsu, S.; Takao, K.; Komine, O.; Endo, F.; Miyakawa, T.; Misawa, H.; Takahashi, R.; Kinoshita, M.; et al. SIRT1 overexpression ameliorates a mouse model of SOD1-linked amyotrophic lateral sclerosis via HSF1/HSP70i chaperone system. Mol. Brain 2014, 7, 62. [Google Scholar] [CrossRef]
- Roh, E.; Kim, M.S. Hypothalamic NAD(+)-Sirtuin Axis: Function and Regulation. Biomolecules 2020, 10, 396. [Google Scholar] [CrossRef]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Canto, C.; Mottis, A.; Jo, Y.S.; Viswanathan, M.; Schoonjans, K.; et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 2013, 154, 430–441. [Google Scholar] [CrossRef]
- Satoh, A.; Imai, S.I.; Guarente, L. The brain, sirtuins, and ageing. Nat. Rev. Neurosci. 2017, 18, 362–374. [Google Scholar] [CrossRef]
- Zhao, Y.; Guan, Y.F.; Zhou, X.M.; Li, G.Q.; Li, Z.Y.; Zhou, C.C.; Wang, P.; Miao, C.Y. Regenerative Neurogenesis After Ischemic Stroke Promoted by Nicotinamide Phosphoribosyltransferase-Nicotinamide Adenine Dinucleotide Cascade. Stroke 2015, 46, 1966–1974. [Google Scholar] [CrossRef]
- Fang, E.F.; Lautrup, S.; Hou, Y.; Demarest, T.G.; Croteau, D.L.; Mattson, M.P.; Bohr, V.A. NAD(+) in Aging: Molecular Mechanisms and Translational Implications. Trends Mol. Med. 2017, 23, 899–916. [Google Scholar] [CrossRef]
- Bai, P.; Canto, C. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab. 2012, 16, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ge, P. Parthanatos in the pathogenesis of nervous system diseases. Neuroscience 2020, 449, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, R.; Kraus, W.L. The PARP side of the nucleus: Molecular actions, physiological outcomes, and clinical targets. Mol. Cell 2010, 39, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Zhang, H.; Ropelle, E.R.; Sorrentino, V.; Mazala, D.A.; Mouchiroud, L.; Marshall, P.L.; Campbell, M.D.; Ali, A.S.; Knowels, G.M.; et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci. Transl. Med. 2016, 8, 361ra139. [Google Scholar] [CrossRef]
- Park, J.H.; Long, A.; Owens, K.; Kristian, T. Nicotinamide mononucleotide inhibits post-ischemic NAD(+) degradation and dramatically ameliorates brain damage following global cerebral ischemia. Neurobiol. Dis. 2016, 95, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.M.; Castoldi, A.; Sanin, D.E.; Flachsmann, L.J.; Field, C.S.; Puleston, D.J.; Kyle, R.L.; Patterson, A.E.; Hassler, F.; Buescher, J.M.; et al. Inflammatory macrophage dependence on NAD(+) salvage is a consequence of reactive oxygen species-mediated DNA damage. Nat. Immunol. 2019, 20, 420–432. [Google Scholar] [CrossRef]
- McGurk, L.; Rifai, O.M.; Bonini, N.M. Poly(ADP-Ribosylation) in Age-Related Neurological Disease. Trends Genet. 2019, 35, 601–613. [Google Scholar] [CrossRef]
- Yu, Y.; Fedele, G.; Celardo, I.; Loh, S.H.Y.; Martins, L.M. Parp mutations protect from mitochondrial toxicity in Alzheimer’s disease. Cell Death Dis. 2021, 12, 651. [Google Scholar] [CrossRef]
- De Flora, A.; Zocchi, E.; Guida, L.; Franco, L.; Bruzzone, S. Autocrine and paracrine calcium signaling by the CD38/NAD+/cyclic ADP-ribose system. Ann. N. Y. Acad. Sci. 2004, 1028, 176–191. [Google Scholar] [CrossRef]
- Aksoy, P.; White, T.A.; Thompson, M.; Chini, E.N. Regulation of intracellular levels of NAD: A novel role for CD38. Biochem. Biophys. Res. Commun. 2006, 345, 1386–1392. [Google Scholar] [CrossRef]
- Takaso, Y.; Noda, M.; Hattori, T.; Roboon, J.; Hatano, M.; Sugimoto, H.; Brenner, C.; Yamamoto, Y.; Okamoto, H.; Higashida, H.; et al. Deletion of CD38 and supplementation of NAD(+) attenuate axon degeneration in a mouse facial nerve axotomy model. Sci. Rep. 2020, 10, 17795. [Google Scholar] [CrossRef] [PubMed]
- Langley, M.R.; Choi, C.I.; Peclat, T.R.; Guo, Y.; Simon, W.L.; Yoon, H.; Kleppe, L.; Lucchinetti, C.F.; Chini, C.C.S.; Chini, E.N.; et al. Critical Role of Astrocyte NAD(+) Glycohydrolase in Myelin Injury and Regeneration. J. Neurosci. 2021, 41, 8644–8667. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Hu, H.; Xu, K.; Cao, T.; Wang, Z.; Nie, J.; Zheng, H.; Luo, H.; Wang, F.; Xiong, C.; et al. CD38 Deficiency Protects Mouse Retinal Ganglion Cells Through Activating the NAD+/Sirt1 Pathway in Ischemia-Reperfusion and Optic Nerve Crush Models. Investig. Ophthalmol. Vis. Sci. 2024, 65, 36. [Google Scholar] [CrossRef] [PubMed]
- Essuman, K.; Summers, D.W.; Sasaki, Y.; Mao, X.; DiAntonio, A.; Milbrandt, J. The SARM1 Toll/Interleukin-1 Receptor Domain Possesses Intrinsic NAD(+) Cleavage Activity that Promotes Pathological Axonal Degeneration. Neuron 2017, 93, 1334–1343.e1335. [Google Scholar] [CrossRef]
- Wan, L.; Essuman, K.; Anderson, R.G.; Sasaki, Y.; Monteiro, F.; Chung, E.H.; Osborne Nishimura, E.; DiAntonio, A.; Milbrandt, J.; Dangl, J.L.; et al. TIR domains of plant immune receptors are NAD(+)-cleaving enzymes that promote cell death. Science 2019, 365, 799–803. [Google Scholar] [CrossRef]
- Horsefield, S.; Burdett, H.; Zhang, X.; Manik, M.K.; Shi, Y.; Chen, J.; Qi, T.; Gilley, J.; Lai, J.S.; Rank, M.X.; et al. NAD(+) cleavage activity by animal and plant TIR domains in cell death pathways. Science 2019, 365, 793–799. [Google Scholar] [CrossRef]
- Figley, M.D.; Gu, W.; Nanson, J.D.; Shi, Y.; Sasaki, Y.; Cunnea, K.; Malde, A.K.; Jia, X.; Luo, Z.; Saikot, F.K.; et al. SARM1 is a metabolic sensor activated by an increased NMN/NAD(+) ratio to trigger axon degeneration. Neuron 2021, 109, 1118–1136.e11. [Google Scholar] [CrossRef]
- Sasaki, Y.; Nakagawa, T.; Mao, X.; DiAntonio, A.; Milbrandt, J. NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD(+) depletion. eLife 2016, 5, e19749. [Google Scholar] [CrossRef]
- Gilley, J.; Ribchester, R.R.; Coleman, M.P. Sarm1 Deletion, but Not Wld(S), Confers Lifelong Rescue in a Mouse Model of Severe Axonopathy. Cell Rep. 2017, 21, 10–16. [Google Scholar] [CrossRef]
- Loreto, A.; Hill, C.S.; Hewitt, V.L.; Orsomando, G.; Angeletti, C.; Gilley, J.; Lucci, C.; Sanchez-Martinez, A.; Whitworth, A.J.; Conforti, L.; et al. Mitochondrial impairment activates the Wallerian pathway through depletion of NMNAT2 leading to SARM1-dependent axon degeneration. Neurobiol. Dis. 2020, 134, 104678. [Google Scholar] [CrossRef] [PubMed]
- Bratkowski, M.; Burdett, T.C.; Danao, J.; Wang, X.; Mathur, P.; Gu, W.; Beckstead, J.A.; Talreja, S.; Yang, Y.S.; Danko, G.; et al. Uncompetitive, adduct-forming SARM1 inhibitors are neuroprotective in preclinical models of nerve injury and disease. Neuron 2022, 110, 3711–3726.e16. [Google Scholar] [CrossRef]
- Murata, H.; Khine, C.C.; Nishikawa, A.; Yamamoto, K.I.; Kinoshita, R.; Sakaguchi, M. c-Jun N-terminal kinase (JNK)-mediated phosphorylation of SARM1 regulates NAD(+) cleavage activity to inhibit mitochondrial respiration. J. Biol. Chem. 2018, 293, 18933–18943. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q.; Du, S.; Xiang, L.; Zhou, S.; Zhu, J.; Chen, Z.; Wang, H.; Pan, X.; Fan, Y.; et al. SARM1 Promotes Neurodegeneration and Memory Impairment in Mouse Models of Alzheimer’s Disease. Aging Dis. 2024, 15, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Pieper, A.A.; McKnight, S.L. Benefits of Enhancing Nicotinamide Adenine Dinucleotide Levels in Damaged or Diseased Nerve Cells. Cold Spring Harb. Symp. Quant. Biol. 2018, 83, 207–217. [Google Scholar] [CrossRef]
- Lundt, S.; Zhang, N.; Polo-Parada, L.; Wang, X.; Ding, S. Dietary NMN supplementation enhances motor and NMJ function in ALS. Exp. Neurol. 2024, 374, 114698. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador, R.; Marchio, P.; Lopez-Blanch, R.; Jihad-Jebbar, A.; Rivera, P.; Valles, S.L.; Banacloche, S.; Alcacer, J.; Colomer, N.; et al. Nicotinamide Riboside and Pterostilbene Cooperatively Delay Motor Neuron Failure in ALS SOD1(G93A) Mice. Mol. Neurobiol. 2021, 58, 1345–1371. [Google Scholar] [CrossRef] [PubMed]
- Roderer, P.; Klatt, L.; John, F.; Theis, V.; Winklhofer, K.F.; Theiss, C.; Matschke, V. Increased ROS Level in Spinal Cord of Wobbler Mice due to Nmnat2 Downregulation. Mol. Neurobiol. 2018, 55, 8414–8424. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Bao, R.; Zhang, N.; Wang, Y.; Polo-Parada, L.; Tarim, A.; Alemifar, A.; Han, X.; Wilkins, H.M.; et al. Deletion of Nampt in Projection Neurons of Adult Mice Leads to Motor Dysfunction, Neurodegeneration, and Death. Cell Rep. 2017, 20, 2184–2200. [Google Scholar] [CrossRef]
- Lundt, S.; Ding, S. Non-cell autonomous effect of neuronal nicotinamide phosphoribosyl transferase on the function of neuromuscular junctions. Neural Regen. Res. 2021, 16, 302–303. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Beal, M.F. Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Ann. Neurol. 2001, 49, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Blacher, E.; Bashiardes, S.; Shapiro, H.; Rothschild, D.; Mor, U.; Dori-Bachash, M.; Kleimeyer, C.; Moresi, C.; Harnik, Y.; Zur, M.; et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 2019, 572, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, Y.; Chen, W.; Li, M.; Yang, M.; Shen, Z.; Zhou, Y.; Wang, L.; Wang, H.; Li, S.; et al. Circulating NAD+ Metabolism-Derived Genes Unveils Prognostic and Peripheral Immune Infiltration in Amyotrophic Lateral Sclerosis. Front. Cell Dev. Biol. 2022, 10, 831273. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Cui, L.Y.; Sun, X.H.; Shen, D.C.; Yang, X.Z.; Liu, Q.; Liu, M.S. Alterations in metabolic biomarkers and their potential role in amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2022, 9, 1027–1038. [Google Scholar] [CrossRef]
- Harlan, B.A.; Killoy, K.M.; Pehar, M.; Liu, L.; Auwerx, J.; Vargas, M.R. Evaluation of the NAD(+) biosynthetic pathway in ALS patients and effect of modulating NAD(+) levels in hSOD1-linked ALS mouse models. Exp. Neurol. 2020, 327, 113219. [Google Scholar] [CrossRef]
- McGurk, L.; Mojsilovic-Petrovic, J.; Van Deerlin, V.M.; Shorter, J.; Kalb, R.G.; Lee, V.M.; Trojanowski, J.Q.; Lee, E.B.; Bonini, N.M. Nuclear poly(ADP-ribose) activity is a therapeutic target in amyotrophic lateral sclerosis. Acta Neuropathol. Commun. 2018, 6, 84. [Google Scholar] [CrossRef]
- Gilley, J.; Jackson, O.; Pipis, M.; Estiar, M.A.; Gan-Or, Z.; Goutman, S.A.; Harms, M.B.; Kaye, J.; Lima, L.; Genomics, Q.S.; et al. Enrichment of SARM1 alleles encoding variants with constitutively hyperactive NADase in patients with ALS and other motor nerve disorders. medRxiv 2021. [Google Scholar] [CrossRef]
- Bloom, A.J.; Mao, X.; Strickland, A.; Sasaki, Y.; Milbrandt, J.; DiAntonio, A. Constitutively active SARM1 variants found in ALS patients induce neuropathy. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lopez-Blanch, R.; Salvador-Palmer, R.; Oriol-Caballo, M.; Moreno-Murciano, P.; Dellinger, R.W.; Estrela, J.M.; Obrador, E. Nicotinamide riboside, pterostilbene and ibudilast protect motor neurons and extend survival in ALS mice. Neurotherapeutics 2024, 21, e00301. [Google Scholar] [CrossRef]
- Harlan, B.A.; Pehar, M.; Sharma, D.R.; Beeson, G.; Beeson, C.C.; Vargas, M.R. Enhancing NAD+ Salvage Pathway Reverts the Toxicity of Primary Astrocytes Expressing Amyotrophic Lateral Sclerosis-linked Mutant Superoxide Dismutase 1 (SOD1). J. Biol. Chem. 2016, 291, 10836–10846. [Google Scholar] [CrossRef]
- Harlan, B.A.; Pehar, M.; Killoy, K.M.; Vargas, M.R. Enhanced SIRT6 activity abrogates the neurotoxic phenotype of astrocytes expressing ALS-linked mutant SOD1. FASEB J. 2019, 33, 7084–7091. [Google Scholar] [CrossRef]
- Tesla, R.; Wolf, H.P.; Xu, P.; Drawbridge, J.; Estill, S.J.; Huntington, P.; McDaniel, L.; Knobbe, W.; Burket, A.; Tran, S.; et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2012, 109, 17016–17021. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Gunay, A.; Chandel, N.S.; Ozdinler, P.H. Mitochondrial dysregulation occurs early in ALS motor cortex with TDP-43 pathology and suggests maintaining NAD(+) balance as a therapeutic strategy. Sci. Rep. 2022, 12, 4287. [Google Scholar] [CrossRef]
- Hamilton, H.L.; Akther, M.; Anis, S.; Colwell, C.B.; Vargas, M.R.; Pehar, M. Nicotinamide Adenine Dinucleotide Precursor Supplementation Modulates Neurite Complexity and Survival in Motor Neurons from Amyotrophic Lateral Sclerosis Models. Antioxid. Redox Signal 2024. [Google Scholar] [CrossRef] [PubMed]
- Zwilling, M.; Theiss, C.; Matschke, V. Caffeine and NAD(+) Improve Motor Neural Integrity of Dissociated Wobbler Cells In Vitro. Antioxidants 2020, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Briese, M.; Saal-Bauernschubert, L.; Luningschror, P.; Moradi, M.; Dombert, B.; Surrey, V.; Appenzeller, S.; Deng, C.; Jablonka, S.; Sendtner, M. Loss of Tdp-43 disrupts the axonal transcriptome of motoneurons accompanied by impaired axonal translation and mitochondria function. Acta Neuropathol. Commun. 2020, 8, 116. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Tang, L.; Zhang, N.; Fan, D. Protective effects of resveratrol through the up-regulation of SIRT1 expression in the mutant hSOD1-G93A-bearing motor neuron-like cell culture model of amyotrophic lateral sclerosis. Neurosci. Lett. 2011, 503, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Nguyen, M.D.; Dobbin, M.M.; Fischer, A.; Sananbenesi, F.; Rodgers, J.T.; Delalle, I.; Baur, J.A.; Sui, G.; Armour, S.M.; et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007, 26, 3169–3179. [Google Scholar] [CrossRef]
- Hor, J.H.; Santosa, M.M.; Lim, V.J.W.; Ho, B.X.; Taylor, A.; Khong, Z.J.; Ravits, J.; Fan, Y.; Liou, Y.C.; Soh, B.S.; et al. ALS motor neurons exhibit hallmark metabolic defects that are rescued by SIRT3 activation. Cell Death Differ. 2021, 28, 1379–1397. [Google Scholar] [CrossRef]
- Alhindi, A.; Boehm, I.; Chaytow, H. Small junction, big problems: Neuromuscular junction pathology in mouse models of amyotrophic lateral sclerosis (ALS). J. Anat. 2022, 241, 1089–1107. [Google Scholar] [CrossRef]
- Verma, S.; Khurana, S.; Vats, A.; Sahu, B.; Ganguly, N.K.; Chakraborti, P.; Gourie-Devi, M.; Taneja, V. Neuromuscular Junction Dysfunction in Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2022, 59, 1502–1527. [Google Scholar] [CrossRef]
- Arnold, F.J.; Putka, A.F.; Raychaudhuri, U.; Hsu, S.; Bedlack, R.S.; Bennett, C.L.; La Spada, A.R. Revisiting Glutamate Excitotoxicity in Amyotrophic Lateral Sclerosis and Age-Related Neurodegeneration. Int. J. Mol. Sci. 2024, 25, 5587. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; del Valle, J.; Modol, L.; Martinez, A.; Granado-Serrano, A.B.; Ramirez-Nunez, O.; Pallas, M.; Portero-Otin, M.; Osta, R.; Navarro, X. Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics 2014, 11, 419–432. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhu, L.; Qiu, W.; Liu, Y.; Yang, F.; Chen, W.; Xu, R. Nicotinamide Riboside Enhances Mitochondrial Proteostasis and Adult Neurogenesis through Activation of Mitochondrial Unfolded Protein Response Signaling in the Brain of ALS SOD1(G93A) Mice. Int. J. Biol. Sci. 2020, 16, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Tossing, G.; Livernoche, R.; Maios, C.; Bretonneau, C.; Labarre, A.; Parker, J.A. Genetic and pharmacological PARP inhibition reduces axonal degeneration in C. elegans models of ALS. Hum. Mol. Genet. 2022, 31, 3313–3324. [Google Scholar] [CrossRef] [PubMed]
- de la Rubia, J.E.; Drehmer, E.; Platero, J.L.; Benlloch, M.; Caplliure-Llopis, J.; Villaron-Casales, C.; de Bernardo, N.; AlarcOn, J.; Fuente, C.; Carrera, S.; et al. Efficacy and tolerability of EH301 for amyotrophic lateral sclerosis: A randomized, double-blind, placebo-controlled human pilot study. Amyotroph. Lateral Scler. Front. Degener. 2019, 20, 115–122. [Google Scholar] [CrossRef]
- Hwang, E.S.; Song, S.B. Nicotinamide is an inhibitor of SIRT1 in vitro, but can be a stimulator in cells. Cell. Mol. Life Sci. 2017, 74, 3347–3362. [Google Scholar] [CrossRef]
- Dutta, T.; Kapoor, N.; Mathew, M.; Chakraborty, S.S.; Ward, N.P.; Prieto-Farigua, N.; Falzone, A.; DeLany, J.P.; Smith, S.R.; Coen, P.M.; et al. Source of nicotinamide governs its metabolic fate in cultured cells, mice, and humans. Cell Rep. 2023, 42, 112218. [Google Scholar] [CrossRef]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef]
- McGurk, L.; Gomes, E.; Guo, L.; Mojsilovic-Petrovic, J.; Tran, V.; Kalb, R.G.; Shorter, J.; Bonini, N.M. Poly(ADP-Ribose) Prevents Pathological Phase Separation of TDP-43 by Promoting Liquid Demixing and Stress Granule Localization. Mol. Cell 2018, 71, 703–717.e9. [Google Scholar] [CrossRef]
- Chen, Y.; Guillemin, G.J. Kynurenine pathway metabolites in humans: Disease and healthy States. Int. J. Tryptophan Res. 2009, 2, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Alarcan, H.; Chaumond, R.; Emond, P.; Benz-De Bretagne, I.; Lefèvre, A.; Bakkouche, S.-e.; Veyrat-Durebex, C.; Vourc’h, P.; Andres, C.; Corcia, P.; et al. Some CSF Kynurenine Pathway Intermediates Associated with Disease Evolution in Amyotrophic Lateral Sclerosis. Biomolecules 2021, 11, 691. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lundt, S.; Ding, S. Potential Therapeutic Interventions Targeting NAD+ Metabolism for ALS. Cells 2024, 13, 1509. https://doi.org/10.3390/cells13171509
Lundt S, Ding S. Potential Therapeutic Interventions Targeting NAD+ Metabolism for ALS. Cells. 2024; 13(17):1509. https://doi.org/10.3390/cells13171509
Chicago/Turabian StyleLundt, Samuel, and Shinghua Ding. 2024. "Potential Therapeutic Interventions Targeting NAD+ Metabolism for ALS" Cells 13, no. 17: 1509. https://doi.org/10.3390/cells13171509
APA StyleLundt, S., & Ding, S. (2024). Potential Therapeutic Interventions Targeting NAD+ Metabolism for ALS. Cells, 13(17), 1509. https://doi.org/10.3390/cells13171509





